Abstract
Lorcaserin, a selective 5-hydroxytryptamine2C (5-HT2C) agonist, has been shown to facilitate weight loss in obese populations. It was assessed for its efficacy in reducing nicotine self-administration in young adult female Sprague-Dawley rats. The effect of short-term doses (subcutaneous) on nicotine self-administration (0.03 mg/kg per infusion) with a fixed ratio 1 schedule was assessed in 3-h sessions. Short-term lorcaserin doses (0.3125–20 mg/kg) were administered in a counterbalanced order. Significant reduction of nicotine self-administration was achieved with all of the short-term doses in this range. Tests of lorcaserin on locomotor activity detected prominent sedative effects at doses greater than 1.25 mg/kg with more modest transient effects seen at 0.625 to 1.25 mg/kg. Long-term effects of lorcaserin on locomotor activity were tested with repeated injections with 0.625 mg/kg lorcaserin 10 times over 2 weeks. This low lorcaserin dose did not cause an overall change in locomotor activity relative to that of saline-injected controls. Long-term lorcaserin (0.625 mg/kg) significantly reduced nicotine self-administration over a 2-week period of repeated injections. Long-term lorcaserin at this same dose had no significant effects on food self-administration over the same 2-week period of repeated injections. These studies support development of the 5-HT2C agonist lorcaserin to aid tobacco smoking cessation.
Introduction
A greater diversity of therapies to aid smoking cessation is needed to provide a tool box of treatments that can be used in an adaptive fashion to tailor treatment to the heterogeneous population of addicted smokers. Pharmacotherapy to aid smoking cessation initially focused on nicotinic treatments. Nicotine skin patches, gums, and sprays do provide help in aiding smoking cessation, but the majority of smokers still relapse to smoking after a cessation attempt. Varenicline and bupropion provide two additional treatments, one nicotinic and one nonnicotinic. However, these also are effective only in a minority of smokers. It may be the case that there is no single “magic bullet” that would successfully aid all smokers to their goal of abstinence. A greater diversity of treatments, which are effective in different subpopulations could, as a group, provide effective treatment for the majority of smokers who want to quit.
Nicotinic acetylcholine receptors interact directly with a variety of neurotransmitter systems. However, the interactions are complex, and the functional importance and therapeutic opportunities are not immediately evident. Dopaminergic interactions with nicotine are the best characterized. Nicotine stimulates the release of dopamine, and dopamine has been extensively characterized as being central to the reinforcing effects of drugs including nicotine. However, to date, dopaminergic antagonists have not been found to effectively reduce smoking or promote cessation. On the contrary, the dopaminergic antagonist haloperidol has been found to increase smoking (McEvoy et al., 1995). Nicotine has also been shown to stimulate a variety of other monoaminergic transmitters such as norepinephrine, histamine, and serotonin. Noradrenergic α2-agonist treatment with clonidine has been found in some studies to help with smoking cessation (Glassman et al., 1988). In a recent study, we have found that the histamine H1 antagonist pyrilamine significantly reduces nicotine self-administration in a rat model (Levin et al., 2010).
Serotonin appears to be involved with nicotine effects as well. Serotonergic systems are quite complex with a wide variety of receptor subtypes. We have found that ketanserin, a 5-HT2A and 5-HT2C antagonist, significantly reduces nicotine self-administration in rats (Levin et al., 2008). The relative involvement of serotonin 5-HT2A and 5-HT2C receptors is not clear from this study, but it is known that 5-HT2C receptors located in the ventral tegmental area provide inhibitory influence over dopaminergic projections that are important for drug reinforcement (Bubar and Cunningham, 2007). Lorcaserin is a relatively selective 5-HT2C agonist (Smith et al., 2008; Thomsen et al., 2008; Fletcher et al., 2009). It has been shown to significantly reduce feeding at high doses in rat models (Thomsen et al., 2008) and has been shown in clinical studies to provide a modest effect in potentiating weight loss among obese people (Smith et al., 2009, 2010). An initial study reported efficacy of lorcaserin for reducing nicotine reward in rats (Higgins et al., 2010). The current set of studies determined lorcaserin effects on nicotine self-administration over a wide range of short-term doses and with long-term administration. Also assessed were potential side effects of lorcaserin on locomotor activity and behavior reinforced by food motivation. This project was pursued to determine the role of 5-HT2C receptors in nicotine reinforcement and to explore the possibility of developing lorcaserin or similar 5-HT2C agonists as a novel smoking cessation treatment.
Materials and Methods
Subjects.
Young adult female Sprague-Dawley rats (Taconic Laboratories, Germantown, NY) were given access to intravenous nicotine self-administration. The rats were housed in approved standard laboratory conditions in a Duke University vivarium facility near the testing room to minimize any stress induced by transporting the rats. They were kept on a 12:12 reverse day/night cycle, so that they were in their active phase during behavioral testing. The rats in the drug intravenous self-administration studies were singly housed to prevent them from damaging each other's catheters. All rats were allowed access to water at all times while in their home cages and fed daily approximately 20 to 30 min after completing the sessions. The studies were conducted in accordance with the regulations outlined by the Duke University Animal Care and Use Committee.
Behavioral Procedures.
Solutions of nicotine bitartrate were prepared biweekly in pyrogen-free glassware in sterilized isotonic saline. The dose used for self-administration (0.03 mg/kg per infusion) was calculated as a function of the nicotine base weight. The pH of the solutions was adjusted to 7.0 using NaOH, and then the solutions were passed through a Nalgene filter (Nalge Nunc International, Rochester, NY) for sterilization. Between sessions, all solutions were kept refrigerated in the dark to prevent the decomposition of nicotine.
Rats had catheters surgically implanted into the jugular vein to enable them to receive nicotine infusions. Surgery was performed aseptically. With the rat under general anesthesia (ketamine and medetomidine, 70/0.3 mg/kg i.p.), the jugular vein was tied off distal to the place of cannula insertion. A small V-shaped incision was made in the jugular. A catheter of silicon rubber tubing (Silastic Medical Grade Tubing; Dow Corning Corporation, Midland, MI) was secured into the right jugular vein with cyanoacrylate adhesive so that the tip was just outside the heart. The portion of the cannula external to the vein was sutured to deep muscle and placed subdermally such that it will exit the body of the dorsal surface between the scapulae. Surgical mesh under the skin in this area anchored the cannula. For the 1st week after surgery, the catheter was flushed with sterile saline and heparin (0.1 ml/day with 5 units USP/ml), along with 40 mg/ml gentamicin for an antibiotic. Catheters were flushed daily, before the sessions began, with a 0.3-ml solution containing 100 units/ml heparinized saline (Baxter Health Corporation, Deerfield, IL). When sessions were over, the nicotine remaining in the ports was drawn out and replaced by a 0.25-ml sterile lock consisting of 500 units/ml heparinized saline with 8 mg/ml gentamicin (American Pharmaceutical Partners, Schaumburg, IL).
The rats were trained to self-administer nicotine (0.03 mg/kg per infusion i.v.) via operant lever response (FR1) with a visual secondary reinforcer. For behavioral training, rats were placed in dual-lever operant chambers (30.5 × 24.1 × 21.0 cm) (Med Associates, St. Albans, VT) with one lever being active for causing the delivery of nicotine on an FR1 schedule and the other lever having no consequence. Pressing the lever on the active side resulted in the activation of the feedback tone for 0.5 s and the immediate delivery of one 50-μl infusion of nicotine in less than 1 s with Med Associates syringe pumps (PHM 100 and PHM 103). Each infusion was immediately followed by a 1-min period in which the cue lights went out, the house light came on, and responses were recorded but not reinforced. The operant events and responses were programmed with MED-PC software. Each session lasted for 3 h. In the three nicotine self-administration studies, there were 4 rats that did not reliably self-administer nicotine, 5 rats that did not maintain catheter patency, and 34 rats that completed the study.
Locomotor Activity Assessment.
Another set of rats was tested for short-term and long-term lorcaserin effects on locomotor activity in a figure-eight maze over the course of a 1-h session. The mazes had continuous enclosed alleys (10 × 10 cm) in the shape of a figure eight (Crofton et al., 1991). The overall dimensions of the apparatus were 70 cm long and 42 cm wide, with a 21 × 16 cm central arena, a 20-cm high ceiling, and two blind alleys extending 20 cm from either side. Eight infrared photobeams, which crossed the alleys, indexed locomotor activity. One photobeam was located on each of the two blind alleys, and three were located on each of two loops of the figure-eight maze. The number of photobeam breaks was tallied during the 1-h session. The measure was repeated for 12 5-min blocks in each session.
Lorcaserin Treatment.
After training for nicotine self-administration on an FR1 schedule (0.03 mg/kg per infusion), for five consecutive sessions, lorcaserin treatment began. Lorcaserin was injected (subcutaneous) in a volume of 1 ml/kg 10 min before testing. Lorcaserin was purchased from Trylead Chemical Co., Inc. (Hangzhou, China) by the National Institute on Drug Abuse and identity was confirmed by NMR and liquid chromatography/mass spectrometry. Liquid chromatography/mass spectrometry confirmed that the masses of protonated parent ions found in all test solutions were consistent with the known structure of the compound. The vehicle, physiological saline, was used for control injections. In all the short-term studies 1 or more days elapsed between consecutive injections in the repeated-measures design with doses administered in a counterbalanced order. There were five experiments in the series.
Experiment 1 assessed short-term and long-term lorcaserin effects on nicotine self-administration. The short-term dosing study used a dose range (5, 10, and 20 mg/kg) that has previously been shown to be effective in reducing food self-administration in rats (Thomsen et al., 2008). The rats (n = 7) were administered these doses in a counterbalanced order two times with saline as the control.
Experiment 2 tested a lower dose range of lorcaserin (0.3125, 0.625, 1.25, and 2.5 mg/kg) in another set of rats (n = 8), using the same repeated-measures counterbalanced testing regimen with saline as control given twice as described above.
Experiment 3 assessed short-term lorcaserin effects on locomotor activity. One set of rats (n = 12) received the full range of lorcaserin to assess its effects on locomotor activity using the figure-eight maze. The doses were given in a repeated-measures counterbalanced design given twice.
In experiment 4, another set of rats (n = 10 controls and 10 lorcaserin-treated) was tested for long-term effects of lorcaserin (0.625 mg/kg) on locomotor activity.
Experiment 5 tested the long-term effects on food-motivated responding of the 0.625 mg/kg lorcaserin dose (n = 10 controls and 10 lorcaserin-treated).
Experiment 6 tested the long-term effects of 0.625 mg/kg lorcaserin on nicotine self-administration over 10 sessions (n = 10 controls and n = 9 treated with 0.625 mg/kg lorcaserin).
Data Analysis.
The data were evaluated with analysis of variance using Superanova/Statview (SAS, Cary, NC). In the short-term studies, lorcaserin doses and saline were administered in a repeated measures, counterbalanced design two times. In the long-term studies, lorcaserin was assessed in a between-subjects design with separate rats receiving lorcaserin and saline injections repeatedly over successive sessions. An α level of p < 0.05 (two-tailed) was used as the threshold for significance. In the short-term dose-effect function studies, planned comparisons were made with each dose compared with control. For comparison, these comparisons were also made with the Dunn post hoc test. Significant interactions were followed up by tests of the simple main effects.
Results
Short-Term Lorcaserin Effects on Nicotine Self-Administration.
Short-term doses of lorcaserin were assessed for efficacy in reducing nicotine self-administration (0.03 mg/kg per infusion) with FR1 in 3-h sessions. There were two studies: one for the higher dose range (5, 10, and 20 mg/kg) and one for the lower dose range (0.3125, 0.625, 1.25, and 2.5 mg/kg), with separate sets of rats for each. The lorcaserin doses and vehicle (saline) were administered in a counterbalanced order twice (phase 1 and phase 2).
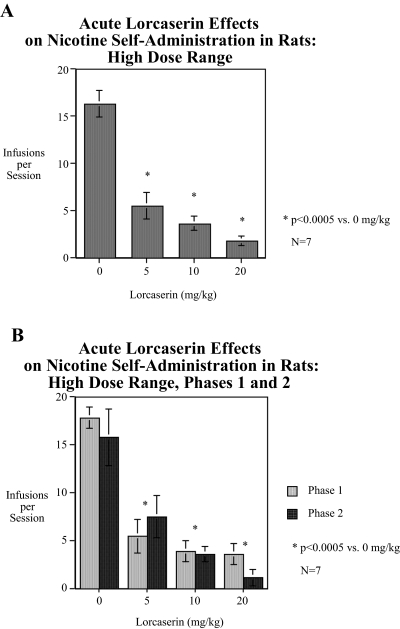
In experiment 1, with the higher dose range, lorcaserin doses caused a significant [F(3,15) = 31.01, p < 0.0005] main effect of decreased nicotine self-administration, reducing nicotine self-administration by more than two-thirds control levels in the average response over the two test phases (Fig. 1A). Each of the three doses caused significant [5 mg/kg, F(1,15) = 42.97, p < 0.0005, Dunn p < 0.005; 10 mg/kg, F(1,15) = 58.74, p < 0.0005, Dunn p < 0.005; and 20 mg/kg, F(1,15) = 76.98, p < 0.0005, Dunn p < 0.005] decreases in nicotine self-administration. The lorcaserin effect did not diminish from the first to the second phase of testing (Fig. 1B). The high dose range of lorcaserin caused a significant [F(1,18) = 57.72, p < 0.0005] linear dose-related reduction in responses on the active lever but had no significant effect on the response to the inactive lever.
Fig. 1.
A, short-term dose-effect function of the higher dose range of lorcaserin on nicotine-self-administration (mean ± S.E.M.). All three of the lorcaserin doses (5, 10, and 20 mg/kg) caused significant (p < 0.0005) decreases in nicotine self-administration relative to controls (n = 7). B, phases 1 and 2 of the short-term dose-effect function of the higher dose range of lorcaserin on nicotine-self-administration (mean ± S.E.M.). No differential effects of lorcaserin were seen in the first and second test phases (n = 7).
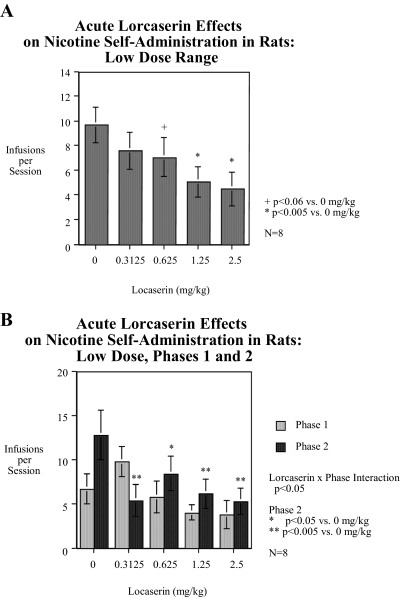
Experiment 2 showed that the lower doses of lorcaserin (0.3125, 0.625, 1.25, and 2.5 mg/kg) also produced a significant reduction in nicotine self-administration. The main effect of lorcaserin was significant [F(4,28) = 5.07, p < 0.005]. As shown in Fig. 2A, averaged across the two phases of the study, the 1.25 and 2.5 mg/kg doses caused clearly significant [1.25 mg/kg, F(1,28) = 12.55, p < 0.005, Dunn p < 0.01 and 2.5 mg/kg, F(1,28) = 15.78, p < 0.0005, Dunn p < 0.005] reductions in nicotine self-administration relative to controls, whereas the 0.625 mg/kg lorcaserin dose caused a nearly significant [F(1,28) = 4.04, p < 0.06, Dunn not significant] reduction in nicotine self-administration. As with the higher dose experiment, this group of rats was tested in two consecutive phases after administration of each of the doses in a counterbalanced repeated measures design. Figure 2B shows the data for the first and second phase. There was a significant [F(4,28) = 3.19, p < 0.05] interaction of test phase and lorcaserin effect. Tests of the simple main effects of lorcaserin in each phase showed significant effects in the second phase in which each of the lorcaserin doses caused significant [0.3125 mg/kg, F(1,28) = 12.06, p < 0.005, Dunn p < 0.05; 0.625 mg/kg, F(1,28) = 4.25, p < 0.05, Dunn not significant; 1.25 mg/kg, F(1,28) = 9.74, p < 0.005, Dunn p < 0.05; and 2.5 mg/kg, F(1,28) = 12.48, p < 0.005, Dunn p < 0.01] reductions in nicotine self-administration, but in not the first phase. The principal effect of phase was that nicotine self-administration increased during the second phase in the control condition with further training, whereas there was no rise in the lorcaserin dose conditions. The low dose range of lorcaserin caused a significant [F(1,28) = 4.34, p < 0.05] linear dose-related reduction in responses on the active lever but had no significant effect on the response to the inactive lever.
Fig. 2.
A, short-term dose-effect function of the lower dose range of lorcaserin on nicotine-self-administration (mean ± S.E.M.). Significant (p < 0.05) decreases in nicotine self-administration were seen with 1.25 and 2.5 mg/kg lorcaserin, whereas 0.625 mg/kg (p < 0.06) just missed a significant decline (n = 8). B, phases 1 and 2 of the short-term dose-effect function of the lower dose range of lorcaserin on nicotine self-administration (mean ± S.E.M.). There was a significant lorcaserin × test phase interaction (p < 0.05). Follow-up tests of the simple main effects showed that in the second phase 0.3125 (p < 0.005), 0.0625 (p < 0.05), 1.25 (p < 0.005), and 2.5 mg/kg (p < 0.005) caused significant decreases in nicotine self-administration relative to control (n = 8).
Short-Term Lorcaserin Effects on Locomotor Activity.
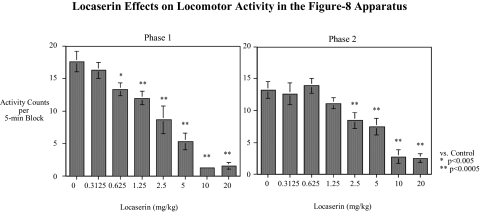
In experiment 3, another cohort of rats (n = 12) was tested for the effects of the complete low- and high-dose ranges of lorcaserin on locomotor activity to investigate possible sedative effects (Fig. 3). The doses were tested in a repeated-measures counterbalanced design twice (test phases 1 and 2). There was a significant [F(7,77) = 3.71, p < 0.005] interaction of lorcaserin × test phase. Tests of the simple main effects showed that in the first phase, 0.625 mg/kg caused significant [F(1,77) = 11.80, p < 0.005, Dunn p < 0.05] slowing, as did all of the higher doses [F(1,77) = 20.21–164.77, p < 0.0005, Dunn p < 0.005]. In the second phase only, doses of 2.5 mg/kg and higher significantly [F(1,77) = 14.54–71.91, p < 0.0005, Dunn p < 0.005] decreased activity.
Fig. 3.
Short-term dose-effect functions of lorcaserin on locomotor activity in the figure-eight apparatus (mean ± S.E.M.) activity counts (photobeam breaks) per 5-min block averaged over the 1-h session (n = 12). There was a significant (p < 0.005) interaction of lorcaserin × test phase. Tests of the simple main effects showed that in the first phase 0.625 mg/kg caused significant (p < 0.005) slowing as did all of the higher doses (p < 0.0005). In the second phase, only doses of 2.5 mg/kg and higher caused significant (p < 0.0005) decreased activity.
Long-Term Lorcaserin Effects on Locomotion.
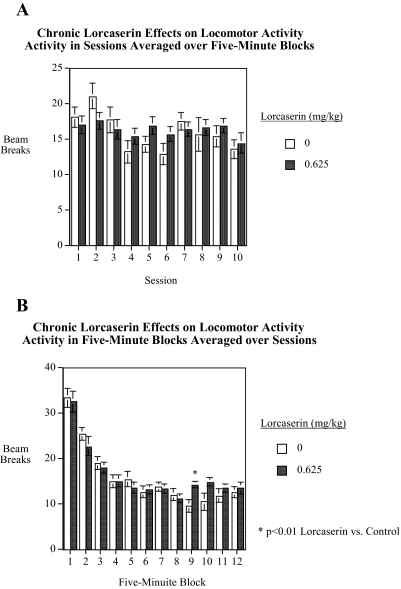
In experiment 4, the effect of long-term administration of 0.625 mg/kg lorcaserin on locomotor activity was tested for 10 sessions over the course of 2 weeks. The main effect of lorcaserin was not significant. The saline-treated controls averaged 15.9 ± 1.2 beam breaks/5-min block, whereas the rats treated with 0.625 mg/kg lorcaserin averaged 16.2 ± 0.9 beam breaks/5-min block. There were significant lorcaserin × session [F(9,162) = 2.36, p < 0.025] and lorcaserin × time block [F(11,198) = 2.77, p < 0.005] interactions. Tests of the effects of lorcaserin at each session did not detect any significant lorcaserin effect at any of the individual sessions (Fig. 4A). As shown in Fig. 4B, tests of the simple main effects of lorcaserin at each of the time blocks within the test session only detected a significant [F(1,18) = 8.59, p < 0.01] lorcaserin effect on the 9th of the 12 5-min session blocks, and at this time there was a lorcaserin-induced (14.2 ± 0.7 beam breaks/5-min block) increase in locomotor speed versus that in vehicle-treated controls (9.6 ± 1.4 beam breaks/5-min block).
Fig. 4.
A, chronic lorcaserin administration effect on locomotor activity over 2 weeks of treatment averaged over 12 5-min blocks (mean ± S.E.M.) (n = 10/treatment group). B, chronic lorcaserin administration effect on locomotor activity averaged over 2 weeks of treatment for each of 12 5-min blocks (mean ± S.E.M.) (n = 10/treatment group). There was a significant (p < 0.005) interaction of lorcaserin and time block (p < 0.01), with elevation in activity with lorcaserin for one time block late in the session.
Long-Term Lorcaserin Effects on Food Self-Administration.
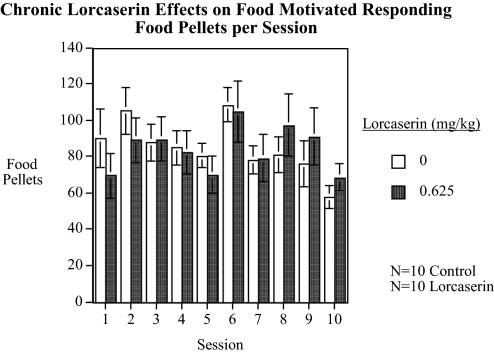
In experiment 5, the same schedule of 10 injections of 0.625 mg/kg lorcaserin over a period of 2 weeks as tested in the locomotor activity apparatus was used to evaluate food-motivated responding in another set of rats (n = 10 controls and n = 10 injected with lorcaserin at a dose of 0.625 mg/kg). Lorcaserin did not significantly affect food-motivated responding. The control group averaged 84.9 ± 8.0 food pellets/session over the 10 sessions, whereas the lorcaserin-treated rats averaged 84.0 ± 10.8 food pellets/session. The more detailed lorcaserin and control data for each session are shown in Fig. 5.
Fig. 5.
Chronic effect of lorcaserin on food-motivated responding (mean ± S.E.M.) (control, n = 10; 0.625 mg/kg lorcaserin, n = 10), showing no significant effects of lorcaserin on food-motivated responding.
Long-Term Lorcaserin Effects on Nicotine Self-Administration.
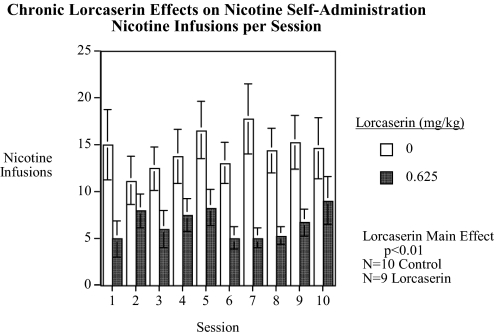
In experiment 6, long-term lorcaserin (0.625 mg/kg) significantly [F(1,17) = 9.17, p < 0.01] reduced nicotine self-administration in a long-term study over a series of 10 injections (Fig. 6). The same series of 10 injections of 0.625 mg/kg lorcaserin as tested in the locomotor activity apparatus and for food self-administration was evaluated in another set of rats (n = 10 controls and n = 9 injected with 0.625 mg/kg lorcaserin). The control group averaged 14.7 ± 2.2 over the 10 sessions, whereas the lorcaserin-treated rats averaged 6.5 ± 1.3, a 55.8% reduction in nicotine self-administration. There was no sign of diminished effectiveness over the course of treatment. Long-term lorcaserin caused a significant [F(1,17) = 4.93, p < 0.05] linear dose-related reduction in responses on the active lever but had no significant effect on the response to the inactive lever.
Fig. 6.
Chronic effect of lorcaserin on nicotine self-administration (mean ± S.E.M.) (control n = 10; 0.625 mg/kg lorcaserin, n = 9), showing a significant (p < 0.025) main effect of lower nicotine self-administration with lorcaserin treatment.
Discussion
Short-term lorcaserin effectively reduced nicotine self-administration over a broad dose range (0.3125–20 mg/kg). The higher end of this dose range caused substantial sedation, but at the lower end of this range (0.3125–0.625 mg/kg), lorcaserin did not cause substantive locomotor hypoactivity. Nonetheless, these doses also caused significant reduction of nicotine self-administration. Long-term studies with a benchmark dose of 0.625 mg/kg lorcaserin showed that this dose significantly reduced nicotine self-administration. This was not merely due to sedation inasmuch as this long-term dose did not cause a significant reduction in locomotor activity, and it was not due to generalized reduction in all motivated behavior inasmuch as there was not an effect seen with food-motivated responding. These studies have identified a dose range of lorcaserin that effectively reduces nicotine self-administration without undue side effects of sedation or generalized reduction in motivated behavior.
The higher dose range of lorcaserin (5–20 mg/kg) was initially selected for investigation for effects on nicotine self-administration because this dose range had previously been found to significantly reduce food consumption (Thomsen et al., 2008). All of the doses in this range significantly reduced nicotine self-administration; however, they all also caused pronounced hypoactivity. Thus, effects on nicotine self-administration and food self-administration may have been secondary to sedative effects. Therefore, we then tested the effects of a lower range of lorcaserin on nicotine self-administration. This dose range, 0.3125 to 2.5 mg/kg, extended from a dose (0.3125 mg/kg), which did not cause any hint of locomotor hypoactivity to doses 1.25 to 2.5 mg/kg, which caused clear hypoactive effects, but not as profound as those caused by 5 to 20 mg/kg lorcaserin. The dose of 0.625 mg/kg lorcaserin was selected for long-term studies because it was clearly above threshold for significant reduction of nicotine self-administration in the short-term study and had very modest and transient effects of reducing locomotor activity. In the chronic test it did not have any effect in producing hypoactivity.
Long-term lorcaserin, at a dose of 0.625 mg/kg, significantly lowered nicotine self-administration over 10 sessions of repeated administration, with no sign of attenuation of effect. Long-term lorcaserin lowered nicotine self-administration to approximately 50% of the control rate. This same dose of 0.625 mg/kg given to another set of rats for 10 sessions had no discernible effect on food-motivated responding.
Serotonergic systems play key roles in actions of nicotine, and a serotonergic approach may hold promise as a novel avenue for smoking cessation treatment. We previously showed in rats that ketanserin, a 5-HT2A and 5-HT2C antagonist, significantly reduces nicotine self-administration (Levin et al., 2008). The differential role of 5-HT2A versus 5-HT2C receptors was not clear from that study. 5-HT2C receptors have been shown to be present in the ventral tegmental area, where they exert inhibition over the dopaminergic neurons, which play a key role for drug reinforcement (Bubar and Cunningham, 2007). Lorcaserin, a selective 5-HT2C agonist (Smith et al., 2008; Thomsen et al., 2008; Fletcher et al., 2009), has shown efficacy in reducing nicotine reward in rats (Higgins et al., 2010) and was clearly effective in reducing nicotine self-administration with both short-term and long-term administration in the current study.
These results demonstrate that 5-HT2C agonist treatment holds promise for development of a new treatment for aiding smoking cessation. The model of reducing ad libitum nicotine self-administration is a good one for vetting compounds for use during the prequit date period. Rose et al. (2006) have found that people who react to nicotine treatment by significantly reducing ad libitum smoking before the quit date have a substantially higher successful cessation rates after the quit date.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant P50-DA027840].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183525.
- 5-HT
- 5-hydroxytryptamine
- FR
- fixed ratio.
Authorship Contributions
Participated in research design: Levin, Johnson, and Rose.
Conducted experiments: Johnson, Slade, Wells, Cauley, and Petro.
Performed data analysis: Levin.
Wrote or contributed to the writing of the manuscript: Levin, Johnson, and Rose.
References
- Bubar MJ, Cunningham KA. (2007) Distribution of serotonin 5-HT2c receptors in the ventral tegmental area. Neuroscience 146:286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Howard JL, Moser VC, Gill MW, Reiter LW, Tilson HA, MacPhail RC. (1991) Interlaboratory comparison of motor activity experiments: implications for neurotoxicological assessments. Neurotoxicol Teratol 13:599–609 [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. (2009) Characterizing the effects of 5-HT2C receptor ligands on motor activity and feeding behaviour in 5-HT2C receptor knockout mice. Neuropharmacology 57:259–267 [DOI] [PubMed] [Google Scholar]
- Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS. (1988) Heavy smokers, smoking cessation, and clonidine: results of a double-blind, randomized trial. JAMA 259:2863–2866 [PubMed] [Google Scholar]
- Higgins GA, Silenieks L, Rizos Z, Noble K, Fletcher PJ. (2010) The selective 5-HT2C receptor agonist, lorcaserin, reduces indices of nicotine reward as well as food intake in the rat, in Proceedings of the 40th Annual Meeting of the Society for Neuroscience; 2010 Nov 13–17; San Diego, CA Abstract 168.19, Society for Neuroscience, Washington, DC [Google Scholar]
- Levin ED, Pruitt M, Cousins V, Slade S, Wells C, Cauley M, Hampton D, Rose JE. (2010) Histamine H1 antagonist treatment with pyrilamine reduces nicotine self-administration in rats. Eur J Pharmacol 212:551–558 [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Johnson M, Petro A, Horton K, Williams P, Rezvani AH, Rose JE. (2008) Ketanserin, a 5-HT2 receptor antagonist, decreases nicotine self-administration in rats. Eur J Pharmacol 600:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, Levin ED, Rose JE. (1995) Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology 119:124–126 [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Kukovich P. (2006) Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res 8:89–101 [DOI] [PubMed] [Google Scholar]
- Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, et al. (2008) Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem 51:305–313 [DOI] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR, and APD356–004 Study Group (2009) Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity 17:494–503 [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, and Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group (2010) Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 363:245–256 [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, et al. (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587 [DOI] [PubMed] [Google Scholar]