Abstract
The most common cause of inherited mental retardation, fragile X syndrome, results from a triplet repeat expansion in the FMR1 gene and loss of the mRNA binding protein, fragile X mental retardation protein (FMRP). In the absence of FMRP, signaling through group I metabotropic glutamate receptors (mGluRs) is enhanced. We previously proposed a mechanism whereby the audiogenic seizures exhibited by FMR1 null mice result from an imbalance in excitatory mGluR and inhibitory GABAB receptor (GABABR) signaling (Mol Pharmacol 76:18–24, 2009). Here, we tested the mGluR5-positive allosteric modulator 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB), the mGluR5 inverse agonist 2-methyl-6-(phenylethynyl)pyridine (MPEP), and GABAB receptor agonists, alone and in combination on receptor protein expression and audiogenic seizures in FMR1 mice. Single doses of MPEP (30 mg/kg), the GABABR orthosteric agonist R-baclofen (1 mg/kg), or the GABABR-positive allosteric modulator N,N′-dicyclopentyl-2-(methylthio)-5-nitro-4,6-pyrimidine diamine (GS-39783) (30 mg/kg), reduced the incidence of seizures. However, when administered subchronically (daily injections for 6 days), MPEP retained its anticonvulsant activity, whereas R-baclofen and GS-39783 did not. When administered at lower doses that had no effect when given alone, a single injection of MPEP plus R-baclofen also reduced seizures, but the effect was lost after subchronic administration. We were surprised to find that subchronic treatment with R-baclofen also induced tolerance to a single high dose of MPEP. These data demonstrate that tolerance develops rapidly to the antiseizure properties of R-baclofen alone and R-baclofen coadministered with MPEP, but not with MPEP alone. Our findings suggest that cross-talk between the G-protein signaling pathways of these receptors affects drug efficacy after repeated treatment.
Introduction
Fragile X syndrome (FXS) is caused by the expansion of a CGG triplet repeat in the 5′ untranslated region of the FMR1 gene, which induces silencing of gene expression and the absence of the mRNA-binding protein fragile X mental retardation protein (FMRP). The “mGluR Theory of Fragile X” (Bear et al., 2004) postulates that, in the absence of FMRP, protein translation downstream of group I metabotropic glutamate receptors (mGluRs) is abnormal, leading to enhanced mGluR signaling, which contributes to many of the phenotypes associated with FXS. The mGluR theory also suggests that reducing group I mGluR (mGluR1 and mGluR5) signaling should alleviate some of the symptoms of FXS (Bear, 2005). Indeed, treatment with mGluR5 antagonists has been shown to reduce seizures and hyperactivity in FMR1 KO mice (Yan et al., 2005), restore dendritic spine defects in cultured neurons lacking FMRP (de Vrij et al., 2008), and correct behavioral deficits in FMR1 null flies (McBride et al., 2005). Genetic reduction of mGluR5 expression in mice also rescues several FXS phenotypes including abnormal ocular dominance plasticity, increased protein synthesis, seizures, and spine defects (Dölen et al., 2007).
Although much focus has been placed on group I mGluRs, evidence for changes in the GABAergic system in FXS has emerged, and both group I mGluR antagonists and GABAB receptor agonists are being tested in clinical trials of FXS (see Hampson et al., 2011 for a review). GABA exerts its effects through two distinct families of receptors. The ionotropic GABAA receptors are pentameric chloride channels that mediate fast inhibition through controlling the movement of chloride across the cell membrane, whereas metabotropic GABAB receptors are heteromeric G protein-coupled receptors that require dimerization of the R1 and R2 subunits to form fully functional receptors. GABAB receptors are localized presynaptically or postsynaptically where they mediate slow inhibition through Giα-mediated inhibition of adenylyl cyclase and/or activation of inwardly rectifying K+ channels. Presynaptic GABAB receptors also regulate the release of glutamate (Vigot et al., 2006).
Decreased GABAA receptor expression and function has been demonstrated in FMR1 knockout mice (El Idrissi et al., 2005; D'Hulst et al., 2006, 2009; Curia et al., 2009; Adusei et al., 2010; Olmos-Serrano et al., 2010). We have also recently demonstrated that GABABR1 expression is down-regulated in the forebrains of immature and mature FMR1 mice compared with wild-type mice (Pacey et al., 2011). This observation is particularly interesting because GABAB receptor expression has also been reported to be reduced in the brains of persons with autism, a disorder related to FXS (Fatemi et al., 2009). However, other studies conducted on FMR1 mice showed increased sensitivity to the locomotor suppressing effects of the orthosteric GABAB receptor agonist R/S-baclofen (Zupan and Toth, 2008), and treatment with R-baclofen prevents audiogenic seizures in these mice (Pacey et al., 2009). In addition, increased GABABR signaling is at least partially responsible for the rescue of audiogenic seizures in FMR1 mice lacking regulator of G protein signaling 4, a protein that normally inhibits GABABR signaling (Pacey et al., 2009, 2011). Finally, drugs that increase GABA signaling rescued diet-induced glutamate lethality in FMR1 null flies (Chang et al., 2008). Taken together, these findings point to the GABAergic system as a promising pharmacological target for treating FXS.
The purpose of the present study was 3-fold. The first goal was to investigate the effectiveness of a combination of (2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) and R-baclofen at low doses that did not block seizures in FMR1 mice when given individually. The second objective was to examine both receptor subunit expression and seizure susceptibility after subchronic administration (6 days) of MPEP, R-baclofen, and combinations of the two drugs. The third objective was to examine the effects of the mGluR5-positive allosteric modulator 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) and the GABAB receptor-positive allosteric modulator N,N′-dicyclopentyl-2-(methylthio)-5-nitro-4,6-pyrimidine diamine (GS-39783) on audiogenic seizures. We found that the combination of a low dose of MPEP together with a low dose of R-baclofen was effective in suppressing seizures in FMR1 mice after a single treatment, but that tolerance to the antiseizure effects of GABAB receptor agonists and a combination of GABAB receptor agonist plus MPEP became apparent after short-term treatment. These findings have implications for the treatment of FXS and possibly for related conditions such as autism spectrum disorders.
Materials and Methods
Animals.
All animal experiments were carried out in accordance with the guidelines set out by the Canadian Council on Animal Care and approved by the University of Toronto Animal Care Committee. FMR1 knockout mice (backcrossed >10 generations on the C57BL/6 background) were generously provided by Dr. William Greenough (University of Illinois, Urbana, IL) and bred at the University of Toronto. All mice were the offspring of homozygous pairings.
Audiogenic Seizure Testing and Drug Injections.
Audiogenic seizure testing was carried out as described previously (Pacey et al., 2009). In brief, FMR1 knockout mice (27–30 days old) were placed individually into the testing apparatus and allowed to explore for 2 min, after which the 135-db bell was rung for 2 min. Animals were tested only once. Seizure testing was carried out between 1:00 and 6:00 PM. Seizure activity was observed and scored using a seizure severity score as follows: wild running, 1; clonic seizure, 2; tonic seizure, 3; status epilepticus/respiratory arrest/death, 4 (Musumeci et al., 2000). For the data analyses the animals that obtained a score of 0 or 1 were classified as “no seizures,” whereas animals with a score of 2 or more were considered as having seizures. Seizure incidence for drug-injected animals was compared with the appropriate vehicle, and Fisher's exact test was used for statistical analysis of seizure susceptibility incidence.
For drug injection studies, an intraperitoneal or subcutaneous injection of drug or vehicle (0.1 ml/10g body weight) was administered 60 min before seizure testing (acute treatment) or daily for 6 days beginning on postnatal day (PND) 25 (subchronic treatment) with the final injection 60 min before testing on PND30. For subchronic administration, mice were injected between 2:00 and 4:00 PM daily. The drugs and vehicles were as follows: R-baclofen (Tocris Bioscience, Ellisville, MO); MPEP (FRAXA Foundation, Newburyport, MA); (3-aminopropyl) (cyclohexylmethyl)phosphinic acid (CGP46381;Tocris Bioscience) in 20% β-cyclodextrin (Sigma-Aldrich, St. Louis, MO); GS-39783 (Tocris Bioscience) suspended in 10% polyethylene glycol (PEG) 200/15% DMSO/75% sterile saline; and CDPPB (Tocris Bioscience) suspended in 50% DMSO/50% sterile saline.
Western Blotting.
Immediately after seizure testing, mice were euthanized with an overdose of ketamine/xylazine, and the brains were removed and frozen on dry ice. Quantitative Western blotting was performed on whole forebrain homogenates as described previously (Adusei et al., 2010). The following antibodies were used: mouse anti-GABABR1 (clone NR3A/49; 1:250; NeuroMab, University of California, Davis, CA/National Institutes of Health, Bethesda, MD); mouse anti-GABABR2 (clone N81/2; 1:750; NeuroMab, University of California, Davis/National Institutes of Health); rabbit anti-mGluR5 (1:500; Millipore Bioscience Research Reagents, Temecula, CA); mouse anti-GAPDH antibody (1:40,000–1:100,000; Sigma-Aldrich); and a goat anti-mouse (The Jackson Laboratory, Bar Harbor, ME) or goat anti-rabbit (Thermo Fisher Scientific, Waltham, MA) horseradish peroxidase-conjugated secondary antibody. The immunoreactive proteins were visualized using the FluorChem MultiImage Light Cabinet (Alpha Innotech, San Leandro, CA). Densitometric analysis was carried out using AlphaEaseFC software (Alpha Innotech). The intensity of the band of interest was normalized relative to the GAPDH band intensity. Protein expression in drug-treated animals is presented as a percentage of expression in vehicle controls. An unpaired Student's t test was used to determine statistical significance.
Results
Acute Administration of MPEP, R-Baclofen, or GS-39783 Reduced Seizures in FMR1 Mice.
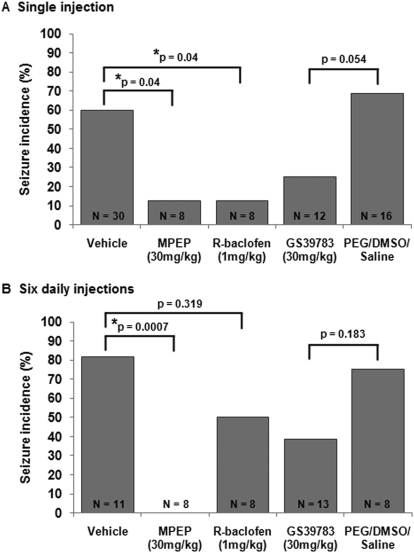
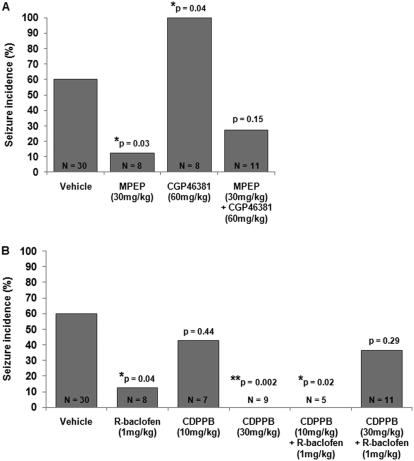
FMR1 knockout mice are susceptible to audiogenic seizures (Chen and Toth, 2001). Because the incidence of audiogenic seizures is very low (∼5%) in wild-type C57BL/6 mice (Pacey et al., 2009), only FMR1 knockout mice were used in this study. Single injections of MPEP (Yan et al., 2005) and R-baclofen (Pacey et al., 2009) have been previously shown to reduce the incidence of audiogenic seizures in FMR1 mice. To confirm these results, FMR1 mice were treated with 30 mg/kg MPEP or 1 mg/kg R-baclofen 60 min before seizure testing (Fig. 1A). Vehicle-treated FMR1 mice had a seizure incidence of 60%. Both 30 mg/kg MPEP and 1 mg/kg R-baclofen significantly reduced seizure incidence to 13% (p = 0.03 compared with vehicle). The GABAB receptor-positive allosteric modulator GS-39783 was also tested for its antiseizure capabilities. GS-39783 (30 mg/kg) reduced seizures in FMR1 mice to 25% compared with vehicle controls (10% PEG/15% DMSO/75% saline), which had a seizure rate of 69%, although this difference did not quite reach statistical significance (p = 0.054; Fig. 1A).
Fig. 1.
The effects of single and repeated doses of MPEP, R-baclofen, and GS-39783 on audiogenic seizures in FMR1 mice. A, when administered as single doses 60 min before testing MPEP (30 mg/kg) and R-baclofen (1 mg/kg) significantly reduced seizure susceptibility in FMR1 mice. The GABAB receptor-positive allosteric modulator GS-39783 produced a nonsignificant reduction in seizure incidence. B, FMR1 mice were injected with vehicle, MPEP, R-baclofen, or GS-39783 daily for 6 days starting on PND25 and tested for audiogenic seizures 60 min after the final injection on PND30. After subchronic treatment, MPEP retained anticonvulsant activity, whereas R-baclofen and GS-39783 did not.
FMR1 Mice Develop Tolerance to R-Baclofen and GS-39783 but Not MPEP after Repeated Administration.
Next, we determined whether these drugs could maintain their anticonvulsant activities after subchronic administration. FMR1 mice were injected with MPEP (30 mg/kg), R-baclofen (1 mg/kg), GS39783 (30 mg/kg), or vehicle daily for 6 days beginning on PND25. Seizure testing was carried out 60 min after the final injection on PND30. After subchronic administration, 82% of vehicle-treated mice had seizures (note that the seizure incidence in the vehicle group was consistently higher after subchronic administration compared with a single injection), whereas none of the eight animals treated with 30 mg/kg MPEP had seizures (p = 0.0007; Fig. 1B). This indicated that MPEP retained its anticonvulsant activity after subchronic administration. In contrast, the anticonvulsant efficacy of R-baclofen and GS-39783 was reduced after subchronic administration compared with single drug injections (Fig. 1B), with 50 and 38% of animals exhibiting seizures, respectively, compared with controls after six daily injections (p = 0.32 and 0.18, respectively, compared with vehicle).
Acute Administration of a Combination of Low-Dose MPEP and R-Baclofen Prevents Seizures in FMR1 KO Mice.
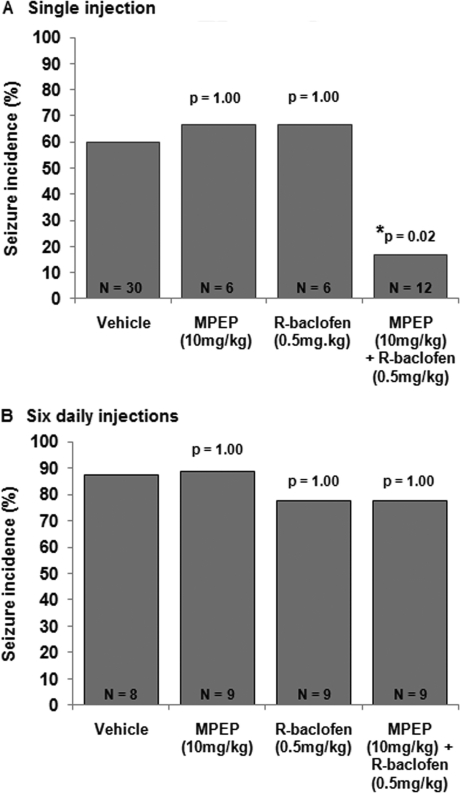
Given that targeting mGluR5 or GABAB receptors alone can alleviate seizures in FMR1 mice, we postulated that treating mice with combined low doses of MPEP and R-baclofen should also reduce seizures and using low doses of both drugs might prevent the tolerance seen with the high dose of R-baclofen. Treatment with 10 mg/kg MPEP alone or 0.5 mg/kg R-baclofen alone did not significantly affect the incidence of audiogenic seizures compared with vehicle controls (67% seizures for MPEP or R-baclofen alone; 60% seizures for vehicle; Fig. 2A). However, when animals were treated simultaneously with a combined dose of 10 mg/kg MPEP plus 0.5 mg/kg R-baclofen the incidence of seizures dropped to 17% (p = 0.03 compared with vehicle). This finding demonstrates that low doses of MPEP and R-baclofen that are not anticonvulsant when administered alone can significantly reduce seizure activity when administered in combination. This finding supports the theory that an imbalance in mGluR and GABAB receptor signaling contributes to audiogenic seizures in FMR1 null mice.
Fig. 2.
The effects of low doses of MPEP and R-baclofen on seizures. A, a single dose of 10 mg/kg MPEP or 0.5 mg/kg R-baclofen had no effect on seizure incidence in FMR1 mice. However, when administered in combination, the drugs significantly reduced the incidence of seizures. B, when administered daily for 6 days, 10 mg/kg MPEP or 0.5 mg/kg R-baclofen alone did not block seizures. A combination of 10 mg/kg MPEP and 0.5 mg/kg R-baclofen also failed to alleviate seizures after subchronic treatment.
FMR1 Mice Develop Tolerance to Combined Low-Dose MPEP Plus R-Baclofen after Repeated Administration.
We also tested whether the combination of 10 mg/kg MPEP plus 0.5 mg/kg R-baclofen maintained its anticonvulsant activity after repeated administrations (Fig. 2B). Similar to a single injection, six daily injections of either 10 mg/kg MPEP or 0.5 mg/kg R-baclofen alone did not affect seizure incidence with 89 and 78% of animals exhibiting seizures, respectively, compared with 88% of vehicle controls (p = 1.0 and 1.0, respectively). We were surprised to find that the combination of 10 mg/kg MPEP plus 0.5 mg/kg R-baclofen no longer prevented seizures after repeated injections, with 78% of animals exhibiting seizures (p = 1.0).
mGluR5 and GABAB Receptor Expression after Repeated Administration of MPEP, R-Baclofen, and GS-39783.
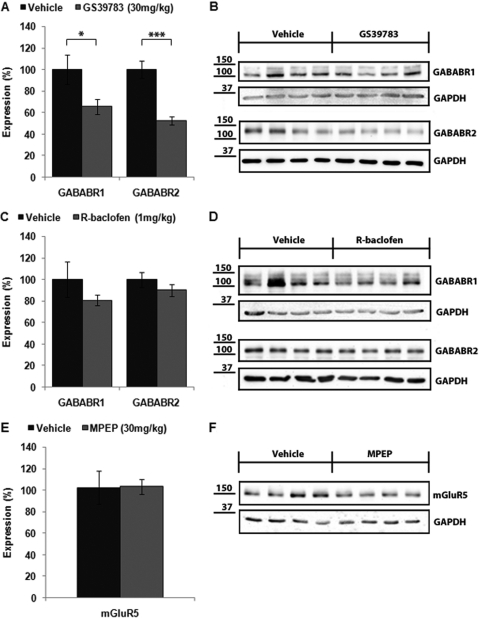
mGluR5 and GABAB receptor subunit expression was examined in the forebrains of mice treated subchronically with 30 mg/kg MPEP, 1 mg/kg R-baclofen, or 30 mg/kg GS-39783. Western blot analysis indicated that there was no difference in mGluR5 expression in the brains of FMR1 mice treated subchronically with 30 mg/kg MPEP compared with vehicle controls (103.4 ± 7.0% of vehicle; p > 0.05; Fig. 3, E and F). Subchronic treatment with R-baclofen induced a small, nonsignificant decrease in GABABR1 (80.4 ± 4.7% of vehicle; p > 0.05) and GABABR2 (90.2 ± 5.6%; p > 0.05; Fig. 3, C and D) compared with vehicle controls. It is noteworthy that mice treated with GS-39783 displayed a dramatic reduction in GABAB receptor expression in the forebrain compared with vehicle-treated mice (GABABR1, 65.7 ± 6.9% of vehicle, p < 0.05; GABABR2, 52.6 ± 3.6%, p < 0.001; Fig. 3, A and B). An analysis of R-baclofen- and GS-39783-treated animals was performed to determine whether GABAB receptor expression might correlate with seizure incidence. However, there was no significant difference in GABABR1 or R2 expression in the brains of mice that had seizures compared with those that did not have seizures after treatment with either R-baclofen or GS-39783 (data not shown).
Fig. 3.
mGluR5 and GABAB receptor expression after subchronic drug treatment. FMR1 mice were injected with drug daily for 6 days starting on PND25, and the expression of GABABR1, R2 and mGluR5 in forebrain was examined by quantitative Western blotting after audiogenic seizure testing on PND30. For each condition, six to eight mice were analyzed. A, FMR1 mice injected with the GABAB-positive allosteric modulator GS-39783 (30 mg/kg) for 6 days showed a significant reduction in GABABR1 and GABABR2 expression in the forebrain. *, p < 0.05, ***, p < 0.001. C, no differences in GABABR1 or GABABR2 expression were observed after subchronic R-baclofen (1 mg/kg) injection. E, mGluR5 protein levels were not altered in mice injected subchronically with MPEP (30 mg/kg) compared with vehicle. B, D, and F, representative Western blots of GABABR1, GABABR2, and mGluR5 expression after subchronic drug treatment.
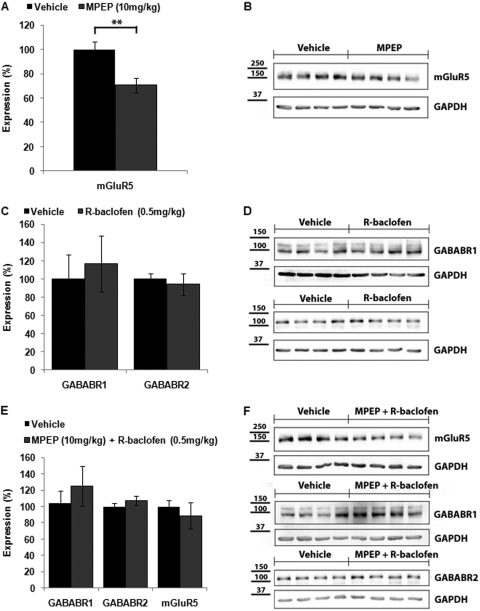
mGluR5 and GABAB receptor expression was also examined in the forebrains of mice treated subchronically with low doses of MPEP (10 mg/kg) and R-baclofen (0.5 mg/kg) alone and in combination. Western blot analysis demonstrated no difference in GABAB receptor expression after treatment with low-dose R-baclofen (Fig. 4, C and D; R1 expression 116.7 ± 30.7% of vehicle; R2 expression 94.0 ± 11.8% of vehicle; p > 0.05 for both). We were surprised to find that treatment with the low-dose MPEP induced a significant reduction in mGluR5 expression after subchronic treatment (Fig. 4, A and B; 70.7 ± 6.1% of vehicle, p < 0.01). Although this result was unexpected given that MPEP is an mGluR5 inverse agonist, it is consistent with the findings of Cowen et al. (2005) who demonstrated a decrease in mGluR5 expression in the olfactory tubercle of rats treated for 12 days with the structurally similar mGluR5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP). As observed with subchronic treatment with low-dose MPEP alone, subchronic treatment with low-dose MPEP plus R-baclofen also reduced expression of mGluR5, although in this case the reduction was not statistically significant (88.8 ± 15.9%; p > 0.05). The combination treatment also did not produce significant changes in GABAB R1 or R2 subunit expression compared with vehicle-treated mice (Fig. 4, E and F; R1 expression 125.2 ± 24.6% of vehicle; R2 expression 107.0 ± 5.8% of vehicle; p > 0.05 for both).
Fig. 4.
Expression of GABABR1, GABABR2, and mGluR5 in forebrain of FMR1 mice injected subchronically (daily for 6 days) with a low dose of MPEP (10 mg/kg), R-baclofen (0.5 mg/kg), or combined MPEP + R-baclofen. For each condition, six to eight mice were analyzed. A, FMR1 mice injected subchronically with low-dose MPEP showed a significant decrease in mGluR5 expression compared with vehicle. **, p < 0.01. C, GABABR1 and GABABR2 expression were not significantly altered after subchronic administration of low-dose R-baclofen. E, no significant differences in GABABR1, GABABR2, or mGluR5 expression were detected after injection with combined low doses of MPEP plus R-baclofen. B, D, and F, representative Western blots of mGluR5, GABABR1, and GABABR2 expression after subchronic drug treatment.
Acute and Subchronic Administration of the mGluR5-Positive Modulator CDPPB.
Based on our theory that audiogenic seizures result from an imbalance in group I mGluR and GABAB receptor signaling, we predicted that reducing GABAB receptor signaling might negate the anticonvulsant effect of MPEP. To test this, FMR1 mice were treated with 30 mg/kg MPEP and 60 mg/kg of the GABAB antagonist CGP46381 60 min before seizure testing (Fig. 5A). Treatment with CGP46381 alone exacerbated seizures in FMR1 mice (100% seizures with CGP46381; p = 0.039 versus vehicle), whereas the seizure incidence after treatment with MPEP plus CGP46381 was not significantly different from vehicle (27% seizure incidence; p = 0.15 compared with vehicle), indicating that CGP46381 reduced the effectiveness of MPEP in alleviating audiogenic seizures.
Fig. 5.
A, the GABAB antagonist CGP46381 dramatically increased seizure incidence in FMR1 knockout mice and seizure incidence after treatment with CGP46381 plus MPEP was not different from vehicle, indicating that CGP46381 partially blocked the anticonvulsant effect of MPEP. B, treatment with a high dose (30 mg/kg) of the mGluR5-positive allosteric modulator CDPPB completely blocked seizures, whereas a lower dose (10 mg/kg) had no effect on seizure incidence in FMR1 mice. Treatment with 10 mg/kg CDPPB plus 1 mg/kg R-baclofen blocked seizures, whereas treatment with 30 mg/kg CDPPB plus 1 mg/kg R-baclofen produced no significant difference in seizure incidence compared with vehicle; this indicates that a high dose of CDPPB partially blocked the anticonvulsant effects of R-baclofen in FMR1 mice.
We also tested whether treatment with the mGluR5-positive allosteric modulator CDPPB could counteract the anticonvulsant effects of R-baclofen. It is noteworthy that treatment with 30 mg/kg CDPPB alone did not exacerbate seizure activity but instead completely blocked seizures (0% seizures with CDPPB; p = 0.002 versus vehicle; Fig. 5B). The seizure incidence after the combined treatment with 30 mg/kg CDPPB and 1 mg/kg R-baclofen (37%) was not different from vehicle (p = 0.29), indicating that the combination of a mGluR5-positive modulator plus GABAB agonist was less effective in preventing seizures than either drug administered alone.
A lower dose of CDPPB (10 mg/kg) administered alone had no effect on seizure incidence (43% seizure incidence; p = 0.44 compared with vehicle), whereas 10 mg/kg CDPPB administered with 1 mg/kg R-baclofen significantly reduced the incidence of seizures (0%) compared with vehicle (p = 0.02; Fig. 5B).
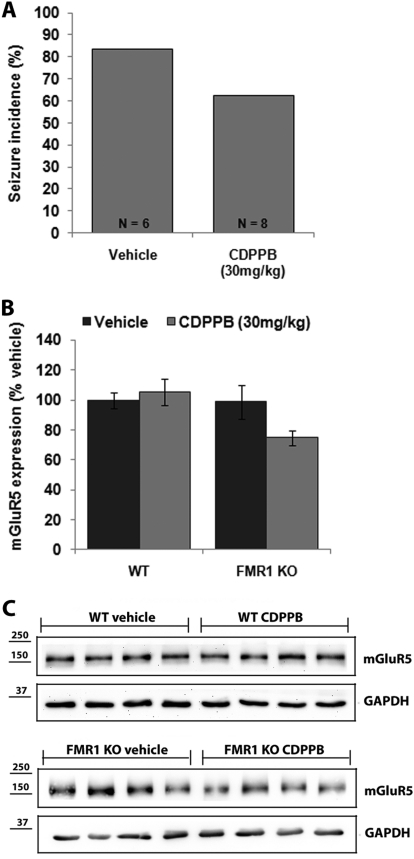
We were intrigued by the finding that a high dose of the mGluR5-positive allosteric modulator CDPPB blocked seizures in FMR1 KO mice when administered acutely. To determine whether this effect persisted after repeated administrations, we treated FMR1 mice for 6 days with 30 mg/kg CDPPB and tested for seizures 1 h after the final dose. After subchronic treatment, 63% of the mice had seizures, which was not different from vehicle (83% seizure incidence; p = 0.58, Fig. 6A); this indicated that tolerance developed to the anticonvulsant effects of CDPPB after 6 days of administration. The levels of mGluR5 protein in the forebrains of mice injected subchronically with CDPPB was measured by quantitative Western blot (Fig. 6, B and C); there was a trend toward decreased mGluR5 expression in FMR1 mice (74.7 ± 4.8% of vehicle; p = 0.056). It is noteworthy that subchronic injection of vehicle or 30 mg/kg CDPPB did not induce seizures in wild-type mice (data not shown) and no changes in mGluR5 expression were detected in the forebrains of CDPPB-injected wild-type animals compared with vehicle controls (105.5 ± 9.0% of vehicle; p = 0.62; Fig. 6, B and C).
Fig. 6.
The effects of subchronic administration of the mGluR5-positive allosteric modulator CDPPB on seizures and expression of mGluR5. A, subchronic treatment for 6 days (PND25–30) with 30 mg/kg CDPPB did not block audiogenic seizures in FMR1 knockout mice (p > 0.58). B, after subchronic treatment with CDPPB expression of mGluR5 was decreased (p = 0.056) in FMR1 knockout but not wild-type (WT; p = 0.62) mice. C, representative Western blots of mGluR5 expression and the GAPDH loading control in the forebrains of wild-type (top) and FMR1 (bottom) mice.
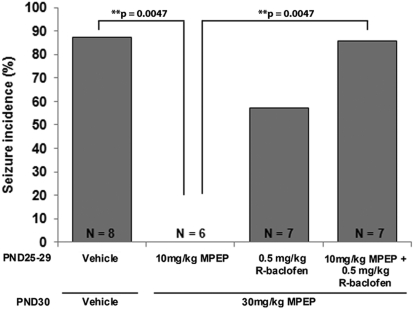
Subchronic Administration of Low-Dose R-Baclofen Modifies the Response of FMR1 Mice to a High Dose of MPEP.
In a final series of experiments, mice were treated for 5 days (PND25–29) with low doses of MPEP (10 mg/kg) or R-baclofen (0.5 mg/kg), alone or in combination, followed by a single high dose of MPEP (30 mg/kg) 1 h before testing on PND30. In mice pretreated with only the low-dose MPEP, a single high dose of MPEP still blocked seizures (0% seizure incidence versus 88% in vehicle-treated mice; p = 0.005; Fig. 7). Unexpectedly, in mice that were pretreated with a combination of low-dose MPEP and low-dose R-baclofen for 5 days a single injection of 30 mg/kg MPEP was no longer effective in preventing seizures (86% seizure incidence; p = 1.00 versus vehicle). Moreover, pretreatment for 5 days with low-dose R-baclofen alone also produced tolerance to the antiseizure effects of high-dose MPEP (57% seizure incidence; p = 0.28 versus vehicle). These results indicate that subchronic treatment with a low dose of R-baclofen can modulate the response to MPEP, suggesting cross-talk between the GABAB and mGluR5 pathways.
Fig. 7.
The anticonvulsant effects of subchronic treatment with low doses of MPEP and/or R-baclofen followed by high-dose MPEP. FMR1 knockout mice were treated for 5 days (PND25–29) with low doses of MPEP (10 mg/kg) or R-baclofen (0.5 mg/kg) alone or in combination and subsequently treated with a high dose of MPEP (30 mg/kg) 60 min before seizure testing on PND30. Mice pretreated with 10 mg/kg MPEP displayed no seizures after injection of high-dose MPEP. However, pretreatment with a low dose of R-baclofen alone or a combination of low-dose MPEP and low-dose R-baclofen produced tolerance to the anticonvulsant effects of high-dose MPEP.
Discussion
Our results show that when administered as single doses MPEP (30 mg/kg), R-baclofen (1 mg/kg), and the GABAB-positive modulator GS-39783 (30 mg/kg) displayed robust suppression of audiogenic seizures in FMR1 mice. However, when FMR1 mice were treated daily for 6 days, MPEP retained its anticonvulsant activity, whereas R-baclofen and GS-39783 did not. Baclofen has previously been shown to induce tolerance in several models of chronic pain (Lehmann et al., 2003; Sands et al., 2003) and in humans after intrathecal infusion (Akman et al., 1993; Nielsen et al., 2002). In the present study, Western blot analysis showed no significant difference in GABAB receptor expression in the forebrains of FMR1 mice after subchronic treatment with R-baclofen. This is consistent with previous findings suggesting that baclofen induces tolerance through mechanisms other than down-regulation of GABAB receptor expression (Lehmann et al., 2003; Sands et al., 2003). However, it is possible that microdissection and analysis of specific brain regions (e.g., the inferior colliculus, a brain region that may mediate audiogenic seizures) might have demonstrated regional changes in GABAB receptor expression after baclofen injection.
We initially hypothesized that targeting GABAB receptors with GS-39783, a positive allosteric modulator, rather than an orthosteric agonist (R-baclofen), might provide a better long-term approach given the overall theoretical advantages of using allosteric modulators as therapeutic drugs (Gregory et al., 2011; Keov et al., 2011) and the specific observations that GS-39783 has been shown to produce fewer side effects (Cryan et al., 2004) and less tolerance (Mombereau et al., 2004) after repeated administration in mouse models of anxiety. In addition, Gjoni and Urwyler (2008) demonstrated that prolonged administration of GS-39783 did not lead to GABAB receptor desensitization in cultured cells. However, in the present study, GS-39783 produced tolerance in FMR1 mice, with an accompanying decrease in GABAB receptor levels. The differences between our results examining the effects of GS-39783 on seizures in FMR1 mice and those of Mombereau et al., (2004) who studied the effects of this drug in tests of anxiety and depression in GABAB R1 null mice may be caused by differences in the strain of mice used (C57/BL6 versus BALB/c), route of administration (intraperitoneal versus oral), length of treatment time (6 versus 21 days), and the doses used (30 mg/kg in the present study versus 0.3–30 mg/kg in Mombereau et al.). In our experiments, doses of GS-39783 below 30 mg/kg were tested but seemed to have minimal efficacy in blocking seizures.
Based on the efficacy of both mGluR antagonists and GABAB agonists in reducing audiogenic seizures after single administrations, we hypothesized that a combination of low doses of MPEP and R-baclofen might prevent seizures while also avoiding the adverse side effects and tolerance seen with higher doses of baclofen. When administered acutely, a combination of MPEP and R-baclofen prevented seizures; however, the effect was lost after repeated administration. A possible explanation for this finding is that, when administered acutely, baclofen might preferentially target presynaptic GABAB receptors to reduce glutamate release thus reducing mGluR5 stimulation and relieving audiogenic seizures. However, after repeated administrations, GABABR desensitization occurs (which may or may not be accompanied by changes in receptor expression) and glutamate release is no longer blocked, thus restoring enhanced mGluR5 signaling and seizures in FMR1 mice. When low doses of MPEP and R-baclofen are combined, the additive effect of reduced glutamate release and decreased mGluR5 activation is responsible for alleviating seizures. We hypothesize that tolerance occurs after repeated administration caused by the loss of the GABAB-mediated reduction in glutamate release, because the receptor blockade induced by the low dose of MPEP alone is not sufficient to prevent seizures. Further experiments are needed to examine this hypothesis.
Our data also demonstrate that R-baclofen promotes tolerance to the anticonvulsant effects of MPEP in FMR1 mice. High-dose MPEP on day 6 of treatment retained anticonvulsant efficacy in mice that had been pretreated for 5 days with low-dose MPEP. However, tolerance ensued when low-dose MPEP was administered together with low-dose R-baclofen over the same 5-day period. Even more surprising was that pretreatment of the mice with low-dose R-baclofen alone for 5 days also induced tolerance to the antiseizure effects of MPEP given on day 6. Similar to the hypothesis above, if R-baclofen-induced GABAB receptor desensitization leads to increased presynaptic glutamate release, the high dose of MPEP used here might not provide sufficient mGluR5 blockade to prevent seizures.
Another intriguing possibility is that the intracellular signaling pathways of both receptors may converge to promote drug tolerance. Group I mGluRs are coupled to Gαq and the activation of phospholipase C and release of intracellular Ca2+ via the inositol trisphosphate receptor. The GABAB receptor is coupled to Gαi/Gαo G-proteins inducing activation of potassium channels and the inhibition of adenylyl cyclase. Rives et al. (2009) studied cross-talk between these two pathways and reported that stimulation of the GABAB receptor strongly potentiates signaling of Gαq coupled mGluR1, most likely via the activation of phospholipase C from the βγ G-proteins subunits generated from complexes with Gαi/Gαo after GABAB receptor stimulation. It is noteworthy that this effect was observed only when the mGluR and GABAB were activated simultaneously, and the mGluR1 potentiation was larger after lower levels of mGluR1 activation compared with higher levels of mGluR1 activation. We propose that the low dose of MPEP used here (Fig. 7) allowed residual signaling through mGluR5 with release of intracellular Ca2+, which was potentiated by R-baclofen stimulation of GABAB and accompanied by additional elevation of intracellular Ca2+, thus inducing tolerance to blockade of mGluR5. Although further studies are needed to rigorously test this idea, the results reported here in FMR1 mice, together with those of Rives et al. (2009), suggest that this mechanism could have wider implications and extend to drug combinations that activate or block other classes of G protein-coupled receptors.
Because group I mGluR signaling is enhanced in FMR1 mice we were surprised to find that treatment with a high dose (30 mg/kg) of the mGluR5-positive allosteric modulator CDPPB alone did not exacerbate seizures as predicted, but rather completely prevented audiogenic seizures in FMR1 mice. This anticonvulsant effect seemed to be dose-dependent because 10 mg/kg CDPPB had no effect on seizure activity. Uslaner et al. (2009) reported an inverted U-shaped dose-response effect whereby improved memory recognition and markers of synaptic plasticity were seen in rats treated with low doses of CDPPB and this effect was lost at the higher dose.
One potential explanation for the observation that CDPPB blocked seizures at high doses in FMR1 mice is that mGluR5 receptor desensitization occurred at the high drug concentration, thereby effectively decreasing mGluR5 signaling and reducing audiogenic seizure susceptibility. However, this is unlikely given that tolerance to the antiseizure effects of high-dose CDPPB developed after subchronic administration despite a reduction in mGluR5 expression (Fig. 6). Another possibility is that the pharmacological actions of CDPPB may convert from a positive modulator at low concentrations to a negative allosteric modulator at high concentrations. CDPPB has been shown to potentiate glutamate stimulation of mGluR5 at very low concentrations with an EC50 of 27 nM (Kinney et al., 2005). Moreover, CDPPB binds to the same site as MPEP in the transmembrane region of mGluR5 (Chen et al., 2007; Rodriguez et al., 2010). We obtained support for this idea by examining the effects of CDPPB on mGluR5 expressed in human embryonic kidney 293 cells. We observed that 10 μM CDPPB blocked glutamate responses in mGluR5-expressing cells, and at 200 nM it reduced the glutamate response (Supplemental Fig. 1). The 200 nM concentration may be pharmacologically relevant because Parmentier-Batteur et al. (2010) have shown that in rats injected with 30 mg/kg CDPPB brain levels of CDPPB were in the range of 400 to 800 nM. We speculate that subtle differences in the precise docking orientation within the pocket of some (but perhaps not all) allosteric modulators at this site may have concentration-dependent effects such that positive versus negative allosteric receptor modulation for some modulators may depend on drug concentration. Together, these findings may have implications for mGluR drug development beyond the treatment of FXS in that positive allosteric modulators of mGluR5 are being developed for use as novel antipsychotics (Gasparini et al., 2008; Uslaner et al., 2009; Rodriguez et al., 2010).
There is presently no cure for FXS, and current therapies are aimed at treating specific symptoms such as anxiety and hyperactivity. Recent advances in understanding the pathobiology of FXS have led to new and exciting drug targets with the potential to treat the disease itself rather than individual symptoms (see Levenga et al., 2010 and Hampson et al., 2011 for reviews). Based on these findings, clinical trials are underway examining the use of several mGluR5-negative modulators and the GABAB agonist arbaclofen in treating FXS (www.clinicaltrials.gov). Taken together, the results of our study indicate that in FMR1 mice tolerance develops rapidly to the anticonvulsant effects of R-baclofen, even at very low doses. In addition, cross-tolerance to MPEP occurred after repeated administration of R-baclofen. These results indicate that caution is warranted and future preclinical drug studies should evaluate the effectiveness of long-term drug administration.
Supplementary Material
This work was supported by the Canadian Institutes for Health Research [Grant MOP-81179]. The monoclonal anti-GABABR1 (clone NR3A/49) and GABABR2 (clone N81/2) antibodies were obtained from the University of California, Davis, CA/National Institutes of Health NeuroMab Facility, which is supported by National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant U24NS050606] and maintained by the Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California, Davis, CA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183327.
- FXS
- fragile X syndrome
- CDPPB
- 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide
- FMRP
- fragile X mental retardation protein
- mGluR
- metabotropic glutamate receptor
- MPEP
- 2-methyl-6-(phenylethynyl)pyridine
- PND
- postnatal day
- GS-39783
- N,N′-dicyclopentyl-2-(methylthio)-5-nitro-4,6-pyrimidine diamine
- GABABR
- GABAB receptor
- CGP46381
- (3-aminopropyl)(cyclohexylmethyl)phosphinic acid
- DMSO
- dimethyl sulfoxide
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- KO
- knockout
- WT
- wild type
- PEG
- polyethylene glycol
- MTEP
- 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine.
Authorship Contributions
Participated in research design: Pacey and Hampson.
Conducted experiments: Pacey and Tharmalingam.
Performed data analysis: Pacey and Tharmalingam.
Wrote or contributed to the writing of the manuscript: Pacey and Hampson.
References
- Adusei DC, Pacey LK, Chen D, Hampson DR. (2010) Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 59:167–171 [DOI] [PubMed] [Google Scholar]
- Akman MN, Loubser PG, Donovan WH, O'Neill ME, Rossi CD. (1993) Intrathecal baclofen: does tolerance occur? Paraplegia 31:516–520 [DOI] [PubMed] [Google Scholar]
- Bear MF. (2005) Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 4:393–398 [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27:370–377 [DOI] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. (2008) Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol 4:256–263 [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M. (2001) Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 103:1043–1050 [DOI] [PubMed] [Google Scholar]
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. (2007) Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol 71:1389–1398 [DOI] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. (2005) The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther 315:590–600 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP. (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 310:952–963 [DOI] [PubMed] [Google Scholar]
- Curia G, Papouin T, Séguéla P, Avoli M. (2009) Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex 19:1515–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. (2006) Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 1121:238–245 [DOI] [PubMed] [Google Scholar]
- D'Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, De Deyn PP, Hassan BA, Kooy RF. (2009) Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res 1253:176–183 [DOI] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. (2008) Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis 31:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. (2007) Correction of fragile X syndrome in mice. Neuron 56:955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. (2005) Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci Lett 377:141–146 [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. (2009) Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum 8:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Bilbe G, Gomez-Mancilla B, Spooren W. (2008) mGluR5 antagonists: discovery, characterization and drug development. Curr Opin Drug Discov Devel 11:655–665 [PubMed] [Google Scholar]
- Gjoni T, Urwyler S. (2008) Receptor activation involving positive allosteric modulation, unlike full agonism, does not result in GABAB receptor desensitization. Neuropharmacology 55:1293–1299 [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Dong EN, Meiler J, Conn PJ. (2011) Allosteric modulation of metabotropic glutamate receptors: structural insights and therapeutic potential. Neuropharmacology 60:66–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DR, Adusei DC, Pacey LK. (2011) The neurochemical basis for the treatment of autism spectrum disorders and fragile X syndrome. Biochem Pharmacol 81:1078–1086 [DOI] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. (2011) Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60:24–35 [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, et al. (2005) A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther 313:199–206 [DOI] [PubMed] [Google Scholar]
- Lehmann A, Mattsson JP, Edlund A, Johansson T, Ekstrand AJ. (2003) Effects of repeated administration of baclofen to rats on GABAB receptor binding sites and subunit expression in the brain. Neurochem Res 28:387–393 [DOI] [PubMed] [Google Scholar]
- Levenga J, de Vrij FM, Oostra BA, Willemsen R. (2010) Potential therapeutic interventions for fragile X syndrome. Trends Mol Med 16:516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, et al. (2005) Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45:753–764 [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. (2004) Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology 29:1050–1062 [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. (2000) Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia 41:19–23 [DOI] [PubMed] [Google Scholar]
- Nielsen JF, Hansen HJ, Sunde N, Christensen JJ. (2002) Evidence of tolerance to baclofen in treatment of severe spasticity with intrathecal baclofen. Clin Neurol Neurosurg 104:142–145 [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. (2010) Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci 30:9929–9938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LK, Doss L, Cifelli C, van der Kooy D, Heximer SP, Hampson DR. (2011) Genetic deletion of regulator of G-protein signaling 4 (RGS4) rescues a subset of fragile X related phenotypes in the FMR1 knockout mouse. Mol Cell Neurosci 46:563–572 [DOI] [PubMed] [Google Scholar]
- Pacey LK, Heximer SP, Hampson DR. (2009) Increased GABA(B) receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol Pharmacol 76:18–24 [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Obrien JA, Doran S, Nguyen SJ, Flick RB, Uslaner JM, Chen H, Finger EN, Williams TM, Jacobson MA, et al. (2010) Differential effects of the mGluR5 positive allosteric modulator CDPPB in the cortex and striatum following repeated administration. Neuropharmacology doi:10.1016/j.neuropharm.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prézeau L. (2009) Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J 28:2195–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, et al. (2010) Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol 78:1105–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands SA, McCarson KE, Enna SJ. (2003) Differential regulation of GABA B receptor subunit expression and function. J Pharmacol Exp Ther 305:191–196 [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. (2009) Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 57:531–538 [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Luján R, Jacobson LH, Biermann B, Fritschy JM, et al. (2006) Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50:589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. (2005) Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49:1053–1066 [DOI] [PubMed] [Google Scholar]
- Zupan B, Toth M. (2008) Inactivation of the maternal fragile X gene results in sensitization of GABAB receptor function in the offspring. J Pharmacol Exp Ther 327:820–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.