Abstract
The formation of adenosine dampens inflammation by inhibiting most cells of the immune system. Among its actions on neutrophils, adenosine suppresses superoxide generation and regulates chemotactic activity. To date, most evidence implicates the Gs protein-coupled A2A adenosine receptor (AR) as the primary AR subtype responsible for mediating the actions of adenosine on neutrophils by stimulating cAMP production. Given that the A2BAR is now known to be expressed in neutrophils and that it is a Gs protein-coupled receptor, we examined in this study whether it signals to suppress neutrophil activities by using 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide (BAY 60-6583), a new agonist for the human A2BAR that was confirmed in preliminary studies to be a potent and highly selective agonist for the murine A2BAR. We found that treating mouse neutrophils with low concentrations (10−9 and 10−8 M) of BAY 60-6583 inhibited formylated-methionine-leucine-phenylalanine (fMLP)-stimulated superoxide production by either naive neutrophils, tumor necrosis factor-α-primed neutrophils, or neutrophils isolated from mice treated systemically with lipopolysaccharide. This inhibitory action of BAY 60-6583 was confirmed to involve the A2BAR in experiments using neutrophils obtained from A2BAR gene knockout mice. It is noteworthy that BAY 60-6583 increased fMLP-stimulated superoxide production at higher concentrations (>1 μM), which was attributed to an AR-independent effect. In a standard Boyden chamber migration assay, BAY 60-6583 alone did not stimulate neutrophil chemotaxis or influence chemotaxis in response to fMLP. These results indicate that the A2BAR signals to suppress oxidase activity by murine neutrophils, supporting the idea that this low-affinity receptor for adenosine participates along with the A2AAR in regulating the proinflammatory actions of neutrophils.
Introduction
Adenosine is a purine nucleoside generated in response to ischemia and inflammation, partly by the extracellular catalysis by ecto-nucleotidases of ATP and ADP secreted by activated or injured cells (Haskó et al., 2008). During acute inflammation, adenosine functions in a autocrine/paracrine manner to suppress activity of essentially all cells of the immune system, including the neutrophil, thereby dampening and resolving the inflammatory reaction (Cronstein, 1994; Haskó et al., 2008). Among other actions, adenosine inhibits the superoxide burst produced by the NADPH complex in response to activating agents including host- and bacterial-derived formylated peptides, a process that is essential for killing phagocytosed bacteria but that also promotes oxidant-induced tissue injury in inflammatory diseases such as asthma, arthritis, inflammatory bowel disease, and ischemia/reperfusion injury (Cronstein, 1994; Haskó et al., 2008).
Among the four adenosine receptor (AR) subtypes (A1, A2A, A2B, and A3; Fredholm et al., 2000), most previous studies have implicated the Gs protein-coupled A2AAR in mediating the suppressive actions of adenosine on stimulated superoxide production (Sullivan et al., 2001; Haskó et al., 2008). However, it has become apparent in recent years that additional AR subtypes probably participate in this action. For example, it is now well established that the A3AR is abundantly expressed in neutrophils, surprisingly at a level that exceeds that of the A2AAR (Chen et al., 2006; van der Hoeven et al., 2008, 2010). Our data demonstrated that the A3AR signals along with the A2AAR to suppress the superoxide burst in murine neutrophils by a mechanism that seems to involve inhibition of the small GTPase Rac (van der Hoeven et al., 2008, 2010).
In our previous studies (van der Hoeven et al., 2008, 2010), we had also detected mRNA expression of the A2BAR subtype at low levels in murine neutrophils that increased 15-fold during systemic inflammation (van der Hoeven et al., 2008). Considering that the A2BAR is a Gs protein-coupled receptor like the A2AAR (Feoktistov and Biaggioni, 1997; Fredholm et al., 2000), in this investigation we tested the hypothesis that the A2BAR couples to inhibition of stimulated superoxide production in neutrophils. We also examined whether the A2BAR regulates neutrophil chemotaxis by using a Boyden chamber migration assay (van der Hoeven et al., 2008). Our studies involved the use of the A2BAR agonist 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide (BAY 60-6385) (Beukers et al., 2004; Eckle et al., 2007) and A2BAR gene knockout/β-galactosidase reporter gene knock-in mice (A2BKO) (Yang et al., 2006). In preliminary studies the pharmacology of BAY 60-6583 for recombinant mouse ARs expressed in HEK 293 cells was extensively characterized in radioligand binding and functional cAMP accumulation assays.
Materials and Methods
Reagents.
Dulbecco's modified Eagle's medium, fetal bovine serum, antibiotics, Hanks' balanced salt solution (HBSS), recombinant mouse tumor necrosis factor α (TNF-α), calcein-AM, and 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazol[1,2-a]pyrazin-3-one, hydrochloride (MCLA) were purchased from Invitrogen (Carlsbad, CA). Percoll was purchased from Amersham Biosciences (Piscataway, NJ), and adenosine deaminase (ADA) was from Roche Applied Science (Indianapolis, IN). 3-Ethyl-1-propyl-8-(1-(3-trifluoromethylbenzyl)-1H-pyrazol-4-yl)-3,7-dihydropurine-2,6-dione (CVT 6883) was purchased from ChemieTek (Indianapolis, IN), 8-[4-[4-(4-chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine (PSB-603) was purchased from Tocris Bioscience (Ellisville, MO), xanthine amine congener (XAC) was from Research Biochemicals International (Natick, MA), and [3H]cAMP and Na[125I] was purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). BAY 60-6583 and 2-[2-(4-aminophenyl)ethylamino]adenosine (APE) were custom-synthesized as described previously (Luthin et al., 1995; Auchampach et al., 2009). All remaining reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Mice.
Male C57BL/6J wild-type mice (10–14 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME). A2BKO mice made congenic to the C57BL/6J mouse strain were as described previously (Yang et al., 2006). All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee at the Medical College of Wisconsin.
Radioligand Binding Assays.
Radioligand binding analysis was conducted with membranes prepared from HEK 293 cells lines expressing mouse ARs (Auchampach et al., 1997; Kreckler et al., 2006). The full-length cDNA sequences were obtained by reverse transcription-polymerase chain reaction using total RNA isolated from mouse brain (Kreckler et al., 2006). The cDNAs were subcloned into pCDNA3.1, transfected into HEK 293 cells using Lipofectamine, and selected with 2 mg/ml (2R,3S,4R,5R,6S)-5-amino-6-[(1R,2S,3S,4R,6S)-4,6-diamino-3-[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-methylaminooxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-2-(1-hydroxyethyl)oxane-3,4-diol (G418). In binding assays, crude membranes prepared from HEK 293 cells expressing mouse ARs (Kreckler et al., 2006) were incubated with various concentrations of radioligand and competitors in 100 μl of binding buffer (20 mM HEPES, 1 mM EDTA, 5 mM MgCl2, 1 unit/ml adenosine deaminase). After incubation at 25°C for 3 h, bound and free radioligands were separated by rapid filtration over glass fiber filters. Nonspecific binding was measured in the presence of 100 μM adenosine-5′-N-ethylcarboxamide (NECA). N6-(4-amino-3-[125I]iodobenzyl)adenosine-5′-N-methylcarboxamide ([125I]I-AB-MECA) (agonist) was used in assays with the mouse A1 and A3 ARs (Kreckler et al., 2006), and [125I]APE (agonist) was used with mouse A2AARs (Luthin et al., 1995; Murphree et al., 2002). The radioligands were prepared by the chloramine-T method and purified by high-performance liquid chromatography (Luthin et al., 1995; Auchampach et al., 1997; Murphree et al., 2002).
In saturation binding assays, Bmax and Kd values were obtained by fitting specific binding data to either single- or two-site binding models using nonlinear least-squares interpolation. An F test was used to identify the model that provided the best fit. In competition assays with compounds binding to a single site, specific binding data were fit to: binding = nonspecific binding + (total binding − specific binding)/(1 + 10×−log IC50). Ki values were calculated from IC50 values using the Cheng-Prusoff equation (Cheng and Prusoff, 1973). In competition assays with compounds competing for two binding sites, two Ki values for competitors were calculated by solving the following four equations simultaneously (Auchampach et al., 1997): LB = (Bmax1[(L/Kd1)/(1 + L/Kd1 + C/Ki1)]) + (Bmax2[(L/Kd2)/(1 + L/Kd2 + C/Ki2)]) + f × L; CB = (Bmax1[(C/Ki1)/(1 + L/Kd1 + C/Ki1)]) + (Bmax2[(C/Ki2)/(1 + L/Kd2 + C/Ki2)]) + f × C; LT = L + LB; CT = C + CB, where LB is radioligand bound, CB is competitor bound, L is free radioligand, C is free competitor, and f is fraction of L or C nonspecifically bound (assumed to be equal). Kd1(high) and Kd2(low) were obtained from equilibrium saturation binding experiments in the absence of competitor. The other values were determined by simultaneously solving the four equations by interpolation within each iteration of nonlinear least-squares analysis (Auchampach et al., 1997).
cAMP Accumulation Assays.
HEK 293 cells expressing recombinant mouse ARs were detached from cell culture plates using phosphate-buffered saline containing 5 mM EDTA, resuspended in serum-free Dulbecco's modified Eagle's medium containing 25 mM HEPES, pH 7.4, 1 unit/ml ADA, and 20 μM 4-[(3-butoxy-4-methoxyphenyl)-methyl]-2-imidazolidinone (Ro 20-1724), and then transferred to polypropylene tubes (200,000 cells/200 μl). The cells were incubated with ligands with or without forskolin (10 μM) for 15 min at 37°C with shaking, after which the assays were terminated by adding 500 μl of 0.15 N HCl. cAMP in the acid extract was determined by a competitive binding assay using [3H]cAMP and protein extracts from bovine adrenal tissue (Nordstedt and Fredholm, 1990; Auchampach et al., 2010). The data were expressed as the amount of cAMP that accumulated during the 15-min incubation time (pmol/15 min/200,000 cells). EC50 values were calculated by fitting the data to: V = Vmin + (Vmax − Vmin)/(1 + 10×−logEC50). The A2BAR antagonists CVT-6883 (300 nM) and PSB-603 (300 nM) were included in assays with cells expressing the A1, A2A, or A3 AR to inhibit A2BARs expressed endogenously in HEK 293 cells (Gao et al., 1999).
Isolation of Mouse Bone Marrow Neutrophils.
Morphologically mature neutrophils were purified from mouse bone marrow by isotonic Percoll gradient centrifugation as described previously (van der Hoeven et al., 2008, 2010). In brief, mice were euthanized by anoxia with carbon dioxide. Bone marrow cells were flushed from tibias and femurs of mice with neutrophil isolation buffer (1× Ca2+- and Mg2+-free HBSS containing 0.4% sodium citrate), washed once with neutrophil isolation buffer, and then layered onto a three-step Percoll gradient (72, 64, 52%). After centrifugation at 1060g for 30 min, cells at the 72/64% interface were removed and washed once with isolation buffer before use in experiments.
Neutrophil Superoxide Production Assay.
Superoxide production was measured using the chemiluminescent probe MCLA (van der Hoeven et al., 2008). Bone marrow neutrophils (7 × 104 cells/ml) were preincubated in HBSS containing 1 unit/ml ADA at 37°C for 30 min with vehicle or BAY 60-6583 at the concentrations indicated. After the addition of MCLA (0.5 μM) and fMLP, chemiluminescence was measured using a luminometer (AutoLumat, LB 953; Berthold Technologies, Bad Wildbad, Germany), and the cumulative relative light units (RLU) over 3 min were obtained for each sample. RLUs of unstimulated cells were deducted from the sample RLUs, and superoxide produced was calculated as a percentage of that produced with vehicle-treated control samples. A cell-free xanthine/xanthine oxidase superoxide-generating system was used to determine the chemilumiscence enhancing/quenching properties of BAY 60-6583. Specificity of the probe for superoxide was verified in all of the assays by adding superoxide dismutase in control reactions (van der Hoeven et al., 2008).
Chemotaxis Assay.
Neutrophil chemotaxis was assessed using a 48-well microchemotaxis chamber (van der Hoeven et al., 2008). Neutrophils were labeled with the fluorescent dye Calcein-AM (3 μM) in neutrophil isolation buffer for 30 min at 37°C. After washing twice, the cells were resuspended in HBSS containing 0.5% bovine serum albumin and 1 unit/ml ADA. Next, 5 × 104 neutrophils (in a volume of 100 μl) were transferred to the upper wells of the chemotaxis chamber, which were separated from the lower chamber by a porous (5 μm) polycarbonate membrane (Neuroprobe, Gaithersburg, MD). The lower wells were filled with media containing fMLP or BAY 60-6583 as indicated. The cells were allowed to migrate at 37°C for 35 min in a humidified cell culture incubator, after which fluorescence emitted by cells that adhered to the underside of the membrane was measured using a fluorimager (Molecular Dynamics Typhoon Imaging System; GE Healthcare). After densitometry analysis using Scion Image software (National Institutes of Health, Bethesda, MD), the chemotaxis index was calculated using the following formula: fluorescence density of cells migrated in the presence of chemoattractant/fluorescence density of cells migrated toward medium (van der Hoeven et al., 2008).
Data Analysis.
Data are reported as means ± S.E.M. Superoxide data were analyzed by a one-way analysis of variance followed by post hoc contrasts using Dunnett's t test. Chemotaxis data were analyzed by a two-way analysis of variance to determine whether there was a main effect of the chemoattractant (i.e., fMLP), a main effect of pretreating with BAY 60-6583 or a chemoattractant-BAY 60-6583 treatment interaction. A p value <0.05 was considered statistically significant.
Results
Characterization of BAY 60-6583 for Mouse ARs.
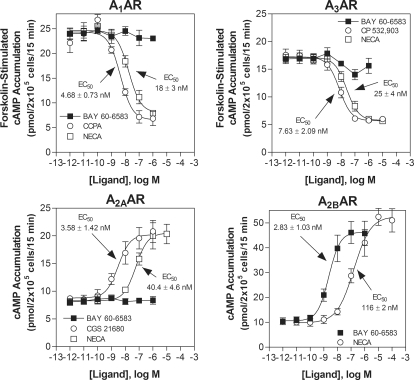
The potency and selectivity of BAY 60-6583 for murine ARs was initially assessed in cAMP accumulation assays and competitive radioligand binding assays using HEK 293 cells stably expressing recombinant mouse ARs. As shown in Fig. 1, BAY 60-6583 potently stimulated cAMP production in HEK 293 cells expressing mouse A2BARs with an EC50 value of 2.83 ± 1.03 nM. In comparison, BAY 60-6583 exhibited no agonist activity in assays using cells expressing A1, A2A, or A3 ARs at concentrations as high as 1 μM. Inclusion of NECA and the subtype-selective agonists 2-chloro-1,3-diethyl-N6-cyclopentyldenosine (CCPA; A1), 2-[p-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine (CGS 21680) (A2A), and (2S,3S,4R,5R)-3-amino-5-[6-(2,5-dichlorobenzylamino)purin-9-yl]-4-hydroxytetrahydrofuran-2-carboxylic acid methylamide (CP-532,903) (A3) were included as comparators in the assays (Fig. 1).
Fig. 1.
The selectivity of BAY 60-6583 for the mouse A2BAR was confirmed in cAMP accumulation assays using HEK 293 cells stably expressing either the mouse A1, A2A, A2B, or A3 ARs. EC50 values calculated for BAY 60-6583 to stimulate cAMP accumulation (A2A and A2B ARs) or inhibit forskolin (10 μM)-stimulated cAMP accumulation are provided within the graphs. The potency of NECA, the selective A1AR agonist CCPA, the selective A2AAR agonist CGS 21680, and the A3AR agonist CP 532,903 are included for the purpose of comparison. n = 3–5.
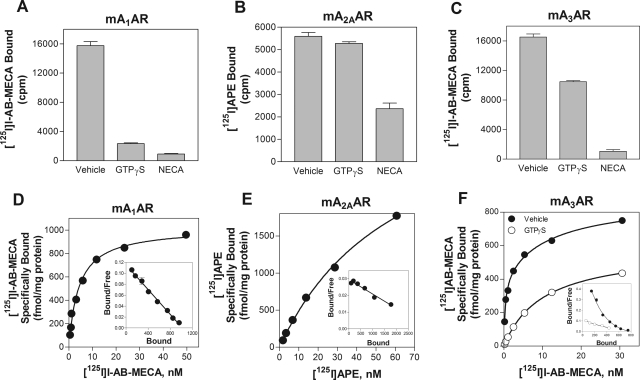
Before conducting competition binding assays, we performed preliminary diagnostic experiments with GTPγS to determine the characteristics of agonist radioligand binding to mouse ARs expressed in HEK 293 cells. As shown in Fig. 2, binding of [125I]I-AB-MECA to the A1AR was reduced significantly in the presence of GTPγS (100 μM). In the case of the A1AR, specific binding was essentially abolished, whereas with the A3AR specific binding was reduced ∼50%. In saturation assays (Fig. 2), specific [125I]I-AB-MECA binding to the mouse A1AR fit best to a one-site binding model with a Kd value of 7.27 ± 1.8 nM and a Bmax value of 956 ± 51 fmol/mg protein. On the other hand, specific binding of [125I]I-AB-MECA to the mouse A3AR fit best to a two-site binding model (Kd(high) = 0.51 ± 0.08 nM; Bmax = 374 ± 24 fmol/mg protein; Kd(low) = 23.8 ± 5.6 nM; Bmax = 504 ± 85 fmol/mg protein) that was converted to a one-site relationship in the presence of GTPγS (Fig. 2). These data indicate that [125I]I-AB-MECA is able to detect the high-affinity, G protein-coupled states of the mouse A1 and A3 ARs when expressed in HEK 293 cells. In the case of the A1AR, [125I]I-AB-MECA exclusively detects the G protein-coupled state of the receptor, whereas it detects both the coupled and uncoupled states of the A3AR. In contrast, specific binding of [125I]APE to the mouse A2AAR was not appreciatively reduced by GTPγS (Fig. 2). Moreover, saturation binding analysis revealed that [125I]APE bound to a single receptor population with relatively low affinity (46 ± 6 nM and a Bmax value of 4235 ± 782 fmol/mg protein). Similar results were obtained with the tritiated radioligand [3H]CGS 2180 (data not shown). Collectively, these data indicate that mouse A2AARs expressed in HEK 293 cells fail to form high-affinity receptor-G protein complexes to an appreciable extend that can be accurately detected with agonist radioligands.
Fig. 2.
Agonist radioligand binding to HEK 293 cells expressing recombinant mouse ARs. A to C, inhibition of agonist radioligand binding to HEK 293 cell membranes expressing recombinant mouse ARs by GTPγS (100 μM). Agonist radioligands were included at a concentration of ∼0.2 nM. D to F, specific binding of the agonist radioligands to HEK 293 cell membranes expressing recombinant mouse ARs in equilibrium saturation binding assays. In studies of the A3AR, the assays were conducted in the presence and absence of 100 μM GTPγS. Scatchard transformation of the specific binding data is presented in the insets. Each assay contained 50 μg of membrane protein, and nonspecific binding was revealed by the presence of NECA (100 μM). n = 3 for the binding assays with GTPγS. For saturation bindings assays, specific binding data from a representative experiment performed in triplicate of a total of three independent experiments is presented.
We subsequently proceeded to assess the affinity of BAY 60-6583 and other ligands used in the investigation for the two mouse AR subtypes in competition binding assays wherein it was possible to detect high-affinity agonist binding (i.e., A1 and A3 ARs). The results of these experiments are shown in Table 1. The Ki values reported for the A3AR represents binding of the competitors for the high-affinity, G protein-coupled state of the mouse A3AR calculated using nontraditional analysis taking into account competition of the radioligand and competitors for two affinity states (Auchampach et al., 1997). The binding data indicate that BAY 60-6583 displays relatively low binding affinity for the functional, G protein-coupled state of the mouse A1 and A3 ARs.
TABLE 1.
Binding affinities (nM) of BAY 60-6583 and other drugs used in the investigation to the high-affinity, G protein-coupled state of recombinant mouse ARs determined by competition radioligand binding analysis using membranes prepared from HEK 293 cells expressing mouse ARs and [125I]I-AB-MECA
| mA1 | mA3 | |
|---|---|---|
| BAY 60-6583 | 232 ± 19 | >10 μM |
| NECA | 0.45 ± 0.13 | 14 ± 7 |
| CCPA | 0.21 ± 0.10 | 17 ± 5 |
| CGS 21680 | 193 ± 38 | N.D. |
| CP 532,903 | 889 ± 43 | 8.5 ± 2.2 |
N.D., not determined.
Activation of the A2BAR Inhibits fMLP-Induced Superoxide Production by Murine Neutrophils.
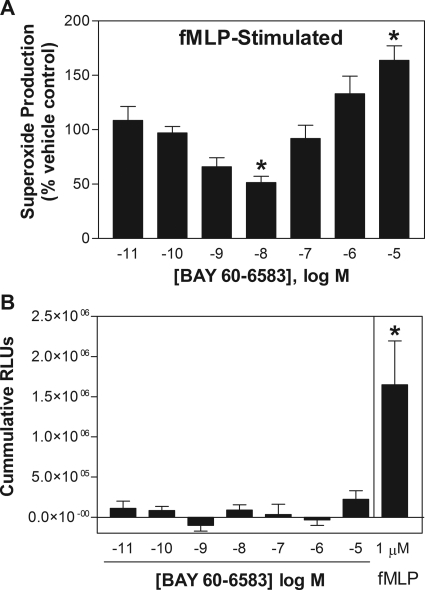
Treating neutrophils with BAY 60-6583 produced a biphasic effect on fMLP-stimulated superoxide production (Fig. 3A). At low concentrations (1–10 nM), BAY 60-6583 reduced fMLP-stimulated superoxide production with a maximal inhibitory effect of ∼50% at 10 nM. At higher concentrations (1–10 μM), however, BAY 60-6583 stimulated fMLP-induced superoxide production, producing a >50% increase over vehicle-treated cells at a concentration of 10 μM. In the absence of fMLP, treating neutrophils with BAY 60-6583 did not directly stimulate superoxide production (i.e., in the absence of fMLP) at any of the concentrations tested (Fig. 3B). BAY 60-6583 (10−11 to 10−5) also did not directly scavenge superoxide anions or interfere with light production by MCLA as determined in a cell-free superoxide-generating system composed of xanthine/xanthine oxidase (data not shown).
Fig. 3.
Effect of increasing concentrations of BAY 60-6583 on superoxide production by murine neutrophils. A, effect of BAY 60-6583 expressed as a percentage of vehicle-treated cells. Neutrophils were pretreated with BAY 60-6583 for 30 min before stimulation with fMLP (1 μM). B, direct effect of treating with BAY 60-6583 (no stimulation with fMLP) on neutrophil superoxide production, expressed as cumulative RLUs. Superoxide production was measured as described under Materials and Methods using the chemiluminescent probe MCLA. *, P < 0.05 versus vehicle-treated cells; n = 4–6.
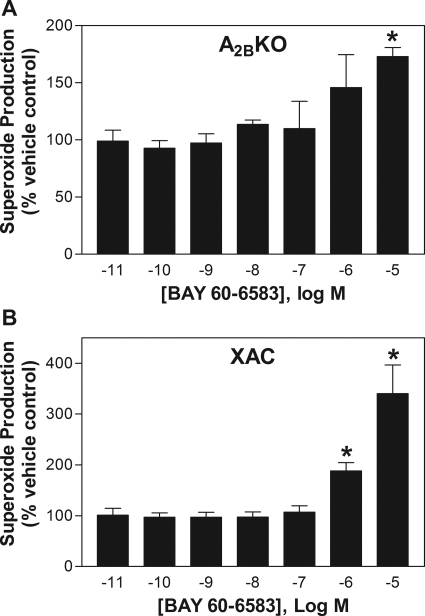
In assays using neutrophils isolated from A2BKO mice, the inhibitory effect of BAY 60-6583 on fMLP-induced superoxide generation observed at low nanomolar concentrations was no longer evident, although it continued to potentiate superoxide production at higher concentrations (Fig. 4A). A similar profile with BAY 60-6583 was observed with wild-type neutrophils in the presence of the nonselective AR antagonist XAC (1 μM; Fig. 4B). These results indicate that the inhibitory effect of BAY 60-6583 on fMLP-stimulated superoxide production at lower concentrations was mediated via the A2BAR, whereas the stimulatory effect at higher concentrations was caused by a nonspecific, AR-independent mechanism.
Fig. 4.
Effect of increasing concentrations of BAY 60-6583 on fMLP-stimulated superoxide production of murine neutrophils obtained from A2BKO mice (A) or neutrophils obtained from wild-type mice in the presence of the nonselective AR antagonist XAC (1 μM) (B). Neutrophils were pretreated with BAY 60-6583 for 30 min before stimulation with fMLP (1 μM). Superoxide production is expressed as the percentage of vehicle-treated cells. *, P < 0.05 versus vehicle-treated cells; n = 3.
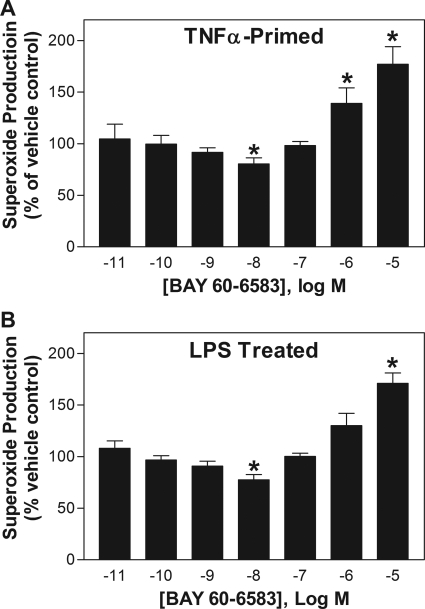
BAY 60-6583 also produced a biphasic response on fMLP-induced superoxide production in assays using TNF-α-primed neutrophils (100 ng/ml for 30 min) or neutrophils isolated from mice pretreated 4 h earlier with 10 mg/kg of lipopolysaccharide (LPS) that results in neutrophil priming and a 10- to 15-fold increase in mRNA expression of A2A and A2B ARs (van der Hoeven et al., 2008; Fig. 5). The maximal inhibitory effect of BAY 60-6583 in these studies (∼20%) was less compared with assays with naive cells (see Fig. 3).
Fig. 5.
Effect of increasing concentrations of BAY 60-6583 on fMLP (1 μM)-stimulated superoxide production of murine neutrophils primed by pretreatment with TNF-α (100 ng/ml for 30 min) (A) or neutrophils isolated from mice treated 4 h earlier with LPS (10 mg/kg i.p.) (B). Neutrophils were pretreated with BAY 60-6583 for 30 min before stimulation with fMLP (1 μM). Superoxide production is expressed as the percentage of vehicle-treated cells. *, P < 0.05 versus vehicle-treated cells; n = 4–6.
Activation of the A2BAR Does Not Influence Chemotaxis.
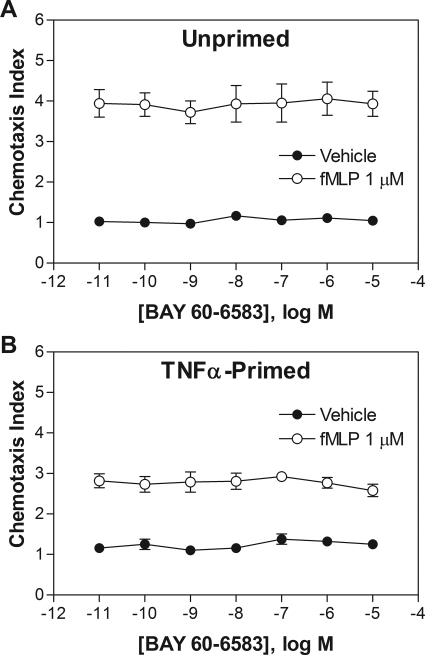
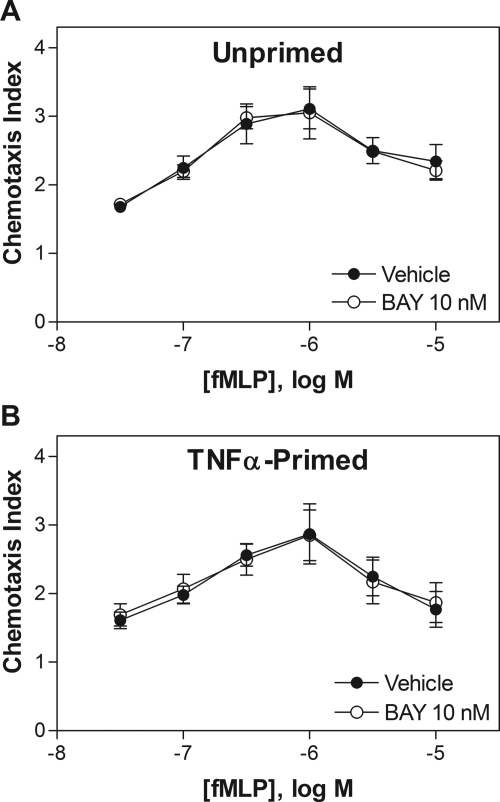
In transwell chemotaxis assays, adding BAY 60-6583 (10−12 to 10−5) into the lower wells did not directly stimulate migration or influence migration toward 1 μM fMLP of either naive or TNF-α-primed neutrophils (Fig. 6). Treating neutrophils with BAY 60-6583 (10 nM for 30 min) before the addition of the cells to upper wells also had no influence on migration toward increasing concentrations of fMLP in lower wells (Fig. 7).
Fig. 6.
Effect of BAY 60-6583 on chemotaxis of mouse neutrophils. Fluorescently labeled naive (A) or TNF-α-primed (100 ng/ml for 30 min) (B) neutrophils were placed into the upper wells of a 48-well chemotaxis chamber separated by a polycarbonate membrane (5-μm pores). The lower wells contained increasing concentrations of BAY 60-6583 in combination with vehicle or fMLP (1 μM). The cells were allowed to migrate at 37°C for 35 min, and the chemotaxis index was calculated as described under Materials and Methods. n = 3–5.
Fig. 7.
Effect of pretreating mouse neutrophils with BAY 60-6583 on chemotaxis induced by fMLP. Fluorescently labeled naive (A) or TNF-α-primed (100 ng/ml for 30 min) (B) neutrophils were incubated with 10 nM BAY 60-6583 for 30 min and then placed into the upper wells of a 48-well chemotaxis chamber separated by a polycarbonate membrane (5-μm pores). The lower wells contained increasing concentrations of fMLP. The cells were allowed to migrate at 37°C for 35 min, and the chemotaxis index was calculated as described under Materials and Methods. n = 4–5.
Discussion
We previously identified expression of the low-affinity A2BAR subtype in murine neutrophils that is transcriptionally induced during systemic inflammation (van der Hoeven et al., 2008). In this study, we demonstrated that the A2BAR signals in murine neutrophils to inhibit superoxide production, but does not seem to modulate chemotaxis. Our results indicate that the A2BAR participates in mediating the inhibitory actions of adenosine on neutrophils. Considering that the A2BAR is a low-affinity receptor for adenosine and its expression is induced in response to hypoxia or inflammatory stimuli (Eckle et al., 2007; van der Hoeven et al., 2008; Haskó et al., 2009), we propose that A2BAR signaling may become increasingly important during inflammation resolution and when adenosine levels in tissues are high.
Based on the results of this investigation and previous work from our group and others, it is now apparent that three different AR subtypes expressed in murine neutrophils (A2A, A2B, and A3) are capable of regulating stimulated superoxide production (Cronstein et al., 1985, 1992; Cronstein, 1994; Fredholm et al., 1996; Sullivan et al., 2001; van der Hoeven et al., 2008, 2010). The participation of multiple different AR subtypes may serve to increase the potency and efficacy of adenosine to dampen neutrophil activity at various stages of the inflammatory reaction. The expression of multiple different AR subtypes in neutrophils also raises the possibility for complex regulation of neutrophil activities through receptor interactions that may involve receptor heterodimeration and cross-talk between signal transduction pathways. It has previously been demonstrated that human neutrophils express the A2BAR, along with the A2A, A3, and potentially the A1 AR (Chen et al., 2006; Fortin et al., 2006; Zhang et al., 2006). It will be important to determine whether the findings from this work with murine cells hold true with human neutrophils.
Neutrophil oxidant production is catalyzed by the multicomponent NADPH oxidase complex composed of cytosolic subunits (p47phox, p67phox, p40phox, and Rac2) that migrate to the membrane and associate with membrane subunits (p22phox and gp91phox) upon activation (Groemping and Rittinger, 2005). The complex catalyzes NADPH-dependent reduction of oxygen to superoxide anion (Groemping and Rittinger, 2005). The extent of neutrophil superoxide production is enhanced anywhere from ∼4- to 15-fold after exposure to TNF-α and a variety of other proinflammatory cytokines, a process that is termed neutrophil “priming” (Dewas et al., 2003; Boussetta et al., 2010). The mechanism of priming by TNF-α is not completely understood, but seems to involve hyperphosphorylation of the cytosolic p47phox subunit of the NADPH complex that may be facilitated after isomerization of a phosphorylated serine residue within the carboxyl-terminal region of the protein (Dewas et al., 2003; Boussetta et al., 2010). Priming serves to amplify the destructive actions of neutrophils selectively within regions that are inflamed. Our data indicate that activation of the A2BAR is able to continue to suppress fMLP-stimulated superoxide by neutrophils that had been primed by TNF-α, albeit to a lesser extent compared with naive cells. With priming, BAY 60-6583 produced a maximal inhibitory effect of ∼25% compared with a maximal inhibitory effect of 50% with nonprimed cells. A similar reduction in efficacy was observed in experiments using neutrophils isolated from LPS-treated mice whereby priming occurs in vivo before isolation (van der Hoeven et al., 2008). In this experiment, the efficacy of BAY 60-6583 was reduced even though expression of the A2BAR was induced. At a molecular level, the reduced efficacy with TNF-α priming may be related to previous reports that AR activation does not inhibit neutrophil superoxide production in response to phorbol esters that directly activate protein kinase C, and that the molecular mechanism of TNF-α priming involving p47phox hyperphosphorylation is protein kinase C-dependent (Cronstein et al., 1985; van der Hoeven et al., 2008; Boussetta et al., 2010). It is notable that in our previous studies of murine neutrophils we also observed that the extent of inhibition of stimulated superoxide production provided by A2A and A3 AR activation was similarly reduced after priming by TNF-α (van der Hoeven et al., 2008).
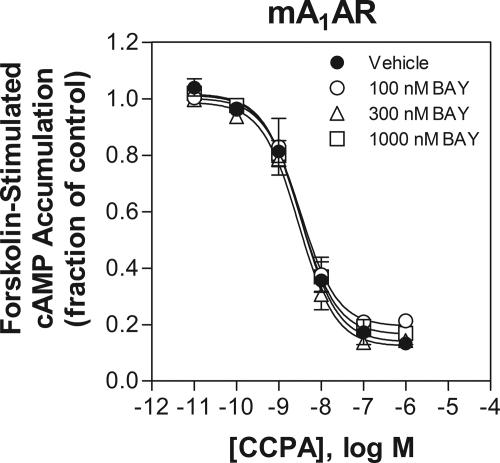
BAY 60-6583 is member of a new series of 6-amino-3,5-dicyano-4-phenylpyridine derivatives that has been identified as selective agonists for the human A2BAR (Beukers et al., 2004; Eckle et al., 2007). In preliminary studies, we carefully examined the binding affinity and functional potency of BAY 60-6583 for murine ARs. Comparisons were made with the adenosine analog NECA, which has been used extensively as a pharmacological agonist for the A2BAR in past studies. We found that BAY 60-6583 potently stimulated cAMP accumulation in cells transfected with the murine A2BAR with an EC50 value of 2.83 ± 1.03 nM while exhibiting no agonist activity for mouse A1, A2A, or A3 ARs at concentrations as high as 1 μM. Correlating with the cAMP data, our radioligand binding data demonstrated that BAY 60-6583 binds to the G protein-coupled, high-affinity states of the A1 and A3 AR with low affinity. In comparison, NECA was essentially equipotent at activating all four subtypes of murine ARs being 40 times less potent than BAY 60-6583 as an agonist of the murine A2BAR. Thus, our data agree with previous work with human ARs (Eckle et al., 2007) and confirm that BAY 60-6583 is a potent and remarkably selective agonist for the murine A2BAR. Our data further reveal that NECA functions as a nonselective agonist for mouse ARs. In fact, among the four mouse AR subtypes, NECA displays the lowest potency for the A2BAR, indicating that this agent should not be used as a selective probe for the A2BAR in mouse studies. One notable observation comparing the binding data in Table 1 and the cAMP data in Fig. 1 is that BAY 60-6583 binds to the mouse A1AR with a Ki value of 232 nM (Table 1), yet it does not inhibit forskolin-stimulated cAMP accumulation in assays with A1AR-overexpressing HEK 293 cells even at a concentration of 1 μM, suggesting that it may act as an antagonist of the murine A1AR. This turns out not to be the case, however, because we observed in additional experiments that including BAY 60-6583 up to a concentration of 1 μM did not shift the concentration-response curve of CCPA to inhibit forskolin-stimulated cAMP accumulation (Fig. 8). A clear explanation for this discrepancy between binding potency and functional potency is not readily apparent. One possibility is that BAY 60-6583 may exert a noncompetitive effect that influences radioligand binding to the A1AR in assays involving isolated membrane preparations that does not occur with intact cells. For instance, BAY 60-6583 at high concentrations may in some way disrupt the formation of A1AR-G protein complexes in isolated membranes.
Fig. 8.
Lack of effect of BAY 60-6583 up to a concentration of 1 μM to inhibit CCPA-mediated inhibition of forskolin (10 μM)-stimulated cAMP accumulation in HEK 293 cells overexpressing the mouse A1AR. n = 3.
We believe it is important to emphasize the results of our binding data with mouse A2ARs. In preliminary assays, we found that agonist binding to mouse A2AARs expressed in HEK 293 cells is almost completely insensitive to GTPγS, indicating that the observed binding is primarily to the low-affinity, G protein-uncoupled form of the receptor. As previously suggested by Murphree et al. (2002), this is probably explained by the limited ability of A2AARs to form receptor-G protein complexes when expressed in HEK 293 cells. As a result, heterologous overexpression of the A2AAR in HEK 293 cells results in a large pool of uncoupled receptors whose presence interferes with detection of the much smaller pool of high-affinity binding sites using readily available radioligands. Poor receptor coupling is probably caused by limited expression of required subtypes of heterotrimeric G proteins (Murphree et al., 2002). Thus, it was not possible to accurately assess the binding affinity of BAY 60-6583 for the murine A2AAR in the present investigation. For this same reason, we believe it is also not possible to accurately assess high-affinity agonist binding to the A2BAR when expressed in HEK 293 cells as described previously (Auchampach et al., 2009).
We did not explore the signaling mechanisms by which the A2BAR inhibits superoxide production. Considering that the A2BAR is coupled to Gs proteins, it is likely that it functions similarly to the A2AAR, namely through either the elevation of cAMP (Sullivan et al., 2001) or Gs protein-mediated uncoupling of chemoattractant receptors (Cronstein et al., 1992). The A2BAR differs from the A2AAR, however, in being capable of dually coupling to both Gs and Gq proteins in some cell types (Feoktistov and Biaggioni, 1997; Fredholm et al., 2000). Thus, the participation of additional or alternative signaling mechanisms remains possible.
Our evidence for involvement of the A2BAR is based on the observation that BAY 60-6583 inhibited fMLP-stimulated superoxide generation at low concentrations that correlated with its potency at activating the murine A2BAR. At concentrations between 1 and 10 nM, BAY 60-6583 inhibited fMLP-stimulated superoxide production of naive cells as well as neutrophils primed with TNF-α or systemic exposure to LPS. This potent effect of BAY 60-6583 was not apparent in studies using neutrophils isolated from A2BKO mice. It is noteworthy, however, that the effect of BAY 60-6583 was biphasic, producing a significant increase in fMLP-stimulated superoxide production at micromolar concentrations; this stimulatory action of BAY 60-6583 persisted in the presence of the nonselective A2BAR antagonist XAC and in studies using neutrophils obtained from A2BKO mice. Thus, BAY 60-6583 may have additional pharmacological actions other than A2BAR agonism.
In addition to suppression of the NADPH oxidase burst, adenosine regulates chemotactic activity of neutrophils. However, this action of adenosine remains equivocal. It has been reported that adenosine functions to both promote and inhibit neutrophil chemotaxis, with the participation of multiple different AR subtypes including A1, A2A, and A3 ARs (Cronstein et al., 1990; Chen et al., 2006; Zhang et al., 2006; van der Hoeven et al., 2008). To address the possible involvement of the A2BAR, we examined the effect of BAY 60-6583 on chemotaxis of murine neutrophils in a standard Boyden chamber assay and found that it had no effect to directly stimulate chemotaxis or modulate chemotactic activity produced by fMLP. Although these data provide evidence that the A2BAR probably does not participate in chemotaxis of murine neutrophils, it will be important to investigate this issue further using dynamic assays that can accurately detect effects on direction sensing and migration velocity (Chen et al., 2006).
The results of this investigation demonstrate that selective activation of the A2BAR in murine neutrophils inhibits fMLP-stimulated superoxide production. This observation supports the concept that the A2BAR probably participates along with other AR subtypes in transducing some of the inhibitory actions of adenosine on neutrophils. Studies using A2BKO mice and BAY 60-6583 have provided evidence that the A2BAR plays a protective role in various models of acute tissue injury in the heart, lungs, kidneys, gastrointestinal tract, and blood vessels (Eckle et al., 2007, 2008a,b; Grenz et al., 2008; Yang et al., 2008; Chen et al., 2009; Eltzschig et al., 2009; Hart et al., 2009; Maas et al., 2010; Schingnitz et al., 2010; Zhou et al., 2011). It has been proposed that the protective action of the A2BAR in these models may involve anti-inflammation related to reduced proinflammatory cytokine expression, inhibition of platelet aggregation, and promotion of barrier function within the vasculature and alveoli (Eckle et al., 2007, 2008a,b; Grenz et al., 2008; Yang et al., 2008; Chen et al., 2009; Eltzschig et al., 2009; Hart et al., 2009; Maas et al., 2010; Schingnitz et al.; Zhou et al., 2011). The data reported herein provide evidence that an additional contributing mechanism may involve suppression of oxidant injury by neutrophils.
Acknowledgments
We thank members of the Cardiovascular Center High-Performance Liquid Chromatography Core for assistance with purification of [125I]I-AB-MECA and [125I]APE.
This research was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL077707, R01-HL93149] and the American Heart Association [Grants 0315274Z, 0615533Z, 0810035Z].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.181792.
- AR
- adenosine receptor
- ADA
- adenosine deaminase
- CCPA
- 2-chloro-1,3-diethyl-N6-cyclopentyldenosine
- CGS 21680
- 2-[p-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine
- CP-532,903
- (2S,3S,4R,5R)-3-amino-5-[6-(2,5-dichlorobenzylamino)purin-9-yl]-4-hydroxytetrahydrofuran-2-carboxylic acid methylamide
- CVT-6883
- 3-ethyl-1-propyl-8-(1-(3-trifluoromethylbenzyl)-1H-pyrazol-4-yl)-3,7-dihydropurine-2,6-dione
- fMLP
- formylated-methionine-leucine-phenylalanine
- [125I]I-AB-MECA
- N6-(4-amino-3-[125I]iodobenzyl)adenosine-5′-N-methylcarboxamide
- APE
- 2-[2-(4-aminophenyl)ethylamino]adenosine
- [125I]APE
- 2-[2-amino-3-[125I]iodophenyl)ethylamino]-adenosine
- KO
- knockout
- MCLA
- 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazol[1,2-a]pyrazin-3-one, hydrochloride
- NECA
- adenosine-5′-N-ethylcarboxamide
- PSB-603
- 8-[4-[4-(4-chlorophenzyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine
- TNF-α
- tumor necrosis factor-α
- XAC
- xanthine amine congener
- BAY 60-6583
- 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]pyridin-2-ylsulfanyl]acetamide
- HEK
- human embryonic kidney
- HBSS
- Hanks' balanced salt solution
- G418
- (2R,3S,4R,5R,6S)-5-amino-6-[(1R,2S,3S,4R,6S)-4,6-diamino-3-[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-methylaminooxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-2-(1-hydroxyethyl)oxane-3,4-diol
- Ro 20-1724
- 4-[(3-butoxy-4-methoxyphenyl)-methyl]-2-imidazolidinone
- RLU
- relative light units
- LPS
- lipopolysaccharide.
Authorship Contributions
Participated in research design: van der Hoeven, Wan, Gizewski, Kreckler, Maas, Van Orman, and Auchampach.
Conducted experiments: van der Hoeven, Wan, Gizewski, Kreckler, Maas, Van Orman, and Auchampach.
Contributed new reagents or analytic tools: Ravid.
Performed data analysis: van der Hoeven, Wan, Gizewski, Kreckler, Maas, Van Orman, and Auchampach.
Wrote or contributed to the writing of the manuscript: van der Hoeven and Auchampach.
References
- Auchampach JA, Gizewski ET, Wan TC, de Castro S, Brown GG, Jr, Jacobson KA. (2010) Synthesis and pharmacological characterization of [125I]MRS5127, a high affinity, selective agonist radioligand for the A3 adenosine receptor. Biochem Pharmacol 79:967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. (1997) Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol 52:846–860 [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Kreckler LM, Wan TC, Maas JE, van der Hoeven D, Gizewski E, Narayanan J, Maas GE. (2009) Characterization of the A2B adenosine receptor from mouse, rabbit, and dog. J Pharmacol Exp Ther 329:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukers MW, Chang LC, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Spanjersberg RF, Brussee J, IJzerman AP. (2004) New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J Med Chem 47:3707–3709 [DOI] [PubMed] [Google Scholar]
- Boussetta T, Gougerot-Pocidalo MA, Hayem G, Ciappelloni S, Raad H, Arabi Derkawi R, Bournier O, Kroviarski Y, Zhou XZ, Malter JS, et al. (2010) The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-α-induced priming of the NADPH oxidase in human neutrophils. Blood 116:5795–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yang D, Carroll SH, Eltzschig HK, Ravid K. (2009) Activation of the macrophage A2B adenosine receptor regulates tumor necrosis factor-α levels following vascular injury. Exp Hematol 37:533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314:1792–1795 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Cronstein BN. (1994) Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol 76:5–13 [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. (1990) The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest 85:1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Haines KA, Kolasinski S, Reibman J. (1992) Occupancy of Gα s-linked receptors uncouples chemoattractant receptors from their stimulus-transduction mechanisms in the neutrophil. Blood 80:1052–1057 [PubMed] [Google Scholar]
- Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. (1985) Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol 135:1366–1371 [PubMed] [Google Scholar]
- Dewas C, Dang PM, Gougerot-Pocidalo MA, El-Benna J. (2003) TNF-α induces phosphorylation of p47(phox) in human neutrophils: partial phosphorylation of p47phox is a common event of priming of human neutrophils by TNF-α and granulocyte-macrophage colony-stimulating factor. J Immunol 171:4392–4398 [DOI] [PubMed] [Google Scholar]
- Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. (2008a) A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111:2024–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Grenz A, Laucher S, Eltzschig HK. (2008b) A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118:3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, et al. (2007) Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115:1581–1590 [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Rivera-Nieves J, Colgan SP. (2009) Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets 13:1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. (1997) Adenosine A2B receptors. Pharmacol Rev 49:381–402 [PubMed] [Google Scholar]
- Fortin A, Harbour D, Fernandes M, Borgeat P, Bourgoin S. (2006) Differential expression of adenosine receptors in human neutrophils: up-regulation by specific Th1 cytokines and lipopolysaccharide. J Leukoc Biol 79:574–585 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. (2000) Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol 362:364–374 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Zhang Y, van der Ploeg I. (1996) Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leucocytes. Naunyn Schmiedebergs Arch Pharmacol 354:262–267 [DOI] [PubMed] [Google Scholar]
- Gao Z, Chen T, Weber MJ, Linden J. (1999) A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. Cross-talk between cyclic AMP and protein kinase c pathways. J Biol Chem 274:5972–5980 [DOI] [PubMed] [Google Scholar]
- Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. (2008) The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. (2005) Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 386:401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. (2009) Cutting Edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182:3965–3968 [DOI] [PubMed] [Google Scholar]
- Haskó G, Csóka B, Németh ZH, Vizi ES, Pacher P. (2009) A2B adenosine receptors in immunity and inflammation. Trends Immunol 30:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Linden J, Cronstein B, Pacher P. (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. (2006) Adenosine inhibits tumor necrosis factor-α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther 317:172–180 [DOI] [PubMed] [Google Scholar]
- Luthin DR, Olsson RA, Thompson RD, Sawmiller DR, Linden J. (1995) Characterization of two affinity states of adenosine A2A receptors with a new radioligand, 2-[2-(4-amino-3-[125I]iodophenyl)ethylamino]adenosine. Mol Pharmacol 47:307–313 [PubMed] [Google Scholar]
- Maas JE, Wan TC, Figler RA, Gross GJ, Auchampach JA. (2010) Evidence that the acute phase of ischemic preconditioning does not require signaling by the A2B adenosine receptor. J Mol Cell Cardiol 49:886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree LJ, Marshall MA, Rieger JM, MacDonald TL, Linden J. (2002) Human A2A adenosine receptors: high-affinity agonist binding to receptor-G protein complexes containing Gβ4. Mol Pharmacol 61:455–462 [DOI] [PubMed] [Google Scholar]
- Nordstedt C, Fredholm BB. (1990) A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Analyt Biochem 189:231–234 [DOI] [PubMed] [Google Scholar]
- Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. (2010) Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 184:5271–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. (2001) Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A2A receptor agonists. Br J Pharmacol 132:1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven D, Gizewski ET, Auchampach JA. (2010) Activation of the A3 adenosine receptor inhibits fMLP-induced Rac activation in mouse bone marrow neutrophils. Biochem Pharmacol 79:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven D, Wan TC, Auchampach JA. (2008) Activation of the A3 adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils. Mol Pharmacol 74:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. (2008) The A2B adenosine receptor protects against vascular injury. Proc Nat Acad Sci U.S.A 105:792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, et al. (2006) The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 116:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Yang D, Dong H, Chen Q, Dimitrova DI, Rogers TJ, Sitkovsky M, Oppenheim JJ. (2006) Adenosine A2A receptors induce heterologous desensitization of chemokine receptors. Blood 108:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR. (2011) Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol 186:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]