Abstract
In this study, metabolism of bupropion, efavirenz, and 7-ethoxy-4-trifluoromethylcoumarin (7-EFC) by CYP2B6 wild type (CYP2B6.1) and six polymorphic variants (CYP2B6.4 to CYP2B6.9) was investigated in a reconstituted system to gain a better understanding of the effects of the mutations on the catalytic properties of these naturally occurring variants. All six variants were successfully overexpressed in Escherichia coli, including CYP2B6.8 (the K139E variant), which previously could not be overexpressed in mammalian COS-1 cells (J Pharmacol Exp Ther 311:34–43, 2004). The steady-state turnover rates for the hydroxylation of bupropion and efavirenz and the O-deethylation of 7-EFC showed that these mutations significantly alter the catalytic activities of CYP2B6. It was found that CYP2B6.6 exhibits 4- and 27-fold increases in the Km values for the hydroxylation of bupropion and efavirenz, respectively, and CYP2B6.8 completely loses its ability to metabolize any of the substrates under normal turnover conditions. However, compared with CYP2B6.1, CYP2B6.8 retains 77% of its 7-EFC O-deethylase activity in the presence of tert-butyl hydroperoxide as an alternative oxidant, indicating that the heme and the active site are catalytically competent. Presteady-state measurements of the rate of electron transfer from NADPH-dependent cytochrome P450 reductase (CPR) to CYP2B6.8 using stopped-flow spectrophotometry revealed that CYP2B6.8 is incapable of accepting electrons from CPR. These observations provide conclusive evidence suggesting that the charge-reversal mutation in the K139E variant prevents CYP2B6.8 from forming a functional complex with CPR. Results from this work provide further insights to better understand the genotype–phenotype correlation regarding CYP2B6 polymorphisms and drug metabolism.
Introduction
Cytochrome P450 2B6 (CYP2B6) participates in the metabolism of a number of clinically important drugs including bupropion (antidepressant), cyclophosphamide (anticancer), efavirenz (antiretroviral), and propofol (anesthetic) among many others (Walsky et al., 2006; Wang and Tompkins, 2008). In particular, bupropion and efavirenz are almost exclusively metabolized by CYP2B6 in humans (Faucette et al., 2000; Ward et al., 2003; Desta et al., 2007; Chen et al., 2010). The importance of CYP2B6 to drug metabolism also lies in its polymorphic nature. CYP2B6 is one of the most polymorphic cytochrome P450 genes because∼100 single-nucleotide polymorphisms have been identified, and 29 of these single-nucleotide polymorphisms result in amino acid substitutions (http://www.cypalleles.ki.se). Some of the CYP2B6 alleles occur with relatively high frequencies across different populations; the allele frequency for CYP2B6*6 is approximately 21% in Mongolians, 28.2% in whites, 32.8% in African-Americans, and 62% in Papua New Guineans (Lang et al., 2001; Mehlotra et al., 2006; Davaalkham et al., 2009). CYP2B6*4 and CYP2B6*5 occur more frequently among the Han Chinese (9.1%) and whites (12.2%), respectively, than other ethnic populations (Jacob et al., 2004; Guan et al., 2006). The occurrence of these genetic mutations in CYP2B6 has been correlated with large interindividual variability in drug exposures among different ethnic populations. A number of studies have indicated that CYP2B6 polymorphism can affect both the protein expression level and the functionality of CYP2B6, resulting in significant effects on drug clearance and efficacy (Ekins et al., 1998; Faucette et al., 2000; Lang et al., 2004).
Based on an analysis of a bank of human liver microsomes using a specific monoclonal antibody against CYP2B6, Ekins et al. (1998) reported that the protein level of CYP2B6 was highly variable in humans because it may vary by up to 100-fold. This variation in protein levels may be caused by a variety of factors such as CYP2B6 polymorphism, induction, etc. It was found that several CYP2B6 alleles were associated with low levels of protein expression. For instance, Hofmann et al. (2008) reported that single-nucleotide polymorphism c.516G>T in allele CYP2B6*6 was alone responsible for aberrant splicing, resulting in high-splice variant 1 and low-CYP2B6 expression phenotype. The protein level of homozygous carriers of CYP2B6.5 was reduced by approximately 8-fold compared with homozygous CYP2B6.1 (Lang et al., 2001; Desta et al., 2007). However, the decrease in the protein levels of CYP2B6.5 did not seem to significantly affect overall drug clearance, which may be compensated for by an increase in enzyme activity caused by the amino acid substitution of R487C (Zanger et al., 2007). As to the effect on the functionality of CYP2B6, it has been reported that CYP2B6 polymorphisms may have profound impacts on the catalytic functions of CYP2B6. We and others have previously demonstrated that CYP2B6.4 (the K262R variant) exhibits alterations in both drug metabolism and inactivation (Bumpus et al., 2005; Shebley and Hollenberg, 2007; Talakad et al., 2009). As reported, the K262→R mutation led to a loss in the mechanism-based inactivation by phencyclidine and the K262R variant was also less susceptible to inhibition by several clinically used drugs including clopidogrel, itraconazole, raloxifene, and sertraline (Shebley and Hollenberg, 2007; Talakad et al., 2009). The combined effects of genetic mutations and/or induction in CYP2B6 on protein expression levels and catalytic activities may cause 20- to 250-fold interindividual variability in exposure to drugs (Wang and Tompkins, 2008). Numerous clinical studies have correlated the c.516G>T polymorphism with high efavirenz plasma concentrations (Owen et al., 2006; Rotger et al., 2006; Mukonzo et al., 2009). HIV-infected patients homozygous for CYP2B6*6 have been shown to be at an increased risk for neurotoxicity because of elevated plasma levels of efavirenz (Telenti and Zanger, 2008).
To establish genotype–phenotype correlations, it is important to understand how CYP2B6 polymorphisms may affect the enzyme activity of CYP2B6. So far, systematic functional characterizations of the CYP2B6 polymorphic variants have been scarce, in part because of the low levels of protein expression in heterologous expression systems for some of the CYP2B6 alleles. Nonetheless, Jinno et al. (2003) reported that the 7-EFC O-deethylase activities of the variants CYP2B6.2 to CYP2B6.7 were increased over that of CYP2B6.1 by using recombinant CYP2B6 preparations from mammalian COS-1 cells. Recently, 26 allelic variants of CYP2B6 (CYP2B6.2–CYP2B6.28 except for CYP2B6.22) were heterologously expressed in mammalian COS-7 cells and their kinetic parameters for the metabolism of 7-EFC and selegiline were reported (Watanabe et al., 2010). Those authors depicted a much more complex picture with respect to the effects of the various amino acid substitutions on the catalytic activities of CYP2B6 polymorphic variants. However, the metabolism of bupropion and efavirenz by the majority of these polymorphic variants has not been characterized in detail and the mechanisms by which the CYP2B6 variants affect the functional activity of CYP2B6 remain poorly understood.
In this study, we investigated the metabolism of buproprion, efavirenz, and 7-EFC by six polymorphic variants of CYP2B6 (CYP2B6.4–CYP2B6.9) in a reconstituted system to examine their catalytic properties. CYP2B6 WT and the six polymorphic variants were each overexpressed in Escherichia coli and purified to homogeneity to give active forms of CYP2B6. Measurements of the steady-state turnover rates for bupropion, efavirenz, and 7-EFC demonstrated that these mutations significantly alter the catalytic activities of CYP2B6. It is noteworthy that CYP2B6.8 completely lost its ability to metabolize these substrates under steady-state conditions in the reconstituted system even though it retained a functional heme. This loss of activity observed in the K139E variant has been attributed to the fact that this variant is incapable of accepting electrons from CPR. Thus it can be concluded that the charge-reversal mutation in the K139E variant abolishes its ability to interact with CPR to form a functional complex, leading to permanent loss of catalytic activities regardless of the substrates used.
Materials and Methods
Mutagenesis, Overexpression, and Purification of the CYP2B6.4 to CYP2B6.9 Polymorphic Variants.
Site-directed mutagenesis was performed using the Quikchange method according to the manufacturer's instructions (Agilent Technologies, Santa Clara, CA). The single mutants, CYP2B6.4, CYP2B6.5, CYP2B6.8, and CYP2B6.9, were prepared using the plasmid of CYP2B6 WT as the template and a pair of complementary mutagenic primers containing the desired mutations as shown in Table 1. The double mutant CYP2B6.6 was prepared using the plasmid of CYP2B6.4 as the template and the mutagenic primers of CYP2B6.9, whereas the triple mutant CYP2B6.7 was prepared using the plasmid of CYP2B6.6 and the mutagenic primers of CYP2B6.5. To confirm these mutations, the entire DNA coding regions of all of the six polymorphic mutants were sequenced at the Biomedical Core Facilities at the University of Michigan.
TABLE 1.
The forward mutagenic primers used for preparation of the polymorphic mutants of CYP2B6
The base pairs that contain the desired mutations are underlined. The single mutants were prepared using the plasmid of CYP2B6*1 as templates, whereas the double and triple mutants were prepared using the plasmids of CYP2B6*4 and CYP2B6*6 as templates, respectively, as described under Materials and Methods.
| CYP2B6 | Amino Acid | Mutagenic Primers |
|---|---|---|
| CYP2B6*4 | K262R | GACCCCAGCGCCCCCCGCGACCTCATCGACACC |
| CYP2B6*5 | R487C | CAACATACCAGATCTGCTTCCTGCCCCGC |
| CYP2B6*6 | Q172H/K262R | CYP2B6*4 + GACCCCACCTTCCTCTTCCATTCCATTACCGC |
| CYP2B6*7 | Q172H/K262R/R487C | CYP2B6*6 + CAACATACCAGATCTGCTTCCTGCCCCGC |
| CYP2B6*8 | K139E | AGGGACTTCGGGATGGGAGAACGGAGTGTGGAG |
| CYP2B6*9 | Q172H | GACCCCACCTTCCTCTTCCATTCCATTACCGC |
CYP2B6 WT and the six variants were overexpressed individually in E. coli C41(DE3) cells and purified as reported previously (Zhang et al., 2009), but with some modifications. The major modification was that the CYP2B6 gene was subcloned into the pLW01 vector containing a T7 promoter as reported by Bridges et al. (1998). CYP2B6 WT and the six variants were expressed as an N-terminally truncated form to increase the expression yield as reported previously (Hanna et al., 2000).
Metabolism of 7-EFC, Bupropion, and Efavirenz by the CYP2B6.4 to CYP2B6.9 Polymorphic Variants.
Metabolism of 7-EFC, bupropion, and efavirenz by CYP2B6 WT and the six polymorphic variants was carried out in a reconstituted system. Dilauroylphosphatidylcholine was absent from the reconstituted systems because it was not required for the catalysis of the truncated form of CYP2B6 (Scott et al., 2001). Measurements of the kinetic parameters, Km and kcat, for the 7-EFC O-deethylase activities were performed on a 96-well microtiter plate as reported previously (Kenaan et al., 2010). CYP2B6 WT and the six polymorphic variants (37.5 pmol) were reconstituted with 75 pmol of CPR on ice for 45 min. The reconstituted enzymes were then supplemented with catalase (15 units) and diluted with 50 mM potassium phosphate buffer, pH 7.4, to a final volume of 150 μl. The final concentrations of 7-EFC varied in the range of 0 to 80 μM. The mixture was allowed to equilibrate at 37°C for 10 min. NADPH was then added to give a final concentration of 0.3 mM to initiate the reactions. The reactions were then terminated after 6 min of incubation by the addition of 50 μl of acetonitrile. The fluorescence emission from the O-deethylated product, 7-hydroxy-4-trifluoromethylcoumarin (7-HFC), was measured at 510 nm with excitation at 410 nm using a VictorII fluorescence plate reader (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Unlike for 7-EFC, the metabolism of bupropion was performed in glass test tubes. After the reconstitution as described above, the reconstituted enzymes were then supplemented with catalase (15 units) and diluted with 50 mM potassium phosphate buffer, pH 7.4, to a final volume of 130 μl. The mixture was allowed to equilibrate at 37°C for 10 min, and 100 μl of the mixture was transferred into the assay buffer containing 0 to 1 mM bupropion and 1 mM NADPH in 50 mM HEPES buffer. The reaction mixture was incubated at 37°C for 30 min, and the metabolism was terminated by adding an equal volume of ice-cold acetonitrile containing 0.1% formic acid. Testosterone (400 pmol) was spiked into each sample as an internal standard. The samples were then centrifuged at 16,000g for 10 min to pellet the proteins. A 100-μl aliquot of the supernatant was loaded onto a RP C18 column (150 × 2.00 mm, Aqua 5μ 125Å; Phenomenex, Torrance, CA). The analytes and internal standards were separated and eluted at a flow rate of 0.3 ml/min on a Shimadzu (Kyoto, Japan) high-performance liquid chromatography system with a gradient of solvent A (0.1% TFA in water) and solvent B (0.1% TFA in acetonitrile): starting with 2% B for 10 min, linearly increasing to 70% B over 20 min, and then held at 70% B for 4 min. The analytes were detected at 214 nm using an ultraviolet detector.
The metabolism of efavirenz was determined using substrate concentrations of 0 to 250 μM as described for bupropion. Efavirenz and its metabolites were resolved on a RP C18 column (Varian Microsorb-MV, 4.6 × 250 mm; Varian, Inc., Palo Alto, CA) isocratically with 45% acetonitrile/55% water containing 0.1% TFA at a flow rate of 0.8 ml/min.
The kinetic parameters for the metabolism of 7-EFC, bupropion, and efavirenz were obtained by fitting the activities obtained at the various substrate concentrations to the Michaelis-Menten equation using Prism software (GraphPad Software Inc., San Diego, CA).
tert-Butyl Hydroperoxide-Supported 7-EFC O-Deethylase Activities of the CYP2B6.4 to CYP2B6.9 Polymorphic Variants.
To examine the effect of mutations on the catalytic activity of CYP2B6 alone, we determined the 7-EFC O-deethylase activities of the polymorphic variants in the absence of CPR, but in the presence of the alternative oxidant tBHP. The reactions were carried out on a microtiter plate in 0.15 ml of 0.1 M potassium phosphate solution, pH 7.4. Each well of the plate contained CYP2B6 (0.38 μM) and a saturating amount of 7-EFC (38 μM). tBHP was added to a final concentration of 0.69 mM to initiate the reaction. The reaction was incubated at 37°C for 20 min, and the fluorescence of 7-HFC was measured using a VictorII plate reader as described above.
The Rate of Electron Transfer from CPR to the Ferric CYP2B6.4 to CYP2B6.9 Polymorphic Variants.
The rate of electron transfer from CPR to the ferric CYP2B6 WT and polymorphic variants was determined at 25°C using an SF61DX2 stopped-flow spectrophotometer (TgK Scientific, Bradford-on-Avon, UK) as described previously (Zhang et al., 2009). To preform the P450–CPR complex, equimolar CYP2B6 and CPR (3 μM each) were incubated on ice overnight in 0.1 M potassium phosphate solution, pH 7.4. The preformed complex was rapidly mixed in the stopped-flow spectrophotometer with an equal volume of 0.1 M potassium phosphate solution, pH 7.4, containing 0.3 mM NADPH. Both solutions had been saturated with carbon monoxide. The kinetics of electron transfer were monitored at 450 nm. The rate constants were obtained by fitting the kinetic traces at 450 nm to exponential functions using KinetAsyst software (TgK Scientific).
Results
Overexpression of the CYP2B6.4 to CYP2B6.9 Polymorphic Variants in E. coli.
Unlike the previous reports where CYP2B6 polymorphic variants were overexpressed in mammalian COS cells or baculovirus-infected insect cells (Jinno et al., 2003; Lang et al., 2004; Watanabe et al., 2010), the six polymorphic variants used in this study were alternatively overexpressed in E. coli and purified to homogeneity. The expression yields were approximately 50–250 nmol per liter of culture (data not shown). Unexpectedly, CYP2B6.8 (the K139E variant), which could not be expressed in mammalian COS-1 cells (Lang et al., 2004), was well expressed in E. coli. The carbonmonoxyl forms of the purified CYP2B6 variants exhibited maximal absorption at 450 nm as shown in Fig. 1. All of the variants except CYP2B6.8 contained minimal amounts of P420 (<10%), the inactive form of P450. It was estimated that CYP2B6.8 contained ∼46% of P420 based on the extinction coefficients of P450 and P420 species as reported by Omura and Sato (1964).
Fig. 1.
The UV-visible difference spectra of the carbonmonoxyl forms of the purified CYP2B6.4 to CYP2B6.9 polymorphic variants. The difference spectra were recorded according the method of Omura and Sato (1964). CYP2B6 WT and variants were reduced with a few grains of dithionite in 0.1 M potassium phosphate buffer, pH 7.4, containing 15% glycerol. The concentrations of CYP2B6 WT and CYP2B6.4 to CYP2B6.9 were 0.41, 0.28, 0.62, 0.60, 0.27, 0.32, and 0.53 μM, respectively.
Steady-State Activities of the CYP2B6.4 to CYP2B6.9 Polymorphic Variants for the Metabolism of 7-EFC, Bupropion, and Efavirenz.
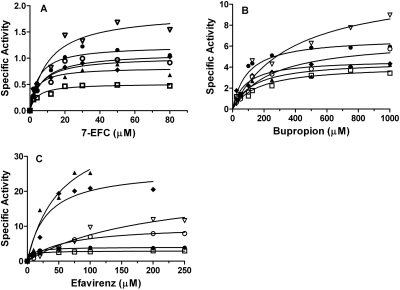
To functionally characterize the six polymorphic variants, the metabolism of three probe substrates, 7-EFC, bupropion, and efavirenz, was investigated. The results are shown in Fig. 2, and the kinetic parameters are summarized in Table 2. As shown in Fig. 2A, all six of the variants except CYP2B6.8 catalyzed the O-deethylation of 7-EFC with decreased catalytic efficiencies as judged by the ratios of kcat/Km (Table 2). The major change in the kinetic parameter was a 2-fold increase in the Km for CYP2B6.6. The steady-state 7-EFC O-deethylase activity of CYP2B6.8 was not detected, as reported previously (Watanabe et al., 2010).
Fig. 2.
The steady-state activities of the CYP2B6.4 to CYP2B6.9 polymorphic variants for the O-deethylation of 7-EFC (A), hydroxylation of bupropion (B), and hydroxylation of efavirenz (C). The activities, expressed as nanomoles of product/minute/nanomoles of P450, were determined at 37°C under steady-state turnover conditions as described under Materials and Methods. The products are 7-HFC, hydroxybupropion, and 8-hydroxyefavirenz for 7-EFC, bupropion, and efavirenz, respectively. ●, CYP2B6.1; □, CYP2B6.4; ▴, CYP2B6.5; ▿, CYP2B6.6; ♦, CYP2B6.7; ○, CYP2B6.9. The solid lines are fits to the Michaelis-Menten equation using Prism 5 (GraphPad Software, Inc.).
TABLE 2.
The Km and kcat for the metabolism of 7-EFC, bupropion, and efavirenz by CYP2B6 WT and CYP2B6.4 to CYP2B6.9 polymorphic variants
The Km and kcat values were obtained by fitting the data shown in Fig. 2 to the Michaelis-Menten equation as described under Materials and Methods. The reported Km and kcat were averages from two separate experiments, each of which was performed in duplicates.
| CYP2B6 | 7-EFC |
Bupropion |
Efavirenz |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Km | kcat | kcat/Km | Km | kcat | kcat/Km | Km | kcat | kcat/Km | |
| μM | min−1 | μM | min−1 | μM | min−1 | ||||
| CYP2B6.1 | 4.8 ± 1.1 | 1.2 ± 0.06 | 0.26 | 95 ± 15 | 6.8 ± 1.1 | 0.072 | 7.3 ± 2.2 | 4.0 ± 0.7 | 0.55 |
| CYP2B6.4 | 3.9 ± 0.42 | 0.52 ± 0.04 | 0.13 | 162 ± 32 | 4.1 ± 0.7 | 0.025 | 5.5 ± 0.8 | 2.9 ± 0.4 | 0.53 |
| CYP2B6.5 | 4.9 ± 1.9 | 0.89 ± 0.04 | 0.18 | 134 ± 7 | 4.5 ± 1.5 | 0.034 | 53 ± 12 | 40.2 ± 4.0 | 0.76 |
| CYP2B6.6 | 9.2 ± 2.1 | 1.7 ± 0.14 | 0.18 | 380 ± 27 | 11.9 ± 0.4 | 0.031 | 198 ± 28 | 22.5 ± 1.4 | 0.11 |
| CYP2B6.7 | 6.1 ± 2.1 | 1.0 ± 0.08 | 0.16 | 83 ± 10 | 4.7 ± 1.2 | 0.057 | 30.4 ± 5.0 | 26.3 ± 7.0 | 0.86 |
| CYP2B6.8 | N.D. | N.D. | N.D. | ||||||
| CYP2B6.9 | 7.0 ± 1.6 | 1.1 ± 0.07 | 0.16 | 244 ± 11 | 6.7 ± 0.9 | 0.027 | 57.5 ± 9.0 | 10.2 ± 2.0 | 0.18 |
N.D., not detected.
Bupropion and efavirenz are hydroxylated by CYP2B6 to give hydroxybupropion and 8-hydroxyefavirenz, respectively, and both hydroxylation reactions are specific for CYP2B6 (Faucette et al., 2000; Ward et al., 2003). Thus bupropion and efavirenz may be considered more appropriate substrates than 7-EFC for evaluation of the effects of mutations on the functional activities of the CYP2B6 polymorphic variants. In the case of bupropion, CYP2B6 WT hydroxylates it with Km and kcat values of 95 μM and 6.8 min−1, respectively, as shown in Fig. 2B and Table 2. This Km value was in the range of 65 to 155 μM as reported for CYP2B6 WT (Faucette et al., 2000; Lang et al., 2004). However, the six polymorphic variants exhibited significantly altered activities for the hydroxylation of bupropion. As observed for 7-EFC, CYP2B6.8 exhibited no ability to hydroxylate bupropion. Among the other variants, CYP2B6.4, CYP2B6.5, CYP2B6.6, and CYP2B6.9 had decreased catalytic efficiencies for bupropion hydroxylation compared with CYP2B6.1. In particular, CYP2B6.6 demonstrated the largest increases in Km and kcat values by 4- and 1.8-fold, respectively, which led to a 60% decrease in the catalytic efficiency. It is noteworthy that CYP2B6.7, which contains three amino acid substitutions (Q172H/K262R/R487C), showed the least alteration in the hydroxylation of bupropion.
To examine the effects of the genetic mutations on efavirenz metabolism, we determined the steady-state activity for the formation of 8-hydroxyefavirenz, the major metabolite of efavirenz formed by CYP2B6 (Ward et al., 2003). As shown in Fig. 2C, the impact of these genetic mutations on the catalytic activities for efavirenz hydroxylation seems more variable than 7-EFC and bupropion. The Km and kcat values of CYP2B6.1 are 7.3 μM and 4.0 min−1, respectively, which are similar to the Km and kcat values of 12.4 μM and 5.2 min−1 as reported for recombinant CYP2B6 by Ward et al. (2003). However, the Km and kcat values of some of the variants show very large deviations from those of the wild type. It is noteworthy that 5- and 6-fold increases in kcat were observed for CYP2B6.5 and CYP2B6.7, respectively, which led to ∼50% increase in the catalytic efficiencies for efavirenz hydroxylation. This increase in the catalytic efficiency was not observed for either of the probe substrates, 7-EFC and bupropion. As observed previously, CYP2B6.6 and CYP2B6.9 showed decreased catalytic efficiencies and the decreases were substantial. Compared with the wild type, the kcat/Km values of CYP2B6.6 and CYP2B6.9 for efavirenz metabolism decreased by 5- and 3-fold, respectively. This is primarily caused by the increase in the Km values. CYP2B6.6 shows a 27-fold increase in the Km compared with that of CYP2B6.1, which is the largest increase in the Km value in this study. Once again, CYP2B6.8 shows no activity for efavirenz hydroxylation.
7-EFC O-Deethylase Activities of the CYP2B6.4 to CYP2B6.9 Polymorphic Variants Supported by tert-Butyl Hydroperoxide.
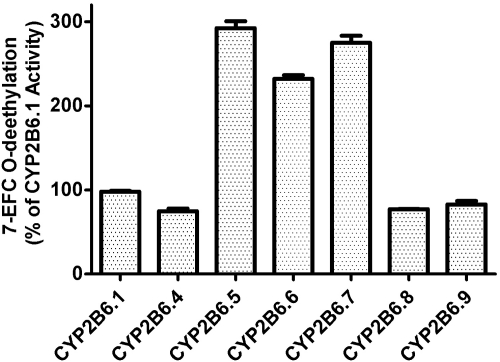
To investigate the effects of the genetic mutations on the heme and the active site of the variants, we used tBHP as an alternative oxidant to support the catalytic activity of CYP2B6, bypassing the electron transfer process involving CPR. The relative 7-EFC O-deethylase activities of CYP2B6.1 and the six variants are presented in Fig. 3. As shown, all the variants are functional in O-deethylating 7-EFC, indicating that the heme and the active site environment of the variants are not adversely affected by these mutations. In fact, CYP2B6.5, CYP2B6.6, and CYP2B6.7 showed ∼2.5-fold increases in the 7-EFC O-deethylase activities. We were surprised to find that CYP2B6.8, which does not exhibit any catalytic activities in the reconstituted system in the presence of NADPH, retained 77% of the 7-EFC O-deethylase activity when supported by the alternative oxidant tBHP. This result demonstrates that the heme and the active site environment of CYP2B6.8 remain largely functional, suggesting that the loss of activities observed under steady-state conditions is caused by impaired electron transfer from CPR.
Fig. 3.
The tert-butyl hydroperoxide-supported 7-EFC O-deethylase activities of the CYP2B6.4 to CYP2B6.9 polymorphic variants. The 7-EFC O-deethylase activity is expressed as the activity relative to that of CYP2B6.1. The details for the measurements of the 7-EFC O-dethylase activities in the presence of tBHP are provided under Materials and Methods.
Rates of Electron Transfer from CPR to the Ferric CYP2B6.4 to CYP2B6.9 Polymorphic Variants.
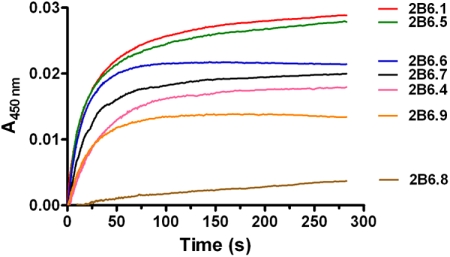
To further investigate the causes for the alterations in the catalytic activities of these polymorphic variants, we investigated the rate of electron transfer from CPR to ferric CYP2B6, a critical step before substrate hydroxylation in the catalytic cycle of P450s. This is especially relevant to understand why CYP2B6.8 loses its catalytic activity when it retains a functional heme and active site. The kinetic traces for the electron transfer are depicted in Fig. 4, and the rate constants and relative amplitudes for the fast and slow phases are summarized in Table 3. As shown, CPR transfers an electron to ferric CYP2B6.1 in the presence of NADPH with a biphasic rate constants of 0.31 (23%) and 0.04 s−1 (67%) for the fast and slow phases, respectively. These rate constants are similar to those reported previously for CYP2B4 in the absence of benzphetamine (Zhang et al., 2009). However, the rate constants for the variants were decreased to various extents compared with the wild type. The most dramatic change was observed with CYP2B6.8. The monophasic rate constant for CYP2B6.8 was 0.01 s−1, which is approximately ∼30-fold slower than that of CYP2B6.1. The other five variants also showed decreases ranging from 3- to 7-fold in the rate constants for the fast phase.
Fig. 4.
Kinetics of electron transfer from CPR to the ferric CYP2B6.4 to CYP2B6.9 polymorphic variants. The kinetics were determined in the absence of substrate at 25°C in a stopped-flow spectrophotometer as described under Materials and Methods. The final concentrations of CYP2B6 and CPR were 1.5 μM each. The kinetic traces from the top to the bottom are those for CYP2B6.1, CYP2B6.5, CYP2B6.6, CYP2B6.7, CYP2B6.4, CYP2B6.9, and CYP2B6.8.
TABLE 3.
The kinetic parameters for the rate of electron transfer from CPR to the ferric CYP2B6.4 to CYP2B6.9 polymorphic variants
k1 and k2 are rate constants for the fast and slow phases, respectively, and A1 and A2 are their respective relative amplitudes. The data were averaged from two separate measurements with S.D. <10%.
| CYP2B6 | Kinetic Parameters |
|||
|---|---|---|---|---|
| A1 | k1 | A2 | k2 | |
| % | s−1 | % | s−1 | |
| CYP2B6.1 | 23 | 0.31 | 67 | 0.04 |
| CYP2B6.4 | 78 | 0.04 | 22 | 0.01 |
| CYP2B6.5 | 51 | 0.13 | 49 | 0.02 |
| CYP2B6.6 | 36 | 0.11 | 64 | 0.04 |
| CYP2B6.7 | 66 | 0.08 | 54 | 0.02 |
| CYP2B6.8 | 100 | 0.01 | ||
| CYP2B6.9 | 8 | 0.42 | 92 | 0.04 |
Discussion
In this work, we have characterized the catalytic properties of six polymorphic variants of CYP2B6 (CYP2B6.4 to CYP2B6.9) in a reconstituted system to gain a better understanding of the mechanism by which these genetic mutations affect the catalytic activities of CYP2B6. Unlike previous in vitro studies where CYP2B6 variants were overexpressed in mammalian COS cells (Jinno et al., 2003; Lang et al., 2004; Watanabe et al., 2010), the six polymorphic variants of CYP2B6 in this study were alternatively overexpressed in E. coli and were used to determine Km and kcat values for the metabolism of three probe substrates, i.e., 7-EFC, bupropion, and efavirenz. This is the first systematic investigation of the in vitro metabolism of bupropion and efavirenz by the CYP2B6.4 to CYP2B6.9 variants under the same experimental conditions. Therefore, the effects of these mutations on the functionality of CYP2B6 can be directly compared.
Our studies showed that variants CYP2B6.4 to CYP2B6.9 exhibit altered catalytic activities for the metabolism of the three probe substrates, and the extent to which the catalytic activities are altered varies not only with individual mutations but also with substrate tested. The six polymorphic variants show the least variation in the metabolism of 7-EFC based on two observations; 1) the largest variation in either Km or kcat is less than 2-fold compared with that of the wild type; and 2) all six variants show an approximately 2-fold or less decrease in the catalytic efficiencies (see Table 2). The results with bupropion are approximately similar. However, the alteration in the metabolism of efavirenz by these variants seems much more pronounced and unpredictable. For instance, the Km and kcat of CYP2B6.6 are increased substantially by 27- and 5.6-fold, respectively, compared with the CYP2B6 WT. Consequently, this large increase in the Km results in the lowest catalytic efficiency of CYP2B6.6 for efavirenz metabolism. It can be anticipated that this low catalytic efficiency resulting from the large increase in the Km would substantially decrease the rate of metabolism for efavirenz at the effective plasma concentration of efavirenz, which has been estimated to be ∼3 to 12 μM (Zanger et al., 2007). This relatively narrow range of effective plasma concentration for efavirenz is one of the factors thought to be responsible for the significant interindividual variability in the clinical efficacy of efavirenz among HIV-infected patients. Similar to CYP2B6.6, CYP2B6.9 also shows significantly decreased catalytic efficiency. However, CYP2B6.5 and CYP2B6.7 show modest gains in the catalytic efficiencies, which are the only gains in catalytic efficiency observed in this study. The increased enzyme activity of CYP2B6.5 for the hydroxylation of efavirenz may account for the clinical observation that the efavirenz plasma concentrations for CYP2B6.5 carriers do not increase significantly even though the protein level of CYP2B6.5 may be decreased by up to 8-fold (Lang et al., 2001; Desta et al., 2007). It is reasonable to assume that the increased enzyme activity of CYP2B6.5 compensates for the decrease in its protein expression level.
The extent of the alteration in the metabolism of bupropion by the six polymorphic variants is somewhere between those observed for 7-EFC and efavirenz. All of the variants show decreased catalytic efficiencies in the hydroxylation of bupropion with CYP2B6.4 and CYP2B6.9 being the least efficient.
The most dramatic impact of the amino acid substitutions on the function of the CYP2B6 variants investigated in this study was observed for CYP2B6.8 (the K139E variant). This variant contains a charge-reversal mutation of Lys139 to Glu. As a result, it loses the catalytic ability to metabolize any of the three substrates. It is noteworthy that the K139E variant is still functional in O-deethylating 7-EFC in the presence of the alternative oxidant tBHP, indicating that the active site of the K139E variant remains largely intact in spite of the mutation. Further investigation revealed that the electron transfer from CPR to the ferric K139E variant is severely impaired (see Fig. 4). This provides conclusive evidence that the loss of the catalytic activity in the K139E variant is caused by the impaired interactions between the K139E variant and CPR, not by modification of the heme or active site. According to the crystal structure of CYP2B6 (Gay et al., 2010), Lys139 is located in the C/D loop region that is known to be involved in the binding of the redox partners of P450. Site-directed mutagenic studies of the interaction between CPR and CYP2B4 revealed that charge-pairing plays an important role in the P450-reductase interaction (Bridges et al., 1998). Those authors identified that a few positively charged residues, including Lys433, Lys139, Arg133, and Arg422, interact with negatively charged Asp or Glu residues on the reductase, contributing to a large extent to the formation of the P450-reductase complex. Furthermore mutation of the Lys139 residue to an alanine led to a 23-fold decrease in the binding affinity for the P450-reductase complex. It is conceivable that the charge-reversal mutation in the K139E variant of CYP2B6 would negate the formation of the complex in a more dramatic fashion because of charge repulsion. Because the loss of activity of CYP2B6.8 occurs before the oxidation of substrates as evidenced by the stopped-flow studies (see Fig. 4), it is clear that CYP2B6.8 must be a phenotypic null variant regardless of other factors that might influence enzyme activities such as transcription, steady-state protein level, identity of the substrates, etc.
As to the other polymorphic variants, the amino acid substitutions as a result of the genetic mutations exert complex effects on the metabolism of substrates. Not only do some of the substitutions alter the interactions of CYP2B6 with CPR, but also affect the active site architecture as evidenced by the tBHP-supported activities (Fig. 3). This complexity is confounded by the fact that these changes are substrate-dependent. More studies are required to investigate the effects of these and other polymorphisms on the functionality of CYP2B6 both in vivo and in vitro to establish a genotype-phenotype correlation to improve our understanding of the efficacy of clinically used drugs primarily metabolized by CYP2B6.
In conclusion, the amino acid substitutions in the CYP2B6.4 to CYP2B6.9 variants significantly alter the metabolism of bupropion and efavirenz. They not only affect the active-site architecture, but also affect the interactions with the P450 reductase as in the case of CYP2B6.8. Based on the functional characterization of the K139E variant, it can be concluded that the charge-reversal mutation in the K139E variant impairs the formation of a functional P450-reductase complex, leading to permanent loss of catalytic activity regardless of the nature of substrates.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA16945] (to P.F.H.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183111.
- 7-EFC
- 7-ethoxy-4-trifluoromethylcoumarin
- 7-HFC
- 7-hydroxy-4-trifluoromethylcoumarin
- P450
- cytochrome P450
- P420
- cytochrome P420
- CPR
- NADPH-dependent cytochrome P450 reductase
- tBHP
- tert-butyl hydroperoxide
- WT
- wild type
- TFA
- trifluoroacetic acid.
Authorship Contributions
Participated in research design: Zhang and Sridar.
Conducted experiments: Zhang, Sridar, Kenaan, and Amunugama.
Contributed new reagents or analytic tools: Ballou.
Wrote or contributed to the writing of the manuscript: Zhang, Sridar, Ballou, and Hollenberg.
References
- Bridges A, Gruenke L, Chang YT, Vakser IA, Loew G, Waskell L. (1998) Identification of the binding site on cytochrome P450 2B4 for cytochrome b5 and cytochrome P450 reductase. J Biol Chem 273:17036–17049 [DOI] [PubMed] [Google Scholar]
- Bumpus NN, Sridar C, Kent UM, Hollenberg PF. (2005) The naturally occurring cytochrome P450 (P450) 2B6 K262R mutant of P450 2B6 exhibits alterations in substrate metabolism and inactivation. Drug Metab Dispos 33:795–802 [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu HF, Liu L, Nguyen K, Jones EB, Fretland AJ. (2010) The in vitro metabolism of bupropion revisited: concentration dependent involvement of cytochrome P450 2C19. Xenobiotica 40:536–546 [DOI] [PubMed] [Google Scholar]
- Davaalkham J, Hayashida T, Tsuchiya K, Gatanaga H, Nyamkhuu D, Oka S. (2009) Allele and genotype frequencies of cytochrome P450 2B6 gene in a Mongolian population. Drug Metab Dispos 37:1991–1993 [DOI] [PubMed] [Google Scholar]
- Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, Flockhart DA, Zanger UM. (2007) Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 8:547–558 [DOI] [PubMed] [Google Scholar]
- Ekins S, Vandenbranden M, Ring BJ, Gillespie JS, Yang TJ, Gelboin HV, Wrighton SA. (1998) Further characterization of the expression in liver and catalytic activity of CYP2B6. J Pharmacol Exp Ther 286:1253–1259 [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230 [PubMed] [Google Scholar]
- Gay SC, Shah MB, Talakad JC, Maekawa K, Roberts AG, Wilderman PR, Sun L, Yang JY, Huelga SC, Hong WX, et al. (2010) Crystal structure of a cytochrome P450 2B6 genetic variant in complex with the inhibitor 4-(4-chlorophenyl)imidazole at 2.0-Å resolution. Mol Pharmacol 77:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S, Huang M, Li X, Chen X, Chan E, Zhou SF. (2006) Intra- and inter-ethnic differences in the allele frequencies of cytochrome P450 2B6 gene in Chinese. Pharm Res 23:1983–1990 [DOI] [PubMed] [Google Scholar]
- Hanna IH, Reed JR, Guengerich FP, Hollenberg PF. (2000) Expression of human cytochrome P450 2B6 in Escherichia coli: characterization of catalytic activity and expression levels in human liver. Arch Biochem Biophys 376:206–216 [DOI] [PubMed] [Google Scholar]
- Hofmann MH, Blievernicht JK, Klein K, Saussele T, Schaeffeler E, Schwab M, Zanger UM. (2008) Aberrant splicing caused by single nucleotide polymorphism c. 516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther 325:284–292 [DOI] [PubMed] [Google Scholar]
- Jacob RM, Johnstone EC, Neville MJ, Walton RT. (2004) Identification of CYP2B6 sequence variants by use of multiplex PCR with allele-specific genotyping. Clin Chem 50:1372–1377 [DOI] [PubMed] [Google Scholar]
- Jinno H, Tanaka-Kagawa T, Ohno A, Makino Y, Matsushima E, Hanioka N, Ando M. (2003) Functional characterization of cytochrome P450 2B6 allelic variants. Drug Metab Dispos 31:398–403 [DOI] [PubMed] [Google Scholar]
- Kenaan C, Zhang H, Hollenberg PF. (2010) A quantitative high-throughput 96-well plate fluorescence assay for mechanism-based inactivators of cytochromes P450 exemplified using CYP2B6. Nat Protoc 5:1652–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Klein K, Fischer J, Nüssler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM. (2001) Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 11:399–415 [DOI] [PubMed] [Google Scholar]
- Lang T, Klein K, Richter T, Zibat A, Kerb R, Eichelbaum M, Schwab M, Zanger UM. (2004) Multiple novel nonsynonymous CYP2B6 gene polymorphisms in Caucasians: demonstration of phenotypic null alleles. J Pharmacol Exp Ther 311:34–43 [DOI] [PubMed] [Google Scholar]
- Mehlotra RK, Ziats MN, Bockarie MJ, Zimmerman PA. (2006) Prevalence of CYP2B6 alleles in malaria-endemic populations of West Africa and Papua New Guinea. Eur J Clin Pharmacol 62:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukonzo JK, Röshammar D, Waako P, Andersson M, Fukasawa T, Milani L, Svensson JO, Ogwal-Okeng J, Gustafsson LL, Aklillu E. (2009) A novel polymorphism in ABCB1 gene, CYP2B6*6 and sex predict single-dose efavirenz population pharmacokinetics in Ugandans. Br J Clin Pharmacol 68:690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T, Sato R. (1964) The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J Biol Chem 239:2379–2385 [PubMed] [Google Scholar]
- Owen A, Pirmohamed M, Khoo SH, Back DJ. (2006) Pharmacogenetics of HIV therapy. Pharmacogenet Genomics 16:693–703 [DOI] [PubMed] [Google Scholar]
- Rotger M, Csajka C, Telenti A. (2006) Genetic, ethnic, and gender differences in the pharmacokinetics of antiretroviral agents. Curr HIV/AIDS Rep 3:118–125 [DOI] [PubMed] [Google Scholar]
- Scott EE, Spatzenegger M, Halpert JR. (2001) A truncation of 2B subfamily cytochromes P450 yields increased expression levels, increased solubility, and decreased aggregation while retaining function. Arch Biochem Biophys 395:57–68 [DOI] [PubMed] [Google Scholar]
- Shebley M, Hollenberg PF. (2007) Mutation of a single residue (K262R) in P450 2B6 leads to loss of mechanism-based inactivation by phencyclidine. Drug Metab Dispos 35:1365–1371 [DOI] [PubMed] [Google Scholar]
- Talakad JC, Kumar S, Halpert JR. (2009) Decreased susceptibility of the cytochrome P450 2B6 variant K262R to inhibition by several clinically important drugs. Drug Metab Dispos 37:644–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A, Zanger UM. (2008) Pharmacogenetics of anti-HIV drugs. Annu Rev Pharmacol Toxicol 48:227–256 [DOI] [PubMed] [Google Scholar]
- Walsky RL, Astuccio AV, Obach RS. (2006) Evaluation of 227 drugs for in vitro inhibition of cytochrome P450 2B6. J Clin Pharmacol 46:1426–1438 [DOI] [PubMed] [Google Scholar]
- Wang H, Tompkins LM. (2008) CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab 9:598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. (2003) The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 306:287–300 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sakuyama K, Sasaki T, Ishii Y, Ishikawa M, Hirasawa N, Hiratsuka M. (2010) Functional characterization of 26 CYP2B6 allelic variants (CYP2B6.2-CYP2B6.28, except CYP2B6.22). Pharmacogenet Genomics 20:459–462 [DOI] [PubMed] [Google Scholar]
- Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann MH, Schwab M. (2007) Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics 8:743–759 [DOI] [PubMed] [Google Scholar]
- Zhang H, Lin HL, Walker VJ, Hamdane D, Hollenberg PF. (2009) tert-Butylphenylacetylene is a potent mechanism-based inactivator of cytochrome P450 2B4: inhibition of cytochrome P450 catalysis by steric hindrance. Mol Pharmacol 76:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]