Abstract
We assessed the relationship between oxidative stress, cytokinetic parameters, and tumor growth in response to novel phospho-nonsteroidal anti-inflammatory drugs (NSAIDs), agents with significant anticancer effects in preclinical models. Compared with controls, in SW480 colon and MCF-7 breast cancer cells, phospho-sulindac, phospho-aspirin, phospho-flurbiprofen, and phospho-ibuprofen (P-I) increased the levels of reactive oxygen and nitrogen species (RONS) and decreased GSH levels and thioredoxin reductase activity, whereas the conventional chemotherapeutic drugs (CCDs), 5-fluorouracil (5-FU), irinotecan, oxaliplatin, chlorambucil, paclitaxel, and vincristine, did not. In both cell lines, phospho-NSAIDs induced apoptosis and inhibited cell proliferation much more potently than CCDs. We then treated nude mice bearing SW480 xenografts with P-I or 5-FU that had an opposite effect on RONS in vitro. Compared with controls, P-I markedly suppressed xenograft growth, induced apoptosis in the xenografts (8.9 ± 2.7 versus 19.5 ± 3.0), inhibited cell proliferation (52.6 ± 5.58 versus 25.8 ± 7.71), and increased urinary F2-isoprostane levels (10.7 ± 3.3 versus 17.9 ± 2.2 ng/mg creatinine, a marker of oxidative stress); all differences were statistically significant. 5-FU's effects on tumor growth, apoptosis, proliferation, and F2-isoprostane were not statistically significant. F2-isoprostane levels correlated with the induction of apoptosis and the inhibition of cell growth. P-I induced oxidative stress only in the tumors, and its apoptotic effect was restricted to xenografts. Our data show that phospho-NSAIDs act against cancer through a mechanism distinct from that of various CCDs, underscore the critical role of oxidative stress in their effect, and indicate that pathways leading to oxidative stress may be useful targets for anticancer strategies.
Introduction
NSAIDs have emerged as significant chemopreventive agents against several cancers (Crew and Neugut, 2006; Baron, 2009). Their limited efficacy and appreciable side effects have motivated several groups to modify the structure of NSAIDs to enhance their efficacy and/or decrease their side effects. Such modifications have centered on covalently modifying NSAIDs at their –COOH moiety, which is a structural feature of nearly all of them (Piazza et al., 2009). We have worked with two classes of modified NSAIDs, namely nitric oxide-donating NSAIDs and phospho-NSAIDs; the chemopreventive efficacy of both has been demonstrated in preclinical models (Rigas, 2007; Zhao et al., 2009; Huang et al., 2010; Mackenzie et al., 2010).
Significant work in the last decade has attempted to unravel the mechanism of action of modified NSAIDs. Modulation by modified NSAIDs of several signaling pathways, some of them critical to the survival of the neoplastic cell, has been reported to mediate their chemopreventive effect (Rigas, 2007). It seems that although such changes are important, none of them represents a dominant mechanism or is sufficient to explain their efficacy. Our recent work suggests as a potentially unifying mechanistic theme the induction of oxidative stress by modified NSAIDs, upon which most or all of the critical signaling changes depend. In particular, when we mapped the temporal sequence of cell signaling changes in response to nitric oxide-aspirin it was clear that increased intracellular levels of reactive oxygen and nitrogen species (RONS) preceded the activation of signaling pathways controlling proliferation and apoptosis (Sun and Rigas, 2008). We also noted that the pathways affected by modified NSAIDs were redox-sensitive. Furthermore, we have demonstrated that pretreatment of cells with antioxidant agents, such as N-acetyl-cysteine (NAC), abrogates the growth inhibitory effect of phospho-NSAIDs (Sun and Rigas, 2008; Zhao et al., 2009; Huang et al., 2010; Mackenzie et al., 2010).
Oxidative stress represents an irreversible state in which the intracellular level of RONS is increased. The idea that particularly high RONS levels may contribute to the antineoplastic effect of several agents has been gaining acceptance (Rigas and Sun, 2008). Given the evidence that phospho-NSAIDs may share as a critical proximal event in their mechanism of action the induction of oxidative stress, we undertook a systematic evaluation of this question. To this end, we studied four structurally diverse phospho-NSAIDs and determined in human colon and breast cancer cell lines the relationship between the induction of oxidative stress and cell growth inhibition. As controls, we evaluated the effect of several conventional chemotherapeutic drugs (CCDs) that are used clinically in the treatment of breast or colon cancer. The mechanism of the anticancer effect of CCDs varies. However, there is a common recognition that oxidative stress is important in modulating such effect and this biochemical property can be exploited for therapeutic benefits (Trachootham et al., 2009).
The cellular levels of RONS are determined by their rate production and also the antioxidant defense mechanism, notably the thioredoxin (Trx) system (Holmgren et al., 2005; Arnér, 2009; Meyer et al., 2009; Circu and Aw, 2010) and GSH, the most important chemical antioxidant agent in mammalian cells (Diaz Vivancos et al., 2010; Jones and Go, 2010). Indeed, we have reported that some of our study compounds suppress the cellular levels of GSH and the Trx system mediates, to a significant extent, their proapoptotic effect (Sun and Rigas, 2008). These compounds convert Trx-1 to its oxidized (inactive) form by suppressing the activity of thioredoxin reductase (TrxR), the enzyme that reduces oxidized Trx-1 (Sun and Rigas, 2008).
Here, we demonstrate that in both breast and colon cancer cell lines phospho-NSAIDs induce oxidative stress, which is of greater intensity compared with that induced by CCDs. In both cases, oxidative stress is accompanied by reduced GSH levels and TrxR activity. We also show that oxidative stress correlates with the induction of apoptosis both in vitro and in vivo, paralleling their antitumor efficacy.
Materials and Methods
Reagents.
We obtained all conventional drugs from Sigma-Aldrich (St. Louis, MO). DCFDA, annexin V, propidium iodide (PI), and JC-1 were from Invitrogen (Carlsbad, CA). BrdU and anti-BrdU-FITC were from BD Biosciences (San Jose, CA). Phospho-sulindac (P-S; OXT-328), phosphor-aspirin (P-A; MDC-43), phosphor-flurbiprofen (P-F; MDC-813), and phosphor-ibuprofen (P-I; MDC-917) were gifts from Medicon Pharmaceuticals, Inc. (Stony Brook, NY). All drugs were dissolved in dimethyl sulfoxide as 100 mM stock solution and diluted to their final concentration in cell culture media. Phospho-NSAIDs in general are stable at 4°C for more than 1 year (Xie et al., 2011 and data not shown).
Cell Culture.
The SW480 human colon cancer cells, established from a lymph node metastasis from a primary adenocarcinoma of the colon (Leibovitz et al., 1976), incubated in 5% CO2 at 37°C, were grown in RPMI medium containing 10% fetal bovine serum and antibiotics and MCF-7 human breast cancer cells (McGrath et al., 1974) in Eagle's minimum essential medium (American Type Culture Collection, Manassas, VA) containing 0.01 mg/ml human insulin, 10% fetal bovine serum, and antibiotics. The MCF-7 cells are human breast epithelial adenocarcinoma cells derived from the metastatic pleural effusion of a patient with breast adenocarcinoma. This cell line retains several characteristics of differentiated mammary epithelium including the ability to process estradiol via cytoplasmic estrogen receptors and the capability of forming domes (Huguet et al., 1994). Both cell lines were obtained from the American Type Culture Collection.
Determination of RONS by FACScalibur.
After treatment with the test drug in six-well plates (0.3 × 106 cells/well) for 1 h, cells were trypsinized and stained with 10 μM DCFDA for 30 min at 37°C, and their fluorescence intensity was analyzed with a FACScalibur (BD Bioscience).
Determination of Cell Death by Annexin V and PI Staining.
After cells were treated with the test drugs in 12-well plates for 16 h, all cell populations (suspended and attached) were collected and stained with annexin V-FITC (100× dilution) and PI (0.5 μg/ml) for 15 min. Annexin V-FITC and PI fluorescence intensities were analyzed with a FACScalibur. Annexin V(+)/PI(−) cells are early apoptotic cells; annexin V(+)/PI(+) cells are late apoptotic cells (i.e., they have undergone secondary necrosis); and annexin V(−)/PI(+) cells are necrotic.
Determination of Mitochondrial Transmembrane Potential by JC-1 Staining.
To determine the mitochondrial transmembrane potential (ΔΨm), after treatment with the test drugs for 3 h, cells were trypsinized and stained with 5 μM JC-1 for 30 min at 37°C and their fluorescence intensity was analyzed with a FACScalibur.
Determination of Proliferation Using BrdU Staining.
Cells were treated with the test drugs for 16 h. Before harvesting them, BrdU was added directly to the culture medium to a final concentration of 10 μM. All cell populations (suspended and attached) were incubated in the CO2 incubator for 30 min at 37°C, harvested, and fixed in 70% ethanol for 30 min on ice. DNA was denatured by incubating the cells with 2 N HCl/Triton X-100 for 30 min, followed by neutralization in 0.1 M Na2B4O7·10H2O. After 30 min of incubation with 20 μl of anti-BrdU-FITC per 106 cells, cells were washed and resuspended in PBS containing 5 μg/ml PI. Cell fluorescence intensities were analyzed with a FACScalibur.
Thioredoxin Reductase Activity Assay.
After treatment with the test drugs for 1 h, cells were lysed in RIPA lysis buffer (Sigma-Aldrich), and TrxR activity was determined in the protein lysate using a commercially available kit according to the instructions of the manufacturer (Cayman Chemical, Ann Arbor, MI). In this assay, TrxR uses NADPH to reduce 5,5′-dithiobis-(2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid (Sun and Rigas, 2008), which produces a yellow product that is measured at 405 to 414; TrxR activity is determined based on the change in absorbance (ΔA412) per minute for 10 min.
Treatment of Nude Mice with Colon Cancer Xenografts.
SW480 cells (1.5 × 106) suspended in 100 μl of PBS were injected subcutaneously in both the left and right flanks of 5- to 6-week-old female Ncr nude mice (Taconic Farms, Germantown, NY). These mice have both BALB/c inbred nude and NIH(S) outbred nude mice stock in their genetic background. Tumor size was monitored by measuring the length (L) and width (W) with a caliper and the volume was calculated according to the formula, L × W × (L + W/2) × 0.56. When the average tumor volume reached ∼100 mm3, mice were treated with P-I at 400 mg/kg/day (1/3 of its maximal tolerated dose; n = 5), 5-FU at 100 mg/kg/week (its maximal tolerated dose; n = 6), or vehicle (PBS; n = 5), all administered intraperitoneally (P-I and 5-FU were suspended in PBS). Mice were euthanized 22 days later. An 18-h urine sample from each mouse was collected on day 10 using individual metabolic cages. The same study was performed on nude mice without SW480 xenografts and urine samples were collected on day 10.
Cell death was determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining sections of paraffin-embedded tissues, as described previously (Ouyang et al., 2006). Cell proliferation was determined by staining sections of paraffin-embedded tissues for Ki-67 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), as described previously (Ouyang et al., 2006). For TUNEL and Ki-67 scoring, for each slide, three to five randomly selected fields (200×) were photographed. TUNEL(+) or Ki-67(+) cells in each photograph were counted using Image J software (National Institutes of Health, Bethesda, MD; http://www.nci.com). Results were expressed as the average of the TUNEL(+) or Ki-67(+) cells/200× field of each tumor.
Urinary F2-Isoprostane Levels.
Urinary F2-isoprostane and creatinine were determined in the urine by enzyme-linked immunosorbent assay (Oxford Biomedical Research, Oxford, MI). F2-isoprostane values, normalized to creatinine levels, were expressed as ng/mg creatinine. The same assay was performed on Ncr nude mice without SW480 xenografts treated with P-I, 5-FU, or PBS for 10 days.
Statistical Analyses.
Results were expressed as mean ± S.E.M. Differences between groups were determined by the Student's t test. The association between data sets was evaluated by correlation analysis and computing the correlation coefficient (r). p < 0.05 was considered statistically significant.
Results
Phospho-NSAIDs Induce Oxidative Stress in Colon and Breast Cancer Cells More Potently than CCDs.
We determined the ability of phospho-NSAIDs and conventional chemotherapeutic agents to induce oxidative stress in SW480 human colon cancer and MCF-7 human breast cancer cells. We studied four phospho-NSAIDs: P-S, P-A, P-F, and P-I (Sun and Rigas, 2008; Zhao et al., 2009; Mackenzie et al., 2010, 2011; Xie et al., 2011); their structures are shown in Supplemental Fig. 1. We also studied six conventional chemotherapeutic agents: 5-FU, irinotecan, oxaliplatin, chlorambucil, paclitaxel, and vincristine.
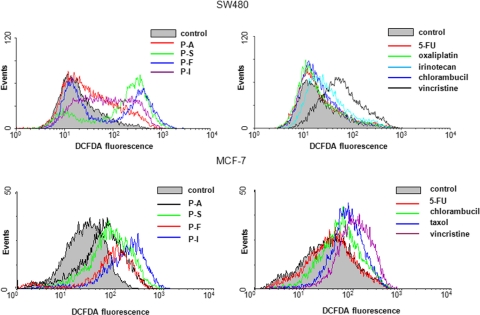
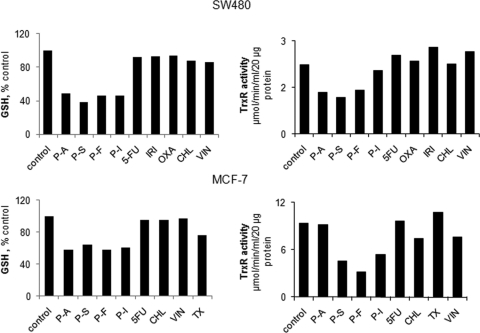
All study compounds inhibited the growth of SW480 and MCF-7 cells (Table 1). SW480 and MCF-7 cells were incubated for 1 h with each of the test compounds at the respective 1.5 × IC50 values for cell growth. Intracellular RONS were detected using DCFDA (Fig. 1), a molecular probe that detects >10 individual reactive species (Bass et al., 1983; LeBel et al., 1992). We also determined the intracellular levels of GSH and the activity of TrxR (Fig. 2).
TABLE 1.
Values of parameters determined in SW480 cells treated with the study compounds
Numbers for all but IC50 are the averages from two individual experiments; values were within 10%.
| Treatment | IC50a | DCFDA | GSH | TrxR | Annexin V(+) | JC-1 | BrdU |
|---|---|---|---|---|---|---|---|
| μM | geometric mean | % control | μmol · min−1 · ml−1 | % total | geometric mean | % total | |
| Control | 23.7 ± 3.1 | 100 ± 0 | 2.24 ± 0.1 | 4.3 ± 0.2 | 132.5 ± 11.5 | 33.2 ± 1.8 | |
| P-A | 37 ± 1.6 | 43.2 | 48.8 | 1.33 | 21.8 | 183 | 1.2 |
| P-S | 98 ± 3.7 | 198.5 | 38.7 | 1.18 | 44.6 | 717 | 1.3 |
| P-F | 65 ± 4.3 | 170.8 | 45.9 | 1.42 | 89.9 | 1295 | 6.0 |
| P-I | 79 ± 2.9 | 88.1 | 46.5 | 2.04 | 67.3 | 235 | 12.7 |
| Mean ± S.E.M. | 125.2 ± 36.0b | 45.0 ± 2.2*** | 1.5 ± 0.2* | 55.9 ± 14.7* | 607.5 ± 258.8* | 5.3 ± 2.7*** | |
| 5-FU | 173 ± 14.2 | 23.01 | 92.4 | 2.54 | 4.9 | 145.5 | 28.5 |
| Oxaliplatin | 61 ± 5.9 | 21.6 | 92.8 | 2.24 | 5.2 | 163 | 19.9 |
| Irinotecan | 167 ± 12.0 | 33 | 93.9 | 2.78 | 12.1 | 217 | 29.1 |
| Chlorambucil | 135 ± 14.9 | 23.4 | 87.6 | 2.35 | 8.1 | 160 | 15.6 |
| Vincristine | 55 ± 7.4 | 65.6 | 86.4 | 1.88 | 7.7 | 251 | 14.2 |
| Mean ± S.E.M. | 33.3 ± 8.3 | 90.6 ± 1.5** | 2.4 ± 0.15 | 7.6 ± 1.3c | 187.3 ± 20.1* | 21.5 ± 3.2* |
n ≥ three different experiments, each done in triplicate.
Compared with control: P = 0.06.
Compared with control: P = 0.063.
P < 0.05;
P < 0.01;
P < 0.001 compared with control.
Fig. 1.
Anticancer agents induce RONS in human colon and breast cancer cells. SW480 colon cancer cells (top) and MCF-7 breast cancer cells (bottom) were treated with each of the study compounds at their respective 1.5 × IC50 values. RONS levels (determined at 1 h) were assayed by FACS after staining with DCFDA as described under Materials and Methods.
Fig. 2.
Cellular antioxidants in response to anticancer agents in colon and breast cancer cells. GSH (left) and TrxR (right) activity were determined as described under Materials and Methods in SW480 (top) and MCF-7 (bottom) cells treated for 3 h with each of the study compounds at their respective 1.5 × IC50 values.
Compared with controls, in SW480 cells RONS levels increased 5.3-fold (p = 0.06) in response to phospho-NSAIDs and only marginally (1.4-fold, p = 0.31) in response to CCDs (Table 1). This effect was accompanied by 1) reduced GSH levels: 55% by phospho-NSAIDs (p < 0.0001) and 9.4% (p = 0.003) by CCDs; and 2) reduced TrxR activity: 33% (p = 0.02) by phospho-NSAIDs and no change by CCDs (Fig. 2 and Table 1). In MCF-7 breast cancer cells we obtained similar results (Figs. 1 and 2; Table 2). Specifically, compared with controls, phospho-NSAIDs increased RONS levels 3.8-fold (p = 0.04) and reduced GSH levels by 39.8% (p < 0.0001) and TrxR activity by 41% (p = 0.059). CCDs had no statistically significant effects on these parameters: RONS levels were increased 1.7-fold (p = 0.07), GSH levels were reduced by only 9.1% (p = 0.16), and TrxR activity did not change. It is noteworthy that in both SW480 and MCF-7 cell lines, compared with CCDs, phospho-NSAIDs induced significantly more RONS (3.8- and 2.1-fold, respectively; p < 0.05 for both), reduced GSH levels 5.9- and 4.4-fold, respectively (p < 0.001 for both), and suppressed TrxR activity whereas CCDs failed to have any effect on it.
TABLE 2.
Values of parameters determined in MCF-7 cells treated with the study compounds
Numbers for all but IC50 are the averages from two individual experiments; values were within 10%.
| MCF-7 | IC50a | DCFDA | GSH | TrxR | Annexin V(+) | JC-1 | BrdU |
|---|---|---|---|---|---|---|---|
| μM | geometric mean | % control | μmol · min−1 · ml−1 | % total | geometric mean | % total | |
| Control | 28.9 ± 2.1 | 100 ± 0 | 9.3 ± 0.4 | 7.8 ± 0.3 | 146 ± 8.2 | 19.9 ± 1.6 | |
| P-A | 23 ± 1.6 | 45.2 | 58.3 | 9.11 | 66.4 | 330 | 3.26 |
| P-S | 62 ± 4.4 | 110.8 | 64.4 | 4.56 | 54.1 | 566 | 3.28 |
| P-F | 65 ± 5.8 | 164.3 | 57.6 | 3.1 | 96.7 | 747 | 3.47 |
| P-I | 79 ± 5.6 | 119.9 | 60.5 | 5.34 | 97.9 | 845 | 4.18 |
| Mean ± S.E.M. | 110.1 ± 24.6* | 60.2 ± 1.5*** | 5.5 ± 1.3b | 78.8 ± 11.0** | 622 ± 113.2* | 3.5 ± 0.2*** | |
| 5-FU | 283 ± 12.4 | 35.3 | 95.3 | 9.6 | 7.7 | 135 | 2.39 |
| Chlorambucil | 58 ± 3.9 | 39.1 | 95.3 | 7.34 | 9.1 | 166 | 4.28 |
| Paclitaxel | 33 ± 4.7 | 69.2 | 96.9 | 10.71 | 14.2 | 135 | 5.11 |
| Vincristine | 37 ± 2.2 | 56.7 | 76.1 | 7.61 | 17.2 | 165 | 7.34 |
| Mean ± S.E.M. | 50.1 ± 7.9c | 90.9 ± 4.9 | 8.8 ± 0.8 | 12.1 ± 2.2 | 150.3 ± 8.8 | 4.8 ± 1.0*** |
n ≥ three different experiments, each done in triplicate.
Compared with control: P = 0.059.
Compared with control: P = 0.07.
P < 0.05;
P < 0.01;
P < 0.001 compared with control.
Phospho-NSAIDs Induce Apoptosis More Potently than CCDs in Colon and Breast Cancer Cells.
We evaluated the effect of both phospho-NSAIDs and CCDs on cytokinetics of the two cell lines. We determined apoptosis using annexin V staining and ΔΨm. Mitochondria, the main source of RONS, are central to cell survival and death (Circu and Aw, 2010). Collapse of ΔΨm, which maintains their physiological function such as ATP production, induces cytochrome c release and activates downstream cell death signaling. To determine ΔΨm we used the molecular marker JC-1; increased JC-1 green fluorescence indicates decreased ΔΨm. We also determined the effect of phospho-NSAIDs and CCDs on cell proliferation by using BrdU incorporation. There is evidence that, under certain conditions, the induction of RONS could inhibit cell proliferation (Chan et al., 2009; Sarsour et al., 2009; Visconti and Grieco, 2009).
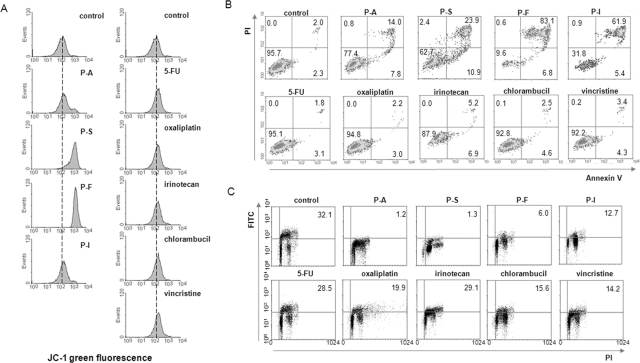
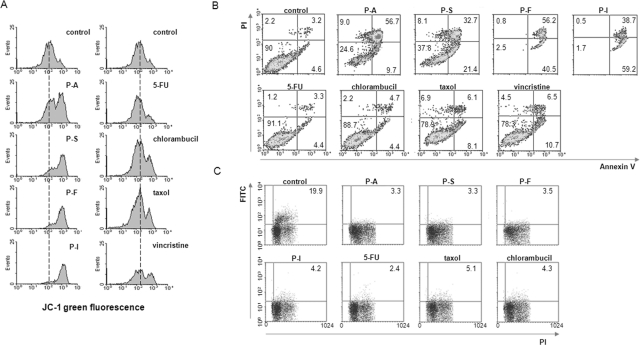
As shown in Fig. 3 and Table 1 in SW480 cells phospho-NSAIDs induced apoptosis strongly, increasing it 13-fold over controls (p < 0.01), whereas CCDs doubled, an effect that was not statistically significant (p = 0.15). Apoptosis includes all annexin V(+) cells, i.e., those showing early [annexin V(+)/PI(−)] and late [annexin V(+)/PI(+)] apoptosis. JC-1 fluorescence also changed in the same manner: phospho-NSAIDs induced apoptosis strongly, increasing it 4.6-fold over controls (p < 0.05), whereas the change by CCDs was marginal and statistically not significant (p = 0.66). In MCF-7 cells the results were similar (Fig. 4 and Table 2). Specifically, compared with control, phospho-NSAIDs increased annexin V(+) cells 10-fold (p < 0.01) and JC-1 green fluorescence 4.3-fold (p < 0.05), whereas CCDs failed to change these parameters in a statistically significant way. When the percentages of annexin V(+) cells and the JC-1 fluorescence intensity of the phospho-NSAID group were compared with those of the CCDs group both were significantly higher (7.4- and 3.2-fold, respectively, in SW480 cells and 6.5- and 4.1-fold, respectively, in MCF-7 cells; p < 0.01 for all).
Fig. 3.
Anticancer agents induce cell death, dissipate the ΔΨm, and inhibit cell proliferation in colon cancer cells. A, SW480 cells treated for 3 h with each of the study compounds at 1.5 × IC50 were stained with JC-1, an indicator of ΔΨm, whose fluorescence were analyzed by FACS. Increased JC-1 green fluorescence indicates ΔΨm dissipation. B and C, SW480 cells were treated for 16 h with each of the study compounds at 1.5 × IC50, and cell death and proliferation were assayed by FACS after staining with annexin V/PI (B) or BrdU (C), as described under Materials and Methods.
Fig. 4.
Anticancer agents induce cell death, dissipate the ΔΨm, and inhibit cell proliferation in breast cancer cells. A, MCF-7 cells treated for 3 h with each of the study compounds at 1.5 × IC50 were stained with JC-1, an indicator of ΔΨm, whose fluorescence were analyzed by FACS. Increased JC-1 green fluorescence indicates ΔΨm dissipation. B and C, MCF-7 cells were treated for 16 h with each of the study compounds at 1.5 × IC50, and cell death and proliferation were assayed by FACS after staining with annexin V/PI (B) or BrdU (C), as described under Materials and Methods.
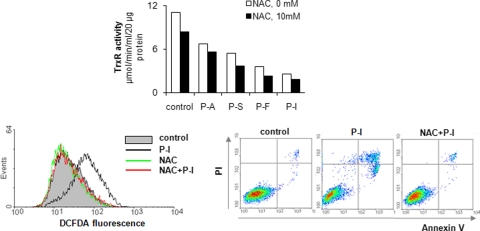
Finally, pretreating these cells with the antioxidant NAC restored RONS to baseline but TrxR activity remained unaffected (Fig. 5). In addition, NAC suppressed the induction of apoptosis by these compounds, indicating that RONS are the agents mediating cell death.
Fig. 5.
The effect of the antioxidant NAC on TrxR, RONS levels, and cell death. MCF-7 cells were pretreated with or without 10 mM NAC for 3 h, followed by 1.5 × IC50 of each of the indicated test compounds for 3 h more, when TrxR activity was determined as described under Materials and Methods (top). In MCF-7 cells treated with P-I as above, we determined RONS levels at 1 h (bottom left) and apoptosis at 16 h (bottom right).
Phospho-NSAIDs and CCDs Inhibit Proliferation in Colon and Breast Cancer Cells.
Both groups of study compounds inhibited cell proliferation. As shown in Fig. 3C and Table 1, in SW480 cells phospho-NSAIDs inhibited proliferation by 84% (p < 0.001) and CCDs by 35% (p < 0.03). The effect of phospho-NSAIDs was significantly stronger than that of CCDs (p < 0.001). In MCF-7 cells phospho-NSAIDs inhibited proliferation by 82% (p < 0.001) and CCDs by 76% (p < 0.001), but the difference between these two groups was not statistically significant (Fig. 4C and Table 2).
A Phospho-NSAID (but Not a CCD) Induces Oxidative Stress in Tumor Xenografts: Association between Oxidative Stress and Apoptosis.
Quantitatively the most pronounced cytokinetic difference between phospho-NSAIDs and CCDs in cultured cells was observed in the induction of RONS and apoptosis. To assess whether this difference also occurs in vivo, we treated with P-I or 5-FU nude mice bearing SW480 xenografts. These two compounds are representative of the phospho-NSAIDs and CCDs, which had an opposite effect on RONS levels in vitro. The level of urinary F2-isoprostane was used as a marker of oxidative stress (Basu, 2008; Tacconelli et al., 2010). We used two control groups: 1) nude mice with xenografts that were treated with vehicle, and 2) nude mice without xenografts that were treated with P-I or 5-FU.
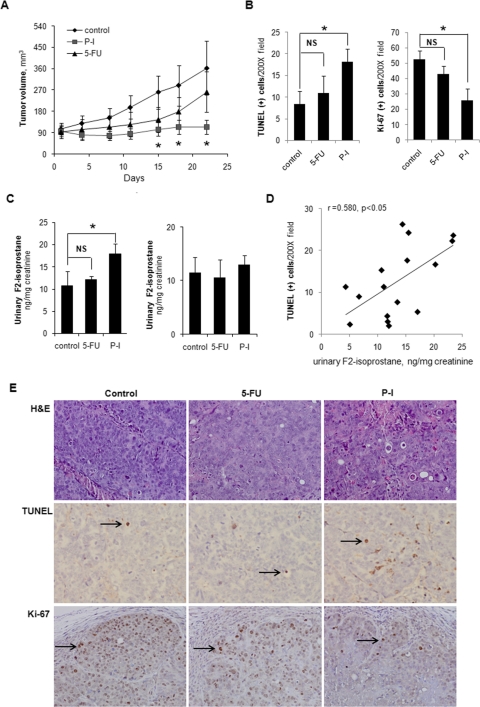
As expected, P-I markedly suppressed the growth of the xenografts, essentially achieving tumor stasis (Fig. 6A); the difference in tumor size from control became statistically significant on day 12 and continued to be so to the end of the study on day 22 (p = 0.03–0.04). In contrast, 5-FU had only a modest inhibitory effect on the growth of the xenografts, which never reached statistical significance (e.g., p = 0.17 on day 22). P-I induced apoptosis in the xenografts, more than doubling the apoptosis index, compared with controls [controls = 8.9 ± 2.7; P-I-treated = 19.5 ± 3.0 (mean ± S.E.M. for this and all subsequent values; p = 0.035; Fig. 6, B and E]. However, no significant induction of apoptosis by 5-FU, was noted (p = 0.81). In terms of cell proliferation (Fig. 6, B and E), P-I inhibited it by 51% (controls = 52.6 ± 5.58; P-I-treated = 25.8 ± 7.71; p = 0.025), whereas the effect of 5-FU was not statistically significant (p = 0.25).
Fig. 6.
Antitumor effect, oxidative stress, and xenograft cytokinetics in vivo. A, SW480 xenografts in nude mice were treated with P-I, 5-FU, or vehicle for 22 days, as described under Materials and Methods, starting when the average tumor volume was ∼100 mm3. Tumor volume values are mean ± S.E.M. *, statistically significantly different from control (p < 0.05). B, apoptosis and cell proliferation were determined in tissue sections of xenografts that were harvested at sacrifice on day 22 by the TUNEL assay (left) and Ki-67 expression (right), respectively. The bar graphs show the mean ± S.E.M. values for each group. *, p < 0.05 compared with control. NS, statistically not significant. C, the levels of F2-isoprostane in 24-h urine of nude mice were determined using an enzyme-linked immunosorbent assay kit, as described under Materials and Methods. Left, results from nude mice bearing SW480 xenografts. Right, results from nude mice bearing no xenografts. Values are mean ± S.E.M. *, p < 0.05 compared with control. NS, statistically not significant. D, the association between apoptosis [TUNEL(+) cells, y-axis] in SW480 xenografts and urinary F2-isoprostane levels (x-axis) in the nude mice of all three study groups is shown. E, studies in xenograft tissues. Samples were stained with hematoxylin and eosin (H&E) (top), TUNEL (middle), or Ki-67 (bottom) as described under Materials and Methods. Representative fields (200×) from each sample are shown.
The levels of urinary F2-isoprostane were significantly elevated in mice treated with P-I (Fig. 6C). On day 10, these levels were 10.7 ± 3.3 ng/mg creatinine in controls and 17.9 ± 2.2 ng/mg creatinine in the PI-treated group, representing a nearly 70% increase over controls (p < 0.05). In contrast, 5-FU failed to significantly increase them (12.1 ± 0.7 ng/mg creatinine; p = 0.6). To ensure that the source of F2-isoprostane was the xenografts, we determined F2-isoprostane levels in nude mice that had no xenografts but were treated with P-I or 5-FU following the same protocol. No appreciable change in F2-isoprostane levels was noted in any of the treated groups (Fig. 6C). Of interest, the urinary F2-isoprostane levels in all mice bearing xenografts (controls plus those treated with P-I or 5-FU) were positively associated with the number of apoptotic cells in the xenografts (r = 0.580, p < 0.05; Fig. 6D). However, no such correlation was observed between urinary F2-isoprostane levels and cell proliferation (Ki-67) (Supplemental Fig. 2). Finally, to determine the specificity of the effect of P-I on apoptosis and proliferation in the SW480 colon cancer xenografts, we determined the effect of P-I and 5-FU on proliferation and apoptosis of the colonic mucosa in nude mice without xenografts. Neither compound changed significantly either parameter (data not shown). There was a correlation between F2-isoprostane levels and tumor growth, which almost reached statistical significance (p = 0.06); this borderline level of significance may reflect our rather small sample size. These findings suggest a potential etiological connection between oxidative stress and the induction of apoptosis in tumor xenografts.
Discussion
Our data establish the induction of oxidative stress as an important mechanism of action of the novel phospho-NSAIDs. Furthermore, they indicate that the apoptotic death of cancer cells is an important consequence of oxidative stress such that it affects tumor growth, i.e., the intended therapeutic outcome of these compounds. These effects were not observed in response to CCDs, indicating a striking difference between these two groups of compounds.
We assessed the relationship between oxidative stress, cytokinetic parameters, and tumor growth in response to phospho-NSAIDs using structurally diverse compounds and two human cancer cell lines that represent two of the commonest human cancers, colon and breast, which differ significantly in both their biology and clinical behavior. The in vitro data were complemented by in vivo studies, all of which used as controls CCDs that are used to treat these two tumors. The sets of CCDs for breast and colon cancer are not identical, overlapping partially, as actually happens in clinical practice. All compounds were studied at equipotent concentrations.
Our study generated several important findings. First, the phospho-NSAIDs induced in both cell lines a state of oxidative stress. This is probably the result of increased production of RONS and a weakened RONS inactivation response, reflected in the suppressed cellular levels of GSH and TrxR, both critical determinants of a cell's ability to contain RONS and the consequent cellular damage. Indeed, our prior detailed work with P-S, one of these compounds, has demonstrated that the thioredoxin system (of which TrxR is a pivotal component (Sun and Rigas, 2008; Mackenzie et al., 2011) mediates most of its growth inhibitory effect.
Second, oxidative stress in response to phospho-NSAIDs is accompanied by two consequential cytokinetic changes, the induction of apoptosis and the suppression of proliferation. The former is quantitatively more pronounced, and, as the study using the antioxidant NAC showed, it totally depends on RONS.
Third, the effects of phospho-NSAIDs are dramatically different from those of CCDs in both cell lines. In both cell lines, CCDs failed to induce oxidative stress, having marginal or no effects on GSH and TrxR. Their main cytokinetic effect was inhibition of proliferation, which seemed to be weaker than that by phospho-NSAIDs.
The animal studies confirmed the in vitro findings and provided an insight into the sequence of events that culminated in the anticancer effect, i.e., tumor growth inhibition. P-I, representing the phospho-NSAIDs, induced oxidative stress, but this occurred only in the tumor tissue and not in normal tissues: F2-isoprostane levels were increased in animals bearing xenografts but not in those without them. Similar to cultured cells, the state of oxidative stress was accompanied by the induction of apoptosis and inhibition of proliferation. However, F2-isoprostane levels correlated only with the induction of apoptosis and this effect led predominantly to the anticancer effect. It was interesting that P-I had no effect on proliferation or apoptosis in the normal colonic mucosa of mice without tumors, indicating the tissue specificity of the effect and its association with oxidative stress.
F2-isoprostane levels did not correlate significantly with the inhibition of proliferation (p = 0.08). The relationship between RONS and proliferation is unclear and at best not uniform. In some cases, RONS increase cell proliferation (Klaunig et al., 1998, 2010; Brown and Bicknell, 2001; Visconti and Grieco, 2009), although in others they inhibit it (Engler et al., 1999; Sasaki et al., 2006; Herring et al., 2007). Given our rather limited sample size, we may have underestimated a potential relationship between oxidative stress and inhibition of proliferation.
Our conclusions on the sequence of events (phospho-NSAIDs → oxidative stress → induction of apoptosis → anticancer effect) are strengthened by the inability of 5-FU to initiate the same and its failure to inhibit the growth of colon cancer xenografts; 5-FU was administered at its maximal tolerated dose.
These data can be interpreted as showing that phospho-NSAIDs act pharmacologically against cancer through a mechanism that is distinct from that of a variety of CCDs. The critical question, of course, is whether this difference in preclinical models translates to a corresponding effect in humans, for which currently no data are available. Besides this distinction, our results emphasize clearly the role of oxidative stress in the pharmacological action of anticancer agents, which, at least for this class of compounds, may be their key mechanistic effect. Indeed, all evidence that we have generated points in that direction.
Although oxidative stress is generally considered a procarcinogenic factor, this is not universally so (Rigas and Sun, 2008). There have also been reports in the literature that chemotherapeutic agents induce RONS as part of their effect on cells. Agents that exhibit antitumor activity via RONS-dependent activation of apoptotic cell death include vinblastine, cisplatin, mitomycin C, doxorubicin, camptothecin, inostamycin, neocarzinostatin, etoposide, and arsenic trioxide (Fang et al., 2007; Rigas and Sun, 2008). Similar to chemotherapeutic agents, irradiation-induced cellular DNA damage is preceded by damage to the plasma membrane, which in turn induces RONS production (Cataldi, 2010). Despite their various antioxidant and detoxifying systems mitochondria are the main source of intracellular RONS; perhaps surprisingly, they are also sensitive targets of the damaging effects of these radicals (Orrenius, 2007). Oxidative stress markedly sensitizes mitochondria toward mitochondria permeability transition; the resultant mitochondrial failure can lead to caspase-dependent apoptosis (Orrenius et al., 2007; Lemasters et al., 2009). The effect of phospho-NSAIDs on ΔΨm shows that they target mitochondria, inducing apoptosis.
In conclusion, our study underscores the potentially critical role of the induction of oxidative stress in the therapeutic effect of phospho-NSAIDs, highlights their striking difference from CCDs in this regard, and indicates that pathways leading to oxidative stress may be useful targets for anticancer strategies.
Supplementary Material
This work was funded by the National Institutes of Health National Cancer Institute [Grants 5R01-CA092423, R01-CA139454, R01-CA154172]; the National Institutes of Health Division of Cancer Prevention and Control [1N01-CN43302WA22]; and the Department of Defense U.S. Army Medical Research Acquisition Activity [Grant W81XWH1010873].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183533.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- NSAID
- nonsteroidal anti-inflammatory drug
- RONS
- reactive oxygen and nitrogen species
- CCD
- conventional chemotherapeutic drug
- Trx
- thioredoxin
- TrxR
- Trx reductase
- P-S
- phospho-sulindac
- P-A
- phospho-aspirin
- P-F
- phospho-flurbiprofen
- P-I
- phospho-ibuprofen
- 5-FU
- 5-fluorouracil
- PBS
- phosphate-buffered saline
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick-end labeling
- BrdU
- 5-bromo-2′-deoxyuridine
- PI
- propidium iodide
- FACS
- fluorescence-activated cell sorting
- DCFDA
- dichlorofluorescin diacetate
- NAC
- N-acetyl cysteine
- ΔΨm
- mitochondrial transmembrane potential
- FITC
- fluorescein isothiocyanate.
Authorship Contributions
Participated in research design: Sun, Huang, Mackenzie, and Rigas.
Conducted experiments: Sun, Huang, and Mackenzie.
Contributed new reagents or analytic tools: Rigas.
Performed data analysis: Sun, Huang, Mackenzie, and Rigas.
Wrote or contributed to the writing of the manuscript: Sun and Rigas.
References
- Arnér ES. (2009) Focus on mammalian thioredoxin reductases–important selenoproteins with versatile functions. Biochim Biophys Acta 1790:495–526 [DOI] [PubMed] [Google Scholar]
- Baron JA. (2009) Aspirin and NSAIDs for the prevention of colorectal cancer. Recent Results Cancer Res 181:223–229 [DOI] [PubMed] [Google Scholar]
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. (1983) Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 130:1910–1917 [PubMed] [Google Scholar]
- Basu S. (2008) F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal 10:1405–1434 [DOI] [PubMed] [Google Scholar]
- Brown NS, Bicknell R. (2001) Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 3:323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldi A. (2010) Cell responses to oxidative stressors. Curr Pharm Des 16:1387–1395 [DOI] [PubMed] [Google Scholar]
- Chan EC, Jiang F, Peshavariya HM, Dusting GJ. (2009) Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther 122:97–108 [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY. (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew KD, Neugut AI. (2006) Aspirin and NSAIDs: effects in breast and ovarian cancers. Curr Opin Obstet Gynecol 18:71–75 [DOI] [PubMed] [Google Scholar]
- Diaz Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH. (2010) A nuclear glutathione cycle within the cell cycle. Biochem J 431:169–178 [DOI] [PubMed] [Google Scholar]
- Engler JA, Gupta A, Li L, Rao RK. (1999) Inhibition of DNA synthesis in Caco-2 cells by oxidative stress: amelioration by epidermal growth factor. Dig Dis Sci 44:1902–1909 [DOI] [PubMed] [Google Scholar]
- Fang J, Nakamura H, Iyer AK. (2007) Tumor-targeted induction of oxystress for cancer therapy. J Drug Target 15:475–486 [DOI] [PubMed] [Google Scholar]
- Herring TA, Cuppett SL, Zempleni J. (2007) Genomic implications of H2O2 for cell proliferation and growth of Caco-2 cells. Dig Dis Sci 52:3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A, Johansson C, Berndt C, Lönn ME, Hudemann C, Lillig CH. (2005) Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans 33:1375–1377 [DOI] [PubMed] [Google Scholar]
- Huang L, Zhu C, Sun Y, Xie G, Mackenzie GG, Qiao G, Komninou D, Rigas B. (2010) Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine dependent effect. Carcinogenesis 31:1982–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet EL, McMahon JA, McMahon AP, Bicknell R, Harris AL. (1994) Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res 54:2615–2621 [PubMed] [Google Scholar]
- Jones DP, Go YM. (2010) Redox compartmentalization and cellular stress. Diabetes Obes Metab 12 (Suppl 2):116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM, Hocevar BA. (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38:96–109 [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, Stevenson DE, Walborg EF., Jr (1998) The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect 106 (Suppl 1):289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231 [DOI] [PubMed] [Google Scholar]
- Leibovitz A, Stinson JC, McCombs WB, 3rd, McCoy CE, Mazur KC, Mabry ND. (1976) Classification of human colorectal adenocarcinoma cell lines. Cancer Res 36:4562–4569 [PubMed] [Google Scholar]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. (2009) Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787:1395–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie GG, Ouyang N, Xie G, Vrankova K, Huang L, Sun Y, Komninou D, Kopelovich L, Rigas B. (2011) Phospho-sulindac (OXT-328) combined with difluoromethylornithine prevents colon cancer in mice. Cancer Prev Res (Phila), doi:10.1158/1940-6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, Johnson F, Komninou D, Kopelovich L, Rigas B. (2010) Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology 139:1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath CM, Grant PM, Soule HD, Glancy T, Rich MA. (1974) Replication of oncornavirus-like particle in human breast carcinoma cell line, MCF-7. Nature 252:247–250 [DOI] [PubMed] [Google Scholar]
- Meyer Y, Buchanan BB, Vignols F, Reichheld JP. (2009) Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu Rev Genet 43:335–367 [DOI] [PubMed] [Google Scholar]
- Orrenius S. (2007) Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev 39:443–455 [DOI] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–183 [DOI] [PubMed] [Google Scholar]
- Ouyang N, Williams JL, Tsioulias GJ, Gao J, Iatropoulos MJ, Kopelovich L, Kashfi K, Rigas B. (2006) Nitric oxide-donating aspirin prevents pancreatic cancer in a hamster tumor model. Cancer Res 66:4503–4511 [DOI] [PubMed] [Google Scholar]
- Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, Thaiparambil J, Coward L, Gorman G, Li Y, et al. (2009) A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2:572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas B. (2007) The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr Opin Gastroenterol 23:55–59 [DOI] [PubMed] [Google Scholar]
- Rigas B, Sun Y. (2008) Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer 98:1157–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. (2009) Redox control of the cell cycle in health and disease. Antioxid Redox Signal 11:2985–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Ikeda H, Sato Y, Nakanuma Y. (2006) Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis. Am J Pathol 169:831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Rigas B. (2008) The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res 68:8269–8277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli S, Capone ML, Patrignani P. (2010) Measurement of 8-iso-prostaglandin F2α in biological fluids as a measure of lipid peroxidation. Methods Mol Biol 644:165–178 [DOI] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–591 [DOI] [PubMed] [Google Scholar]
- Visconti R, Grieco D. (2009) New insights on oxidative stress in cancer. Curr Opin Drug Discov Dev 12:240–245 [PubMed] [Google Scholar]
- Xie G, Sun Y, Nie T, Mackenzie GG, Huang L, Kopelovich L, Komninou D, Rigas B. (2011) Phospho-ibuprofen (MDC-917) is a novel agent against colon cancer: Efficacy, metabolism and pharmacokinetics in mouse models. J Pharmacol Exp Ther 337:876–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Mackenzie GG, Murray OT, Zhang Z, Rigas B. (2009) Phosphoaspirin (MDC-43), a novel benzyl ester of aspirin, inhibits the growth of human cancer cell lines more potently than aspirin: a redox-dependent effect. Carcinogenesis 30:512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.