Abstract
Covalent binding to proteins to form neoantigens is thought to be central to the pathogenesis of penicillin hypersensitivity reactions. We have undertaken detailed mass spectrometric studies to define the mechanism and protein chemistry of hapten formation from benzylpenicillin (BP) and its rearrangement product, benzylpenicillenic acid (PA). Mass spectrometric analysis of human serum albumin exposed to BP and PA in vitro revealed that at low concentrations (drug protein molar ratio 0.001:1) and during short time incubations BP and PA selectively target different residues, Lys199 and Lys525, respectively. Molecular modeling showed that the selectivity was a function of noncovalent interaction before covalent modification. With increased exposure to higher concentrations of BP and PA, multiple epitopes were detected on albumin, demonstrating that the multiplicity of hapten formation is a function of time and concentration. More importantly, we have demonstrated direct evidence that PA is a hapten accounting for the diastereoisomeric BP antigen formation in albumin isolated from the blood of patients receiving penicillin. Furthermore, PA was found to be more potent than BP with respect to stimulation of T cells from patients with penicillin hypersensitivity, illustrating the functional relevance of diastereoisomeric hapten formation.
Introduction
β-Lactams such as the penicillins remain a very important group of antibiotics for the treatment of a wide variety of infections. Unfortunately, penicillins can cause serious adverse drug reactions (ADRs), which vary in severity from mild skin rashes to much more severe conditions such as anaphylaxis (Gruchalla and Pirmohamed, 2006). Up to 10% of patients receiving penicillin report allergic reactions, but the incidence of genuine hypersensitivity reactions is found to be only 1 to 2% (Solensky, 2003). In the United Kingdom, 26% of fatal drug-induced anaphylaxis and 11% of all cases of fatal anaphylaxis are caused by β-lactam antibiotics (Solensky, 2003; Pumphrey, 2004; Fitzharris, 2008). It is therefore important to improve the early diagnosis of these ADRs to prevent patients from progressing to life-threatening reactions, ensure sensitive patients are not exposed inadvertently, and ensure that nonallergic patients are not misdiagnosed, leading to the prescribing of other more expensive antibiotics. To develop novel diagnostic assays with high sensitivity and specificity, we need to better understand the mechanism underlying penicillin-mediated ADRs.
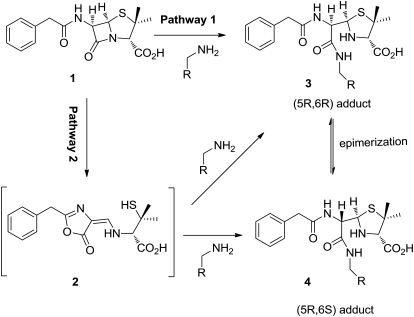
The mechanism of penicillin-mediated ADRs has not been fully elucidated; however, it has been postulated that covalent binding to proteins to form neoantigens plays a crucial role in these ADRs (Levine and Ovary, 1961; Brander et al., 1995; Park et al., 1998). Benzylpenicillin (BP) has been shown to form protein conjugates in vitro and in vivo, and six amino acids of human serum albumin (HSA) have been claimed to be penicilloylated on the basis that these lysine residues were absent from the N-terminal sequences of tryptic peptides (Yvon et al., 1989, 1990). However, the precise structure of these conjugates and the chemical mechanism of this conjugation reaction have not been elucidated completely. In particular, it is unclear whether conjugates are formed via the direct aminolysis of BP (pathway 1; Fig. 1) (Batchelor et al., 1965; Schneider and De Weck, 1965) or via the reaction of penicillenic acid (PA), an intermediate formed from the rearrangement of penicillin (pathway 2).
Fig. 1.
Scheme showing the two potential pathways by which BP covalently bound to protein. Pathway 1: direct binding of BP (1) with epsilon amino groups of lysine residues by opening of the β-lactam ring. Pathway 2: rearrangement of BP (1) to PA (2) followed by nucleophilic attack on lysine residues to form diastereoisomeric adducts (3) and (4).
PA has been postulated as a possible intermediate for penicillin antigen formation on the basis of the observation that aqueous solutions of BP exhibited a strong and distinctive UV absorption at 322 nm, which is characteristic of PA (Neftel et al., 1982; Christie et al., 1988). PA was found to be highly immunogenic both in vitro and in experimental animals (Levine and Price, 1964; Christie and Park, 1989). However, substantive evidence that PA is involved in penicillin antigen formation in patients is lacking, and the origins of the greater immunogenicity of PA remain to be determined. Defining the chemistry of antigen formation is a critical step in understanding the mechanism of penicillin hypersensitivity, and full characterization of the penicillin hapten formed in patients is essential for the design and synthesis of antigens for use in a diagnostic assay. The aims of this study were therefore 1) to determine whether PA is involved in penicillin antigen formation in patients, 2) to define the precise structures of the penicillin antigenic determinants, and 3) to investigate the role of PA in penicillin hypersensitivity reactions.
Materials and Methods
Reagents.
The following products were purchased from Sigma-Aldrich (Gillingham, UK): Hanks' balanced salt solution, penicillin-streptomycin, l-glutamine, HEPES, RPMI medium 1640, human AB serum, HSA (97–99%), and benzylpenicillin. Invitrogen (Paisley, UK) provided fetal bovine serum. Radiolabeled thymidine was obtained from Moravek International Limited (Brea, CA). Trypsin was obtained from Promega (Madison, WI).
Synthesis of Penicillenic Acid.
The synthesis of PA was achieved by coupling oxazolone with d-penicillamine as described previously (Livermore et al., 1948) with modifications (Supplemental Fig. 1A). A full description of methods and characterization of compounds (Supplemental Fig. 1B) is provided in Supplemental Methods.
Rearrangement of Benzylpenicillin to Penicillenic Acid.
BP (4.8 mg/ml) was incubated in phosphate buffer (10 mM, pH 7.4), HSA, and denatured HSA (20 mg/ml) at 37°C. At each time interval (0.5, 1, 3, 5, or 16 h), a 50-μl aliquot was taken out and extracted with ethyl acetate. The solution obtained was then dried in a speed vac, and the products were reconstituted in absolute ethanol and analyzed by LC-MS. Denatured HSA was prepared by incubating HSA with dithiothreitol at 37°C for 15 min and then with iodoacetamide at room temperature for another 15 min. The protein was purified by methanol precipitation and then resuspended in phosphate buffer, pH 7.4.
Preparation and Isolation of Modified HSA.
The time- and concentration- dependent modification of human serum albumin was investigated in vitro. HSA (40 mg/ml, 1 mM) in phosphate buffer (10 mM, pH 7.4) was incubated at 37°C with BP or PA at molar ratios of drug to HSA of 0.01:1, 0.1:1, 1:1, 10:1 and 50:1 for 24 h and at 10:1 for 0.5, 1, 3, 5, and 16 h. In patients, the molar ratio of BP to HSA is approximately 0.1:1 after administration of a single dose of 1.2 g of BP by continuous infusion over 2 h (O'Grady et al., 1997). Thus, the conditions used in in vitro studies were consistent with those that would be encountered in vivo. The protein was precipitated by the addition of nine volumes of ice-cold methanol followed by centrifugation at 14,000g and 4°C for 15 min. To ensure the removal of noncovalently bound drug, the precipitation was repeated and protein pellets were washed with ice-cold methanol. The efficiency of washing was confirmed as detailed previously (Jenkins et al., 2009) using radiolabeled benzylpenicillin. The concentration of HSA was determined by Bradford assay (Bradford, 1976), and aliquots were prepared in serum-free RPMI medium for application in T cell assays, in 50 mM ammonium bicarbonate for mass spectrometric analysis and in Laemmli sample buffer for Western blotting. Before mass spectrometry, all samples were processed as described previously (Jenkins et al., 2009).
A pool of the plasma samples was prepared. HSA was isolated by affinity chromatography as described previously (Jenkins et al., 2009). In brief, a POROS anti-HSA affinity cartridge (Applied Biosystems, Foster City, CA) attached to a Vision Workstation (Applied Biosystems) was used to affinity-capture HSA, which was then eluted with 12 mM HCl. Protein was methanol-precipitated, processed as described previously, and analyzed by reversed-phase LC-MS.
Mass Spectrometric Analysis of Penicillin Hapten.
Analyses were performed on a 5500 QTRAP hybrid triple-quadrupole/linear ion trap instrument with Nanospray II source (Applied Biosystems/MDS Sciex, Foster City, CA). MRM transitions specific for drug-modified peptides were selected as follows: the m/z values for all possible modified peptides with a missed cleavage at the modified lysine residue were used together with a fragment mass of 160 corresponding to the cleaved thiazolidine ring of the drug. Notwithstanding the disparity in the ionisation efficiency of the peptides, relative MRM peak heights for each of the modified peptides were determined by MultiQuant software version 2.0 (Applied Biosystems/MDS Sciex) to provide an “epitope profile” that is characteristic for each drug. The total ion count for the whole digest for each sample was normalized to that of the BP-HSA conjugate formed at a molar ratio of drug to protein of 10:1 for 16 h; in this way, the MRM signals were adjusted for differences in sample loading on-column. Further details of MS method are provided in Supplemental Methods.
In Vitro Reaction of BP or PA with N-Acetyl-l-Lysine.
BP or PA was incubated with N-acetyl-l-lysine methyl ester in 50% ethanol in phosphate buffer (10 mM, pH 7.4) at a molar ratio of 1:1 for 16 h. Aliquots were extracted with ethyl acetate, and the products were analyzed by LC-MS. Further details of the MS method are provided in Supplemental Methods.
In Vitro Kinetic Studies of Diastereomers of Penicillin Hapten.
Drug-pulsed experiments were performed as follows. BP (2 mM) or PA (400 μM) was freshly dissolved in phosphate buffer and then added to a solution of HSA (40 μM, 0.25 ml). The mixture was incubated at 37°C for 1 h, then the protein was precipitated in methanol to remove unbound drug and then resuspended in phosphate buffer to continue the incubation at 37°C. At 3, 5, and 16 h, an aliquot (50 μl) was removed and processed for MS analysis as described above.
Detection of BP and PA Antigens by Western Blotting.
Five micrograms of protein was separated by electrophoresis on a 10% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane by electroblotting. The nitrocellulose membrane was washed in deionized water and blocked in Tris/saline/Tween (TST) buffer (150 mM NaCl, 10 mM Tris-HCl, 0.05% Tween 20, pH 8.0) containing 10% nonfat dry milk for 16 h at 4°C. The membrane was then washed in TST buffer and incubated with primary antipenicillin antibody (mouse antipenicillin monoclonal antibody; Serotec, Oxford, UK) in TST buffer and 5% nonfat dry milk for 1 h. The blot was washed repeatedly in TST buffer and incubated with horseradish peroxidise conjugated anti-mouse IgG antibody (Abcam plc, Cambridge, UK) in TST buffer and 5% nonfat dry milk for an additional 1 h. After repeated washes, signal was detected by enhanced chemiluminescence (Western Lightning; PerkinElmer Life and Analytical Sciences, Waltham, MA).
Computer Modeling of the Noncovalent Binding of BP and PA to HSA.
BP and PA were subjected to an in silico docking procedure using Autodock (Morris et al., 1998) and an associated suite of programs. For the calculations, the structure of HSA (Protein Data Bank code 2BXM with myristate and indomethacin removed) was held rigid, and BP and PA were modeled in their neutral form. The center of the volume that was searched for favorable binding poses was either Lys199 or Lys525 as appropriate. The most popular docking poses for each molecule, as clustered by their root mean square deviation, were identified and examined. Details of the modeling method are provided in Supplemental Methods.
Drug-Specific Lymphocyte Transformation Test.
Freshly isolated peripheral blood mononuclear cells from heparinized venous blood were dispensed into a 96-well U-bottom culture plate [0.15 × 106 cells per well in 200 μl of cell culture medium (RPMI 1640 supplemented with 25 mM HEPES, 2 mM l-glutamine, 10% pooled human AB serum, and 12.5 mg transferrin]. BP or PA was first tested from 5 μM to 2 mM. Tetanus toxoid (0.5 μg/ml) was used as a positive control. Cell cultures were incubated in a CO2-ventilated (5%) incubator at 37°C for 6 days. On the fifth day 0.5 μCi of [3H]thymidine was added to each well. Cells were harvested onto filter membranes, and the amount of incorporated radioactivity was measured (cpm) using a β-counter (MicroBeta Trilux; PerkinElmer, Cambridge, UK).
Patient Details.
Patients (n = 8) receiving intravenous BP for cellulitis at either 1.2 or 2.4 g four times daily were recruited. The age range of the patients was 33 to 87 years (median 60); there were three females and five males, and the course of treatment lasted 3 to 7 days. None of the patients exhibited hypersensitivity reactions to BP or any other drugs being given concurrently. Venepuncture samples were taken no more than 8 h after a prior dose of BP and extracted into heparinized tubes. Samples were placed immediately on ice and centrifuged at 2000g and 4°C within 15 min. Small aliquots were prepared and stored at −80°C.
Patients (n = 2) with clinical histories of allergic reactions to either BP or amoxicillin were recruited for the T cell stimulation studies. These patients had immediate reactions to amoxicillin, and the clinical symptoms included facial swelling and erythematory rash. The studies were approved by Liverpool Local Research Ethics Committee, and informed consent was obtained from all subjects before carrying out the studies.
Results
Rearrangement of Benzylpenicillin to Penicillenic Acid Catalyzed by HSA.
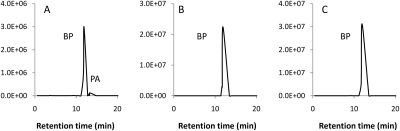
The degradation of BP in different media was monitored to confirm whether PA is an intermediate involved in this process. Our data have shown that the degradation of BP was influenced dramatically by the reaction medium used. PA was formed in the presence of native HSA within 1-h incubation with BP, but was undetectable in phosphate buffer, pH 7.4, even after 16-h incubation (Fig. 2, A and B), clearly indicating the formation of PA was catalyzed by HSA. Only a small amount of PA was detected, but this may not reflect the actual amount of PA formed in the system because PA has a short half-life and could either covalently bind to HSA or hydrolyze to penicilloic acid. Further study suggested that the degree of the catalytic effect depended on the nature of HSA. As seen in Fig. 2C, denatured HSA lost its catalytic effect on BP degradation, indicating that the protein pocket of HSA is essential to facilitate this function.
Fig. 2.
Analysis of BP degradation in different aqueous media. Degradation of BP (4.8 mg/ml) at 37°C in HSA (A), phosphate buffer (B), and denatured HSA (C) was analyzed by LC-MRM-MS after 1-h incubation.
Characterization of Penicillin Hapten Formed by BP and PA In Vitro.
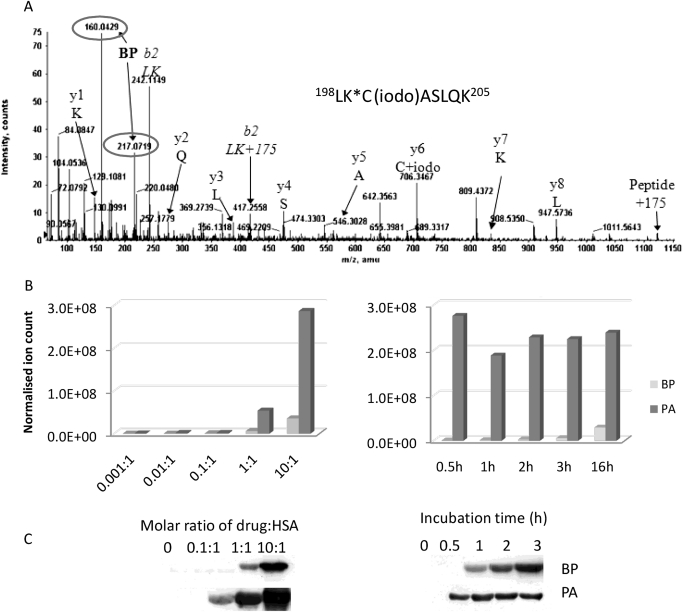
Mass spectrometric analysis revealed that both BP and PA covalently bind to lysine residues in HSA through opening of the β-lactam ring, mainly forming penicilloyl adducts in vitro. At a molar ratio of drug to protein of 10:1, 14 penicilloylated lysine residues were detected when BP was incubated with HSA, whereas 18 penicilloylated lysine residues were detected after incubation with PA (Table 1). As an example, an MS/MS spectrum of carboxamidomethylated tryptic peptide 198LK*C(iodo)ASLQK205 is shown with covalent modification of Lys199 with a penicilloyl group (Fig. 3A). The characteristic fragment ions (both circled in Fig. 3A) at m/z 160 (cleavage of the thiazolidine ring) and m/z 217 are derived from the anticipated fragmentation of a BP or PA hapten, providing firm evidence for modification. In addition, a missed cleavage at the proposed site of modification and the presence of b2 ions corresponding to the N-terminal dipeptide plus 175 atomic mass units (the moiety remaining after cleavage of the thiazolidine ring) provided further evidence of penicilloylation at Lys199.
TABLE 1.
Penicilloylated tryptic peptides of HSA identified in vitro and in vivo
| Lysine | Peptidea | PAb | BPc | In patient |
|---|---|---|---|---|
| 20 | FK*DLGEENFK | + | + | + |
| 137 | K*YLYEIAR | + | + | + |
| 159 | HPYFYAPELLFFAK*R | + | + | + |
| 162 | YK*AAFTECCQAADK | + | − | − |
| 190 | LDELRDEGK*ASSAK | + | + | + |
| 195 | ASSAK*QR | + | + | + |
| 199 | LK*CASLQK | + | + | + |
| 212 | AFK*AWAVAR | + | + | + |
| 351 | LAK*TYETTLEK | + | + | + |
| 372 | VFDEFK*PLVEEPQNLIK | + | − | + |
| 432 | NLGK*VGSK | + | + | + |
| 436 | VGSK*CCK | + | − | + |
| 475 | VTK*CCTESLVNR | + | − | − |
| 525 | K*QTALVELVK | + | + | + |
| 541 | ATK*EQLK | + | + | + |
| 545 | EQLK*AVMDDFAAFVEK | + | + | + |
*indicates modification site.
Incubation at PA HSA molar ratio of 1:1.
Incubation at BP HSA molar ratio of 10:1.
Fig. 3.
MS/MS and Western blotting analysis of penicilloylated HSA peptides identified in vitro. A, MS/MS spectrum of tryptic peptide 198LK*C(iodo)ASLQK205 modified with BP at the Lys199 marked by *. Dominant fragment ions from the penicilloyl group are circled. B and C, time and concentration-dependent binding of BP and PA to HSA in vitro analyzed by mass spectrometric analysis (B) and Western blotting (C).
The penicilloylation of HSA by BP and PA was found to be time- and concentration-dependent. The epitope profile measured by LC-MRM-MS revealed that PA modification was 40 to 60 times greater than BP modification (Fig. 3B). These findings were also mirrored by Western blot data (Fig. 3C). There was an approximately linear relationship between the ratio of drug to protein and the normalized ion count for each modified peptide, which is illustrated by the data acquired for peptides containing Lys199 (Supplemental Fig. 2A). A linear relationship was also observed between the level of modified peptide and incubation time (Supplemental Fig. 2B).
Selective Modification of HSA by BP and PA In Vitro.
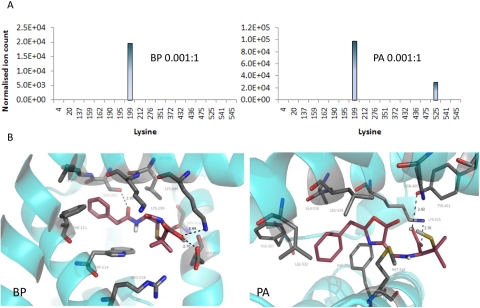
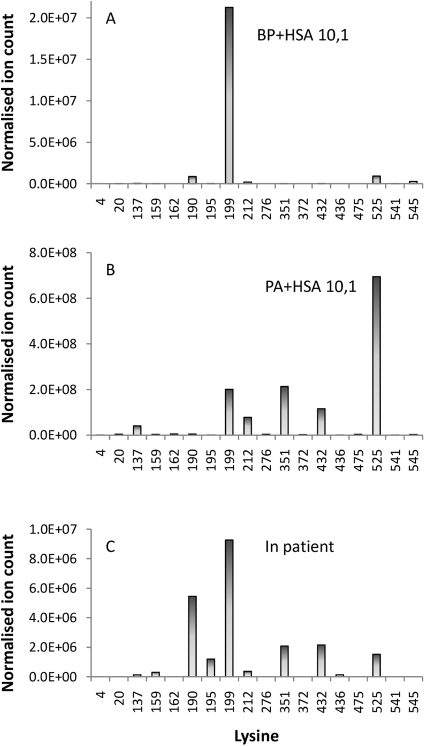
BP and PA seemed to selectively target different lysine residues in HSA when incubated with HSA at low concentrations (drug protein molar ratio 0.001:1) and short incubation times. BP exhibited a marked preference for Lys199, whereas PA preferentially targeted Lys199 and Lys525 (Fig. 4A). Molecular modeling via docking of BP and PA into the structure of HSA revealed the favorable binding poses adjacent to Lys199 and Lys525, respectively (Fig. 4B). Further “focused” docking experiments were performed in which the search volume was restricted around Lys199 and Lys525, respectively (see Materials and Methods for details).The most popular binding pose of BP and PA had a predicted binding energy of −7.88 and −7.07 kcal/mol, respectively. However, at greater exposure, multiple epitopes were observed for both BP and PA (Fig. 5).
Fig. 4.
Selective binding of BP or PA to HSA identified in vitro. A, at low concentration BP preferentially bound to Lys199, whereas PA bound to Lys199 and Lys525. B, molecular modeling of noncovalent interaction of drug with HSA revealed the best poses by docking BP and PA into HSA, showing the key proximity between Lys199 and the β-lactam carbonyl group for BP, and Lys525 and the oxazolone ring for PA. Proteins are rendered as cyan ribbons, amino acid residues close to the guest molecule are rendered as sticks (carbon, gray; nitrogen, blue; oxygen, red), and guest molecules are rendered as sticks (carbon, violet; nitrogen, blue; oxygen, red; polar hydrogens, white).
Fig. 5.
Mutiple epitope profile identified in vitro and in vivo. Notwithstanding the differences in the ionization efficiency of the peptides, epitope profile generated by relative MRM peak heights revealed that multiple epitopes were formed at high concentrations of BP (A) and PA (B) incubated with HSA in vitro and in patients (C).
Characterization of Diastereoisomeric Penicillin Hapten Formed In Vitro and In Vivo.
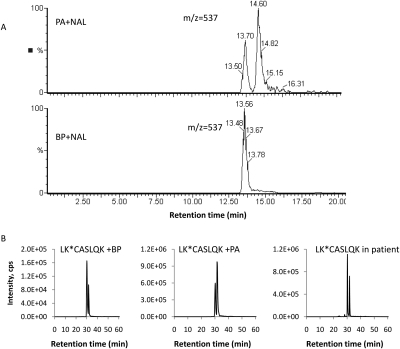
To probe the mechanism of penicillin hapten formation in vitro and in vivo, the aminolysis reactions of BP or PA with N-acetyl lysine were first studied. As shown in Fig. 6A, only one adduct was detected (retention time 13.6 min, m/z 537 [M+H]+) when BP was reacted with N-acetyl lysine methyl ester at pH 7.4. We hypothesize that this adduct resulted from the direct binding of BP to N-acetyl lysine methyl ester and therefore that it retains the 5R,6R configuration. In contrast, two adducts were formed from the reaction of PA under identical conditions (adduct 1, retention time 13.7 min, m/z 537 [M+H], and adduct 2, retention time 14.6 min, m/z 537 [M+H]+). The MS fragmentation patterns observed of the two adducts are identical, indicating that they are diastereoisomers (Supplemental Fig. 3). As the thiazolidine ring of PA would prefer to be 1,3-trans, which would lead to a (5R)-configuration upon ring closure of PA, we therefore postulate that adduct 1 was in 5R,6R configuration, whereas adduct 2 was in 5R,6S configuration.
Fig. 6.
MS/MS analysis of diastereoisomeric penicillin adducts. A, mass chromatogram ([MH+]) of penicilloyl adducts formed by BP or PA with N-acetyl lysine methyl ester. B, mass chromatograms of distereomers of penicilloylated HSA tryptic peptides identified in incubation of BP (left) or PA (center) with HAS and mass chromatogram of penicilloylated peptide diastereomers identified in albumin isolated from plasma of patients receiving BP (right).
It is noteworthy that when BP was incubated with HSA in vitro two isomeric adducts at each of the modified lysine residues were observed (Fig. 6B), in contrast to the single adduct that was formed with N-acetyl lysine methyl ester. The same isomeric adducts were also produced when PA was incubated with HSA. The relative amount of the two diastereomers varied: BP seemed to predominantly form the diastereomer 1 at most sites, whereas PA preferentially yielded diastereomer 2. This pattern was observed for the majority of modified peptides (Supplemental Table 1). An exception was found with the modified peptide containing Lys525: diastereomer 2 was present at slightly higher abundance than diastereomer 1 irrespective of whether HSA was incubated with BP or PA (Supplemental Table 1). This may be caused by competition for the site and the preferential binding of PA to Lys525 observed in Fig. 3A.
The Diastereoisomeric Penicillin Hapten Formation Depends on PA.
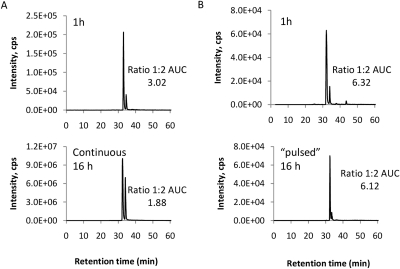
To determine how BP forms diastereoisomeric haptens in the presence of protein, the dynamics of diastereomer formation was monitored over the time course of incubation. During the continuous and prolonged incubation of BP with HSA, an increase in the relative abundance of diastereomer 2 was observed (Fig. 7A). Diastereomer 2 could result from PA, which was formed spontaneously in solution, but could also result from epimerization of diasteromer 1 because HSA has been shown previously to have a catalytic effect on the rearrangement of reactive metabolites (Smith et al., 1989). To determine which pathway has occurred in the presence of HSA, a “pulsed” experiment was designed in which drug was removed after 1-h incubation with protein, and the protein was incubated for another 16 h in the absence of drug. Under these conditions, the ratio of diastereomer 1 to diastereomer 2 does not change over time (Fig. 7B). The same profile was observed for the majority of modified peptides (Supplemental Fig. 4). These data suggested that epimerization of diastereomer 1 to diastereomer 2 does not take place after the drug has become covalently bound to the protein. Therefore, for incubation of HSA with BP, diastereomer 2 can be formed only via a two-step sequential reaction: rearrangement of BP to PA followed by covalent modification of lysine residues by PA (pathway 2), rather than BP modification of lysine residues followed by epimerization of diastereomer 1 to form diastereomer 2 (pathway 1).
Fig. 7.
Kinetic profile of penicilloylated peptide diastereomers formed in the in vitro incubation of BP with HSA. A, mass chromatograms of penicilloylated peptide diastereomers derived from continuous incubation with BP. B, in the pulsed incubation, HSA was incubated with BP for 1 h, and the incubation was continued for 16 h in the absence of drug.
Penicillin Hapten Formed in Patients.
HSA was extracted by affinity chromatography from a pool of plasma samples donated by patients undergoing antibiotic therapy and analyzed on a 5500 QTRAP instrument using the MIDAS approach (Unwin et al., 2005). Fourteen sites of penicilloylation were detected (Table 1), indicating that the qualitative profile of protein modification was similar to that observed in the samples modified with BP and PA in vitro. The ion current epitope profile (Fig. 5) displayed some similarity to those obtained with BP and PA but did not map exactly onto either of them. In addition, diastereoisomeric haptens were detected on albumin isolated from plasma from patients receiving BP (Fig. 6B). It must be noted that most of the patients were being treated with more than one penicillin, and indeed haptens formed by flucloxacillin and amoxicillin were detected in the pooled sample.
Drug-Specific Lymphocyte Transformation Test.
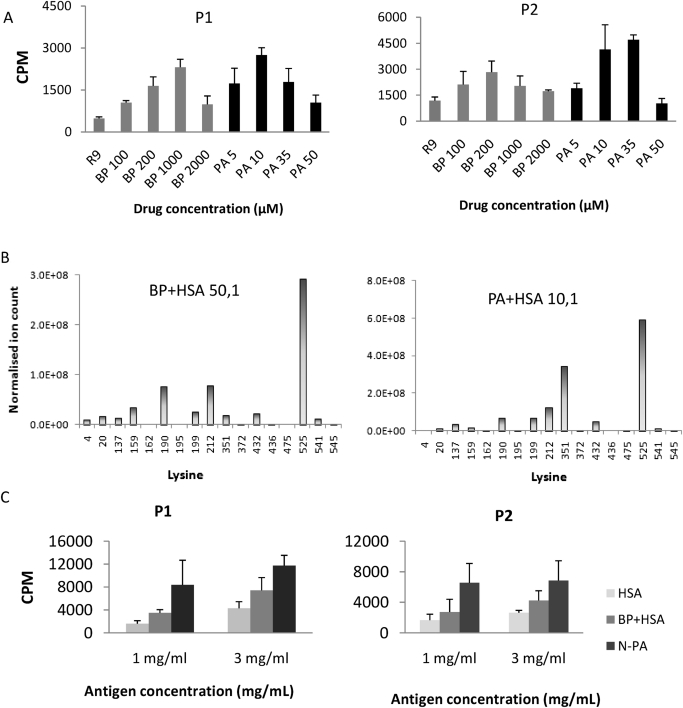
Lymphocytes from BP-hypersensitive patients proliferated in the presence of BP or PA. For BP, a maximal stimulation was achieved at 1 mM, with no stimulation being observed under 100 μM. In contrast, maximal stimulation was achieved by PA at a much lower concentration (approximately 20 μM; Fig. 8A), with higher concentrations of PA being toxic to the patients' T cells. To investigate why PA seemed to be more immunogenic than BP, a normalized synthetic HSA conjugate of BP and PA were prepared. BP-HSA conjugate was generated in vitro at a molar ratio of drug to protein of 50:1, giving a total MRM signal for all modified peptides of 5.49 × 108 cps. PA-HSA conjugate generated at a molar ratio of drug to protein of 10:1 resulted in a total MRM signal of 1.35 × 109 cps. The PA-HSA conjugate formed at 10:1 was therefore diluted with unmodified HSA at a ratio of 1:2.45 to normalize the MRM signals. In addition, the epitope profiles at these drug concentrations were similar for BP and PA (Fig. 8B). Control HSA was processed in the same manner as the conjugates (overnight incubation followed by two rounds of methanol precipitation). T cells from hypersensitive patients were then challenged with the normalized conjugates. At concentrations of 1 and 3 mg/ml (with a background of 3.5 mg/ml HSA from the serum-supplemented medium), the stimulation of PA conjugate was still greater than BP conjugate (Fig. 8C), indicating the 5R,6S diastereomer adducts formed preferentially by PA may be more immunogenic.
Fig. 8.
Penicillin-specific stimulation of lymphocytes from hypersensitive patients. A, positive responses were observed by stimulation of peripheral blood mononuclear cells from penicillin allergic patient 1 (P1) and patient 2 (P2) with BP and PA. B, epitope profile of synthetic BP-HSA conjugate and normalized PA-HSA conjugate generated in vitro. C, proliferation of lymphocytes with synthetic conjugate. RPMI medium supplemented with 10% HSA was used as control (R9).
Discussion
Covalent binding to proteins to form neoantigens is thought to be central to the pathogenesis of penicillin hypersensitivity (Levine and Fellner, 1965; Park et al., 1998; Pichler et al., 1998). It has been postulated that penicillenic acid is a possible intermediate for penicillin antigen formation and may contribute to the immunogenicity of benzylpenicillin in patients. However, solid evidence to support this hypothesis has been lacking. In this study, we used mass spectrometry to define the reactivity of benzylpenicillin and its rearrangement product, benzyl penicillenic acid, and have confirmed that BP and PA selectively bind to lysine residues in HSA in vitro. More importantly, we have proven, for the first time, that penicillenic acid is a hapten accounting for the formation of diastereoisomeric penicillin antigens in patients and the diastereomer formed preferentially via PA may be the causative immunogen of BP in patients.
Studies described herein have demonstrated that BP and PA bind selectively to lysine residues on HSA through opening of the β-lactam ring, yielding penicilloyl lysine adducts. No evidence of other modified amino acid residues could be found. A possible explanation is that the adducts resulted from other nucleophiles such as serine, histidine, and cysteine may be too labile to be detected under current analytical conditions or a further transacylation may have occurred (Tsuji et al., 1975). In addition, only penicilloyl lysyl antigenic determinants were detected in this study; minor determinants, such as penicillanyl and penicillamine derivatives (Levine, 1960; Schneider et al., 1973), were not observed. It has been shown that some patients are more sensitive to the minor determinants (Weltzien and Padovan, 1998). However, the formation of minor determinants requires either further degradation of BP, for example, penicillamine derivatives, or the involvement of particular bioactivation pathway, for example, production of the penicillanyl adducts derived from the free carboxyl group of penicillin. Thus these adducts may not be formed in plasma.
Furthermore, the penicilloylation of lysine residues by BP and PA seemed to be concentration- and time-dependent. At low concentrations, BP and PA were shown to bind selectively to Lys199 and Lys525, respectively. Molecular modeling via docking of BP and PA into the structure of HSA revealed favorable binding poses in the proximity of Lys199 and Lys525, respectively, closely mirroring the experimental data. It is worth noting the remarkable proximity between Lys199 and the BP β-lactam carbonyl group, a pose clearly favorable to penicilloylation; whereas in the case of PA, the best pose reveals the key proximity between the oxazolone ring and Lys525, which is conducive to covalent binding. Thus the preference of the drugs for different lysine residues in HSA is driven at least in part by the noncovalent interaction with protein, with noncovalent interaction positioning the drugs in favorable orientations to facilitate covalent binding with adjacent lysine residues (Qiu et al., 1998; Szapacs et al., 2006). The three-dimensional shape of the drug, as well as its inherent chemical reactivity, will therefore determine selectivity of covalent binding as demonstrated in this study. However, at high concentrations and with prolonged incubation, multiple epitopes were detected for both BP and PA, indicating the multiplicity of the epitope is a function of concentration and reaction time. Because the half-life of human serum albumin is approximately 19 days (Müller et al., 2010), and consequently the modified protein is likely to accumulate over the course of the therapeutic intervention, which is usually 7 days in duration, it is perhaps not surprising that a similar multiple epitope profile was observed in patients receiving BP, establishing the physiological relevance of the in vitro studies.
More importantly, we have demonstrated that BP can form diastereoisomeric haptens in vitro and in patients via its rearrangement intermediate, penicillienic acid. Two isomeric adducts at each of the modified lysine residues were observed when BP was incubated with HSA in vitro, in contrast to the single adduct formed with N-acetyl lysine methyl ester in phosphate buffer, pH 7.4. Because diastereomer 2 was proven to be formed exclusively from PA, this led to the hypothesis that PA is an intermediate partially accounting for the formation of diastereoisomeric penicillin antigen. This hypothesis was further supported by the observation that BP had indeed undergone rearrangement to PA when incubated with HSA but not in phosphate buffer. Comparable diastereoisomeric penicilloyl albumin adducts were also detected in patients receiving penicillin, suggesting that the same reaction pathway could be essential for the formation of penicillin antigens in patients. It must be noted that most of the patients were on multiple medications, so it is perhaps not surprising that the ratio of diastereomers of penicilloylated peptides observed in vivo is slightly different from those obtained in vitro.
The finding that PA is involved in penicillin antigen formation in patients is of considerable clinical significance because it has been shown that PA is highly reactive and immunogenic (Christie et al., 1988). Although only a small amount of PA may be formed in patients, the greater reactivity of PA could have a significant impact on the antigenic determinants found on protein. Furthermore, the diastereoisomeric penicilloyl haptens derived from PA could also have an impact on penicillin ADRs because the immune system can discriminate between penicillin stereoisomers (Nagata et al., 1986). These two diastereomers may bind differently to the MHC binding grove on antigen-presenting cells, which may subsequently affect the binding affinity and T cell recognition, leading to differences in immunogenicity.
Our data have shown that PA is a more potent stimulator of T cell proliferation than BP. There are several possible explanations for the observed difference: PA may form a higher level of protein adduct because of its greater reactivity; the modified protein may be processed differently because of the preference of PA for specific lysine residues; or the diastereomeric adducts formed preferentially by PA may be inherently more immunogenic than the one formed by BP (Nagata et al., 1986). To investigate the latter possibility, synthetic HSA conjugates of BP and PA were normalized to minimize the effect of lysine residue preference and the total level of antigen. Normalized PA-HSA conjugate was a more potent stimulator of T cell proliferation than BP-HSA conjugate, indicating that the diastereomeric adducts formed preferentially by PA are more antigenic.
In conclusion, we have demonstrated that BP and PA can selectively bind to lysine residues in HSA at low concentrations, whereas at higher concentrations and in patients multiple lysine adducts have been identified. In addition, we have demonstrated direct evidence that PA is an intermediate involved in diastereoisomeric BP antigen formation in patients and that PA forms bona fide antigens that could be responsible for penicillin hypersensitivity reactions. Furthermore, we have found that PA is a more potent stimulator of T cell proliferation than BP. The greater potency of PA could, of course, be a function of hapten density or the epitope multiplicity that has been observed in vitro, but could also be a function of stereochemistry.
Supplementary Material
Acknowledgments
We thank research nurses Margaret Little and Anita Hanson for recruiting patients and acquiring samples and Dr. Joseph Sanderson for help with Western blottings.
This work was funded by the Centre for Drug Safety Science, which is supported by the Medical Research Council [Grant G0700654]. X.M. is supported by the National Institute for Health Research Biomedical Research Centre in Microbial Diseases.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183871.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- ADR
- adverse drug reaction
- BP
- benzylpenicillin
- PA
- benzylpenicillenic acid
- HSA
- human serum albumin
- LC
- liquid chromatography
- MRM
- multiple reaction monitoring
- MS
- mass spectrometry
- TST
- Tris/saline/Tween
- AUC
- area under the curve.
Authorship Contributions
Participated in research design: Meng, Jenkins, Stachulski, French, Naisbitt, Pirmohamed, and Park.
Conducted experiments: Meng, Jenkins, Berry, Maggs, and Farrell.
Contributed new reagents or analytic tools: Meng, Jenkins, and Lane.
Performed data analysis: Meng, Jenkins, Berry, Farrell, and Naisbitt.
Wrote or contributed to the writing of the manuscript: Meng, Jenkins, Berry, Maggs, Stachulski, French, Naisbitt, Pirmohamed, and Park.
References
- Batchelor FR, Dewdney JM, Gazzard D. (1965) Penicillin allergy: the formation of penicilloyl determinant. Nature 206:362–364 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Brander C, Mauri-Hellweg D, Bettens F, Rolli H, Goldman M, Pichler WJ. (1995) Heterogeneous T cell responses to β-lactam-modified self-structures are observed in penicillin-allergic individuals. J Immunol 155:2670–2678 [PubMed] [Google Scholar]
- Christie G, Coleman JW, Park BK. (1988) Drug-protein conjugates–XVII. The effect of storage on the antigenicity and immunogenicity of benzylpenicillin in the rat. Biochem Pharmacol 37:4121–4128 [DOI] [PubMed] [Google Scholar]
- Christie G, Park BK. (1989) Disposition and immunogenicity of penicillin in the rabbit. Int Arch Allergy Appl Immunol 89:162–168 [DOI] [PubMed] [Google Scholar]
- Fitzharris P. (2008) Penicillin allergy: updating the role of skin testing in diagnosis. Postgrad Med J 84:505–506 [DOI] [PubMed] [Google Scholar]
- Gruchalla RS, Pirmohamed M. (2006) Clinical practice. Antibiotic allergy. N Eng J Med 354:601–609 [DOI] [PubMed] [Google Scholar]
- Jenkins RE, Meng X, Elliott VL, Kitteringham NR, Pirmohamed M, Park BK. (2009) Characterisation of flucloxacillin and 5-hydroxymethyl flucloxacillin haptenated HSA in vitro and in vivo. Proteomics Clin Appl 3:720–729 [DOI] [PubMed] [Google Scholar]
- Levine BB. (1960) Formation of d-penicillamine-cysteine mixed disulphide by reaction of d-benzylpenicilloic acid with cystine. Nature 187:940–941 [DOI] [PubMed] [Google Scholar]
- Levine BB, Fellner MJ. (1965) Immune responses to penicillin in man and penicillin allergy. J Clin Invest 44:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BB, Ovary Z. (1961) Studies on the mechanism of the formation of the penicillin antigen. III. The N- (d-α-benzylpenicilloyl) group as an antigenic determinant responsible for hypersensitivity to penicillin G. J Exp Med 114:875–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BB, Price VH. (1964) Studies on the immunological mechanisms of penicillin allergy. II. Antigenic specificities of allergic wheal-and-flare skin responses in patients with histories of penicillin allergy. Immunology 7:542–556 [PMC free article] [PubMed] [Google Scholar]
- Livermore AH, Carpenter FH. (1948) Studies on crystalline dl-benzylpenicillenic acid. J Biol Chem 175:721–726 [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662 [Google Scholar]
- Müller N, Schneider B, Pfizenmaier K, Wajant H. (2010) Superior serum half life of albumin tagged TNF ligands. Biochem Biophys Res Commun 396:793–799 [DOI] [PubMed] [Google Scholar]
- Nagata N, Hurtenbach U, Gleichmann E. (1986) Specific sensitization of Lyt-1+2− T cells to spleen cells modified by the drug d-penicillamine or a stereoisomer. J Immunol 136:136–142 [PubMed] [Google Scholar]
- Neftel KA, Walti M, Spengler H, Deweck AL. (1982) Effect of Storage of Penicillin-G Solutions on Sensitization to Penicillin-G after Intravenous Administration. Lancet 1:986–988 [DOI] [PubMed] [Google Scholar]
- O'Grady F, Lambert HP, Finch RG, Greenwood D. eds (1997) Antibiotic and Chemotherapy: Anti-Infective Agents and Their Use In Therapy. Churchill Livingstone, New York [Google Scholar]
- Park BK, Pirmohamed M, Kitteringham NR. (1998) Role of drug disposition in drug hypersensitivity: a chemical, molecular, and clinical perspective. Chem Res Toxicol 11:969–988 [DOI] [PubMed] [Google Scholar]
- Pichler WJ, Schnyder B, Zanni MP, Hari Y, von Greyerz S. (1998) Role of T cells in drug allergies. Allergy 53:225–232 [DOI] [PubMed] [Google Scholar]
- Pumphrey R. (2004) Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol 4:285–290 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Burlingame AL, Benet LZ. (1998) Mechanisms for covalent binding of benoxaprofen glucuronide to human serum albumin. Studies By tandem mass spectrometry. Drug Metab Dispos 26:246–256 [PubMed] [Google Scholar]
- Schneider CH, De Weck AL. (1965) A new chemical spect of penicillin allergy: the direct reaction of penicillin with epsilon-amino-groups. Nature 208:57–59 [DOI] [PubMed] [Google Scholar]
- Schneider CH, Pfeuti C, de Weck AL. (1973) Aspects of formation of the d-penicillamine-antigenic determinant from penicilloyl compounds. Helv Chim Acta 56:1235–1243 [DOI] [PubMed] [Google Scholar]
- Smith BA, Gutmann HR, Springfield JR. (1989) Catalytic effect of serum albumin on the O-rearrangement of N-sulfooxy-2-acetylaminofluorene, a potential hepatocarcinogen in the rat, to nonmutagenic sulfuric acid esters of O-amidofluorenols. Biochem Pharmacol 38:3987–3994 [DOI] [PubMed] [Google Scholar]
- Solensky R. (2003) Hypersensitivity reactions to β-lactam antibiotics. Clin Rev Allergy Immunol 24:201–220 [DOI] [PubMed] [Google Scholar]
- Szapacs ME, Riggins JN, Zimmerman LJ, Liebler DC. (2006) Covalent adduction of human serum albumin by 4-hydroxy-2-nonenal: kinetic analysis of competing alkylation reactions. Biochemistry 45:10521–10528 [DOI] [PubMed] [Google Scholar]
- Tsuji A, Yamana T, Miyamoto E, Kiya E. (1975) Chemical reactions involved in penicillin allergy: kinetics and mechanism of penicillin aminolysis. J Pharm Pharmacol 27:580–587 [DOI] [PubMed] [Google Scholar]
- Unwin RD, Griffiths JR, Leverentz MK, Grallert A, Hagan IM, Whetton AD. (2005) Multiple reaction monitoring to identify sites of protein phosphorylation with high sensitivity. Mol Cell Proteomics 4:1134–1144 [DOI] [PubMed] [Google Scholar]
- Weltzien HU, Padovan E. (1998) Molecular features of penicillin allergy. J Invest Dermatol 110:203–206 [DOI] [PubMed] [Google Scholar]
- Yvon M, Anglade P, Wal JM. (1989) Binding of benzyl penicilloyl to human serum albumin. Evidence for a highly reactive region at the junction of domains 1 and 2 of the albumin molecule. FEBS Lett 247:273–278 [DOI] [PubMed] [Google Scholar]
- Yvon M, Anglade P, Wal JM. (1990) Identification of the binding sites of benzyl penicilloyl, the allergenic metabolite of penicillin, on the serum albumin molecule. FEBS Lett 263:237–240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.