Abstract
Recent studies suggest that retinoids may be effective in the treatment of Alzheimer's disease, although exposure to an excess of retinoids during gestation causes teratogenesis. Cholesterol is essential for brain development, but high levels of cholesterol have been associated with Alzheimer's disease. We hypothesized that retinoic acid may affect cholesterol homeostasis in rat astrocytes, which regulate cholesterol distribution in the brain, through the up-regulation of cholesterol transporters ATP binding cassette (Abc)a1 and Abcg1. Tretinoin, 13-cis retinoic acid (13-cis-RA), 9-cis-RA, and the selective retinoid X receptor (RXR) agonist methoprene significantly increased cholesterol efflux induced by cholesterol acceptors and protein levels of Abca1 by 2.3- (±0.25), 3.6- (±0.42), 4.1- (±0.5), and 1.75- (±0.43) fold, respectively, and Abcg1 by 2.1- (±0.26), 2.2- (±0.33), 2.5- (±0.23), and 2.2- (±0.21) fold, respectively. 13-cis-RA and 9-cis-RA also significantly increased mRNA levels of Abca1 (maximal induction 7.3 ± 0.42 and 2.7 ± 0.17, respectively) and Abcg1 (maximal induction 2.0 ± 0.18 and 1.8 ± 0.09, respectively), and the levels of membrane-bound Abca1 (2.5 ± 0.3 and 2.5 ± 0.40-fold increase, respectively), whereas they significantly decreased intracellular cholesterol content without affecting cholesterol synthesis. The effect of 9-cis-RA on cholesterol homeostasis in astrocytes can be ascribed to the activation of RXR, whereas the effects of 13-cis-RA and tretinoin were independent of either RXRs or retinoic acid receptors. These findings suggest that retinoids affect cholesterol homeostasis in astrocytes and that this effect may be involved in both their therapeutic and teratogenic actions.
Introduction
Retinoids are biologically active derivatives of vitamin A (retinol), which play an important role in brain development, when they are involved in neuronal patterning, differentiation, and axonal outgrowth, and in adulthood, when they are involved in nerve regeneration, adult neuronal plasticity, and adult neural stem cell proliferation and differentiation into neurons (Maden and Hind, 2003; Tafti and Ghyselinck, 2007).
The activation of two families of nuclear receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs), is responsible for most of the biological activities of retinoids (Germain et al., 2006a,b). RARs form heterodimers with RXRs; RAR agonists activate RAR/RXR heterodimers and modulate transcription of target genes even in the absence of RXR agonists, whereas RXR agonists cannot activate RAR/RXR heterodimers in the absence of RAR agonists (Germain et al., 2006a). RXRs partner with numerous other nuclear receptors, some of which are permissive partners, allowing the activation of the heterodimers by agonists of both RXR and the partner receptor independently, whereas others are nonpermissive partners and do not allow the activation of heterodimers by RXR agonists. As partners of several nuclear receptors, RXRs can modulate the transcription of a very large array of genes (Germain et al., 2006b).
Retinoid isomers differentially activate RARs and/or RXRs; in this study, we used tretinoin (also known as all-trans retinoic acid), which activates RARs; 9-cis retinoic acid (9-cis-RA), a potent activator of RXRs but also an agonist of RARs; and 13-cis-RA, which is neither a RAR nor a RXR agonist (Germain et al., 2006a,b).
Retinoids also exert biological effects that are independent of activation of RXR and RAR; for example, tretinoin activates the peroxisome proliferator-activator receptors β/δ (Shaw et al., 2003; Schug et al., 2007), and several retinoids modulate protein kinase C and cAMP response element-binding protein activity with a mechanism independent of genomic activation (Aggarwal et al., 2006).
Tretinoin and 13-cis-RA have been found in several tissues, including the brain, and their levels are up-regulated by a diet enriched in vitamin A (Kane et al., 2008); the presence of 9-cis-RA in mammalian cells and tissues remains controversial (Germain et al., 2006b; Wolf, 2006).
In the central nervous system, tretinoin and 13-cis-RA have been used for the treatment of brain tumors (Yung et al., 1996; Kaba et al., 1997); these retinoids are also currently under investigation for the treatment of Alzheimer's disease (AD) (Lee et al., 2009; Shudo et al., 2009). The mechanism(s) involved in the therapeutic effects of retinoids in AD have not been fully elucidated. The hypothesis that retinoids may increase cholesterol clearance from central nervous system cells through the up-regulation of ATP binding cassette (ABC) cholesterol transporters and lipoprotein release has not been explored and is the focus of this study. Recent studies have suggested that members of the ABC transporter family, ABCA1 and ABCG1 in particular, may represent therapeutic targets for the treatment of AD, because they may contribute to the reduction of intracellular cholesterol levels and amyloid-β deposition in brain cells (Hirsch-Reinshagen and Wellington, 2007). Furthermore, ABC cholesterol transporters are involved in the biogenesis of lipoproteins by lipidating apolipoprotein (apo)AI and apoE (Oram and Heinecke, 2005). An apoE polymorphism has been implicated in AD, because apoE4 is the major genetic risk factor in the late onset of AD.
Astrocytes are major producers of cholesterol in the brain and also are involved in cholesterol redistribution and/or elimination, because they produce nascent lipoproteins containing apoE that can extract cholesterol from brain cells (LaDu et al., 1998). The cholesterol transporter ABCA1 (human)/Abca1 (rat) mediates cholesterol efflux to lipid-poor apolipoproteins as well as apoE release, leading to the formation of nascent lipoproteins. Another cholesterol acceptor, ABCG1 (human)/Abcg1 (rat), induces cholesterol efflux to nascent lipoproteins, leading to their further lipidation and remodeling (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004; Karten et al., 2006).
The general hypothesis of this study is that retinoids may affect cholesterol homeostasis in astrocytes by affecting cholesterol transporter expression, cholesterol efflux, and intracellular cholesterol content, events that may be involved in the therapeutic effects of retinoids.
Materials and Methods
Materials.
Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS), trypsin, TRIzol reagent, Amplex Red Cholesterol Assay Kit, and all of the tissue culture supplies were from Invitrogen (Carlsbad, CA). [3H]Cholesterol and [3H]acetate were from PerkinElmer Life and Analytical Sciences (Waltham, MA). ApoAI and high-density lipoproteins (HDL) were purchased from EMD Biosciences (San Diego, CA). The protease inhibitor cocktail and protein A-agarose beads were purchased from Roche Applied Science (Indianapolis, IN). Polyvinylidene difluoride membranes were from Millipore Corporation (Billerica, MA); Abca1 and Abcg1 antibodies were from Novus Biologicals (Littleton, CO). Sulfo-N-hydroxysuccinimide-biotin was from Thermo Fisher Scientific (Waltham, MA). The GeneAmp RNA PCR Kit and rat Abca1 and Abcg1 primers were from Applied Biosystems (Foster City, CA). Thin-layer chromatography plates were purchased from Macherey-Nagel (Düren, Germany). Time-pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Tretinoin, 13-cis-RA, 9-cis-RA, 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid (TTNPB), and methoprene were from Enzo Life Sciences (Farmingdale, NY). All of the other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Rat Astrocyte Cultures.
Pregnant rats were euthanized with carbon dioxide, and the uterine horns were removed by using a protocol approved by the University of Washington Institutional Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Institute of Laboratory Animal Resources, 1996). Primary cultures of cortical astrocytes, prepared from fetuses at gestational day 21, as described previously (Guizzetti et al., 2007), were maintained in DMEM (low glucose) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (FBS/DMEM). Nine days after preparation, astrocytes were harvested and plated in 24-well plates for cholesterol efflux and cholesterol synthesis experiments or in 100-mm dishes for all of the other experiments. All of the treatments were carried out in serum-free DMEM supplemented with 0.1% bovine serum albumin (BSA), 100 U/ml penicillin, and 100 μg/ml streptomycin (BSA/DMEM).
Cholesterol Efflux.
Cholesterol efflux was measured as described previously (Guizzetti et al., 2007). In brief, astrocytes were labeled for 24 h with 1 μCi/ml [3H]cholesterol in fetal bovine serum/DMEM followed by 24-h treatments in serum-free BSA/DMEM in the presence or absence of tretinoin, 9-cis-RA, 13-cis-RA, TTNPB, methoprene, or retinol. Cells were chased subsequently for 6 h in the presence or absence of the cholesterol acceptors (apoAI and HDL) in BSA/DMEM. [3H]Cholesterol content was quantified in the medium and in the cellular lipids, extracted in hexane/isopropyl alcohol (3:2, v/v), using a scintillation counter (LS 6000; Beckman Coulter, Fullerton, CA). Cholesterol efflux was expressed as a percentage of total cholesterol by dividing medium [3H]cholesterol by total (medium + cell) [3H]cholesterol.
Western Blot Analysis.
At the end of each incubation, cells were scraped in ice-cold lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1.0 mM EDTA, 1.0 mM EGTA, 1.0% Triton X-100, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 10 mM sodium β-glycerophosphate, 1.5 mM sodium orthovanadate] supplemented with a protease inhibitor cocktail, as described previously (Guizzetti et al., 2007). Samples were sonicated, rocked for 40 min at 4°C, and centrifuged at 14,000g for 15 min; the supernatant then was collected and analyzed. Intracellular proteins were quantified by the Bradford (Bradford, 1976) method, and 25 or 50 μg of proteins were separated on a 7.5% (Abca1) or a 4 to 10% (Abcg1) SDS polyacrylamide gel, transferred to polyvinylidene difluoride membranes, labeled with polyclonal antibodies against Abca1 or Abcg1 followed by the appropriate horseradish peroxidase (HRP)-conjugated IgG, and detected by chemiluminescence. Abca1 and Abcg1 bands were quantified by densitometry and normalized to those of β-actin.
Biotinylation of Surface Proteins and Immunoprecipitation.
For selective labeling of plasma membrane proteins, astrocytes were incubated for 30 min at room temperature with PBS containing 1 mg/ml sulfo-N-hydroxysuccinimide-biotin, as described previously (Guizzetti et al., 2007). Cells were solubilized in the presence of 1% Nonidet P40 and protease inhibitors, and protein levels were quantified by bicinchoninic acid protein assay. Equal amounts of proteins from cell lysates were incubated with a polyclonal antibody to Abca1 for 1 h at 4°C under constant mixing by inversion. Fifty microliters of protein A-agarose beads were added to the mixture and incubated at 4°C overnight under constant mixing. The beads were washed several times, and total Abca1 was eluted from the beads by boiling in sample buffer for 3 min. The samples were separated by SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Total Abca1 and biotinylated Abca1 were detected using an Abca1 polyclonal antibody followed by HRP-conjugated secondary antibody and a streptavidin-HRP conjugate, respectively.
RNA Extraction and Real-Time Polymerase Chain Reaction Analysis.
RNA was extracted using the TRIzol reagent following the directions of the manufacturer. Total RNA concentration and its purity were determined by spectrophotometry. Electrophoresis in 1.5% agarose gels was used to ensure good mRNA integrity. Levels of mRNA were determined by real-time reverse transcription polymerase chain reaction by the Functional Genomics Core Facility (Department of Environmental and Occupational Health Sciences, University of Washington) using TaqMan reverse transcription kits and an ABI 7900 HT Sequence Detection System (Applied Biosystems). β-Actin was used as the reference gene in all of the experiments.
Cholesterol Synthesis and Endogenous Cholesterol Efflux.
Cholesterol synthesis was measured as described previously (Guizzetti et al., 2007), with some modifications. Astrocytes were labeled with 25 μCi/ml [3H]acetate for 4 h in DMEM containing 5% lipoprotein-deficient serum, followed by washout and a 24-h treatment with 9-cis-RA or 13-cis-RA in the presence or absence of HDL. Culture media were collected, centrifuged at 800g for 10 min to remove floating cells and cell debris, and used to quantify the endogenously synthesized cholesterol released in the medium. Lipids in the medium and in the cells were extracted in hexane/isopropyl alcohol (3:2, v/v) for 1 h at room temperature. After solvent evaporation, lipids then were dissolved in 55 μl of chloroform. Five microliters of each sample was counted on a scintillation counter to determine the total 3H radioactivity incorporated into cell lipids; the remaining lipid extracts were spotted on activated silica gel thin-layer chromatography plates and developed in hexane/diethyl ether/methanol/acetic acid (80:20:6.5:1). The plates were allowed to dry and stained with iodine vapor. Cholesterol bands, identified by the comigration of cholesterol standards, were excised from the plates, and the associated radioactivity was quantified by scintillation counting. The relative rate of cholesterol synthesis was expressed as the sum of the 3H radioactivity (cpm) associated with intracellular and medium cholesterol divided by the 3H radioactivity associated with the total lipid mixture. The relative efflux of endogenously synthesized cholesterol was expressed as a percentage of total cholesterol by dividing medium [3H]cholesterol by total (medium + cell) [3H]cholesterol.
Cholesterol Mass.
Astrocytes were lysed in radioimmunoprecipitation assay buffer [150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate, 0.5% SDS, and 50 mM Tris-HCl (pH 7.5)]. Protein content was quantified using the bicinchoninic acid protein assay, and cholesterol was measured by an enzymatic method using the Amplex Red Cholesterol Assay Kit according to the directions of the manufacturer and read with a fluorescence microplate reader (563 nm absorbance and 587 nm emission). Cholesterol content was normalized to protein content and expressed as microgram of cholesterol per milligram of protein.
Cell Viability.
Cell viability was measured using the metabolic dye 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT). In brief, after incubation with 9-cis-RA, 13-cis-RA, methoprene, or TTNPB for 24 h at 37°C, astrocytes were incubated for an additional 30 min at 37°C with fresh medium containing 0.5 mg/ml MTT followed by extraction in dimethyl sulfoxide (250 μl). Sample signal was acquired by subtracting the absorbance read at 630 nm (background) from absorbance read at 570 nm (signal).
Statistical Analysis.
All of the statistical tests were carried out using KaleidaGraph 4.0 software (Synergy Software, Reading, PA) on a Macintosh personal computer. The Student's t test (for single comparisons) or one-way analysis of variance followed by the Dunnett's test (when comparing multiple treatments to one control) was used to determine significant differences from controls.
Results
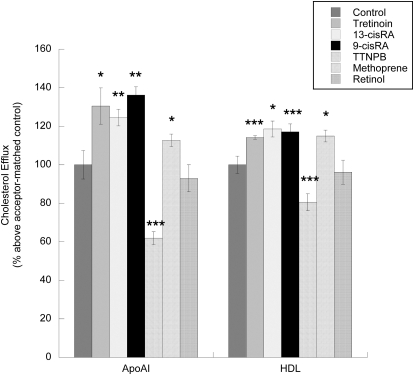
We first investigated the effects of tretinoin, 9-cis-RA, 13-cis-RA, their parent compound retinol, the selective synthetic RAR ligand TTNPB, and the selective RXR agonist methoprene on cholesterol efflux in astrocytes. 9-cis-RA, 13-cis-RA, tretinoin, and methoprene increased cholesterol efflux to both acceptors; these compounds increased apoAI-induced cholesterol efflux by 36.3, 24.5, 30.5, and 12.7%, respectively, and HDL-induced cholesterol efflux by 17.1, 18.6, 14.2, and 15%, respectively. In contrast, the RAR agonist TTNPB inhibited cholesterol efflux mediated by apoAI by 38.1% and by HDL by 19.4%; retinol was ineffective. Results shown in Fig. 1 represent the relative increase in the efflux of [3H]cholesterol caused by the pretreatment of astrocytes with the tested compounds and then chased for 6 h with apoAI (Fig. 1, left) and HDL (Fig. 1, right). The efflux of cholesterol from untreated cells in the absence of acceptors was 2.5 ± 0.14% of total cholesterol; in the presence of 10 μg/ml apoAI and 50 μg/ml HDL, cholesterol efflux was 5.04 ± 0.36 and 11.6 ± 0.65% of total cholesterol, respectively (p < 0.001), similar to that reported previously (Guizzetti et al., 2007).
Fig. 1.
Effects of retinoids on cholesterol efflux from astrocytes. Fetal rat cortical astrocytes labeled with 1 μCi/ml [3H]cholesterol were incubated for an additional 24 h in the presence of tretinoin, 13-cis-RA, 9-cis-RA, TTNPB, methoprene, or retinol (all at 10 μM). [3H]Cholesterol released in the medium during the following 6 h in the presence of cholesterol acceptors (10 μg/ml apoAI or 50 μg/ml HDL) was quantified. Each value is the mean (±S.E.) of three to five independent experiments. *, p < 0.05, **, p < 0.01, and ***, p < 0.0001 compared with an acceptor-matched control.
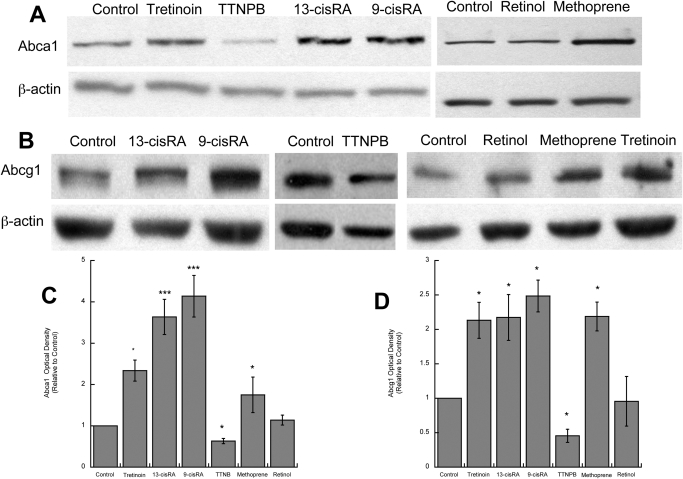
Cholesterol efflux to lipid-poor apolipoproteins, such as apoAI, is mediated by the cholesterol transporter Abca1, which initiates the lipidation of apolipoproteins, leading to the formation of nascent lipoproteins (Oram and Heinecke, 2005). In accordance with these observations, we found that 9-cis-RA, 13-cis-RA, tretinoin, and methoprene increased the levels of Abca1 by 4.1, 3.6, 2.3, and 1.7 fold above that of the control, respectively, whereas the synthetic RAR agonist TTNPB decreased Abca1 levels by 37% and retinol had no effect (Fig. 2, A and C).
Fig. 2.
Effects of retinoids on Abca1 and Abcg1 protein levels. Fetal rat cortical astrocytes were incubated for 24 h in the absence or presence of tretinoin, 13-cis-RA, 9-cis-RA, TTNPB, methoprene, or retinol (all at 10 μM). Proteins were extracted, quantified, separated by electrophoresis, and analyzed by Western blot for Abca1 and Abcg1 proteins. A and B, representative immunoblots labeled with Abca1 (A), Abcg1 (B), and β-actin (loading control) antibodies. C and D, levels of Abca1 (C) and Abcg1 (D) quantified by densitometry, normalized to β-actin, and expressed as optical density relative to controls. Each value is the mean (±S.E.) of three independent experiments. *, p < 0.05 and ***, p < 0.001 compared with control.
Another ABC cholesterol transporter, ABCG1, has been involved in cholesterol efflux to lipoproteins, such as HDL, by acting sequentially to ABCA1 and further lipidating lipoproteins generated by ABCA1 (Vaughan and Oram, 2006). Similar to that observed for Abca1, 9-cis-RA, 13-cis-RA, tretinoin, and methoprene increased Abcg1 levels 2- to 3-fold above that of the control; TTNPB decreased ABCG1 levels by 55%, and retinol was ineffective (Fig. 2, B and D).
Altogether, these results indicate that in astrocytes 9-cis-RA, 13-cis-RA, tretinoin, and methoprene increased cholesterol efflux to apoAI and HDL through the induction of Abca1 and Abcg1, respectively; in contrast, the selective activation of RAR by TTNPB inhibited cholesterol efflux and reduced the levels of Abca1 and Abcg1. None of these compounds, at the concentration used in these experiments, induced cytotoxicity in astrocytes, as determined by the MTT assay (data not shown).
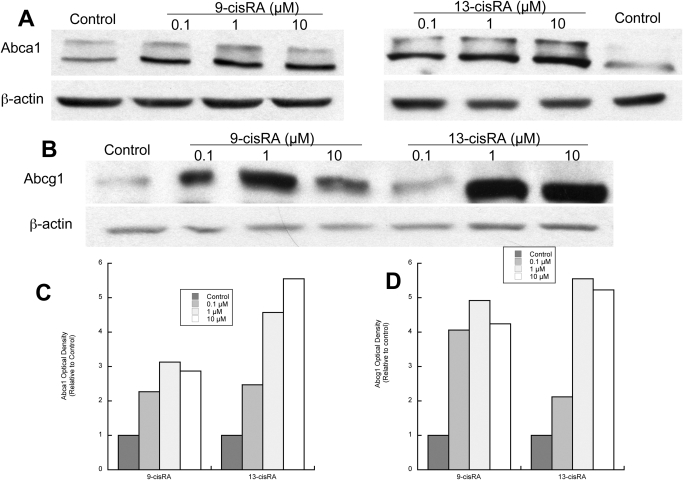
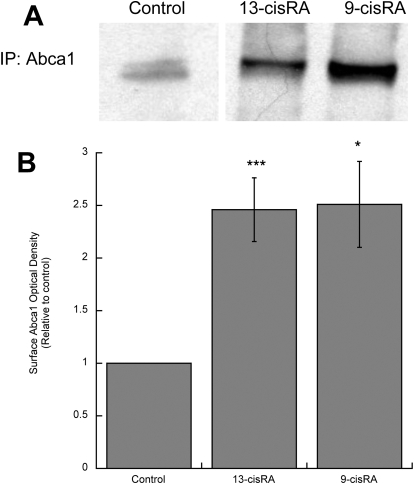
To further characterize the effects of retinoids on cholesterol homeostasis in astrocytes, we focused on two of the six compounds initially tested: 9-cis-RA and 13-cis-RA. 9-cis-RA, a strong activator of RXR and also an agonist of RARs, is not used as a systemic drug because of its side effects; 13-cis-RA, neither a RAR nor a RAR agonist, is used in the treatment of several conditions and was selected as prototype of “therapeutic retinoids” but also because it is a potent teratogen. Two lower concentrations (0.1 and 1 μM) of each compound were effective in increasing Abca1 and Abcg1 levels (Fig. 3). To verify that 9-cis-RA and 13-cis-RA up-regulated the active Abca1, we measured the levels of Abca1 in the plasma membrane, where it can interact directly with apolipoproteins for cholesterol removal (Oram and Heinecke, 2005). After astrocyte exposure to 9-cis-RA and 13-cis-RA, membrane proteins were biotinylated, and Abca1 was immunoprecipitated and detected by streptavidin chemiluminescence. As shown in Fig. 4, A and B, 9-cis-RA and 13-cis-RA increased the levels of membrane-bound Abca1 with similar potency (2.5-fold increase compared with that of the control).
Fig. 3.
Concentration response of 9-cis-RA and 13-cis-RA on Abca1 and Abcg1 protein levels. Fetal rat cortical astrocytes were incubated for 24 h in the presence of 0.1, 1, or 10 μM 9-cis-RA or 13-cis-RA. Proteins were extracted, quantified, separated by electrophoresis, and analyzed by Western blot for Abca1 and Abcg1 proteins. A and B, representative immunoblots labeled with Abca1 (A), Abcg1 (B), or β-actin (loading control) antibodies and detected by chemiluminescence. C and D, levels of Abca1 (C) and Abcg1 (D) quantified by densitometry, normalized to β-actin, and expressed as optical density relative to controls. Similar results were obtained in an additional two experiments.
Fig. 4.
Effects of 9-cis-RA and 13-cis-RA on Abca1 membrane localization. Fetal rat cortical astrocytes were incubated for 24 h in the absence or presence of 10 μM 9-cis-RA or 13-cis-RA. Membrane-bound Abca1 was determined by labeling membrane proteins with sulfo-N-hydroxysuccinimide-biotin followed by protein extraction, Abca1 immunoprecipitation, separation by electrophoresis, and detection by Western blot using a streptavidin HRP-conjugated antibody. A, representative immunoblot of membrane-bound Abca1. B, average optical density of Abca1 normalized to control. Results represent the mean (±S.E.) of three independent experiments. *, p < 0.05 and ***, p < 0.001 compared with control.
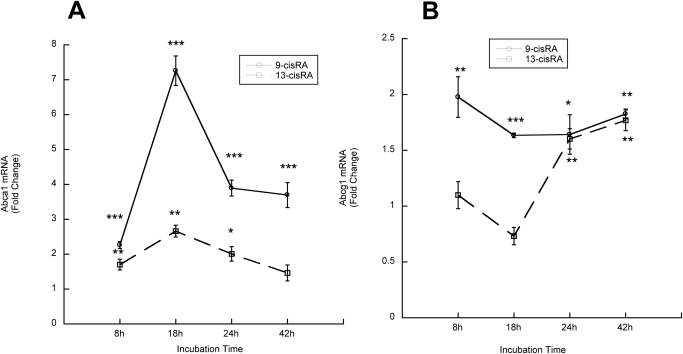
We also assessed whether 9-cis-RA and 13-cis-RA increased Abca1 and Abcg1 transcription by measuring their mRNA levels by real-time reverse transcription polymerase chain reaction. In time course experiments, we observed a significant increase in Abca1 and Abcg1 mRNA at all of the tested time points after incubation with 9-cis-RA. 13-cis-RA induced a more modest increase in Abca1 transcription after 8-, 18-, and 24-h incubations and increased Abcg1 mRNA after 24 and 42 h (Fig. 5, A and B). The maximal Abca1 induction was 7.3-fold for 9-cis-RA and 2.7-fold for 13-cis-RA; maximal Abcg1 induction was 2-fold for 9-cis-RA and 1.8-fold for 13-cis-RA.
Fig. 5.
Effects of 9-cis-RA and 13-cis-RA on Abca1 and Abcg1 mRNA levels. Fetal rat cortical astrocytes were incubated in the presence of 10 μM 9-cis-RA or 13-cis-RA for 8, 18, 24, or 42 h. A and B, mRNA was extracted, and Abca1 (A) and Abcg1 (B) mRNA levels were quantified by real-time reverse transcription polymerase chain reaction. Results were normalized to β-actin mRNA and were expressed as fold changes relative to time-matched controls. Each value is the mean (±S.E.) of three independent experiments. *, p < 0.05, **, p < 0.01, and ***, p < 0.001.
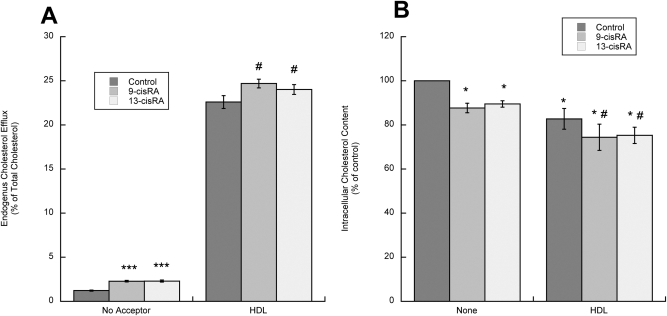
It has been suggested that the efflux of endogenously synthesized cholesterol may be regulated differently from the efflux of cholesterol derived from extracellular uptake (Slotte et al., 1987; Oram et al., 1991). We thus investigated the effects of 9-cis-RA and 13-cis-RA on the efflux of endogenously synthesized cholesterol in astrocytes labeled with the cholesterol precursor [3H]acetate and incubated in the presence or absence of the cholesterol acceptor HDL for 24 h. Similar to that found for exogenously added cholesterol, the efflux of endogenous cholesterol from the astrocytes also was increased significantly by 9-cis-RA and 13-cis-RA, either in the absence of acceptors or in the presence of HDL (Fig. 6A). The finding that a 24-h incubation with retinoids up-regulated cholesterol efflux even in the absence of cholesterol acceptors is not surprising, because astrocytes release lipoproteins acting as “autocrine” cholesterol acceptors. Abca1 and Abcg1 are involved in the biogenesis and maturation of lipoproteins (Oram and Heinecke, 2005), and retinoids up-regulate lipoprotein release by astrocytes (J. Chen, L.G. Costa, and M. Guizzetti, manuscript in preparation), thereby increasing cholesterol efflux.
Fig. 6.
Effects of 9-cis-RA and 13-cis-RA on endogenous cholesterol efflux and cholesterol content in astrocytes. A, fetal rat cortical astrocytes labeled with 25 μCi/ml [3H]acetate were incubated with 10 μM 9-cis-RA or 13-cis-RA in the presence or absence of HDL (50 μg/ml) for 24 h. Endogenous cholesterol efflux and cholesterol synthesis (data not shown) were measured by thin-layer chromatography after lipid extraction from the medium and the cell monolayer. [3H]Cholesterol efflux is expressed as the percentage of total (medium + cell) [3H]cholesterol. Each value is the mean (±S.E.) of three independent determinations. ***, p < 0.001 compared with control; #, p < 0.05 compared with HDL. B, fetal rat cortical astrocytes were incubated for 24 h with or without 50 μg/ml HDL in the presence or absence of 10 μM 9-cis-RA or 13-cis-RA. At the end of the incubation, lipids were extracted and total cholesterol was determined using a spectrophotometric kit. Results represent the mean (±S.E.) of three independent experiments *, p < 0.05 compared with control; #, p < 0.05 compared with HDL.
The increased cholesterol efflux exerted by 9-cis-RA and 13-cis-RA in astrocytes was not compensated by an increase in cholesterol synthesis, when measured either in the presence or absence of HDL (data not shown).
An increase in cholesterol efflux without an increase in cholesterol synthesis would be expected to lead to decreased levels of intracellular cholesterol. Indeed, after a 24-h treatment, 9-cis-RA or 13-cis-RA caused a significant decrease of cholesterol levels in astrocytes incubated in the absence of acceptors (12 and 10% decease, respectively) and in the presence of HDL (approximately 9% decrease by both compounds; Fig. 6B).
Discussion
The main new finding of this study is that retinoic acid isomers (9-cis-RA, 13-cis-RA, and tretinoin) increase the levels of Abca1 and Abcg1 proteins and up-regulate cholesterol efflux in astrocytes. The effects of at least two of the three isomers, namely tretinoin and 13-cis-RA, appear to be independent from RAR and RXR activation. RAR activation by the selective RAR agonist TTNPB reduced the levels of Abca1 and Abcg1 and inhibited cholesterol efflux from astrocytes (Figs. 1 and 2), without affecting cell viability (data not shown); in contrast, RAR activation increased Abca1 protein levels and cholesterol efflux in macrophages (Costet et al., 2003), suggesting that cholesterol homeostasis is regulated differently in different cell types. It has been reported that tretinoin may exert opposite effects in different cell types; indeed, although the activation of RARs is associated generally with the inhibition of cell growth and survival, in some tissues tretinoin stimulates cell survival and hyperplasia, effects that are mediated by the activation of peroxisome proliferator-activator receptors β/δ (Schug et al., 2007). These observations may explain the opposite effects that tretinoin and TTNPB, a selective RAR agonist, have on cholesterol regulation in astrocytes.
9-cis-RA is a potent activator of RXR, which has been shown to increase Abca1 and cholesterol efflux in the intestine by inducing the activation of RXR/liver X receptor (LXR) heterodimers (Repa et al., 2000). The involvement of RXR/LXR heterodimers in the effects of 9-cis-RA is supported by the fact that the selective RXR agonist methoprene up-regulated Abca1 and Abcg1 and induced cholesterol efflux to apoAI and HDL (Figs. 1 and 2) and by the fact that 9-cis-RA acted synergistically with the LXR agonist 22(R)-hydroxycholesterol in increasing the levels of cholesterol transporters and cholesterol efflux (data not shown). The therapeutic use of 9-cis-RA in the United States is limited to the topical treatment of Kaposi's sarcoma, because this retinoid affects the transcription of numerous genes, leading to a plethora of difficult-to-control side effects. Therefore, the effects of 9-cis-RA, though important to elucidate mechanisms of cholesterol homeostasis, may not be relevant to the therapeutic and toxic effects of retinoids in humans.
The third isomer tested, 13-cis-RA, which also increased Abca1, Abcg1, and cholesterol efflux, is neither a RXR nor a RAR agonist. The effects of 13-cis-RA may be due to its isomerization to tretinoin (Veal et al., 2002) and peroxisome proliferator-activator receptor β/δ activation (Shaw et al., 2003; Schug et al., 2007) or to the activation, by this retinoid, of nongenomic signaling pathways (including several protein kinase C isoforms and cAMP response element-binding protein) (Aggarwal et al., 2006).
Additional experiments carried out with 9-cis-RA and 13-cis-RA showed that both compounds up-regulated Abca1 and Abcg1 also at lower concentrations (0.1 and 1 μM), increased membrane-bound (active) Abca1, increased Abca1 and Abcg1 mRNA levels, increased the efflux of endogenously synthesized cholesterol without affecting cholesterol synthesis, and reduced total cellular cholesterol levels (Figs. 3–6). These findings strongly indicate that, in astrocytes, retinoids effectively alter cholesterol trafficking by regulating ABC cholesterol transporter expression. The observed decrease in cholesterol content, though small, may drastically modify the physical characteristics of astrocyte plasma membranes. Furthermore, the effects of retinoids on astrocytes may affect cholesterol homeostasis in the whole brain, because astrocyte Abca1 and Abcg1 are involved in the biogenesis of lipoproteins, which regulate the redistribution of cholesterol between different cell types and cholesterol excretion from the brain (Yu et al., 2010); we found indeed that, as a result of Abca1 and Abcg1 up-regulation, 13-cis-RA increases the release of astrocyte lipoproteins, which extract cholesterol from neurons (J. Chen, L.G. Costa, and M. Guizzetti, manuscript in preparation).
From these results emerges that the activation of RXR appears to be involved in the effect of 9-cis-RA, whereas the mechanisms involved in the effects of tretinoin and 13-cis-RA remain to be elucidated. It is possible that tretinoin and 13-cis-RA may be isomerized to 9-cis-RA and thus may activate RXRs; however, this hypothesis is unlikely because 9-cis-RA, which was identified initially as the endogenous ligand for RXR (Heyman et al., 1992), was not found in mammalian cells and tissues in later studies (Germain et al., 2006b; Wolf, 2006), and it remains controversial whether 9-cis-RA is generated in biological systems. Isomerization of retinoids in the culture medium also has been reported, although it was considered to be negligible up to 72 h in vitro (Veal et al., 2002). Interestingly, although 13-cis-RA and 9-cis-RA were equally potent in increasing Abca1 and Abcg1 protein levels, Abca1 membrane localization, and cholesterol efflux (Figs. 1–4), Abca1 and Abcg1 transcription was regulated differently by these two compounds (Fig. 5), confirming that they increased Abca1 and Abcg1 protein levels through different mechanisms. Indeed, although the effect of 9-cis-RA on Abca1 and Abcg1 protein levels correlated very well with its effect on their transcription, 13-cis-RA was less potent in triggering Abca1 and Abcg1 mRNA transcription than in increasing protein levels, suggesting that this retinoid also may have an effect on the stabilization of these cholesterol transporters, therefore reducing their rate of degradation.
Tretinoin and 13-cis-RA (isotretinoin) are used as systemic therapeutic drugs and have been used in the treatment of brain tumors, particularly gliomas and neuroblastomas (Yung et al., 1996; Kaba et al., 1997). Because up-regulation of ABCA1 and increased cholesterol efflux are associated with the inhibition of cell proliferation (Yvan-Charvet et al., 2010), the effectiveness of retinoic acids in the treatment of brain tumors may be in part due to their effects on cholesterol transporters and cholesterol efflux.
Retinoids have been investigated recently as treatments for AD, though the underlying mechanism(s) remain elusive (Lee et al., 2009; Shudo et al., 2009). Recent evidence suggests that cholesterol metabolism participates in the pathogenesis of AD, and astrocyte-released apoE is the main lipid carrier in the brain and the best-established risk factor for late-onset AD. Furthermore, intracellular cholesterol levels influence the generation of amyloid-β, the toxic peptide whose deposition in the brain parenchyma and blood vessels is one of the hallmarks of AD (Hirsch-Reinshagen and Wellington, 2007). ABCA1 and ABCG1 are implicated in the lipidation of apoE and in removing cholesterol from the cells, and it therefore has been hypothesized that their overexpression may exert a protective role in AD (Hirsch-Reinshagen and Wellington, 2007). Various studies have reported that ABCA1 overexpression increases apoE lipidation in the central nervous system and reduces the formation of amyloid plaques (Fan et al., 2009; Koldamova et al., 2010).
13-cis-RA and tretinoin are also teratogens (Alles and Sulik, 1989; Alles and Sulik, 1992). Cholesterol is essential for brain development and a lack, or decreased levels, of cholesterol can cause severe syndromes characterized by brain malformation and mental retardation (Cohen and Shiota, 2002). The effects of retinoids on cholesterol homeostasis, which are similar to those of ethanol (Guizzetti et al., 2007), may represent a relevant mechanism for the teratogenicity and developmental neurotoxicity of these compounds.
In conclusion, we have shown for the first time that various isomers of retinoic acid up-regulate cholesterol transporters and cholesterol efflux in primary astrocytes. These effects may be involved in the teratogenic effects of retinoids as well as in their therapeutic actions in brain tumors and AD.
These results were obtained in an in vitro system and therefore need to be confirmed in vivo; further studies also need to be carried out to verify that the beneficial effects of retinoids in the treatment of AD are due to the up-regulation of cholesterol transporters and the induction of cholesterol efflux in astrocytes. Primary rodent (mouse and rat) cultures of astrocytes have been important tools used to investigate the regulation of cholesterol homeostasis in the brain. The role of astrocytes in the formation of lipoproteins and the role of Abca1 and Abcg1 in cholesterol efflux to lipid-poor apolipoproteins and HDL and lipoprotein formation as well as the involvement of these processes in AD were extrapolated largely from results obtained in primary astrocyte cultures (LaDu et al., 1998; Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004; Abildayeva et al., 2006; Karten et al., 2006). In a previous study, we compared ABC cholesterol homeostasis in primary cultures of rat and human astrocytes. We found that apoAI and HDL increase cholesterol efflux from human and rat cultures and that in both cultures ethanol, RXR/LXR agonists, and 8-Br-cAMP increase Abca1/ABCA1 levels and cholesterol efflux (Guizzetti et al., 2007), indicating that, in vitro, cholesterol homeostasis is similarly regulated by ABC cholesterol transporters, and exert similar functions in human and rat astrocytes.
Although recent studies suggest that retinoids may be used in the therapy of AD, no clinical studies have been carried out yet. However, retinoids have been used in the treatment of brain tumors. In a clinical trial carried out on children with neuroblastoma, it was shown that at peak serum concentrations lower than 10 μM 13-cis-RA induced toxicity only in a small percentage of children and that the range of concentrations reached in the study were between 4.9 and 8.9 μM (Khan et al., 1996), suggesting that the concentrations of retinoids used in this study are tolerated by humans and are of clinical relevance.
Acknowledgments
We acknowledge the assistance of Khoi Dao in some experiments. We remember Dr. John F. Oram (deceased) who introduced us to the investigation of cholesterol transporters.
This work was supported by the National Institutes of Health National Institute of Alcoholism and Alcohol Abuse [Grant AA017180].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.182196.
- RXR
- retinoid X receptor
- ABC
- ATP binding cassette
- ABCA1
- ATP binding cassette A1 (human)
- ABCG1
- ATP binding cassette G1 (human)
- Abca1
- ATP binding cassette a1 (rat)
- Abcg1
- ATP binding cassette g1 (rat)
- AD
- Alzheimer's disease
- apo
- apolipoprotein
- BSA
- bovine serum albumin
- 9-cis-RA
- 9-cis retinoic acid
- 13-cis-RA
- 13-cis retinoic acid
- FBS
- fetal bovine serum
- HDL
- high-density lipoproteins
- DMEM
- Dulbecco's modified Eagle's medium
- HRP
- horseradish peroxidase
- LXR
- liver X receptor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide
- RAR
- retinoic acid receptor
- TTNPB
- 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid.
Authorship Contributions
Participated in research design: Chen, Costa, and Guizzetti.
Conducted experiments: Chen and Guizzetti.
Performed data analysis: Chen and Guizzetti.
Wrote or contributed to the writing of the manuscript: Chen, Costa, and Guizzetti.
References
- Abildayeva K, Jansen PJ, Hirsch-Reinshagen V, Bloks VW, Bakker AH, Ramaekers FC, de Vente J, Groen AK, Wellington CL, Kuipers F, et al. (2006) 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J Biol Chem 281:12799–12808 [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. (2006) Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell 17:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles AJ, Sulik KK. (1989) Retinoic-acid-induced limb-reduction defects: perturbation of zones of programmed cell death as a pathogenetic mechanism. Teratology 40:163–171 [DOI] [PubMed] [Google Scholar]
- Alles AJ, Sulik KK. (1992) Pathogenesis of retinoid-induced hindbrain malformations in an experimental model. Clin Dysmorphol 1:187–200 [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Shiota K. (2002) Teratogenesis of holoprosencephaly. Am J Med Genet 109:1–15 [DOI] [PubMed] [Google Scholar]
- Costet P, Lalanne F, Gerbod-Giannone MC, Molina JR, Fu X, Lund EG, Gudas LJ, Tall AR. (2003) Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol Cell Biol 23:7756–7766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Donkin J, Wellington C. (2009) Greasing the wheels of Abeta clearance in Alzheimer's disease: the role of lipids and apolipoprotein E. Biofactors 35:239–248 [DOI] [PubMed] [Google Scholar]
- Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. (2006a) International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev 58:712–725 [DOI] [PubMed] [Google Scholar]
- Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. (2006b) International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev 58:760–772 [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Chen J, Oram JF, Tsuji R, Dao K, Möller T, Costa LG. (2007) Ethanol induces cholesterol efflux and up-regulates ATP-binding cassette cholesterol transporters in fetal astrocytes. J Biol Chem 282:18740–18749 [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. (2007) Cholesterol homeostasis in the developing brain: a possible new target for ethanol. Hum Exp Toxicol 26:355–360 [DOI] [PubMed] [Google Scholar]
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. (1992) 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68:397–406 [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Wellington CL. (2007) Cholesterol metabolism, apolipoprotein E, adenosine triphosphate-binding cassette transporters, and Alzheimer's disease. Curr Opin Lipidol 18:325–332 [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. (2004) Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem 279:41197–41207 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Kaba SE, Kyritsis AP, Conrad C, Gleason MJ, Newman R, Levin VA, Yung WK. (1997) The treatment of recurrent cerebral gliomas with all-trans-retinoic acid (tretinoin). J Neurooncol 34:145–151 [DOI] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Wang C, Napoli JL. (2008) Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem 80:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten B, Campenot RB, Vance DE, Vance JE. (2006) Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J Biol Chem 281:4049–4057 [DOI] [PubMed] [Google Scholar]
- Khan AA, Villablanca JG, Reynolds CP, Avramis VI. (1996) Pharmacokinetic studies of 13-cis-retinoic acid in pediatric patients with neuroblastoma following bone marrow transplantation. Cancer Chemother Pharmacol 39:34–41 [DOI] [PubMed] [Google Scholar]
- Koldamova R, Fitz NF, Lefterov I. (2010) The role of ATP-binding cassette transporter A1 in Alzheimer's disease and neurodegeneration. Biochim Biophys Acta 1801:824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDu MJ, Gilligan SM, Lukens JR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM. (1998) Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem 70:2070–2081 [DOI] [PubMed] [Google Scholar]
- Lee HP, Casadesus G, Zhu X, Lee HG, Perry G, Smith MA, Gustaw-Rothenberg K, Lerner A. (2009) All-trans retinoic acid as a novel therapeutic strategy for Alzheimer's disease. Expert Rev Neurother 9:1615–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M, Hind M. (2003) Retinoic acid, a regeneration-inducing molecule. Dev Dyn 226:237–244 [DOI] [PubMed] [Google Scholar]
- Oram JF, Heinecke JW. (2005) ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev 85:1343–1372 [DOI] [PubMed] [Google Scholar]
- Oram JF, Mendez AJ, Slotte JP, Johnson TF. (1991) High density lipoprotein apolipoproteins mediate removal of sterol from intracellular pools but not from plasma membranes of cholesterol-loaded fibroblasts. Arterioscler Thromb 11:403–414 [DOI] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. (2000) Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529 [DOI] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. (2007) Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N, Elholm M, Noy N. (2003) Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem 278:41589–41592 [DOI] [PubMed] [Google Scholar]
- Shudo K, Fukasawa H, Nakagomi M, Yamagata N. (2009) Towards retinoid therapy for Alzheimer's disease. Curr Alzheimer Res 6:302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte JP, Oram JF, Bierman EL. (1987) Binding of high density lipoproteins to cell receptors promotes translocation of cholesterol from intracellular membranes to the cell surface. J Biol Chem 262:12904–12907 [PubMed] [Google Scholar]
- Tafti M, Ghyselinck NB. (2007) Functional implication of the vitamin A signaling pathway in the brain. Arch Neurol 64:1706–1711 [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Oram JF. (2006) ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res 47:2433–2443 [DOI] [PubMed] [Google Scholar]
- Veal GJ, Errington J, Redfern CP, Pearson AD, Boddy AV. (2002) Influence of isomerisation on the growth inhibitory effects and cellular activity of 13-cis and all-trans retinoic acid in neuroblastoma cells. Biochem Pharmacol 63:207–215 [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. (2004) ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem 279:40987–40993 [DOI] [PubMed] [Google Scholar]
- Wolf G. (2006) Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev 64:532–538 [DOI] [PubMed] [Google Scholar]
- Yu C, Youmans KL, LaDu MJ. (2010) Proposed mechanism for lipoprotein remodelling in the brain. Biochim Biophys Acta 1801:819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WK, Kyritsis AP, Gleason MJ, Levin VA. (1996) Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoic acid. Clin Cancer Res 2:1931–1935 [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, et al. (2010) ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328:1689–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]