Abstract
Heparan sulfate (HS) proteoglycans regulate a number of biological functions in many systems. Most of the functions of HS are attributed to its unique structure, consisting of sulfated and non-sulfated domains, arising from the differential presence of iduronyl and glucuronyl residues along the polysaccharide chain. A single glucuronyl C5-epimerase enzyme acts on heparan sulfate precursor, converts glucuronyl residues into iduronyl residues and modulates subsequent biosynthetic steps in vivo. The ratios of non-sulfated epimers within the polysaccharide chain have been calculated by resolving radiolabeled GlcA-AManR and IdoA-AManR disaccharides using a tedious paper chromatography technique. Radioactive assay, based on measuring either the release or incorporation of 3H at C5 carbon of uronyl residues of 3H-labeled HS precursor substrate, has been in use over three decades to characterize the action of HS C5-epimerase. We have developed a non-radioactive assay to estimate the epimerase activity through resolving GlcA-AManR and IdoA-AManR disaccharides on HPLC in conjunction with hydrogen/deuterium exchange upon epimerization protocol-liquid chromatography mass spectrometry (DEEP-LC-MS). Utilizing this new, non-radioactive based assay, DEEP-LC-MS, we were able to determine the extent of both forward and reverse reaction on the same substrate catalyzed by C5-epimerase. Results from this study also provide insights into the action of C5-epimerase and provide an opportunity to delineate snapshots of biosynthetic events that occur during the HSPG assembly in the Golgi.
Keywords: Heparan sulfate, Heparin, C5 epimerase, LC-MS, Hydrogen/Deuterium exchange, Proteoglycan biosynthesis
Introduction
Heparan sulfate (HS) proteoglycans regulate a number of physiological and pathological processes through interaction with a wide array of proteins [1–3]. The structural diversity of HS is in part due to incomplete reaction of HS biosynthetic enzymes and to some extent endosulfatase action after completion of the biosynthesis. The domain organization and subtle variations in the sulfation pattern of HS can be ascribed to the earlier biosynthetic events that are catalyzed by NDST, C5-epi and 2OST. Earlier studies have extensively documented both in vitro enzyme kinetics and in vivo knockout experiments of HS C5-epimerase enzyme. The C5-epimerase gene knockout experiments in mice led to severe early developmental defects and neonatal lethality [4]. Also, the mast cells deficient of the C5-epimerase enzyme do not produce normal heparin [5]. The substrate specificity studies of the C5-epimerase conducted using 3H-labeled heparin like molecules clearly demonstrated that the C5-epimerase reacts only with oligo/polysaccharides containing GlcNS-GlcA-GlcNS and GlcNS-GlcA-GlcNAc but not GlcNAc-GlcA-GlcNS and GlcNAc-GlcA-GlcNAc sequences [6]. However, the C5-epimerase follows different kinetics in cellular and cell free systems. In cell free systems, the recombinant or purified C5-epimerase follows reversible reaction kinetics with a preference for iduronic acid, whereas in the cellular system it is irreversible [7]. This is perceived to be a result of the coupling of epimerization with concurrent sulfotransferase reactions during HS biosynthesis [8,9].
The percentage of glucuronic/iduronic acid epimer content in the HS and heparin determines its fine structure, and consequently the biological functions. Thus, the determination of epimer content is crucial for understanding the structure-function relationships. The nitrous acid mediated deaminative cleavage of HS and heparin, developed by Shively and Conrad, is the most widely used method for calculating the epimer content [10,11]. This tedious and time consuming procedure requires the use of radioactive 3H tracer and paper chromatography technique for the separation of GlcA-AManR and IdoA-AManR disaccharides so that one can estimate the percentage of each epimer in the given HS sample. Here, for the first time, we developed a novel, ion-pairing reverse phase liquid chromatography coupled to mass spectrometry (IPRP-LC-MS) technique for online separation and detection of nitrous acid cleaved products GlcA-AManR and IdoA-AManR disaccharides, without using radiochemicals. This technique is rapid and sensitive to quantify nitrous acid cleaved disaccharide products obtained from epimerase treated or untreated N-sulfoheparosan. This method is also utilized in measuring the in vitro activity of HS C5-epimerase without using radioisotopes and provides further insights into the nature of action of this key biosynthetic enzyme.
Experimental procedures
Materials
The completely desulfated, N-sulfated heparin (CDSNS) was purchased from Seikagaku USA (Cape Cod, MA). N-sulfo heparosan was prepared by chemical N-deacetylation-N-sulfation as described earlier [12]. Baculovirus system that expresses HS C5-epimerase was kindly provided by Prof. Robert D. Rosenberg (Massachusetts Institute of Technology, Cambridge, USA) [13]. The DEAE-sepharose gel was purchased from Amersham Biosciences. The HPLC C18 column for IP-RP-HPLC analysis was purchased from Vydac. Commercially available HS disaccharide standards, from Iduron or Sigma-Aldrich, were used to determine migration positions and elucidate disaccharide composition of HS precursor structures. All other reagents and solvents were from Sigma-Aldrich. Epimerase reactions were performed in the buffer containing 25 mM MES (pH 7.0), 0.02 % Triton X-100, 2.5 mM MgCl2, 2.5 mM MnCl2, 1.25 mM CaCl2, and 0.75 mg/ml BSA. Hydrogen/deuterium exchange experiments with C5-epimerase were performed in D2O using the deuterated buffer (pD 7.0) having the above composition. All pH and pD values reported were taken directly from the pH meter and were not corrected for isotope effects.
Purification of C5-epimerase
15 ml of C5-epimerase viral stock was added to 2 × 109 Sf21 or Sf9 cells. Infected cells were shaken at 90 rpm at 28 °C for 4 days. The cell suspension was then centrifuged at 1000×g for 30 min to pellet cells. The supernatant was diluted 1:2 with 10 mM, PIPES, titrated to pH 7.0, chilled on ice for 30 min, centrifuged at 4000×g for 30 min and phenylmethylsulfonyl fluoride in 10 ml isopropanol was added to a final concentration of 1 mM. The solution was filtered and loaded onto a 100-ml column of ToyoPearl AF heparin 650 M. The column was washed with 600 ml of PCG-50 (10 mM PIPES, pH 7.0, 2% glycerol, 0.6% CHAPS, 50 mM NaCl) and eluted with a 450-ml linear gradient of 50 – 1000 mM NaCl in PCG. Aliquots of selected fractions were analyzed for epimerase activity as described in the following section; the positive fractions were then pooled and concentrated using Amicon YM-10 membrane.
Measuring the activity of C5-epimerase using [3H]-labeled N-sulfoheparosan
The 3H-labeled N-sulfoheparosan (5 μg, 1.5 ×105 CPM) was reacted with 20 μl aliquots of various selected fractions containing C5-epimerase enzyme, eluting from heparin column, in 50 μl reaction buffer. The reactions were incubated for 24 h at 37 °C and terminated by heating for 2 min at 96 °C. The samples were diluted with one volume of 0.016% Triton X-100 and loaded onto a 0.2 ml DEAE-sepharose mini column that had been pre-equilibrated with 2 ml of wash buffer (20 mM NaOAc, 0.1 M NaCl and 0.01 % Triton X-100, pH 6.0). After washing with 6 ml of wash buffer, the bound polysaccharide was eluted with 1.2 ml of elution buffer (20 mM NaOAc, 1 M NaCl, pH 6.0). 50 μl of the eluates were diluted with 5 ml of scintillation mixture and the total radioactivity was measured using a scintillation counter.
Measuring the activity of C5-epimerase using non-radioactive assay
N-sulfoheparosan (50 μg) was reacted with aliquots of various fractions containing HS C5-epimerase in 1 mL of D2O containing the reaction buffer at 37 °C. After 24 h, reaction mixtures were filtered through a 3 KDa cut off Microcon (Millipore, Inc.) and washed five times with 1 mL water. The freeze dried samples were subjected to low pH nitrous acid mediated deaminative cleavage followed by desalting using mini Sephadex-G10 column (1 cm × 10 cm). The flow rate of the desalting column was set at 0.2 ml/min. Fractions of 0.5 mL volume were collected and concentrated using a speed vac system under reduced pressure. In the initial experiments, all of the fractions with the exception of void volume fractions, determined by bromophenol blue migration, were subjected to LC-MS analysis. For subsequent experiments, fractions containing sample disaccharides were pooled together and lyophilized. The desalted samples were finally analyzed by directly infusing into mass spectrometry or by IPRP-LC-MS. The reactions were also performed for various time periods (0, 12, 24, 36 and 48 h) to investigate the activity and stability of C5-epimerase over two days.
Calculation of the epimer content
A typical extracted ion chromatogram for m/z 340.1 showed two peaks corresponding to GlcA-AManR (first peak) and IdoA-AManR (second peak) disaccharides, where as an extracted ion chromatogram for m/z 339.1 showed only a single peak. This is primarily due to the fact that the peak for the m/z 340.1 extracted ion chromatogram is contributed to by both the monoisotopic mass for DUA-AManR and the second isotopic mass (M+1) of HUA-AManR, which is approximately 15 % (observed; theoretical value is 13.1 %) of first isotopic peak (339.1). Therefore, we calculated the relative ratio of the epimeric disaccharides from the total ion counts of first peak (from m/z 339.1) and second peak (m/z 340.1). However, since some of the deuterium-incorporated iduronic acids (DIdoA-AManR) are converted back to glucuronic acids (DGlcA-AManR), the above calculation does not truly reflect the total activity of C5-epimerase. This back converted product can be calculated from the increase in the intensity of second isotopic peak of the spectra for the H/DGlcA-AManR peak using the following equation, total [DUA-AManR] = [DIdoA-AManR] + {[H/DGlcA-AManR] × [% increase in 340.1]}; the % increase in 340.1 is calculated using the formula: ({[340.1]/[339.1+340.1]}−15). With these two equations, one can calculate the back converted product intensity.
IPRP-liquid chromatography–mass spectrometry
All separations were performed on a C18 column (4.6 × 150 mm, Vydac, USA) using a Waters Aliance 6250 HPLC system or Dionex HPLC system. A gradient elution (0 min 95% A; 25 min 100% B; 30 min 95% A) was performed at a flow rate of 1.0 mL/min for 30 min, using a binary solvent system composed of water (eluent A) and 70% aqueous acetonitrile (eluent B), both containing 8 mM acetic acid and 5 mM dibutylamine (DBA) as an ion-pairing agent [14,15]. All mass spectrometric data were acquired using high pressure HPLC coupled to an electrospray ionization time-of-flight (ESI-TOF) mass spectrometer either from Waters Corporation (Milford, MA) or from Bruker Daltonics (Bellerica, MA). In the negative-ion mode, the instrument was calibrated according to manufacturer’s instructions. Nitrogen was used as a desolvation gas as well as a nebulizer. Conditions for ESI-MS were as follows: cone gas flow 50 L/h, nozzle temperature 130 °C, drying gas (N2) flow 450 L/h, spray tip potential 2.3 kV, nozzle potential 35 V. Negative ion spectra were analyzed using either MassLynx V4.1 software (Waters Corporation) or DataAnalysis V4.0 software (Bruker Daltonics).
Results
Primary objectives of this study were to a) develop a non-radioactive assay for measuring the total activity of C5-epimerase and b) quantify the extent of reverse reaction catalyzed by C5-epimerase using a non-radioactive, DEEP-LC-MS technique.
Separation of GlcA-AManR and IdoA-AManR disaccharides by IPRP-LC-MS
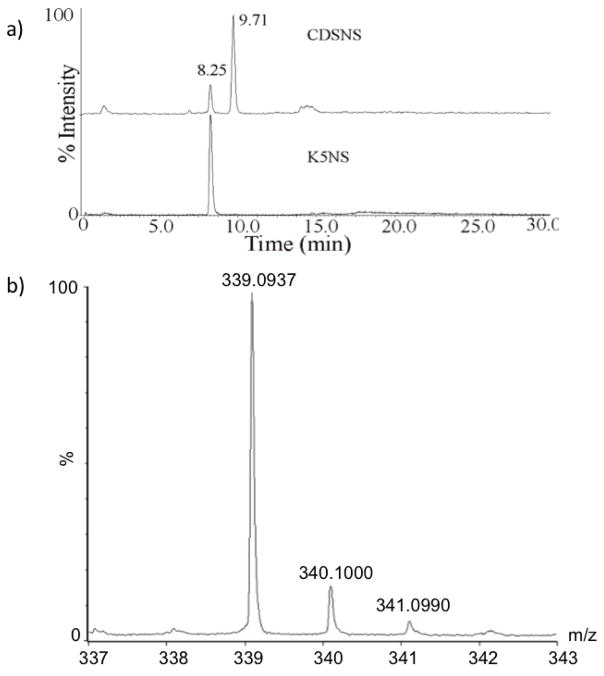
The percentage of the glucuronyl and iduronyl residues, two epimers present in heparin and heparan sulfate, determines the subsequent sulfation events that ultimately dictate their biological functions. Currently, radioactive 3H-labeling coupled to paper chromatography is the only method used for determining the epimer content of HS chains [10]. In order to develop a non-radioacitve LC-MS based method for the separation and quantification of epimers, K5NS and CDSNS polysaccharides were deaminatively cleaved by low pH nitrous acid method. The fully N-sulfated heparosan (K5NS) consists of only GlcA-GlcNS disaccharide units whereas the completely desulfated re-N-sulfated heparin (CDSNS-heparin) contains both GlcA-GlcNS and IdoA-GlcNS disaccharide units. The deaminative disaccharide products of K5NS and CDSNS-heparin were subjected to IPRP-liquid chromatographic separation followed by online mass spectrometric detection. The extracted ion chromatogram (for m/z 339.10 [M-H]-1) of K5NS sample yielded a single peak corresponding to GlcA-AManR as shown in Figure 1A (lower panel) whereas the CDSNS-heparin yielded two peaks corresponding to GlcA-AManR (peak eluting first) and IdoA-AManR (second peak eluting later) disaccharides as shown in the upper panel of Figure 1A. Isotopic pattern of the molecular ion with natural abundance is shown in the Figure 1B. The major ion is found at m/z 339.1. This is the first time that two disaccharides, GlcA-AManR and IdoA-AManR, could be resolved using any HPLC method. Both the glucuronic and iduronic acid containing disaccharides eluted out with no difference in the eluent composition and therefore, the disaccharides should have a very similar extent of ionization in the mass spectrometer. Hence, the total ion counts of each of the epimer peaks can be utilized for estimating their relative abundance. CDSNS heparin contains about 20% of glucuronic acid and 80% of iduronic acid residues, which is very similar to what has been reported in the literature. Thus, the current online LC-MS technique can be used to rapidly quantify the GlcA-AManR and IdoA-AManR disaccharides without using radiochemicals and thus, the percentage of each epimer that is present within the given HS/heparin sample.
Fig. 1.
A) Separation of disaccharides, GlcA-AManR and IdoA-AManR and estimation of epimer percentage in K5NS and CDSNS-heparin polysaccharides. Extracted ion chromatograms for m/z 339.1 of nitrous acid cleaved deaminative products of CDSNS-heparin (top) and K5NS (bottom). Both K5NS and CDSNS substrates were subjected to low pH nitrous acid mediated deaminative cleavage followed by sodium borohydride reduction and the resulting disaccharides were desalted before analysis by LC-MS. B) Mass spectrum of the disaccharides. Isotopic pattern of molecular ions corresponding to the disaccharides, HGlcA-AManR or HIdoA-AManR, carrying natural isotopic abundance is shown. Both the disaccharides have the same monoisotopic mass at m/z 339.1.
DEEP-MS technique for the quantitation of the reaction catalyzed by HS C5-epimerase
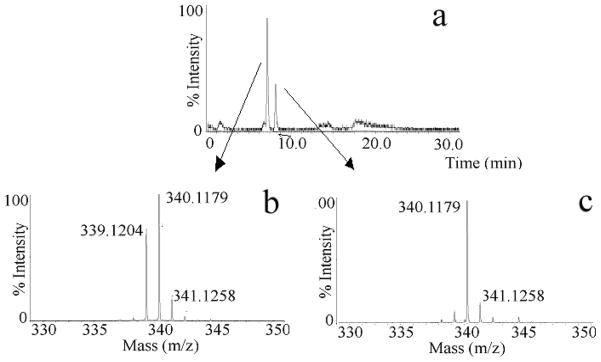
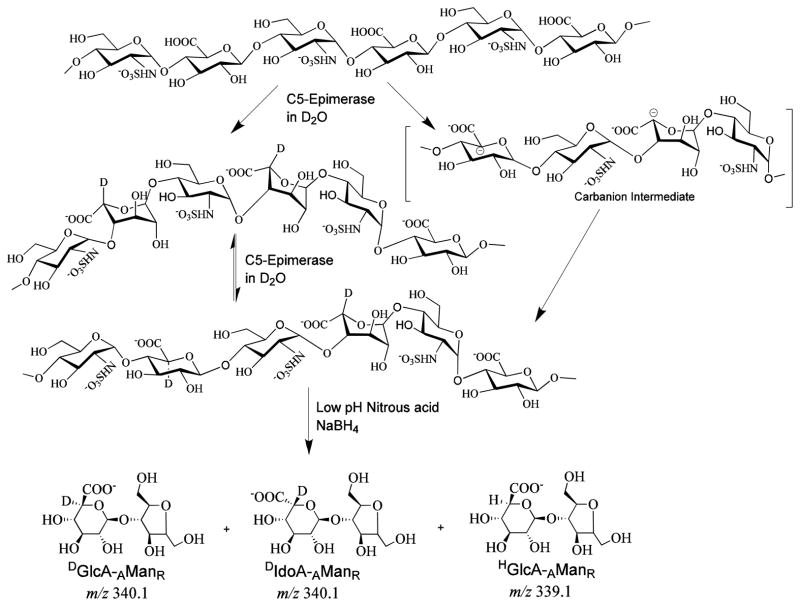
The LC-MS technique, described in the earlier section, was utilized to measure the activity of one of the important enzymes involved in HS and heparin biosynthesis, glucuronyl C5-epimerase, and also applied to estimate the extent of the reverse reaction catalyzed by the same enzyme. Initially, the 100 % K5NS was reacted with C5-epimerase at 37 °C and the resulting product was subjected to low pH nitrous acid deaminative cleavage followed by NaBH4 reduction and IPRP-LC-MS analysis. As expected, two peaks were obtained in the extracted ion chromatogram for m/z 339.1. As mentioned in the introduction, the HS C5-epimerase follows a reversible kinetics in cell free systems, i.e. some of the newly generated iduronic acid residues are converted back to glucuronic acid residues. Therefore, the integration of peak areas of extracted ion chromatograms for GlcA-AManR and IdoA-AManR does not reflect the total activity and does not provide the extent of reverse reaction catalyzed by the C5-epimerase. A similar limitation arises when a 3H-labled K5NS is used to study the enzyme kinetics of C5-epimerase through measuring 3H release as 3H2O [16–18]. To overcome this limitation, we have developed a Deuterium Exchange upon Epimerization Protocol coupled to LC-MS (DEEP-LC-MS) technique. In this DEEP-LC-MS technique, the C5-epimerase reaction was carried out in D2O and the deaminative products were analyzed by LC-MS (see experimental section). This technique allowed us to distinguish and measure both DIdoA-AManR (m/z 340.1) disaccharide formation and its back conversion to DGlcA-AManR (m/z 340.1) disaccharide, by measuring the relative changes in the intensity of mono isotopic ions at m/z 339.1 and m/z 340.1 of the MS spectrum for the first peak, corresponding to both HGlcA-AManR and DGlcA-AManR, eluting from the LC (Figure 2). Thus, the extent of reverse reaction was easily and quantitatively detected based on the incorporation of solvent deuterium at C5-carbon of glucuronic acid residues that would accompany reverse epimerization of newly generated iduronic acid residues.
Fig. 2. DEEP-LC-MS analysis to characterize the action of HS C5-epimerase.
Total ion chromatogram of epimerase treated K5NS in D2O is shown. After reaction with C5-epimerase the product was subjected to low pH nitrous acid treatment followed by sodium borohydride reduction. The resulting anhydromannitol disaccharide mixture was desalted using a mini Sephadex-G10 column (1cm × 10 cm) before MS analysis. The first and second peaks correspond to GlcA-AManR and IdoA-AManR, respectively (A, top panel); Mass spectrum of the first peak corresponding to the mixture of HGlcA-AManR and DGlcA-AManR (B, bottom left) and the second peak corresponding to DIdoA-AManR (C, bottom right). Isotopic pattern of a given molecular ion facilitated the estimation of reacted/unreacted disaccharide species.
Application of DEEP-LC-MS as a non-radioactive assay to measure the total activity of C5-epimerase
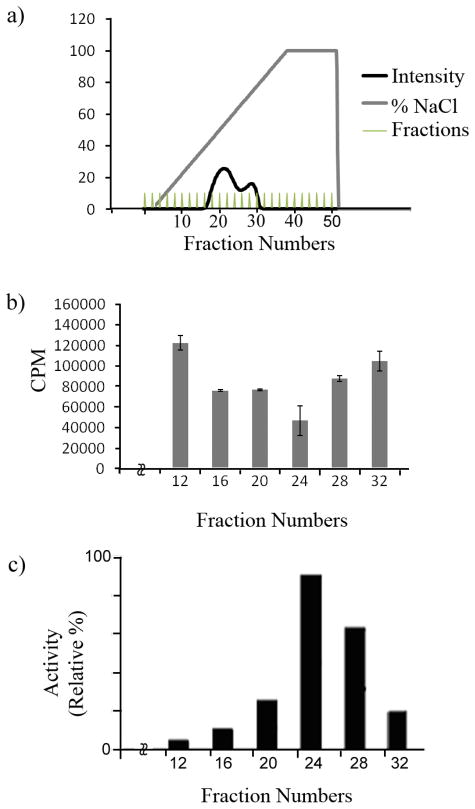
We have developed a non-radioactive DEEP-LC-MS assay to measure the activity of C5-epimerase. For this experiment the C5-epimerase enzyme was expressed and purified using a baculovirus expression system. The crude enzyme preparation was purified on a ToyoPearl AF heparin column and the fractions were tested for the C5-epimerase activity at 37 °C using K5NS as described in the experimental section. Using the DEEP-MS technique, we have calculated the percentage of iduronic acid formation as well as the total uronic acid produced by each enzyme fraction as shown in figures 3 and 4. As is evident from the plot, the total amount of deuterium incorporated uronic acid formed by the epimerase enzyme can easily be correlated to the activity of the enzyme. We also performed the [3H] release assay, a traditional radioactive assay (Figure 3). The results from these two independent assays were comparable. Fraction 24 had the maximal activity in both the amount of 3H released and the amount of deuterium incorporated into the uronyl residues. Moreover, we utilized this technique to investigate the effect of time on C5-epimerase activity. The reactions were performed for various time periods: 0, 12, 24, 36 and 48 h (Figure 4). The DEEP-LC-MS results indicate the increase of deuterium incorporation over time suggesting the enzyme activity and stability. These results clearly demonstrate that the developed technique, DEEP-LC-MS, can be utilized in a routine manner.
Fig. 3.
A) Purification of C5-epimerase on a heparin-Toyopearl affinity chromatography column. The insect cell supernatant medium was applied to a heparin-Toyopearl column as described under “Experimental Section”. The elution of protein from the affinity column was closely monitored by on-line UV detector at 280 nm (dark trace). The salt gradient is indicated by the gray line. The vertical lines indicate column fractions that were collected for further purification after measuring the enzymatic activity. B) Measurement of C5-epimerase activity based on radioactivity assay. Aliquots (20 μl) of indicated fractions were assayed for the C5-epimerase activity with [3H]-labeled N-sulfoheparosan polysaccharide as a substrate (1.5 × 105 CPM). After incubating with the enzyme for 24 hr, the sample was passed through a DEAE-Sepharose column (0.2 ml) to separate the N-sulfoheparosan polysaccharide from free 3H2O. The radioactivity of isolated polysaccharide was measured in the scintillation counter. C) DEEP-MS based assay to measure C5-epimerase activity during enzyme purification. The same fractions that were used in the radioactivity assay were utilized in the newly developed DEEP-MS assay for comparison. The aliquots of specific fractions were incubated with N-sulfoheparosan in D2O as described in the experimental section for 24 hr and then were treated with HNO2 at pH 1.5. The resultant glucuronyl/iduronyl-anhydromannose disaccharides were reduced with NaBH4 reagent, desalted on the mini Sephadex G10 column (1 cm ×10 cm) that was previously calibrated with known disaccharide standards and then analyzed by direct infusion into ESI-TOF-MS instrument to detect the molecular ions and their relative intensity. The relative activity of C5-epimerase was calculated based on the incorporation of deuterium into the substrate by measuring ion counts for DUA-AManR/HUA-AManR and plotted as a function of eluted fractions.
Fig. 4. Monitoring the activity of C5-epimerase over various time periods using mass spectrometry.
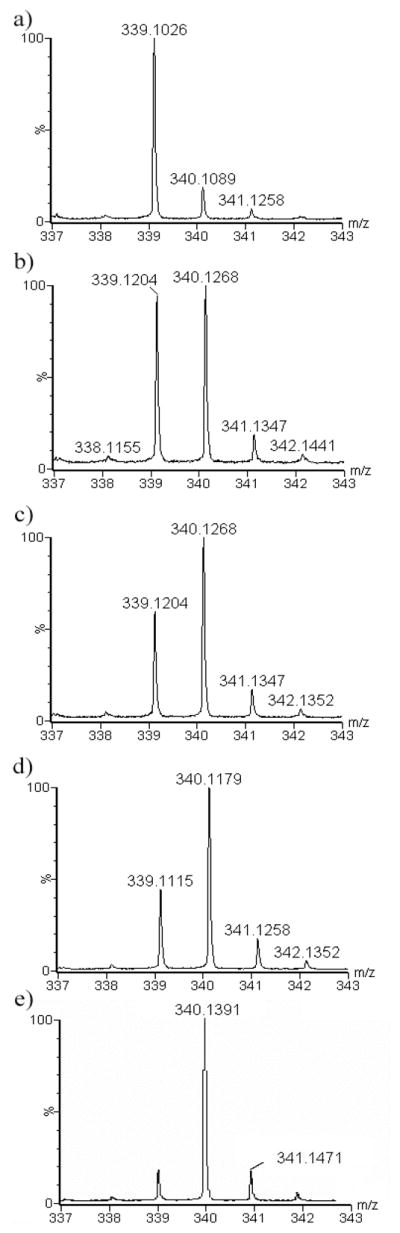
Comparison of mass spectral profile of disaccharides obtained from N-sulfoheparosan treated with C5-epimerase in D2O for various time periods, A) 0 hr, B) 12 hr, C) 24 hr, D) 36 hr and E) 48 hr, as described in the experimental section.
Discussion
Epimerization at C5-carbon of D-glucuronic acid residues to generate L-iduronic acid residues is a key step in the generation of biologically active heparin and heparan sulfate structures that bind a wide array of proteins. Therefore, analysis of epimer content is of great importance in the structural biology studies of heparan sulfate and heparin. The deaminative cleavage of HS and heparin by nitrous acid is the only procedure available for determining the percentage of each epimer found in the HS/heparin sample. However, the utility of this protocol is limited by i) a lack of a chromophore on anhydromannitol disaccharides so as to necessitate radioisotope labeling, and ii) an inability to resolve unsulfated disaccharides, GlcA-AManR and IdoA-AManR, under HPLC conditions. Here, we developed a LC-MS method to separate and quantify epimer content of K5NS and CDSNS-heparin. This method has several advantages over existing laborious paper chromatography technique that requires radiochemicals.
By developing a new separation technique combined with hydrogen/deuterium exchange upon epimerization protocol-mass spectrometry (DEEP-LC-MS), we have not only calculated the percentage of glucuronic acid and iduronic acid contents but also estimated the extent of reverse-epimerization. Thus, the products of both forward and reverse reactions were easily quantified based on the incorporation of solvent deuterium into C5-carbon of glucuronic acid residues that would accompany reverse epimerization of newly generated iduronate residues that carry deuterium at C5-carbon as well. Under the experimental conditions chosen, our observation, consistent with earlier studies, indicated that the D-gluco configuration is favored over L-ido configuration with an equilibration ratio of ~65 % glucuronic acid / ~35% iduronic acid. It is interesting to note that the forward epimerization reaction cannot proceed to 100% completion, i.e., conversion of all glucuronic acid residues to iduronic acid residues. One explanation is that a conformational change may occur in the polymer chain during the forward reaction, which favors the reverse reaction more readily than the forward reaction and thus, resulting in incomplete modification.
Measuring the activity of C5-epimerase has been a cumbersome process as compared to other enzymes involved in the biosynthesis of heparan sulfate. While radioactive 35S-PAPS has been in use to measure the activity of NDST and sulfotransferases, 3H-labeled N-sulfoheparosan polysaccharide has been commonly used to measure the activity of HS C5-epimerase. One of the potential problems of using 3H-labeled K5NS as substrate is that scrambling or loss of 3H-atom occurs during the biosynthesis of 3H-labeled N-acetyl heparosan polysaccharide in E. Coli. Moreover, the percentage of 3H-loss from the substrate does not reflect the total activity of the C5-epimerase enzyme. Earlier studies also examined the relative incorporation of radioactive sulfate catalyzed by sulfotransferases in the presence of C5-epimerase and provided evidence for the action of C5-epimerase. However, it is important to note that action of epimerase may be influenced by sulfotransferase enzymes and thus, it may not provide accurate action of C5-epimerase [19,20]. In contrast, the environmentally benign, MS based, non-radioactive assay system developed here not only measures the conversion of glucuronic acid to iduronic acid but also allows one to quantify the reverse epimerization of iduronic acid to glucuronic acid, which has never been demonstrated before.
Previous studies have shown that radiolabeled disaccharides can be digested with β-D-glucuronidase to elucidate/identify the epimer. A number of groups have also utilized the differential oxidation of uronyl residues by periodate to estimate epimer content or locate the presence of the specific epimer within the target sequence [7,21,22]. Furthermore, other analytical methods, such as NMR, cannot easily determine the extent of epimerization due to the overlapping of C5-H proton signals of glucuronic acid/iduronic acid with signals from other ring protons [12]. Our lab has been focusing on the development of isotope enriched HS to advance the knowledge of HS’s structure-function relationships [23–25].
In conclusion, we have developed an online-LC-MS technique for rapid determination of epimer content and also a non-radioactive, DEEP-MS technique for measurement of HS C5-epimerase activity. These two techniques either alone or in combination will become a powerful tool for a broad range of applications in the structural analysis of HS and heparin.
Scheme 1.
Heparan sulfate C5-epimerase catalyzes both the forward and reverse reaction in vitro.
Acknowledgments
This work was supported by National Institutes of Health grants (GM075168 & NS057144), Mizutani Foundation for Glycoscience Award and American Heart Association National Scientist Development Award to BK. We thank Ms. Vy Tran for experimental assistance with mass spectrometry.
References
- 1.Raman K, Kuberan B. Chemical Tumor Biology of Heparan Sulfate Proteoglycans. Current Chemical Biology. 2010;4:20–31. doi: 10.2174/187231310790226206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 3.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 4.Li JP, Gong F, Hagner-McWhirter A, Forsberg E, Abrink M, Kisilevsky R, Zhang X, Lindahl U. Targeted disruption of a murine glucuronyl C5-epimerase gene results in heparan sulfate lacking L-iduronic acid and in neonatal lethality. J Biol Chem. 2003;278 (31):28363–28366. doi: 10.1074/jbc.C300219200. [DOI] [PubMed] [Google Scholar]
- 5.Feyerabend TB, Li JP, Lindahl U, Rodewald HR. Heparan sulfate C5-epimerase is essential for heparin biosynthesis in mast cells. Nat Chem Biol. 2006;2 (4):195–196. doi: 10.1038/nchembio777. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsson I, Lindahl U, Jensen JW, Roden L, Prihar H, Feingold DS. Biosynthesis of heparin. Substrate specificity of heparosan N-sulfate D-glucuronosyl 5-epimerase. J Biol Chem. 1984;259 (2):1056–1063. [PubMed] [Google Scholar]
- 7.Hagner-McWhirter A, Hannesson HH, Campbell P, Westley J, Roden L, Lindahl U, Li JP. Biosynthesis of heparin/heparan sulfate: kinetic studies of the glucuronyl C5-epimerase with N-sulfated derivatives of the Escherichia coli K5 capsular polysaccharide as substrates. Glycobiology. 2000;10 (2):159–171. doi: 10.1093/glycob/10.2.159. [DOI] [PubMed] [Google Scholar]
- 8.Merry CL, Gallagher JT. New insights into heparan sulphate biosynthesis from the study of mutant mice. Biochem Soc Symp. 2002;69:47–57. doi: 10.1042/bss0690047. [DOI] [PubMed] [Google Scholar]
- 9.Pinhal MA, Smith B, Olson S, Aikawa J, Kimata K, Esko JD. Enzyme interactions in heparan sulfate biosynthesis: uronosyl 5-epimerase and 2-O-sulfotransferase interact in vivo. Proc Natl Acad Sci U S A. 2001;98 (23):12984–12989. doi: 10.1073/pnas.241175798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shively JE, Conrad HE. Stoichiometry of the nitrous acid deaminative cleavage of model amino sugar glycosides and glycosaminoglycuronans. Biochemistry. 1970;9 (1):33–43. doi: 10.1021/bi00803a005. [DOI] [PubMed] [Google Scholar]
- 11.Shively JE, Conrad HE. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976;15 (18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- 12.Naggi A, Torri G, Casu B, Oreste P, Zoppetti G, Li JP, Lindahl U. Toward a biotechnological heparin through combined chemical and enzymatic modification of the Escherichia coli K5 polysaccharide. Semin Thromb Hemost. 2001;27 (5):437–443. doi: 10.1055/s-2001-17954. [DOI] [PubMed] [Google Scholar]
- 13.Kuberan B, Beeler DL, Lech M, Wu ZL, Rosenberg RD. Chemoenzymatic synthesis of classical and non-classical anticoagulant heparan sulfate polysaccharides. J Biol Chem. 2003;278 (52):52613–52621. doi: 10.1074/jbc.M305029200. [DOI] [PubMed] [Google Scholar]
- 14.Kuberan B, Lech M, Zhang L, Wu ZL, Beeler DL, Rosenberg RD. Analysis of heparan sulfate oligosaccharides with ion pair-reverse phase capillary high performance liquid chromatography-microelectrospray ionization time-of-flight mass spectrometry. J Am Chem Soc. 2002;124 (29):8707–8718. doi: 10.1021/ja0178867. [DOI] [PubMed] [Google Scholar]
- 15.Jones CJ, Membreno N, Larive CK. Insights into the mechanism of separation of heparin and heparan sulfate disaccharides by reverse-phase ion-pair chromatography. J Chromatogr A. 2010;1217 (4):479–488. doi: 10.1016/j.chroma.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 16.Backstrom G, Hook M, Lindahl U, Feingold DS, Malmstrom A, Roden L, Jacobsson I. Biosynthesis of heparin. Assay and properties of the microsomal uronosyl C-5 epimerase. J Biol Chem. 1979;254 (8):2975–2982. [PubMed] [Google Scholar]
- 17.Campbell P, Hannesson HH, Sandback D, Roden L, Lindahl U, Li JP. Biosynthesis of heparin/heparan sulfate. Purification of the D-glucuronyl C-5 epimerase from bovine liver. J Biol Chem. 1994;269 (43):26953–26958. [PubMed] [Google Scholar]
- 18.Li J, Hagner-McWhirter A, Kjellen L, Palgi J, Jalkanen M, Lindahl U. Biosynthesis of heparin/heparan sulfate. cDNA cloning and expression of D-glucuronyl C5-epimerase from bovine lung. J Biol Chem. 1997;272 (44):28158–28163. doi: 10.1074/jbc.272.44.28158. [DOI] [PubMed] [Google Scholar]
- 19.Kuberan B, Lech MZ, Beeler DL, Wu ZL, Rosenberg RD. Enzymatic synthesis of antithrombin III-binding heparan sulfate pentasaccharide. Nat Biotechnol. 2003;21 (11):1343–1346. doi: 10.1038/nbt885. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Bethea HN, Liu J. Using engineered 2-O-sulfotransferase to determine the activity of heparan sulfate C5-epimerase and its mutants. J Biol Chem. 2010;285 (15):11106–11113. doi: 10.1074/jbc.M109.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransson LA, Huckerby TN, Nieduszynski IA. alpha-L-iduronate ring conformations in heparin and heparin derivatives. 13-C Nuclear-magnetic-resonance analysis and titration data for variously desulphated and periodate-oxidized heparins. Biochem J. 1978;175 (1):299–309. doi: 10.1042/bj1750299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue S. The determination of iduronic acid and glucuronic acid in heparins by a new technique. Reevaluation of the variable hexuronic acid composition of mammalian heparins. Biochim Biophys Acta. 1973;329 (2):264–270. doi: 10.1016/0304-4165(73)90291-2. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TK, Tran VM, Victor XV, Skalicky JJ, Kuberan B. Characterization of uniformly and atom-specifically (13)C-labeled heparin and heparan sulfate polysaccharide precursors using (13)C NMR spectroscopy and ESI mass spectrometry. Carbohydr Res. 2010;345 (15):2228–2232. doi: 10.1016/j.carres.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigulinsky C, Babu P, Victor XV, Kuberan B. Preparation and characterization of (15)N-enriched, size-defined heparan sulfate precursor oligosaccharides. Carbohydr Res. 2010;345 (2):250–256. doi: 10.1016/j.carres.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran VM, Nguyen TK, Raman K, Kuberan B. Applications of isotopes in advancing structural and functional heparanomics. Anal Bioanal Chem. 2011;399 (2):559–570. doi: 10.1007/s00216-010-4166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]