Abstract
POU transcription factors participate in cell-identity decisions during nervous system development, yet little is known about the regulatory networks controlling their expression. We report all known Drosophila POU genes require castor (cas) for correct CNS expression. drifter and I-POU depend on cas for full expression, whereas pdm-1 and pdm-2 are negatively regulated. cas encodes a zinc finger protein that shares DNA-binding specificity with another pdm repressor: the gap segmentation gene regulator Hunchback (Hb). Our studies reveal that the embryonic CNS contains sequentially generated neuroblast sublineages that can be distinguished by their expression of either Hb, Pdm-1, or Cas. Hb and Cas may directly silence pdm expression in early and late developing sublineages, given that pdm-1 cis-regulatory DNA contains ⩾32 Hb/Cas-binding sites and its enhancer(s) are ectopically activated in cas− neuroblasts. In addition, the targeted misexpression of Cas in all neuroblast lineages reduces Pdm-1 expression without altering Hb expression. By ensuring correct POU gene expression boundaries, hb and cas maintain temporal subdivisions in the cell-identity circuitry controlling CNS development.
Keywords: Castor, Hunchback, zinc finger proteins, CNS POU gene regulation
Nervous system development employs a cascade of integrated regulatory networks to generate specific cell lineages and, ultimately, create unique cellular phenotypes. A detailed understanding of these pathways remains a central goal of neurobiology. Cell-identity decisions during neural development are rooted in cell–cell communications that utilize both extrinsic and intrinsic cues to establish cell fate programs within stem cells called neuroblasts (NBs) (for review, see Artavanis-Tsakonas et al. 1995; Jan and Jan 1995; Bier 1997; Morrison et al. 1997). In the developing Drosophila CNS, different populations of NBs follow sequential ectoderm-delamination schedules, generating successive waves of NBs entering a subepidermal proliferative zone (Hartenstein and Campos-Ortega 1984; Bossing et al. 1996). Shortly after their arrival, most NBs initiate a series of 5–10 asymmetric divisions, producing a progenitor cell with each event—the ganglion mother cell (GMC). GMC divisions yield either neurons or glia (for reviews, see Campos-Ortega 1993; Goodman and Doe 1993; Lin and Schagat 1997).
Although much is known about genes that participate in NB formation, that is, the proneural and neurogenic genes (for review, see Campos-Ortega 1993; Jan and Jan 1994), very little is understood about subsequent identity decisions, particularly those that establish differences between early and late developing NB sublineages. Underpinning the orchestrated arrival of NBs to their proliferative zone are cell fate decisions most likely controlled by temporally integrated transcriptional regulatory circuits. For example, the precise timing of neural identity gene expression is crucial for specific NB lineages (Yang et al. 1993; Bhat and Schedl 1994). In addition, the activation of certain sublineage identity genes has been linked to cell cycle or cytokinesis-dependent mechanisms (Cui and Doe 1995; Weigmann and Lehner 1995). Studies reported here reveal that during embryonic CNS development, NBs give rise to sequentially generated subpopulations of progeny distinguished by their selective expression of neural identity gene regulators.
Members of the POU homeobox gene family (Herr et al. 1988; for review, see Ryan and Rosenfeld 1997) play essential roles in establishing neuronal phenotypes in most, if not all, multicellular organisms, yet essentially nothing is known about the regulatory circuits controlling their dynamic CNS expression. The Drosophila genome contains four known POU genes: pdm-1/dPOU-19/nubbin, pdm-2/dPOU-28/miti-mere, drifter/Cf1-a, and I-POU, all of which are expressed in the developing CNS (Johnson and Hirsh 1990; Billin et al. 1991; Dick et al. 1991; Lloyd and Sakonju 1991; Treacy et al. 1991; Prakash et al. 1992; Bhat et al. 1994; Ng et al. 1995). In vivo functional analysis of three of the four Drosophila POU genes has shown that each is involved in cell-identity decisions during CNS development (for review, see Ryan and Rosenfeld 1997). The structurally related and coexpressed pdm genes are expressed in most, if not all, neuroectoderm cells during NB delaminations. Their expression during lineage development, however, is dynamic and maintained only in a subset of cells that make up each of the CNS ganglia (Billin et al. 1991; Dick et al. 1991; Bhat et al. 1994, 1995; Yeo et al. 1995). Unlike the identical expression patterns of the pdm genes, drifter (drf) and I-POU are activated relatively late in overlapping subsets of NB sublineages (Treacy et al. 1991; Anderson et al. 1995).
In previous reports, we and others have identified a sublineage CNS neuronal precursor gene, cas (also known as ming) (Cui and Doe 1992; Mellerick et al. 1992). Coding for a 792-amino-acid protein with a centrally located zinc finger domain, cas mRNA is expressed in many, if not all, late delaminating S3 through S5 NBs and in early S1–S2 NBs, but only after they have undergone several rounds of GMC-producing divisions (for NB delamination schedules, see Broadus et al. 1995; Bossing et al. 1996). Although cas may not be required for early NB sublineage development, its function is essential for many late developing sublineages, as evident from the reduced axon numbers observed in all CNS ganglia of late stage cas− embryos (Cui and Doe 1992; Mellerick et al. 1992).
To better understand the role of cas during CNS development, we have undertaken the biochemical analysis of its encoded protein, studied the dynamics of its NB lineage distribution, and have shown that cas function is required for the correct temporal expression of neural identity POU genes. DNA-binding studies have led to the unexpected finding that Cas shares DNA-binding specificity with another structurally different Drosophila zinc finger protein: Hunchback (Hb) (Tautz et al. 1987; Stanojevic et al. 1989; Treisman and Desplan 1989). During cellular blastoderm development, Hb functions as a repressor of pdm gene expression (Lloyd and Sakonju 1991; Cockerill et al. 1993). Studies reported here strongly support a role for both Hb and Cas as pdm repressors in early and in late CNS NB sublineages, respectively. We find that NBs sequentially produce subpopulations of progeny that can be identified by their expression of either Hb, Pdm, or Cas. Multiple Hb/Cas DNA recognition sites are part of the pdm-1 cis-regulatory DNA, which also contains in vivo cas responsive enhancer(s), suggesting that Hb and Cas are direct transcriptional repressors of pdm expression. Finally, our studies show that cas function is required for proper expression of all known POU genes, and the effects of cas are both negative, repressing pdm expression, and positive, as drf and I-POU require cas for full expression. This differential control over neural identity genes suggests that Cas may have dual regulatory roles, functioning as an activator to ensure the expression of determinants that control cell fates in late forming NB sublineages and as a repressor to insulate their identity programs from factors that dictate earlier fates.
Results
Cas and Hb have smilar DNA-binding preferences
Multiple features within the Cas primary structure suggest it may function as a DNA-binding transcription factor. It contains a centrally located zinc finger domain made up of four consecutive C2-H2C2-H2 repeats (Cui and Doe 1992; Mellerick et al. 1992). The second C2-H2 of each repeat closely resembles fingers of the Xenopus TFIIIA C2-H2 class (Miller et al. 1985). Flanking this repeat are motifs that may constitute either transcription transactivation or repression domains (Mellerick et al. 1992). In addition, UV-induced protein–DNA cross-linking in vivo studies revealed Cas binds genomic DNA (R. Kambadur and W.F. Odenwald, unpubl.).
To determine if Cas is a sequence-specific DNA-binding protein, we used the cyclic amplification of selected targets protocol (Wright et al. 1991). By use of a bacterially expressed Cas protein and anti-Cas antibodies, Cas–DNA complexes were immuno-selected from an unbiased set of degenerate dsDNA oligonucleotides (see Materials and Methods). After six rounds of selection/amplification, sequencing of cloned fragments revealed that all had at least one sequence motif in common and some contained two core recognition sequences (Fig. 1A). DNA fragments containing one site homologous to the consensus (Fig. 1C) produced a single prominent Cas–DNA gel shift and a fragment with two generated two complexes (Fig. 1A,B). Addition of Cas-specific antisera caused a supershift of the Cas–DNA complex (Fig. 1B); whereas addition of preimmune serum did not result in a supershift (data not shown). A randomly selected fragment that lacked the 10-bp core consensus failed to produce protein–DNA complexes with the recombinant Cas (Fig. 1B).
Figure 1.
Cas and Hb share consensus DNA-binding sites. Cas DNA-binding sites were identified from an unbiased degenerate population of dsDNA fragments by use of the cyclic amplification of selected target DNA-binding site procedure (see Materials and Methods). After six cycles of selection and PCR amplification, which included four rounds of immunoprecipitation followed by two rounds of gel shifts, bound fragments were subcloned and sequenced. (A) Alignment of common DNA sequences (upper strand shown) present in Cas–DNA-bound fragments. Note, clone 4 contains two recognition sequences (4a,b) separated by 8 bp. (B) Gel mobility-shift assays with radiolabeled fragments containing either one binding site (clone 1 and 2), two Cas recognition sites (clone 4a+b), or a randomly-selected fragment with no core-recognition homology (clone 3). Antibody supershifting of fragment 1 DNA–Cas complexes with anti-Cas antibodies establishes Cas involvement in the protein–DNA complexes. Note, the sequence specificity of Cas DNA-binding was confirmed by competition assays and base-pair substitutions (see Fig. 7H). Also note, no Cas protein was added to the samples run in lanes marked (−). (C) Alignment of Cas and Hb consensus DNA-binding sites. Hb consensus DNA recognition sequences were obtained from Stanojevic et al. 1989 (Hb1) and Treisman and Desplan 1989 (Hb2).
A search of known transcription factor DNA-binding sites showed that the Cas recognition sequence is almost identical to that of the Drosophila zinc finger protein Hb (Fig. 1C). The Cas consensus matches 9 bp out of 10 bp for the reported Hb sites (Stanojevic et al. 1989; Treisman and Desplan 1989). To determine if Cas binds Hb sites, we carried out gel-shift experiments by use of DNA fragments with exact sequence matches to Hb targets and found that Cas does bind to these sites (Fig. 7H, below; data not shown). The sequence-specificity of Cas–DNA binding to Hb recognition sites was further tested by competition assays and base-pair substitutions (Fig. 7H, below). Taken together, these experiments show that Cas can bind to the same DNA sites as Hb, raising the possibility that it modulates transcriptional activities of genes also regulated by Hb.
Figure 7.
Cas silences pdm-1 neuroblast enhancer(s). (A,B) Whole-mount in-situ mRNA localizations in transformant embryos shows that a 5.3-kb fragment of pdm-1 regulatory DNA can activate the divergently transcribed reporter genes, mini-white (A) and GAL4 (B), in a manner similar to endogenous cellular blastoderm pdm-1 expression (anterior, left; see Fig. 7G and Materials and Methods for P-element vector details). Note, pdm cellular blastoderm expression boundaries are set by Hb repression (Lloyd and Sakonju 1991; Cockerill et al. 1993). (C–F) In situ hybridizations performed on wild-type (C,E) and cas− (D,F) transformant embryos reveal that the mini-white (C,D) and Gal4 (E,F) reporter genes, are activated in cas− CNS NBs. Shown are dorsal views of stage 13 cephalic lobes (anterior, up; focal plane passes through the dorsal surface of the cephalic lobes). (G) The pdm-1 regulatory DNA/reporter gene P-element construct showing locations (solid ovals) of 32 potential Cas/Hb DNA-binding sites. These sites share 8 of 10 bp with the consensus Hb-binding site and all contain the core A/T-rich sequence (see Fig. 1C for consensus DNA-binding sequences). Core recognition sequences (upper strand) for sites 6 and 12 are shown plus a mutated site (m12) used to test Cas-DNA binding sequence-specificity (see H). Both sites were selected because they match known Hb recognition sites (Stanojevic et al. 1989; Treisman and Desplan 1989). Dashes in m12 represent same bases as in the wild-type site. The pdm-1 embryonic transcriptional start site is shown as a rightward-facing arrow. (H) Electrophoretic mobility-shift assays show that Cas binds specifically to sites in pdm-1 regulatory DNA. Shown are gel-shift assays with 30-bp dsDNA fragments matching either Hb recognition site 6 or 12. Increasing concentrations (5- and 50-fold) of cold competitor DNA (6 or 12) reduce labeled DNA–Cas complexes, whereas the mutated fragment (m12) fails to bind Cas or compete (5- and 50-fold) with wild-type 12 DNA–Cas binding. Note, no recombinant Cas was added to the samples run in lanes marked −.
Hb, Pdm-1, and Cas expressions identify sequentially layered subpopulations of neuroblast progeny
Previous studies on Hb have shown that in addition to its early regulatory functions during segmentation (Struhl et al. 1992; for review, see Rivera-Pomar and Jackle 1996), it is also expressed in the developing nervous system (Jimenez and Campos-Ortega 1990). The precise role of Hb during neurogenesis or the dynamics of its CNS expression, however, have not been fully studied. One possible CNS regulatory target for Hb is the POU gene pdm-1. Hb regulates pdm-1 expression at the cellular blastoderm stage (Lloyd and Sakonju 1991; Cockerill et al. 1993), and may play a similar role in the CNS. To explore the possibility that Hb and Cas regulate the same genes via their shared DNA recognition sites, we compared the CNS expression dynamics of Hb, Cas, and Pdm-1. The embryonic distribution of the three proteins was examined by whole-mount immunostaining with polyclonal antibodies (described in Materials and Methods). Differential interference contrast (DIC) and fluorescent confocal microscopic views of staged immunostained embryos observed either in whole-mount, flattened, or serially cross-sectioned revealed both temporal and spatial differences between the expression patterns of all three proteins (Figs. 2, 3, 4; for details of their expression dynamics, see legends).
Figure 2.
Cas expression during embryonic CNS development. Dissected fillets or transverse sections (6 μm thick) of whole-mount immunostained embryos reveal Cas expression dynamics during embryonic CNS development (embryo staging according to Hartenstein and Campos-Ortega 1984; anterior up in A–E, G, and H; ventral up in F). The same magnification was used in A–E; scale bar in E represents 80 μm. In F, panels 1–5, the same scale bar represents 25 μm, in G it equals 20 μm, and in H it is equivalent to 35 μm. (A) Stage 9 embryos stained with either Cas (main panel) or Hb (inset) antisera. Cas protein is detected first in segmental clusters of ventral midline mesectodermal cells (arrow) and in cells that line the anterior midgut primordium (arrowhead). At this time, no Cas expression is detected in either cephalic lobe or ventral cord NBs (see also panel F, 1). The initial reduced expression observed in the thoracic and first abdominal midline cells reflects the delayed onset of cas mRNA expression in these segments (data not shown). (Inset) By late stage 9, NB delaminations from the lateral ventral cord neurogenic regions have produced three rows of Hb-positive NBs flanking the midline in all gnathal, thoracic, and abdominal segments (see also Fig. 3). Note, NBs were identified on the basis of their large cell body diameters and on their position underlining the ectoderm. (B) During stage 10, bilaterally symmetrical subsets of cephalic lobe and ventral cord NBs initiate Cas expression. The first ventral cord NBs to express Cas are the late delaminating NB6-1s located on the posterior edge of the gnathal, thoracic, and abdominal a1–8 segments (also see F, panel 2). At this time, some of the NB6-1s have produced Cas-positive GMCs (arrows). (Inset) Confocal colocalization views of Hb (green) and Cas (red) reveal no coexpression in NB6-1s or in midline precursors. (C) By early stage 11, the number of cephalic lobe Cas-positive NBs has nearly doubled and the number of Cas-expressing ventral cord NBs has increased to 5–7 per hemisegment (see also F, panel 3). (Inset) Following activation of Cas expression in NB6-1s, the next NBs to express Cas are the earlier delaminating (S1) NB5-2s (arrow). At this time, NB5-2s have terminated Hb expression and their Hb positive sublineages take up dorsal positions relative to ventrally located Cas-positive NBs (see Fig. 3). (D) During late stage 11, Cas expressing NBs in ventral cord neuromeres have now increased to 9–10 and 7–8 per thoracic and abdominal hemisegments, respectively. (E) Stage 12, germ-band contraction; by late stage 11/early stage 12, most CNS neuroblasts express Cas. Cas-expressing NB sublineages are positioned on the outer surface of the cephalic lobes and on the ventral/ventral–lateral surfaces of the developing subesophageal ganglion (arrow) and ventral cord neuromeres (see also F, panels 4 and 5; and Fig. 4). Also note, no Cas expression is detected outside the CNS. (F) Transverse sections (6 μm thick) through abdominal segments of stage 9–13 embryos (1–5, respectively) reveal that Cas-expressing NBs, and their sublineages take up ventral/ventral–lateral positions in the developing neuromeres. (G) Cas is concentrated in GMCs during NB cytokinesis. Shown is a ventral view of a stage 11 ventral cord showing Cas-positive NBs and GMCs in the second and third thoracic and first three abdominal segments. Note, the more intense immunostained GMCs (arrows) relative to their weaker stained NBs. Also note, nuclear-located Cas in nondividing NBs (arrowhead). In situ mRNA localizations show that cas is not expressed in GMCs (data not shown; Mellerick et al. 1992). (H) By stage 15, Cas immunopositive sublineages are evenly distributed on the outer cephalic lobe surfaces and on the ventral/ventral-lateral regions of the subesophageal and ventral cord ganglia.
Figure 3.

CNS neuroblasts produce layered sublineages distinguished by their expression of Hb, Pdm, or Cas. The dynamics of Hb (A,C,E,G) and Pdm-1 (B,D,F,H) expression during ventral cord development are shown in transverse (6 μm thick) sections through abdominal segments of stage 9 (A,B), stage 10 (C,D), stage 11 (E,F), and stage 12 (G,H) embryos immunostained in whole-mount with either Hb or Pdm-1 antibodies. (A,B) Sections through stage 9 embryos reveal that most, if not all, fully delaminated NBs express Hb (see also Fig. 2A, inset) whereas Pdm-1 expression is restricted to neuroectoderm cells. (C,D) By stage 10, many NBs and GMCs are immunostained with Hb antibodies, however, not all NBs contain detectable levels of Hb (arrow). In addition to its high levels of neuroectoderm expression, low levels of Pdm-1 immunostaining are now detected in a small subset of NBs and GMCs. (E,F) Starting at late stage 10, and through stage 11, there is a progressive reduction in the number of NBs containing detectable levels of Hb, whereas many GMCs and/or their progeny maintain high levels of Hb immunostaining. Loss of Hb expression in NBs parallels the activation of Pdm-1 NB expression. The number of GMCs containing detectable levels of Pdm-1 also rises during stage 11 and follows that observed in NBs. Given their close apposition to Pdm-1-positive NBs, many of these GMCs were most likely products of Pdm-1-expressing NBs. (G,H) Stage 12; during this period, Pdm-1 NB expression is reduced such that only low levels of Pdm-1 immunostaining are found in NBs. Hb and Pdm-1-positive sublineages are now positioned dorsal to late dividing NBs. Scale bar in H is equivalent to 28 μm and also applies to A–G. (I) By stage 13, Hb and Pdm-1 immunopositive sublineages form layered subpopulations of cells in all CNS neuromeres. In the ventral cord, Pdm-1 positive sublineages are juxtaposed to the more dorsal/internal Hb sublineages. Shown are lateral, confocal fluorescent views from a double-labeled, Hb (green) and Pdm-1 (red), stage 13 embryo (anterior is left and ventral up). (J) Confocal immunofluorescent views of stage 12 and older embryos reveal that Hb (green) and Cas (red) positive sublineages do not overlap and occupy the dorsal and ventral neuromere surfaces, respectively. Shown is a stage 13 ventral cord (lateral view; anterior is left and ventral up). Note the more ventral position of the Cas-expressing sublineages compared with the internal Pdm-1 positive cells in I. (K) Triple-labeling [Hb (blue), Pdm-1 (green), and Cas (red)] reveals that by stage 13 most, if not all, sublineages express one of these transcription factors. For confocal ventral views of adjacent Pdm-1- and Cas-expressing sublineages, see Fig. 4. Note, the arrow in K indicates a NB that coexpresses Pdm-1 and Cas. Scale bar in J is 30 μm and also applies to I and K.
Figure 4.
By mid-CNS development, Cas and Pdm-1 colocalizations reveal little overlap in their expression. Confocal immunofluorescent views of embryos doubled-labeled for Cas (red) and Pdm-1 (green) reveal that the activation of Cas expression in CNS NBs coincides with the loss of Pdm-1 NB expression. (A–C) Shown are ventral to dorsal (left to right) optical section views of a late stage 12 ventral cord (anterior, up). Note the more ventral/ventral–lateral position of the Cas-positive cells relative to the deeper/internal Pdm-1 immunostained sublineages. (D–F) Cephalic lobe expression of Cas and Pdm-1; shown are dorsal to ventral (left to right), serial views of a late stage 12 brain. Arrow in E points to a NB immunostained for both Cas and Pdm-1. Note, the red staining between and below the cephalic lobes is caused by the nonspecific binding of the rhodamine-tagged secondary antibody to yolk cells. Same magnification in all panels. Scale bar in A, 50 μm.
During the initial S1 and S2 waves of NB delaminations, Pdm-1 is expressed in most, if not all, neuroectoderm cells (Billin et al. 1991; Dick et al. 1991; Bhat et al. 1994). No Pdm-1 immunostaining, however, was detected in fully delaminated NBs, and during stage 9, only a small subset of ventral cord GMCs express detectable levels (Fig. 3B and D). At this time, Hb expression is detected in all fully delaminated NBs and in many of their GMCs but not in neuroectoderm cells (Figs. 2A, inset, and 3, A and C). Starting at late stage 9, Hb immunoreactivity is progressively lost from NBs such that by late stage 10 only a small subset of ventral cord NBs express Hb (Fig. 3E). Hb is detected in many GMC, however, and in their progeny generated during the first rounds of GMC production. These early sublineages reside predominantly along the inner/dorsal surfaces of the developing ganglia (Fig. 3C–J). The reduction in Hb NB expression coincides with the activation of Pdm-1 NB expression such that by late stage 10, Pdm-1 is detected in many cephalic lobe and ventral cord NBs and in GMCs (Fig. 3F; data not shown). Similar to the dynamics of Hb expression, Pdm-1 NB expression is transient. Many GMCs and their progeny arising from the Pdm-expressing NBs, however, maintain high levels of Pdm-1 (Fig. 3F,H). Onset of Cas expression in both ventral cord and cephalic lobe NBs parallels the loss of Pdm-1 NB expression, suggesting a transient overlap in their expression. NBs containing detectable levels of both Pdm-1 and Cas were observed during this period (Fig. 3K, Fig. 4D–F). No Pdm-1/Cas coexpression, however, was detected in GMCs or in their progeny. Hb/Pdm-1 coexpression was also detected at similar frequency in early S1 and S2 NBs but not in their progeny. By stage 11, ventral cord Pdm-1-expressing cells are juxtaposed to the more dorsal or internal Hb-positive sublineages and flanked on their ventral/ventral-lateral side by the superficially positioned Cas-positive NBs and GMCs (Figs. 3 and 4). The same relative positioning of Hb, Pdm-1, and Cas subpopulations was also observed in the cephalic lobes, as Cas-expressing NBs and their offspring predominantly cover the outer flanks of Pdm-1 sublineages (Fig. 4D–F) whereas Hb-positive cells occupy deeper internal positions (data not shown). Although Hb and Cas immunopositive cells together make up >50% of the cells present in stage 12 ganglia, no Hb/Cas coexpressing cells were detected in NBs or in their progeny (Fig. 3J,K). In fact, no cell at any stage of embryonic development was observed coexpressing these zinc finger proteins. Simultaneous labeling of Hb, Pdm-1, and Cas reveals that most, if not all, NB lineages express at least one of these transcription factors (Fig. 3K; data not shown). The absence of prolonged overlap between Hb/Pdm-1 coexpression or Pdm-1/Cas coexpression in early and late sublineages, respectively, suggests that Hb and Cas may control, via repression, the temporal boundaries of pdm expression during CNS development.
In situ mRNA localizations show that cas expression is predominantly restricted to CNS NBs, with little or no message detected in GMCs and no detectable mRNA observed in newly formed neurons or glia (Mellerick et al. 1992). The embryo immunostaining data presented here, however, reveal that Cas protein persists significantly longer than its message and is found in the nuclei of many cells generated during late sublineage development (for message/protein dynamics, cf. Fig. 2 with Figs. 4 and 5 of Mellerick et al. 1992). Cas-positive nuclei in stage 14 and older embryos are detected in all CNS ganglia (Fig. 2H) and many most likely belong to nascent neurons. No immunostaining was observed in cas null embryos, which confirms that our anti-Cas polyclonal antibodies are specific for Cas and do not bind to structurally related proteins, (Fig. 9B, below; and data not shown). Like Cas, Hb, and Pdm-1 are also detected in all ganglia of stage 14 and older embryos (data not shown), suggesting that the regulatory functions of all three transcription factors may be required in many neurons and glia until their functional phenotypes have been achieved.
Figure 9.
Both Drf and I-POU require cas for full-expression. (A,B) Confocal views of Cas (red) and Drf (green) simultaneous localizations in whole-mount immunostained stage 13 wild-type (A) and cas− (B) embryos shows: (1) many late developing NB sublineages coexpress Drf and Cas (yellow cells in A); and (2) loss of cas results in reduced numbers of Drf expressing NB sublineages (B). Shown are lateral views with focal planes passing through the right cephalic lobes (dorsal is to the right, anterior is up). Note, Drf expression in the epidermis of cas− embryos is unaltered. Also note, the absence of Cas immunostaining in cas− embryos confirms the specificity of the Cas antisera. (C,D) In situ mRNA localization of I-POU expression in wild-type (C) and cas− (D) embryos show that cas function is necessary for full I-POU expression. Shown are ventral focal plane views of dissected stage 13 ventral cords (third thoracic and first three abdominal segments; anterior, up). Note, I-POU expression in the medial–lateral cluster (arrowhead in C) is significantly diminished in the cas− ventral cord.
Hb and Cas are repressors of Pdm CNS expression
Given the apparent transient overlap between Hb and Pdm-1 expression in the CNS and Hb’s established role as a repressor of pdm expression in the cellular blastoderm, it is likely that Hb also silences pdm expression during early NB sublineage development. Embryos lacking Hb function suffer multiple defects (for review, see Lindsley and Zimm 1992). During CNS development, hb− embryos fail to develop labial and thoracic ganglia and gaps form between the subesophageal maxillary neuromeres and the abdominal ganglia. In addition, the seventh and eighth abdominal segments are fused because of the absence of parasegment 13 (White and Lehmann 1986). To further characterize the phenotypic consequences triggered by the loss of Hb and compare them with defects caused by loss of Cas, we examined the axon fascicle organization in hb null embryos. As expected, immunostains with the BP102 Mab axon marker revealed that hb− embryos have severe axon guidance defects. Missing are the highly ordered ventral cord axon scaffolds made up of longitudinal connective and commissural fascicles (Fig. 5A,B). Although axon fascicles do not form properly, however, the intensity of BP102 immunoreactivity in hb− ganglia was similar to that observed in wild-type embryos, indicating that significant numbers of neurons still generate axons, albeit misguided, in hb− embryos (Fig. 5A,B). Because many axon-guiding glia and pathfinding neurons are born from early NB sublineages, hb function may be essential for establishing correct axon guidance cues in these sublineages. Unlike the hb− phenotype and consistent with its late NB expression, loss of cas does not disrupt the formation of axon connective or commissure fascicles, but it does reduce the number of late forming axons that participate in these fascicles (Cui and Doe 1992; Mellerick et al. 1992).
Figure 5.
Loss of hb disrupts axon fasciculation and triggers ectopic Pdm-1 expression in early developing neuroblast sublineages. (A,B) Correct formation/organization of both longitudinal and commissural axon fascicle tracks requires hb function. Shown are dorsal views of dissected wild-type (A) and hb4 null mutant (B) stage 13 ventral cords immunostained in whole-mount with an axon marker, the BP102 mAb (anterior is up). Note the lack of longitudinal connectives and missing or disorganized commissures in the hb− ventral cord. (C–F) In hb− mutant embryos, early delaminating CNS NBs (S1 and S2 waves) fail to terminate Pdm-1 expression. Comparisons between wild-type (C) and the hb4 null allele (D) late stage 12 embryos reveal increased numbers of NB sublineages expressing Pdm-1 in all CNS ganglia of the hb mutant. Shown are dissected flattened fillets of whole-mount immunostained embryos (anterior is up). Transverse sections through abdominal segments of late stage 9 (E) and stage 12 (F) hb4 embryos reveal ectopic Pdm-1 expression in early delaminating NBs and in their sublineages. Note the increased numbers and thicker dorsal/ventral field of Pdm-1-positive cells in the stage 12 mutant ventral cord (F) when compared with the Pdm-1 immunoreactive cells present in a similar transverse section from a wild-type embryo shown in Fig. 3H. (G,H) Shown are a transverse section through the second abdominal segment (G) and a dissected fillet (H) both from late stage 12 hb4 embryos immunostained with Cas antibodies. In hb4 mutants, late developing NB sublineages express Cas. When compared with wild-type Cas expression patterns, however, its onset is delayed in certain sublineages (data not shown) and the overall position of Cas-expressing cells is altered in hb− disrupted neuromeres. In hb− embryos, Cas-positive late sublineages are still found on the outer ventral surfaces of the ventral cord neuromeres.
Pdm-1 immunolocalizations performed on hb− embryos confirmed the hypothesis that Hb also functions as a repressor during CNS development. Comparisons between hb− and wild-type Pdm-1 immunostaining patterns showed that in the absence of Hb, Pdm-1 is ectopically expressed in all CNS ganglia (Fig. 5C and D). Transverse sections of Pdm-1 immunostained hb− embryos also revealed that early NBs that normally do not maintain Pdm-1 expression after their ectoderm delaminations fail to terminate Pdm-1 expression (Fig. 5E). The presence of ectopic Pdm-1 is also found in many early GMCs and by stage 12, Pdm-1-positive cells occupy both dorsal and inner regions of the developing ventral cord neuromeres (Fig. 5F). The expanded dorsal-ventral zone of Pdm-1 expression in hb− ventral cords indicates that many early GMCs and their progeny, normally marked by Hb expression, now ectopically maintain Pdm-1. Ectopic Pdm-1 expression, however, is not detected throughout the developing ganglia. After stage 11 in hb− embryos there is no Pdm-1 expression in the most ventral regions of the ventral cord ganglia (Fig. 5F). Similarly, no ectopic Pdm-1 was detected along the outer/superficial surfaces of hb− cephalic lobes after stage 11 (data not shown). This suggests that other mechanism(s) are regulating pdm expression in late developing sublineages.
Interestingly, loss of Hb did not markedly perturb Cas expression (Fig. 5H). Although activation of Cas in many NBs may be slightly delayed, the overall dynamics of its expression and order of NB activation was similar to that observed in wild-type cephalic lobe and abdominal ganglia. As in wild-type embryos, activation of Cas in hb− embryos is first observed in ventral cord midline cells followed by the NB6-1s and then in NB5-2s (data not shown). In addition, the overall number of late developing sublineages in cephalic lobes and in abdominal ganglia of hb− embryos appeared to be equivalent of that of wild-type embryos, as judged from Cas immunostaining in late stage 12 embryos (Fig. 5H).
To determine if Cas is also a pdm repressor, we next examined Pdm-1 and Pdm-2 expression in cas null embryos (Mellerick et al. 1992). In stage 9 and in younger embryos, no differences were detected between the cas− and wild-type expression patterns of Pdm-1 or -2. Starting at stage 10, however, we observed that NBs failed to terminate expression of both Pdms (Fig. 6). Ectopic Pdm expression was observed in most, if not all, late developing sublineages in all CNS ganglia. The sustained Pdm expression is most likely caused by transcriptional derepression, as pdm-1 mRNA in situ hybridizations also reveal that its message persists in cas− late NBs (data not shown). In addition, cas function is required to silence pdm-1 enhancer(s) (see below), whereas loss of cas function had no detectable effect on Hb expression (data not shown).
Figure 6.

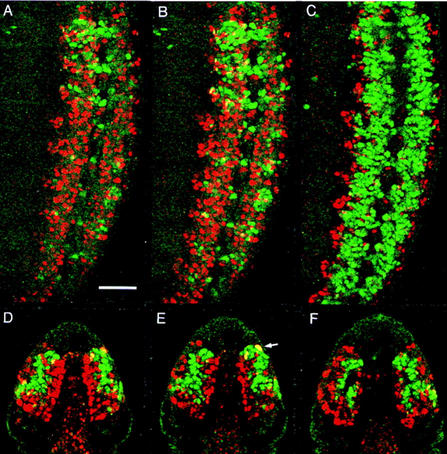
Loss of castor results in ectopic Pdm-1 and Pdm-2 expression in late developing neuroblast sublineages. (A,B) Comparisons of Pdm-1 immunostaining in wild-type (A) and cas− null (B) stage 13 ventral cords reveals ectopic pdm expression in late-stage cas− NBs and in their progeny. Pdm-1 misexpression is particularly evident in the ventral–lateral regions of the cas− ventral cord. In wild-type ventral cords, these ventral–lateral areas are predominantly made up of Cas-expressing sublineages (see Fig. 4C). Shown are the third thoracic through third abdominal neuromeres of early stage 13 embryos (anterior is up). (C,D) Ectopic Pdm-1 expression is also evident in late developing cephalic lobe NB sublineages. Shown are dorsal views of wild-type (C) and cas− (D) stage 13 Pdm-1 immunostained embryos (anterior is up). (E,F) Ni-enhanced, Pdm-2 immunostaining of wild-type (E) and cas− (F) embryos also reveals ectopic Pdm-2 expression in late-stage cas− cephalic lobe NBs. Note, focal planes are similar to C and D.
cas controls pdm-1 by deactivating its neuroblast enhancer(s)
In vivo analysis of pdm-1 genomic DNA has identified the main cis-regulatory elements controlling its embryonic expression (Fig. 7; K. Cockerill and S. Poole, unpubl.). These control elements lie within a 6.3-kb DNA fragment flanking the 5′ side of its transcribed sequence. Analysis of transformant embryos containing a P[mini-white.pdm-1.GAL4] vector (Fig. 7G) showed that the pdm-1 regulatory DNA contains enhancer(s) that can drive the expression of both the mini-white and GAL4 reporter genes in the same cells in which pdm-1 is normally expressed (Fig. 7A,B). As with the endogenous pdm genes, in a cas− background both of the divergently transcribed transgenes are ectopically expressed in NBs during late sublineage development. This result shows that the enhancer(s) within the 6.3-kb regulatory DNA are negatively regulated by Cas (Fig. 7C–F).
To explore the possibility that Cas may play a direct role in silencing pdm gene expression, we carried out Cas–DNA immunoprecipitation with pdm-1 promoter fragments to test for potential Cas DNA-binding sites (data not shown). DNA sequence analysis of bound fragments revealed 32 potential DNA-binding sites, all sharing at least 8 of the 10 bp with the Hb consensus sites (Fig. 7G). Gel-shift analysis and competition assays with pdm-1 fragments containing exact matches to the Hb consensus sequences confirmed that Cas binds to these sites (Fig. 7H). In addition, base-pair substitutions in one of the sites showed that the A/T-rich core sequence is essential for Cas binding (Fig. 7H). All together, the results suggest that Hb and Cas regulate pdm expression by interacting directly with their cis regulators to deactivate controlling enhancer(s), with Hb repressing the pdm genes early and Cas silencing late in CNS development.
To test if Cas can silence Pdm-1 expression outside of its endogenous sublineage boundaries, we studied the effects of misexpressing Cas earlier in CNS development. By use of the GAL4–UAS targeted misexpression strategy (Brand and Perrimon 1993), we compared Pdm-1 expression in P[prospero.GAL4]/P[UAS.cas] embryos with that of wild-type controls. prospero is expressed in all NBs shortly after they delaminate (Doe et al. 1991; Vaessin et al. 1991); thus Gal4, expressed from the prospero.GAL4 transgene, activates UAS.cas expression in both early and late delaminating NBs. Indicating that Cas can act as a pdm repressor outside of its normal late expression boundaries, the temporally misexpressed Cas significantly reduced Pdm-1 expression when compared with wild-type embryos stained under identical conditions (Fig. 8). The inability of the ectopic Cas to completely suppress Pdm-1 expression may be caused by the lack of sufficient levels of Cas necessary to completely override endogenous pdm transactivators. Ectopic Cas had no effect on the dynamics or levels of Hb expression (data not shown).
Figure 8.

Targeted misexpression of Cas in all neuroblast lineages reduces Pdm-1 expression. (A) Activation of a UAS.cas transgene by the prospero.GAL4 driver results in Cas misexpression in most, if not all, NB lineages in both the CNS and in the PNS (arrows). Shown is a transverse section through an abdominal segment of a stage 13 prospero.GAL4/UAS.cas transformant embryo immunostained in whole-mount with Cas antibodies (ventral is up). Scale bar equals 25 μm. (C,D) Comparisons of ventral cord fillets from Pdm-1 immunostained wild-type (C) and prospero.GAL4/UAS.cas (D) stage 13 embryos, processed under identical conditions, reveal that ectopic Cas reduces, but does not eliminate, Pdm-1 expression. Shown are the second and third thoracic neuromeres plus abdominal neuromeres 1–5. The focal plane for both C and D passes through the layers containing the highest density of Pdm-1-positive cells.
drf and I-POU require cas for full CNS expression
The role of Cas control over the pdm genes raises the possibility that it may regulate expressions of other POU genes. To test this, we compared the expression domains of Cas and Drf, and examined Drf expression in cas− embryos. In addition to its established role in midline glia and tracheal development, Drf is also expressed in a subset of NB progeny in both the developing brain and ventral cord (Anderson et al. 1995). We find that many Cas-expressing NB sublineages also express Drf (Fig. 9A). Thus, it appears that Cas does not repress Drf expression. On the contrary, a marked reduction in late-lineage Drf expression was observed in cas− embryos (Fig. 9B), suggesting Cas either directly or indirectly plays a role in activating and/or sustaining Drf expression in these sublineages. To test if Cas could ectopically activate drf outside of its endogenous expression boundaries, we examined Drf expression in the prospero.GAL4/UAS.cas embryos and found that ectopic Cas had no effect on Drf expression (data not shown).
In situ localization of I-POU mRNA in wild-type, cas− and prospero.GAL4/UAS.cas embryos yielded findings similar to drf. In the absence of cas function, I-POU expression is lost in a subset of ventral cord cells (Fig. 9C,D), but ectopic Cas has no effect on the I-POU wild-type expression pattern (data not shown). At this time, we do not know if Cas is a direct activator of drf and/or I-POU. The data indicates, however, that if Cas is playing a direct activator role, it most likely requires cofactors that are not expressed outside of its normal domain.
Discussion
The principal finding of this study is that the zinc finger proteins Hb and Cas act in a cooperative, nonoverlapping manner to control POU gene expression during Drosophila CNS development. By silencing pdm expression in early and late NB sublineages, Hb and Cas establish three pan–CNS compartments whose cellular constituents are marked by the expression of either Hb, Pdm, or Cas. Optical sections through stage 13 and older embryos simultaneously immunostained for Hb, Pdm-1, and Cas reveal that these expression compartments encompass most, if not all, NB lineages. We hypothesize that these sequentially formed transcription factor expression domains represent temporal branch points in the regulatory network controlling cell identities. The fact that cells within a given domain share the expression of one of these transcription factors suggests that subsets of cells in a given expression domain may also have overlapping repertoires of transcriptionally active genes that ultimately produce shared or interrelated cellular functions. The loss of axon fascicle organization in hb− embryos may represent an example of this shared transcription factor interdependence. Descendants of early NB sublineages most likely depend on Hb regulation for correct expression of axon pathfinding signals and/or the machinery to accurately interpret these guidance cues. The transcription factor expression domains reported here bear notable similarity to temporally orchestrated events that occur during mammalian brain development. For example, in the developing cerebral cortex of mammals, neurons are generated in an orderly layered inside-out progression (for review, see McConnell 1988). Populations of cortical neurons within a given layer share birthdates, coexpress certain POU and/or homeobox genes (Frantz et al. 1994a,b), have common functional properties, and display similar connectivity (for review, see McConnell 1995).
Although a detailed understanding of the mechanism(s) that generate the Drosophila CNS expression domains awaits further analysis, the tightly choreographed NB expressions of Hb, Pdm, and Cas suggest temporally integrated processes participate in their formation. Clonal analysis of ventral cord NB lineages has revealed that many early delaminating NBs produce lineages that span most of the ventral cord’s dorsal/ventral axis (Prokop and Technau 1994; Buenzow and Holmgren 1995; Bossing et al. 1996). For example, the NB5-2, one of the first NBs to delaminate, generates a ventral-dorsal column of 17–26 cells (Bossing et al. 1996). The dynamics of Hb, Pdm-1, and Cas expression in NBs indicates that many of the early S1 and S2 delaminating NBs may sequentially express all three and thereby produce lineages spanning all three compartments. Two such ventral cord candidates are the early NB5-2s and NB7-4s. Shortly after their delamination, during early stage 9, they activate Hb expression (Fig. 2A, inset), whereas later, after several rounds of GMC divisions, they activate Cas expression (Fig. 2C, inset; Cui and Doe 1995). The fact that we detect NBs coexpressing Hb/Pdm-1 or Pdm-1/Cas, but never Hb/Cas, further suggests that at least some of the early NBs make the Hb → Pdm → Cas transition. Not all NBs undergo these transitions, however. This is particularly evident in NBs that enter the proliferative zone during later delamination waves. For example, the first ventral cord NBs to express Cas, the S3 NB6-1s, activate Cas shortly after delaminating from the ectoderm and do not express Hb (Fig. 2B, inset).
Previous studies reveal Hb to be a highly versatile regulator of gene expression (for review, see Rivera-Pomar and Jackle 1996). The dynamic expression of Hb in early sublineages may allow for pdm expression at specific stages during sublineage development. For example, the pdm genes are known to collaborate in the S2 NB4-2 → GMC-1 → RP2 neuron lineage by specifying GMC-1 identity (Bhat et al. 1995; Yeo et al. 1995). Interestingly, pdm expression is detected only in GMC-1 and not in its NB. The lack of Hb/Pdm-1 overlap in GMCs or in their progeny suggests Hb may dynamically regulate pdm expression by first silencing their NB expression and then, in absentia, permitting pdm reactivation in the GMC-1. Recent studies on the annelid ortholog of hb, Lzf2, also suggest that it may play a temporal regulatory role during leech nervous system development (Savage and Shankland 1996).
Our studies reveal that late developing sublineages rely on cas to both insulate cell-fate programs and to secure the expression of factors that likely play key roles in their cell identity decisions. Cas carries out the first of these regulatory roles by selectively silencing the expression of pdm genes. This is evidenced by the fact that late developing NB sublineages exhibit sustained, ectopic Pdm-1 and Pdm-2 expression in cas− embryos, pdm-1 enhancer(s) are activated in cas− late NBs; and in contrast to its repressor role, cas is required for full-expression of Drf and I-POU. The process of reinforcing specific cell fates by silencing the expression of regulators that dictate alternative fates is one that is employed during many developmental decisions. For example, shortly after NB commitment, the pan-neural zinc finger protein Scratch promotes neural development by silencing regulators that dictate mesoderm cell fates (Roark et al. 1995). Like scratch, hb and cas participate in cell fate branch points positioned downstream of the initial proneural and neurogenic events.
Our efforts to characterize Cas DNA-binding specificity led to the unexpected observation that it selectively binds to DNA recognized by Hb. Although nothing is known about the high-resolution details of how Hb or Cas interact with DNA, comparisons drawn from data compiled on other C2-H2 finger-DNA complexes may be informative. Crystallographic analyses of different protein–DNA complexes reveal that not all fingers dock with DNA in the same way; hence, no simple code for base-pair specificity exists (Fairall et al. 1993; Pavletich et al. 1993; Elrod-Erickson et al. 1996). All finger–DNA structures resolved thus far, however, show that within a finger’s α-helical reading head, two to four residues play key roles in conferring DNA-binding specificity. Secondary structure predictions of the Cas finger domain indicate only the first and third of its TFIIIA-like fingers contain α-helices. The relative positions of these α-helices and their predicted lengths in the fingers agree with helices in previously analyzed finger–DNA complexes. cas evolutionary comparisons identify these α-helices as having the highest conservation (100% identity between Drosophila melanogaster and Drosophila virilis; R. Kambadur, C. Stivers, and W.F. Odenwald, unpubl.). Interestingly, optimal alignment of Cas and Hb fingers reveal that the first and third α-helices of Cas share the highest homology with the corresponding α-helices of Hb (33%/66% identity/similarity for the first and 27%/80% for the third). Although speculative, their shared DNA-binding preferences may, in part, be caused by the shared residues found in these predicted reading heads. Outside of their zinc fingers, Hb and Cas show no obvious sequence similarities. This may indicate that they interact with different cofactors and thus modulate the same target genes in similar or different ways depending on the presence or absence of specific ancillary factors. Future studies aimed at comparing the regulatory functions of these finger proteins will enhance our understanding of the genetic pathways leading to neuronal diversity.
Materials and methods
Drosophila P-element transformants and stocks
Standard Drosophila husbandry procedures were used (Ashburner 1989). Ectopic expression of cas in all NB lineages was accomplished by use of the GAL4–UAS strategy (Brand and Perrimon 1993). The P[prospero.GAL4] transformant line was kindly provided by F. Matsuzaki (NIN, Tokyo, Japan). A UAS.cas transgene was constructed with a 2.4-kb Vent polymerase (New England Biolabs) amplified cas cDNA fragment, containing only the cas ORF, inserted into the EcoRI site of the pUAST vector to produce P[UAS.cas]. The orientation and reading frame of the cas sequence was confirmed by DNA-sequence analysis. Germ-line transformants were isolated by standard techniques with the yw67c23 stock (Spradling 1986). Phenotypic analysis of cas mutants was performed on the cas− H23A alleles, Δ1 and Δ3, generated by imperfect excisions of the P[HlacZ]vector (Mellerick et al. 1992). For identification of homozygous cas− embryos, these recessive lethal alleles were balanced over the third chromosome TM3,Sb,P[ftz/lacZ] (Hiromi et al. 1985; Nambu et al. 1990). To examine the effect of a cas null background on pdm-1 cis-regulatory DNA/reporter gene expression, a P[pdm-1.GAL4] reporter vector was constructed. The 2.8-kb KpnI–NotI fragment from pGATB (Brand and Perrimon 1993) containing the GAL4 coding sequence was subcloned into the pCaSpeR4 vector polylinker and a 5.3-kb EcoRI fragment from the pdm-1 promoter region was inserted into the EcoRI site of the pCaSpeR4 polylinker. Germ-line transformants were prepared and a P[pdm-1.GAL4] located on the third chromosome (for construct map, see Fig. 7G) was mobilized with Δ2-3 as a genomic source of transposase (Robertson et al. 1988). Second chromosome jumps were saved, made homozygous, and selected for mini-white/GAL4 expression that matched endogenous pdm-1 expression patterns. Males from one of these lines (no. 11), containing the third chromosome Ser/TM-3,Sb,P[ftz/lacZ] balancers, were mated to virgins with the following second and third chromosomes: CyO/Sco;Δ1,H23A/TM-3,Sb,P[ftz/lacZ]. Sibling crosses were carried out with flies possessing the following chromosomes: 11P[pdm-1A.GAL4]/CyO;Δ1, H23A/TM-3,Sb P[ftz/lacZ]. Progeny, homozygous for the 11P[pdm-1A.Gal4] second chromosome, were crossed to establish a stock containing the third chromosomes: Δ1,H23A/TM-3,Sb P[ftz/lacZ]. Phenotypic analysis of loss of hb function was carried out with the hb4 null allele. Balancer chromosomes used above are described in Lindsley and Zimm (1992).
mRNA localization
Localization of mRNA by whole-mount in situ hybridization of fixed embryos was performed with digoxigenin-labeled DNA probes according to Tautz and Pfeifle (1989). Probes were generated by the random primer method with the Genius Kit (Boehringer Mannheim). Templates for probes used included: a 1.6-kb pdm-1 cDNA fragment; a mini-white 2-kb SacI fragment from pCaSpeR vector (Pirrotta 1988); an I-POU 4.2-kb EcoRI–HindIII genomic fragment (kindly provided by S. Certel and W. Johnson, University of Iowa, Iowa City); and a GAL4 3.1-kb BamHI fragment from the pGATB vector (Brand and Perrimon 1993).
Recombinant Cas protein production and purification
A bacterially expressed, histidine-tagged Cas fusion protein was prepared by the pET expression system (Studier et al. 1990) according to the manufacturer’s protocol (Novagen). Briefly, an overnight Escherichia coli culture [strain BL21 (DE3)] harboring the pET 16b plasmid containing a cas cDNA BamHI restriction fragment (coding for the 239 amino acid zinc finger repeat plus 196 and 120 amino- and carboxy-terminal residues, respectively) was diluted and grown up to an OD of 0.8 in 1 liter of LB medium plus 50 μg/ml ampicillin. Induction of the recombinant Cas was accomplished by the addition of 1 mm IPTG to the culture and continued incubation for 2 hr. Bacteria were collected by centrifugation, resuspended in 40 ml of lysis buffer [6 m guanidine hydrochloride, 20 mm Tris (pH 8.1), 5 mm 2-mercaptoethanol], cooled to 4°C, and sonicated for 5 min with intermittent cooling. The lysate was centrifuged at 10,000g for 30 min and the supernatant was saved for Cas purification by the Ni-agarose affinity protocol of Shirakata et al. (1993). Column fractions containing Cas were pooled; dialyzed against two changes of 50 mm Tris-HCl (pH 8.0) containing 200 mm KCl, 10% glycerol overnight; aliquoted; and stored at −80°C. A detailed description of this protocol is available upon request.
Cas polyclonal antibody production
Mouse anti-Cas sera was raised against the recombinant protein by use of the protocols described in the Harlow and Lane antibody manual (1988). Briefly, BALB/c female adult mice were injected subcutaneously at multiple sites with 50 μg of purified recombinant protein in 2 ml of PBS/RIBI adjuvant system [1:1 (vol/vol) RIBI Immunochem Research Inc.]. Fifteen days later, the mice were boosted with the same inoculum and 1 month after the initial inoculation, a third boost was administered by intraperitoneally injecting ∼50 μg of Cas protein/mouse. Fifteen days after this final boost, mice were exanimated by bleeding out, and serum was stored at −80°C for immunohistochemistry. To raise polyclonal anti-Cas antibodies in rabbits, New Zealand white virgin females were given multiple subcutaneous injections of the purified recombinant Cas (500 μg/rabbit) mixed with the RIBI adjuvant (1:1). Rabbits were boosted with the same inoculation 30 and 45 days later. A final boost containing just Cas (500 μg/rabbit) was administered on day 60, and 10 days later the rabbits were terminally bled, their serum collected, aliquoted, and stored at −80°C. The specificity of both anti-Cas antibodies was confirmed by the absence of immunostaining in cas null embryos (see Fig. 8B; data not shown).
DNA-binding studies and molecular biology techniques
The consensus DNA-binding site for Cas was determined from an unbiased set of degenerate oligonucleotides by the cyclic amplification and selection of targets (CASTing) protocol of Wright et al. (1991). Preparation of the double stranded 75-mer oligonucleotides, recombinant Cas–DNA binding conditions, and immuno-affinity selection were performed according to their methods with the following exceptions. The complete sequence of the selected/Cas-bound DNA fragments is available upon request. Approximately 200 ng of the recombinant Cas was used for each binding reaction that was carried out in 50 mm Tris-HCl (pH 8.0), 400 mm KCl, 5 mm MgCl, 2 mm 2-mercaptoethanol, 10% glycerol, and 10 μm ZnCl2. Protein–DNA complexes were immuno-selected by incubating with rabbit anti-Cas serum (final dilution 1:500) for 30 min at 4°C followed by the addition of protein A-coated magnetic beads (Dynal). Prior to their use, protein A beads were preincubated with 0.2% BSA in phosphate buffered saline (pH 7.4), 0.05% triton X-100 (PBT). Protein A–antibody complexes were recovered by a magnetic field. Following three washes with PBT, the beads were resuspended in 50 μl of 1× PCR buffer and a 30-μl aliquot of this mixture was denatured at 95°C and subjected to 20 PCR cycles (92°C for 1 min, 65°C for 1 min, and 72°C for 1 min with a final extension time of 10 min). A 10-μl sample of amplified DNA was used to initiate the next CASTing cycle. CASTing cycles of 2, 3, and 4 were subjected to 15, 10 and 9 cycles of PCR, respectively. After four cycles of CASTing, 1 μg of amplified DNA was used as a template to generate a radiolabeled probe for band shifts by incorporating [32P]dCTP during three cycles of PCR in the absence of cold dCTP by use of the forward and reverse primers as described by Wright (1991). Electrophoretic mobility-shift gel retardation assays with 200 ng of the purified recombinant Cas were performed as in Odenwald et al. (1989) with the following modifications. Prior to their use, probes were purified by electrophoresis through 8% polyacrylamide TBE gels by use of standard procedures (Sambrook et al. 1989). A final concentration of 400 mm KCl was used in the protein–DNA-binding reactions. Following electrophoresis of protein–DNA complexes, shifted bands were excised from the gel and the probe eluted into 200 μl of PBS by vigorous shaking of gel pieces at 37°C for 5 hr. A 25-μl aliquot of the eluted DNA was used as a template for the next round of PCR amplification. After an additional CASTing cycle with band-shift as the selection, amplified fragments were directly cloned into the pCR II vector (Invitrogen) and sequenced by the chain-termination sequencing method (Sanger et al. 1977). For antibody supershift assays, protein–DNA complexes were incubated with the rabbit anti-Cas (final dilution 1:500) for an additional 30 min at 4°C prior to electrophoresis. To test the binding specificity of Cas, 5- and 50-fold excesses (as determined by optical density measurements) of unlabeled cold DNA fragments were mixed with labeled probes prior to the addition of the recombinant Cas. The immunoprecipitation of Cas bound pdm-1 genomic fragments was as follows: upstream pdm-1 DNA genomic fragments were end-labeled with [32P]γATP and T4 polynucleotide kinase according to standard protocols (Sambrook et al. 1989). Labeled DNA fragments were purified by use of SR 200 Microspin columns (Pharmacia), and ∼1 ng of the end-labeled fragments were added to binding buffer (same as the CASTing buffer) and incubated with 200 ng of the recombinant Cas on ice for 30 min. Protein–DNA complexes were immunoprecipitated as above and resolved by electrophoresis through standard agarose gels. DNA sequencing of the pdm-1 regulatory DNA was accomplished by standard chain-termination procedures. The complete sequence of pdm-1 DNA fragments used in the gel-shift assays is available upon request.
Immunocytochemistry
The antibody staining protocol was based on a procedure developed by N. Patel (1994), by use of the Vectastain ABC second antibody avidin/biotin HRP visualization reagents (Vector Labs) for light microscopy. For immunofluorescent confocal microscopy, FITC-, Rho-, Cy2-, or Cy5-conjugated secondary antibodies (Jackson Labs) were used. Detailed protocols are available upon request. To diminish background staining, the mouse and rabbit anti-Cas sera (1:50 dilutions) were preabsorbed with embryos (an overnight collection) for 48 hr at 4°C with one change of embryos. Final dilutions for the anti-Cas mouse and rabbit sera were 1:500 and 1:5000, respectively. Purified rabbit anti-Pdm-1 and Pdm-2 antibodies (Cockerill et al. 1993) were used at 1:25 and 1:10 dilutions, respectively. To enhance the Pdm-2 immunostaining, NiCl2 and CoCl2 were added to the diaminobenzidine chromogen solution according to Kania et al. (1990). Rat anti-Drf serum (kindly provided by M. Anderson and W. Johnson, University of Iowa) was preabsorbed as above and used at a final dilution of 1:3000. The rat anti-Hb (a gift from P. Macdonald) was also preabsorbed and used at a final dilution of 1:15000. The BP102 mouse mAb (a gift from Nipam Patel) was used at a final dilution of 1:20. cas− embryos were identified by the absence of lacZ expression from the balancer TM-3,Sb,P[ftz/lacZ] chromosome with either rabbit anti-β-gal (Cappel, 1:2000) or mouse anti-β-gal Mab (Promega, 1:2000). Stained embryos were mounted in 70% glycerol for observation, and in some cases, flattened embryo fillets or dissected ventral cords were prepared with fine needles (Hamilton, cat. no. 90033). Embryos were also sectioned following whole-mount immunohistochemistry by first post-fixing in 2% glutaraldehyde in PBS and then individually encasing the embryos in albumin plugs to facilitate handling during the plastic embedding (Mellerick et al. 1992). Mounted embryos or dissected fillets were photographed on a Nikon Optiphot microscope with DIC/Nomarski optics. Immunofluorescent images were collected by confocal microscopy on a Zeiss LSM 410 equipped with a krypton/argon laser.
Acknowledgments
We thank Drs. Wayne Johnson, Michael Anderson, and Sarah Certel for drf and I-POU reagents and Dr. Paul Macdonald for the anti-Hb antibody. We also thank Dr. Nipam Patel for the BP102 mAb. Dr. Fumio Matsuzaki kindly provided us the P[prospero.GAL4] line. We thank Drs. Jin Kim and Lynn Hudson for reagents and advice concerning the CASTing procedure. We also thank Dr. Samuel Kunes for advice concerning triple-immunolabeling and acknowledge the support of the NINDS Light Imaging Facility and Dr. Carolyn Smith for her advice on confocal microscopy. For suggestions and comments on the manuscript, we are indebted to Drs. Chris Doe, Judy Kassis, Brian Mozer, and Howard Nash. We are also indebted to Dr. Harold Gainer for his advice and support throughout the duration of these studies.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Note added in proof
The GenBank accession no. for the genomic DNA containing pdm-1 cis-regulatory sequences is AF035264.
Footnotes
E-MAIL ward@codon.nih.gov; FAX (301) 496-1339.
References
- Anderson MG, Perkins GL, Chittick P, Shrigley RJ, Johnson WA. drifter, a Drosophila POU-domain transcription factor, is required for correct differentiation and migration of tracheal cells and midline glia. Genes & Dev. 1995;9:123–137. doi: 10.1101/gad.9.1.123. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas SA, Matsuno K, Fortini ME. Notch Signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A laboratory manual. Cold Sping Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Bellefroid EJ, Poncelet DA, Lecocq PJ, Revelant O, Martial JA. The evolutionarily conserved Kruppel-associated box domain defines a subfamily of eukaryotic multifingered proteins. Proc Natl Acad Sci. 1991;88:3608–3612. doi: 10.1073/pnas.88.9.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat K, Schedl P. The Drosophila miti-mere gene, a member of the POU family, is required for the specification of the RP2/sibling lineage during neurogenesis. Development. 1994;120:1483–1501. doi: 10.1242/dev.120.6.1483. [DOI] [PubMed] [Google Scholar]
- Bhat K, Poole SJ, Schedl P. The miti-mere and pdm-1 genes collaborate during specification of the RP2/sib lineage in Drosophila neurogenesis. Mol Cell Biol. 1995;15:4052–4063. doi: 10.1128/mcb.15.8.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E. Anti-Neural-Inhibition: A conserved mechanism for neural induction. Cell. 1997;89:681–684. doi: 10.1016/s0092-8674(00)80250-0. [DOI] [PubMed] [Google Scholar]
- Billin AN, Cockerill KA, Poole SJ. Isolation of a family of Drosophila POU domain genes expressed in early development. Mech Dev. 1991;34:75–84. doi: 10.1016/0925-4773(91)90045-8. [DOI] [PubMed] [Google Scholar]
- Bossing T, Udolph G, Doe CQ, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster: 1. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol. 1996;179:41–64. doi: 10.1006/dbio.1996.0240. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Broadus J, Skeath JB, Spana EP, Bossing T, Technau GM, Doe CQ. New neuroblast markers and the origin of the aCC/pCC neurons in the Drosophila central nervous system. Mech Dev. 1995;53:393–402. doi: 10.1016/0925-4773(95)00454-8. [DOI] [PubMed] [Google Scholar]
- Buenzow DE, Holmgren R. Expression of the Drosophila gooseberry locus defines a subset of neuroblast lineages in the central nervous system. Dev Biol. 1995;170:338–349. doi: 10.1006/dbio.1995.1219. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. Early neurogenesis in Drosophila melanogaster. In: Bate M, Arias AM, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1091–1131. [Google Scholar]
- Cockerill KA, Billin AN, Poole SJ. Regulation of expression domains and effects of ectopic expression reveal gap gene-like properties of the linked pdm genes of Drosophila. Mech Dev. 1993;41:139–153. doi: 10.1016/0925-4773(93)90044-x. [DOI] [PubMed] [Google Scholar]
- Cui X, Doe CQ. ming is expressed in neuroblast sublineages and regulates gene expression in the Drosophila central nervous system. Development. 1992;116:946–953. doi: 10.1242/dev.116.4.943. [DOI] [PubMed] [Google Scholar]
- ————— The role of the cell cycle and cytokinesis in regulating neuroblast sublineage gene expression in the Drosophila CNS. Development. 1995;121:3233–3243. doi: 10.1242/dev.121.10.3233. [DOI] [PubMed] [Google Scholar]
- Dick T, Yang X, Yeo S, Chia W. Two closely linked Drosophila POU domain genes are expressed in neuroblasts and sensory elements. Proc Natl Acad Sci. 1991;88:7645–7649. doi: 10.1073/pnas.88.17.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ, Chu-LaGraff Q, Wright DM, Scott MP. The prospero gene specifies cell fates in the Drosophila central nervous system. Cell. 1991;65:451–464. doi: 10.1016/0092-8674(91)90463-9. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson M, Rould MA, Nekludova L, Pabo CO. Zif268 protein-DNA complex refined at 1.6A: A model system for understanding zinc finger-DNA interactions. Structure. 1996;4:1171–1180. doi: 10.1016/s0969-2126(96)00125-6. [DOI] [PubMed] [Google Scholar]
- Fairall L, Schwabe JWR, Chapman L, Finch JT, Rhodes D. The crystal structure of a two zinc-finger peptide reveals an extension to the rules for zinc-finger/DNA recognition. Nature. 1993;366:483–487. doi: 10.1038/366483a0. [DOI] [PubMed] [Google Scholar]
- Frantz GD, Bohner AP, Akers RM, McConnell SK. Regulation of the POU domain gene SCIP during cerebral cortical development. J Neurosci. 1994a;14:472–485. doi: 10.1523/JNEUROSCI.14-02-00472.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz GD, Weimann JM, Levin ME, McConnell SK. Otx1 and Otx2 define layers ond regions in developing cerebral cortex and cerebellum. J Neurosci. 1994b;14:5725–5740. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CS, Doe CQ. Embryonic development of the Drosophila central nervous system. In: Bate M, Arias AM, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1131–1206. [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hartenstein V, Campos-Ortega JA. Early neurogenesis in wild type Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1984;193:308–325. doi: 10.1007/BF00848159. [DOI] [PubMed] [Google Scholar]
- Herr W, Sturm RA, Clerc RG, Corcoran LM, Baltimore D, Sharp PA, Ingraham HA, Rosenfeld MG, Finney M, Ruvkun G, Horvitz HR. The POU domain: A large conserved region in the mammalian pit-1, oct-1, oct-2 and Caenorhabditis elegans unc-86 gene products. Genes & Dev. 1988;2:1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Hiromi Y, Kuroiwa A, Gehring WJ. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985;43:603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Jan JN, Jan LY. Genetic control of cell fate specification in Drosophila peripheral nervous system. Annu Rev Genet. 1994;28:373–393. doi: 10.1146/annurev.ge.28.120194.002105. [DOI] [PubMed] [Google Scholar]
- ————— Maggot’s hair and bug’s eye: Role of cell interactions and intrinsic factors in cell fate specification. Neuron. 1995;14:1–5. doi: 10.1016/0896-6273(95)90235-x. [DOI] [PubMed] [Google Scholar]
- Jimenez F, Campos-Ortega JA. Defective neuroblast commitment in mutants of the achaete-scute complex and adjacent genes of D. melanogaster. Neuron. 1990;5:81–89. doi: 10.1016/0896-6273(90)90036-f. [DOI] [PubMed] [Google Scholar]
- Johnson W, Hirsh J. Binding of a Drosophila POU-domain protein to a sequence element regulating gene expression in specific dopaminergic neurons. Nature. 1990;343:467–470. doi: 10.1038/343467a0. [DOI] [PubMed] [Google Scholar]
- Kania MA, Bonner AS, Duffy JB, Gergen PJ. The Drosophila segmentation gene runt encodes a novel nuclear regulatory protein that is also expressed in the developing nervous system. Genes & Dev. 1990;4:1701–1713. doi: 10.1101/gad.4.10.1701. [DOI] [PubMed] [Google Scholar]
- Lin H, Schagat T. Neuroblasts: A model for the asymmetric division of stem cells. Trends Genet. 1997;13:33–39. doi: 10.1016/s0168-9525(96)10050-0. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Lloyd A, Sakonju S. Characterisation of two Drosophila POU domain genes, related to oct-1 and oct-2, and the regulation of their expression patterns. Mech Dev. 1991;36:87–102. doi: 10.1016/0925-4773(91)90075-h. [DOI] [PubMed] [Google Scholar]
- McConnell SK. Development and decision-making in the mammalian cerebral cortex. Brain Res Rev. 1988;13:1–23. doi: 10.1016/0165-0173(88)90002-1. [DOI] [PubMed] [Google Scholar]
- ————— Constructing the cerebral cortex: Neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Mellerick DM, Kassis JA, Zhang S-D, Odenwald WF. castor encodes a novel Zinc finger protein required for the development of a subset of CNS neurons in Drosophila. Neuron. 1992;9:789–803. doi: 10.1016/0896-6273(92)90234-5. [DOI] [PubMed] [Google Scholar]
- Miller J, MacLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIa from Zenopus oocytes. EMBO J. 1985;4:1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Nambu JR, Franks RG, Hu S, Crews ST. The single-minded gene of Drosophila is required for the expression of genes important for the development of CNS midline cells. Cell. 1990;63:63–75. doi: 10.1016/0092-8674(90)90288-p. [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Cohen SM. Nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development. 1995;121:589–599. doi: 10.1242/dev.121.2.589. [DOI] [PubMed] [Google Scholar]
- Odenwald WF, Garbern J, Arnheiter H, Tournier-Lasserve E, Lazzarini RA. The Hox 1.3 homeobox protein is a sequence-specific DNA-binding phosphoprotein. Genes & Dev. 1989;3:158–172. doi: 10.1101/gad.3.2.158. [DOI] [PubMed] [Google Scholar]
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 1994;44:445–487. doi: 10.1016/s0091-679x(08)60927-9. [DOI] [PubMed] [Google Scholar]
- Pavletich NP, Pabo CO. Crystal structure of a five-finger GLI-DNA complex: New perspectives on zinc fingers. Science. 1993;261:1701–1707. doi: 10.1126/science.8378770. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. Vectors for P-element transformation in Drosophila. In: Rodriguez RL, Denhardt DT, editors. Vectors, a survey of molecular cloning vectors and their uses. Boston, MA: Butterworths; 1988. pp. 437–456. [Google Scholar]
- Prakash K, Fang X-D, Engelberg D, Behal A, Parker CS. dOct2, a Drosophila Oct transcription factor that functions in yeast. Proc Natl Acad Sci. 1992;89:7080–7084. doi: 10.1073/pnas.89.15.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Technau GM. Early tagma-specific commitment of Drosophila CNS progenitor NB1-1. Development. 1994;120:2567–2578. doi: 10.1242/dev.120.9.2567. [DOI] [PubMed] [Google Scholar]
- Rivera-Pomar R, Jackle H. From gradients to stripes in Drosophila embryogenesis: filling in the gaps. Trends Genetics. 1996;12:478–483. doi: 10.1016/0168-9525(96)10044-5. [DOI] [PubMed] [Google Scholar]
- Roark M, Sturtevant MA, Emery J, Vaessin H, Grell E, Bier E. scratch, a pan-neural gene encoding a zinc finger protein related to snail, promotes neuronal development. Genes & Dev. 1995;9:2384–2398. doi: 10.1101/gad.9.19.2384. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Preston CR, Phillis RW, Johnson-Shiltz DM, Benz WK, Engels WR. A stable genomic source of P-element transposase in Drosophila melanogaster. Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AK, Rosenfeld MG. POU domain family values: Flexibility, partnerships, and developmental codes. Genes & Dev. 1997;11:1207–1225. doi: 10.1101/gad.11.10.1207. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci. 1977;74:54–63. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage RM, Shankland M. Identification and characterization of a hunchback orthologue, Lzf2, and its expression during Leech embryogenesis. Dev Biol. 1996;175:205–217. doi: 10.1006/dbio.1996.0108. [DOI] [PubMed] [Google Scholar]
- Shirakata M, Friedman FK, Wei Q, Paterson BM. Dimerization specificity of myogenic helix-loop-helix DNA-binding factors directed by nonconserved hydrophilic residues. Genes & Dev. 1993;7:2456–2470. doi: 10.1101/gad.7.12a.2456. [DOI] [PubMed] [Google Scholar]
- Spradling AC. P element-mediated transformation. In: Roberts D B, editor. Drosophila: A practical approach. Oxford, UK: IRL Press; 1986. pp. 175–197. [Google Scholar]
- Stanojevic D, Hoey T, Levine M. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Kruppel in Drosophila. Nature. 1989;341:331–335. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- Struhl G, Johnston P, Lawrence PA. Control of Drosophila body pattern by the hunchback morphogen gradient. Cell. 1992;69:237–249. doi: 10.1016/0092-8674(92)90405-2. [DOI] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Meth Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tautz D, Lehmann R, Schnurch H, Schuh R, Seifert E, Kienlin A, Jones K, Jackle H. Finger protein of novel structure encoded by hunchback, a second member of the gap class of Drosophila segmentation genes. Nature. 1987;327:383–389. [Google Scholar]
- Treacy MN, He X, Rosenfeld MG. I-POU: A POU domain protein that inhibits neuron-specific gene activation. Nature. 1991;350:577–584. doi: 10.1038/350577a0. [DOI] [PubMed] [Google Scholar]
- Treisman J, Desplan C. The products of the Drosophila gap genes hunchback and Kruppel bind to the hunchback promoters. Nature. 1989;341:335–337. doi: 10.1038/341335a0. [DOI] [PubMed] [Google Scholar]
- Vaessin H, Grell E, Wolff E, Bier E, Jan LY, Jan YN. Prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell. 1991;67:941–953. doi: 10.1016/0092-8674(91)90367-8. [DOI] [PubMed] [Google Scholar]
- Weigmann K, Lehner CF. Cell fate specification by even-skipped expression in the Drosophila nervous system is coupled to cell cycle progression. Development. 1995;121:3713–3721. doi: 10.1242/dev.121.11.3713. [DOI] [PubMed] [Google Scholar]
- White RAH, Lehmann R. A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell. 1986;47:311–321. doi: 10.1016/0092-8674(86)90453-8. [DOI] [PubMed] [Google Scholar]
- Wright WE, Binder M, Funk W. Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Mol Cell Biol. 1991;11:4104–4110. doi: 10.1128/mcb.11.8.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yeo S, Dick T, Chia W. The role of a Drosophila POU homeo domain gene in the specification of neural precursor cell identity in the developing embryonic central nervous system. Genes & Dev. 1993;7:504–516. doi: 10.1101/gad.7.3.504. [DOI] [PubMed] [Google Scholar]
- Yeo SL, Lloyd A, Kozak K, Dinh A, Dick T, Yang X, Sakonju S, Chia W. On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes & Dev. 1995;9:1223–1236. doi: 10.1101/gad.9.10.1223. [DOI] [PubMed] [Google Scholar]