Abstract
Chemical investigations of the Dongsha Atoll soft coral Lobophytum durum resulted in the isolation of five new cembranolides, durumolides M–Q (1–5). The structures of compounds 1–5 were characterized by the interpretation of extensive spectroscopic analysis. Compound 4 exhibited cytotoxicity against P-388 (mouse lymphocytic leukemia) cell line with an ED50 of 3.8 μg/mL. Moreover, compound 5 showed significant antiviral activity against human cytomegalovirus with an IC50 of 5.2 μg/mL.
Keywords: soft coral, Lobophytum durum, cytotoxicity, antiviral activity
1. Introduction
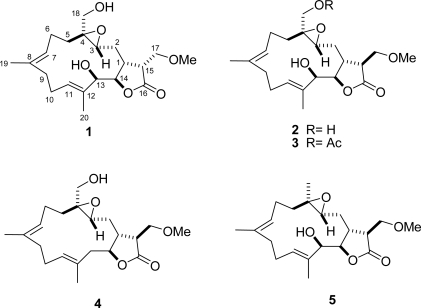
Various natural products, distributed mainly in marine soft corals of the genus Lobophytum (Alcyoniidae) [1–21], have attracted much attention from chemists specializing in natural products due to their structural complexity and remarkable pharmacological activities such as cytotoxicity [2–9], antibacterial activities [10], anti-inflammatory properties [10–12], and HIV-inhibitory activity [13]. Our previous investigations of the soft coral L. durum (Figure 1) from the Dongsha Atoll have resulted in the purification of 15 cembranolides, durumolides A–L and durumhemiketalolides A–C, several of which showed antibacterial and anti-inflammatory activities [10–12]. Our continuing chemical examinations of the bioactive substances of this organism led to the isolation of five new cembranolides, designated as durumolides M–Q (1–5) (Figure 2). The structures of compounds 1–5 were determined on the basis of detailed 1D and 2D NMR experiments, mainly employing COSY, DEPT, HMBC, HSQC, and NOESY spectra. Moreover, durumolides M–Q were evaluated in vitro for the cytotoxicity against A-459 (human lung adenocarcinoma), HT-29 (human colon adenocarcinoma), and P-388 (mouse lymphocytic leukemia) cancer cell lines, and antiviral activity against human cytomegalovirus.
Figure 1.
Soft coral Lobophytum durum
Figure 2.
Structures of compounds 1–5.
2. Results and Discussion
Specimens of L. durum were frozen immediately after collection. Conventional extraction procedures were used, and the acetone extract was exhaustively partitioned between EtOAc and H2O to afford the EtOAc-soluble fraction, which was evaporated under vacuum to yield a dark brown gum (30 g). The concentrated residue was subjected to column chromatography and high-performance liquid chromatography (HPLC), leading to the purification of 1–5.
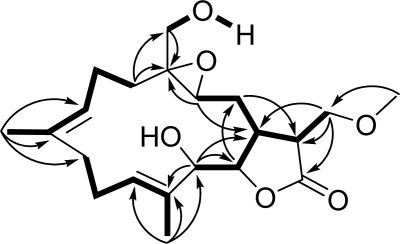
Durumolide M (1), appeared as a colorless oil, exhibited a high resolution electrospray ionization mass spectrometry (HR-ESI-MS) pseudomolecular ion peak at m/z 403.2099 [M + Na]+, corresponding to a molecular formula of C21H32O6 and six degrees of unsaturation. Comparison of the NMR data (Tables 1 and 2) of 1 with those of sinularolide B [22] revealed that 1 was determined to be a 17-methoxylated analogue of sinularolide B, coinciding with methoxyl protons at δH 3.30 (3H, s) correlated to the oxymethine carbon at δC 70.0 (C-17), and oxymethylene protons at δH 3.81 (1H, dd, J = 9.2, 2.8 Hz, H-17a) and 3.73 (1H, dd, J = 9.2, 2.8 Hz, H-17b) correlated to the methine carbons at δC 40.8 (C-1), and 44.5 (C-15), as well as the lactone carbonyl carbon at δC 176.9 (C-16) in the HMBC spectrum (Figure 3). Thus, the planar structure of durumolide M, possessing an α-methoxymethyl-γ-lactone ring fused to a 14-membered ring at C-1 and C-14, was, with certainty, assigned as 1.
Table 1.
1H NMR data for compounds 1–3.
| 1a | 2b | 3b | |
|---|---|---|---|
| 1 | 2.28 m | 2.41 m | 2.40 m |
| 2 | 1.75 dt (14.8, 2.4) c | 1.97 ddd (14..5, 2.0, 2.0) | 2.00 br d (14.5) |
| 1.67 ddd (14.8, 7.6, 2.4) | 1.37 ddd (14.5, 8.0, 2.5) | 1.21 ddd (14.5, 8.5, 1.5) | |
| 3 | 2.67 dd (7.6, 2.4) | 2.73 dd (8.0, 2.5) | 2.69 dd (8.5, 1.5) |
| 5 | 2.43 m | 2.39 m | 2.38 m |
| 1.11 ddd (14.8, 13.2, 3.6) | 1.11 ddd (14.5, 13.0, 3.5) | 1.08 ddd (15.0, 13.5, 3.5) | |
| 6 | 2.31 m | 2.32 m | 2.29 m |
| 2.10 m | 2.14 m | 2.15 m | |
| 7 | 5.04 dd (7.2, 4.4) | 5.08 dd (7.5, 4.5) | 5.07 dd (7.0, 4.0) |
| 9 | 2.39 m | 2.35 m | 2.37 m |
| 2.13 m | 2.16 m | 2.20 m | |
| 10 | 2.55 m | 2.51 m | 2.53 m |
| 2.16 m | 2.19 m | 2.17 m | |
| 11 | 5.45 dd (6.8, 4.0) | 5.49 dd (6.8, 3.5) | 5.50 dd (6.5, 3.0) |
| 13 | 3.97 d (8.8) | 4.11 d (8.5) | 4.08 d (8.5) |
| 14 | 4.20 dd (9.2, 8.8) | 4.06 t (8.5) | 4.04 dd (9.0, 8.5) |
| 15 | 2.86 dt (9.2, 2.8) | 2.50 dt (10.5, 2.5) | 2.49 dt (9.5, 2.5) |
| 17 | 3.81 dd (9.2, 2.8) | 3.81 dd (10.5, 2.5) | 3.81 dd (9.5, 2.5) |
| 3.73 dd (9.2, 2.8) | 3.79 dd (10.5, 2.5) | 3.79 dd (9.5, 2.5) | |
| 18 | 3.87 dd (12.0, 6.0) | 3.84 d (12.0) | 4.44 d (12.5) |
| 3.57 dd (12.0, 4.8) | 3.54 d (12.0) | 3.77 m | |
| 19 | 1.62 s | 1.62 s | 1.64 s |
| 20 | 1.71 s | 1.70 s | 1.70 s |
| 17-OMe | 3.30 s | 3.39 s | 3.38 s |
| 18-OAc | 2.13 s |
Spectra were measured in CDCl3 (400 MHz);
Spectra were measured in CDCl3 (500 MHz);
J values (in Hz) are in parentheses.
Table 2.
13C NMR data for compounds 1–5.
| 1a | 2b | 3b | 4a | 5a | |
|---|---|---|---|---|---|
| 1 | 40.8 | 38.6 | 38.7 | 40.8 | 38.7 |
| 2 | 25.0 | 31.4 | 31.4 | 31.0 | 32.2 |
| 3 | 64.3 | 63.9 | 63.1 | 63.7 | 63.8 |
| 4 | 62.1 | 62.2 | 59.9 | 62.5 | 59.5 |
| 5 | 33.3 | 33.5 | 32.9 | 33.3 | 38.3 |
| 6 | 24.1 | 24.2 | 24.3 | 24.7 | 24.9 |
| 7 | 124.5 | 124.5 | 124.3 | 124.4 | 124.6 |
| 8 | 135.0 | 135.1 | 135.2 | 135.3 | 134.6 |
| 9 | 39.0 | 38.9 | 38.9 | 39.3 | 38.9 |
| 10 | 24.7 | 24.7 | 24.8 | 23.8 | 24.8 |
| 11 | 132.0 | 131.9 | 132.2 | 130.2 | 132.3 |
| 12 | 132.4 | 132.0 | 132.0 | 129.9 | 131.9 |
| 13 | 82.0 | 80.9 | 81.2 | 43.7 | 81.4 |
| 14 | 84.1 | 82.2 | 81.9 | 80.1 | 82.2 |
| 15 | 44.5 | 49.4 | 49.3 | 49.8 | 49.5 |
| 16 | 176.9 | 175.4 | 175.3 | 176.2 | 175.6 |
| 17 | 70.0 | 68.1 | 67.8 | 68.5 | 67.9 |
| 18 | 61.8 | 61.5 | 63.7 | 61.5 | 16.3 |
| 19 | 15.3 | 15.2 | 15.3 | 15.0 | 15.2 |
| 20 | 12.2 | 12.3 | 12.1 | 17.1 | 12.0 |
| 17-OMe | 59.1 | 59.3 | 59.3 | 59.2 | 59.3 |
| 18-OAc | 20.8 | ||||
| 170.9 |
Spectra were measured in CDCl3 (100 MHz);
Spectra were measured in CDCl3 (125 MHz).
Figure 3.
Selected 1H–1H COSY (▬) and HMBC (→) correlations of 1.
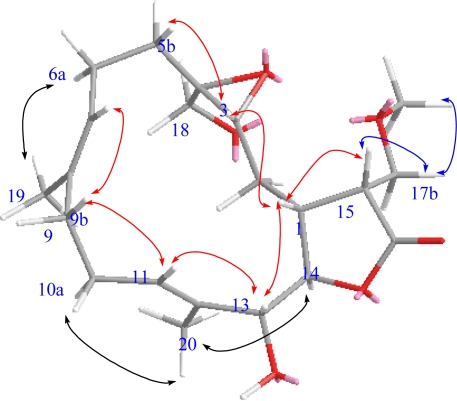
The geometries of the two trisubstituted olefins were proposed as both E based on the γ-effect of the olefinic methyl signals for C-19 and C-20 (less than 20 ppm) and the NOESY correlations between H-7/H-9b and H-11/H-13 (Figure 4). Compound 1 possessed the same configurations as sinularolide B at the C-1, C-3, C-4, C-13, and C-14 stereocenters due to the similar NOESY correlations between H-1/H-3, H-1/H-13, H-11/H-13, H-11/H-9b (δ 2.13), H-3/H-5b (δ 1.11), H-14/H-2a (δ 1.75), H-7/H-9b, and H-14/H3-20. Moreover, the large coupling constant (J = 9.2 Hz) between H-1 and H-15 suggested that the vicinal protons were either in an anticoplanar or eclipse relationship. The latter relationship should be correct because the signal at δH 2.86 (1H, dt, J = 9.2, 2.8 Hz, H-15) showed a strong NOESY correlation with δH 2.28 (1H, m, H-1), implying the R configuration at C-15. On the basis of the above-mentioned observations, the structure of durumolide M (1) was characterized as (1R*,3R*,4S*, 13R*,14R*,15R*,7E,11E)-13,18-dihydroxy-17-methoxy-3,4-epoxycembra-7,11-dien-16,14-olide.
Figure 4.
Selected NOESY correlations of 1.
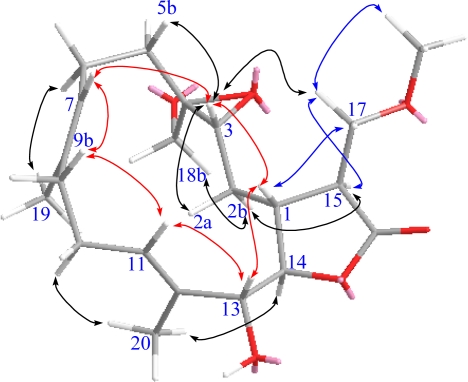
Compounds 1 and 2 had the same molecular formula (C21H32O6). Analysis of their 1H- and 13C-NMR data (Tables 1 and 2) revealed that they shared the same cembranolide skeleton, differing only in the configuration of C-15 in 2. The configuration of C-15 was proposed to be S, as identified by its NOESY correlations between H-15/H-2b, H-14/H-2b, and H2-17/H-1 (Figure 5). Therefore, compound 2 was determined to be 15-epimer of 1. According to the above findings, the structure of durumolide N (2) was unambiguously established and elucidated as (1R*,3R*,4S*,13R*,14R*, 15S*,7E,11E)-13,18-dihydroxy-17-methoxy-3,4-epoxycembra-7,11-dien-16,14-olide.
Figure 5.
Selected NOESY correlations of 2.
Durumolide O (3) was analyzed for a molecular formula of C23H34O7 by its HR-ESI-MS coupled with the DEPT and 13C NMR spectroscopic data (Table 2). Analysis of the 1H–1H COSY and HMBC correlations were diagnostic in determining that the planar framework of durumolide O, having a 14-membered ring fused to an α-methoxymethyl-γ-lactone ring at C-1 and C-14, was proposed as 3. The structure of 3 was identical to that of 2 except that the hydroxy group attached to C-18 was replaced by an acetoxy group. This assumption was confirmed through the key HMBC correlations from H2-18 to C-3, C-4, C-5, and the carbonyl carbon of 18-OAc. All the NMR data (Tables 1 and 2) of 3, assigned by COSY, HMBC, HSQC, and NOESY correlations, were satisfactorily consistent with the structure shown as (1R*,3R*,4S*,13R*,14R*,15S*,7E,11E)-13-hydroxy-18-acetoxy-17-methoxy-3,4-epoxycembra-7,11-dien-16,14-olide.
The positive HR-ESI-MS of durumolide P (4) exhibited a pseudomolecular ion peak at m/z 387.2148 [M + Na]+, consistent with a molecular formula of C21H32O5, which is 16 mass units smaller than that of 1. Comparison of the NMR data (Tables 2 and 3) of both compounds showed that 4 exhibited the same framework of an α-methoxymethyl-γ-lactone-containing cembranolide as 2, with the exception of signals assigned to C-13, where the oxymethine in 2 was replaced by a methylene [δH 2.54 (1H, m) and 2.45 (1H, m); δC 43.7 (CH2)] in 4. The observed COSY correlation between H-13 and H-14 and the key HMBC correlations from H3-20 to C-13 confirmed the location of the methylene group. The relative stereochemistry of 4 was in agreement with that of 2 due to the similar NOESY correlations. Judging from the undisputable evidence, the structure of 4 was unambiguously determined and deduced as (1R*,3R*,4S*,14S*,15S*,7E,11E)-18-hydroxy-17-methoxy-3,4-epoxycembra-7,11-dien-16,14-olide.
Table 3.
1H NMR data a for compounds 4 and 5.
| 4 | 5 | |
|---|---|---|
| 1 | 2.51 m | 2.41 m |
| 2 | 1.95 ddd (14.4, 4.0, 2.0) b | 1.98 d (14.4) |
| 1.45 ddd (14.4, 7.2, 2.4) | 1.09 m | |
| 3 | 2.90 dd (7.2, 4.0) | 2.52 m |
| 5 | 2.37 m | 2.08 m |
| 1.21 ddd (14.8, 12.8, 4.0) | 1.14 ddd (11.6, 10.4, 2.4) | |
| 6 | 2.33 m | 2.29 m |
| 2.15 m | 2.11 m | |
| 7 | 5.09 dd (6.4, 4.4) | 5.05 br d (9.6) |
| 9 | 2.29 m | 2.35 m |
| 2.13 m | 2.17 m | |
| 10 | 2.26 m | 2.54 m |
| 2.13 m | 2.17 m | |
| 11 | 5.17 t (7.6) | 5.50 dd (7.6, 2.8) |
| 13 | 2.54 m | 4.08 d (8.8) |
| 2.45 m | ||
| 14 | 4.13 ddd (10.0, 8.4, 3.2) | 4.03 dd (8.8, 8.4) |
| 15 | 2.48 m | 2.49 t (2.8) |
| 17 | 3.81 dd (9.6, 3.2) | 3.83 dd (9.6, 2.8) |
| 3.77 dd (9.6, 3.2) | 3.79 dd (9.6, 2.8) | |
| 18 | 3.85 dd (12.0, 5.2) | 1.21 s |
| 3.55 dd (12.0 5.2) | ||
| 19 | 1.60 s | 1.63 s |
| 20 | 1.68 s | 1.72 s |
| 17-OMe | 3.39 s | 3.39 s |
Spectra were measured in CDCl3 (400 MHz);
J values (in Hz) are in parentheses.
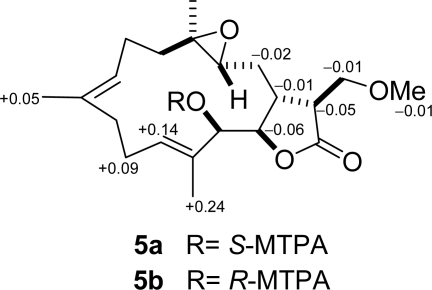
The molecular formula of C21H32O5 was assigned to 5 from its HR-ESI-MS and 13C NMR data (Table 2), indicating six degrees of unsaturation. The NMR data (Tables 2 and 3) of 5 were highly compatible with those obtained for durumolide J [11], with the replacement of the α-methylene-γ-lactone with an α-methoxymethyl-γ-lactone being the most noticeable difference. The methoxymethyl moiety attached to C-15 was inferred from the 1H–1H COSY and HMBC correlations. Moreover, the 15S configuration was confirmed by the key NOESY correlation between H-17/H-1 and H-1/H-3. The appropriate stereochemistry of 5 was determined by spectroscopic method according to Mosher’s acylation for absolute configuration determination of chiral alcohols [10]. Analysis of the ΔδS−R values (Figure 6) according to the Mosher model pointed to an R configuration for C-13 of 5, because H2-10, H-11, Me-19, and Me-20 of (S)-MTPA ester 5a were less shielded by the phenyl ring of MTPA products. Therefore, the absolute stereochemistry of 5 was unambiguously established as (1R,3R,4R,13R,14R,15S,7E,11E)-13-hydroxy-17-methoxy-3,4-epoxycembra-7,11-dien-16,14-olide.
Figure 6.
1H-NMR chemical shift differences (Δδ = δS − δR) of the MTPA esters in pyridine-d5.
The determination of the configurations of γ-lactone ring fused to 14-membered ring at C-1 and C-14 based on the coupling constant between the lactonic methine protons (3J1,14) is dubious [8]. Notably, all of the cis-fused lactones at C-1 and C-14 of cembranolides from the Caribbean gorgonians Eunicea succinea and Eunicea mammosa for which an X-ray analysis has been performed [23] and all trans-fused lactones of durumolides M–Q (1–5) were confirmed after determination of the ring junction of durumolides A–L and durumhemiketalolides A–C using X-ray data and biogenetic considerations [10–12].
The possibility that 1 and 2 were furnished via Micheal addition [24,25] of sinularolide B [22] in MeOH resulting in addition of a methoxy group to α-methylene-γ-lactone should not be excluded. In order to ascertain 1 and 2, having an α-methoxymethyl-γ-lactone ring, to be natural or artificial products from sinularolide B through the isolation process, the following experiments were conducted. A solution of sinularolide B was kept at room temperature for 3 days in the presence of Si-60 or RP-18 gel in MeOH. The formation of 1 and 2 were not observed. Apparently, compounds 1 and 2 are natural products. Similarly, compounds 3–5, possessing the same functionality, may also be natural products.
Compounds 1–5 in the present study were evaluated in vitro for cytotoxicity against P-388, A-459 and HT-29 cancer cell lines using the MTT assay, and antiviral activity against human cytomegalovirus. Preliminary cytotoxic screening revealed that compound 4 exhibited cytotoxicity against P-388 (mouse lymphocytic leukemia) cell line with an ED50 of 3.8 μg/mL. Moreover, compound 5 showed significant antiviral activity against human cytomegalovirus with an IC50 of 5.2 μg/mL.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were determined with a JASCO P1020 digital polarimeter. Ultraviolet (UV) and infrared (IR) spectra were obtained on a JASCO V-650 and JASCO FT/IR-4100 spectrophotometers, respectively. The NMR spectra were recorded on a Varian MR 400 NMR spectrometer at 400 MHz for 1H and 100 MHz for 13C or on a Varian Unity INOVA 500 FT-NMR spectrometer at 500 MHz for 1H and 125 MHz for 13C, respectively. Chemical shifts are expressed in δ (ppm) referring to the solvent peaks δH 7.27 and δC 77.0 for CDCl3, respectively, and coupling constants are expressed in Hz. ESI-MS were recorded by ESI FT-MS on a Bruker APEX II mass spectrometer. Silica gel 60 (Merck, Germany, 230–400 mesh) and LiChroprep RP-18 (Merck, 40–63 μm) were used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) and precoated RP-18 F254s plates (Merck) were used for thin-layer chromatography (TLC) analysis. High-performance liquid chromatography (HPLC) was carried out using a Hitachi L-7100 pump equipped with a Hitachi L-7400 UV detector at 220 nm together with a semi-preparative reversed-phase column (Merck, Hibar LiChrospher RP-18e, 5 μm, 250 × 25 mm).
3.2. Animal Material
The soft coral L. durum was collected by hand using SCUBA along the coast reefs offshore from the Dongsha Atoll in June 2007, at a depth of 8 m, and was stored in a freezer for two months until extraction. Identification was kindly verified by Prof. Chang-Feng Dai, Institute of Oceanography, National Taiwan University, Taiwan. A voucher specimen (TS-13) was deposited in the Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Taiwan.
3.3. Extraction and Isolation
The frozen soft coral (1 kg) was chopped into small pieces and extracted exhaustively by maceration with fresh acetone for 24 h at room temperature. The quantity of solvent used for each extraction (2 L) was at least three times the amount of the soft coral material used. The acetone extracts were filtered and concentrated under vacuum to yield a brownish oily residue, which was subsequently partitioned between EtOAc and H2O. The resulting EtOAc-soluble residue (30 g) was subjected to column chromatography on silica gel using n-hexane with increasing amounts of EtOAc, and finally 100% MeOH as elution, to obtain roughly 30 fractions on the basis of 1H NMR data and TLC analyses. Fraction 18 (2.16 g) eluted with n-hexane–EtOAc (1:8) was subjected to a silica gel column using n-hexane–EtOAc mixtures of increasing polarity for elution, to furnish 14 subfractions. A subfraction 18-8 (507 mg) eluted with n-hexane–EtOAc (1:2) was fractionated to column chromatography on an ODS column using aq MeOH to afford six subfractions. Subsequently, a subfraction 18-8-1 (47 mg) eluted with MeOH–H2O (45:55) was further purified by HPLC (RP-18) using 70% aq MeOH to give 1 (2 mg) and 2 (1 mg). Similarly, 3 (1 mg), and 5 (3 mg) were obtained by separation of a subfraction 18-8-2 (267 mg) eluted with MeOH–H2O (55:45) on HPLC (RP-18) using 70% MeOH in H2O. Similarly, a subfraction 18-8-4 (46 mg) eluted with MeOH–H2O (65:35) was further applied to HPLC (RP-18) using 70% MeOH in H2O to provide 4 (2 mg).
Durumolide M (1): colorless oil; [α]25 D −45 (c 0.1, CHCl3); IR (KBr) νmax 3394, 2924, 1766, 1449, 1027, 765 cm−1; 1H-NMR (CDCl3, 400 MHz) and 13C-NMR (CDCl3, 100 MHz) data, see Tables 1 and 2; ESI-MS m/z 403 [M + Na]+ HR-ESI-MS m/z 403.2099 (calcd for C21H32O6Na, 403.2096).
Durumolide N (2): colorless oil; [α]25 D −98 (c 0.1, CHCl3); IR (KBr) νmax 3436, 2926, 1766, 1386, 1178, 1033 cm−1; 1H-NMR (CDCl3, 500 MHz) and 13C-NMR (CDCl3, 125 MHz) data, see Tables 1 and 2; ESI-MS m/z 403 [M + Na]+; HR-ESI-MS m/z 403.2094 (calcd for C21H32O6Na, 403.2096).
Durumolide O (3): colorless oil; [α]25 D −94 (c 0.1, CHCl3); IR (KBr) νmax 3425, 2924, 1773, 1460, 1181, 1035 cm−1; 1H-NMR (CDCl3, 500 MHz) and 13C-NMR (CDCl3, 125 MHz) data, see Tables 1 and 2; ESI-MS m/z 445 [M + Na]+; HR-ESI-MS m/z 445.2199 (calcd for C23H34O7Na, 445.2202).
Durumolide P (4): colorless oil; [α]25 D −121 (c 0.1, CHCl3); IR (KBr) νmax 3460, 2926, 1769, 1445, 1181, 1031 cm−1; 1H-NMR (CDCl3, 400 MHz) and 13C-NMR (CDCl3, 100 MHz) data, see Tables 1 and 2; ESI-MS m/z 387 [M + Na]+; HR-ESI-MS m/z 387.2148 (calcd for C21H32O5Na, 387.2147).
Durumolide Q (5): colorless oil; [α]25 D −99 (c 0.2, CHCl3); IR (KBr) νmax 3446, 2924, 1773, 1383, 1177, 1031 cm−1; 1H-NMR (CDCl3, 400 MHz) and 13C-NMR (CDCl3, 100 MHz) data, see Tables 2 and 3; ESI-MS m/z 387 [M + Na]+; HR-ESI-MS m/z 387.2146 (calcd for C21H32O5Na, 387.2147).
Preparation of Mosher’s Esters of 5: In separate NMR tubes, duplicate (1.0 mg) samples of 5 were dissolved in 0.6 mL of pyridine-d5 and allowed to react for 3 hr at room temperature with (R)- and (S)-MTPA chloride (one drop) to yield (S)-MTPA ester 5a and (R)-MTPA ester 5b, respectively. Selected 1H-NMR (pyridine-d5, 300 MHz) of 5a: δH 7.41–7.61 (5H, m, Ph), 5.93 (1H, br d, J = 8.9 Hz, H-13), 5.74 (1H, m, H-11), 5.00 (1H, br d, J = 9.1 Hz, H-7), 4.44 (1H, t, J = 8.9 Hz, H-14), 4.01 (2H, m, H2-17), 3.26 (3H, s, OMe), 2.88 (1H, m, H-15), 2.82 (1H, br s, H-1), 2.47 (2H, m, H2-10), 2.20 (2H, m, H2-6), 1.95 (2H, m, H2-2), 1.78 (3H, s, Me-20), 1.54 (3H, s, Me-19), 1.21 (3H, s, Me-18); Selected 1H-NMR (pyridine-d5, 300 MHz) of 5b: 7.42–7.63 (5H, m, Ph), 5.83 (1H, br d, J = 8.4 Hz, H-13), 5.60 (1H, m, H-11), 4.94 (1H, br d, J = 8.9 Hz, H-7), 4.50 (1H, t, J = 8.9 Hz, H-14), 4.02 (2H, m, H2-17), 3.27 (3H, s, OMe), 2.93 (1H, m, H-15), 2.83 (1H, m, H-1), 2.38 (2H, m, H2-10), 2.19 (2H, m, H2-6), 1.97 (2H, m, H2-2), 1.54 (3H, s, Me-20), 1.49 (3H, s, Me-19), 1.16 (3H, s, Me-18).
3.4. Cytotoxicity Assay
Cytotoxicity was determined against P-388 (mouse lymphocytic leukemia), HT-29 (human colon adenocarcinoma), and A-549 (human lung epithelial carcinoma) tumor cells using a modification of the MTT colorimetric method. The provision of the P-388 cell line was supported by J. M. Pezzuto, formerly of the Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago. HT-29 and A-549 cell lines were purchased from the American Type Culture Collection. The experimental details of this assay were carried out according to a previously described procedure [11,26,27].
3.5. Anti-HCMV Assay
To determine the effects of natural products upon the HCMV cytopathic effect (CPE), confluent human embryonic lung (HEL) cells grown in 24-well plates will be incubated for 1 h in the presence or absence of various concentrations of tested natural product. Then, cells will be infected with HCMV at an input of 1000 plaque forming units (pfu) per well of a 24-well dish. Antiviral activity is expressed as IC50 (50% inhibitory concentration), or the compound concentration required to reduce the virus induced CPE by 50% after 7 days as compared with the untreated control. To monitor the cell growth upon treating with natural products, an MTT-colorimetric assay was employed [28].
4. Conclusions
Comparison of cytotoxicity of durumolides M–Q (1–5) with those of cembranolides previously isolated by our group from Lobophtum michaelae [29], showed that methoxylation at C-17 reduced cytotoxicity against the same cancer cell lines.
Acknowledgments
This research was financially supported by grants from the National Science Council (NSC99-2628-B-110-002-MY3) and Ministry of Education of Taiwan awarded to C.-Y.D.
Footnotes
Samples Availability: Not Available.
References
- 1.Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2009;26:170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- 2.Wang S-K, Duh C-Y, Wu Y-C, Wang Y, Cheng M-C, Soong K, Fang L-S. Cytotoxic cembranolides from the soft coral Lobophytum michaelae. J. Nat. Prod. 1992;55:1430–1435. doi: 10.1021/np50088a007. [DOI] [PubMed] [Google Scholar]
- 3.Coval SJ, Patton RW, Petrin JM, James L, Rothofsky ML, Lin SL, Patel M, Reed JK, McPhil AT, Bishop WR. A cembranolide diterpene farnesyl protein transferase inhibitor from the marine soft coral Lobophytum cristagalli. Bioorg. Med. Chem. Lett. 1996;6:909–912. [Google Scholar]
- 4.Higuchi R, Miyamoto T, Yamada K, Komori T. Cytotoxic and ichthyotoxic compounds from marine opisthobranchia and soft coral. Toxicon. 1998;36:1703–1705. doi: 10.1016/s0041-0101(98)00163-9. [DOI] [PubMed] [Google Scholar]
- 5.Matthee GF, König GM, Wright AD. Three new diterpenes from the marine soft coral Lobophytum crassum. J. Nat. Prod. 1998;61:237–240. doi: 10.1021/np970458n. [DOI] [PubMed] [Google Scholar]
- 6.Duh C-Y, Wang S-K, Huang B-T, Dai C-F. Cytotoxic cembrenolide diterpenes from the Formosan soft coral Lobophytum crassum. J. Nat. Prod. 2000;63:884–885. doi: 10.1021/np990620h. [DOI] [PubMed] [Google Scholar]
- 7.Chao C-H, Hang H-C, Wu Y-C, Lu C-K, Dai C-F, Sheu J-H. Glycolipids from the Formosan soft coral Lobophytum crassum. Chem. Pharm. Bull. 2007;55:1720–1723. doi: 10.1248/cpb.55.1720. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Krohn K, Ding J, Miao Z-H, Zhou X-H, Chen S-H, Pescitelli G, Salvadori P, Kurtan T, Guo Y-W. Structural and stereochemical studies of α-methylene-γ-lactone-bearing cembrane diterpenoids from a south China sea soft coral Lobophytum crassum. J. Nat. Prod. 2008;71:961–966. doi: 10.1021/np800081p. [DOI] [PubMed] [Google Scholar]
- 9.Lin S-T, Wang S-K, Cheng S-Y, Duh C-Y. Lobocrasol, a new diterpenoid from the soft coral Lobophytum crassum. Org. Lett. 2009;14:3012–3014. doi: 10.1021/ol901070e. [DOI] [PubMed] [Google Scholar]
- 10.Cheng S-Y, Wen Z-H, Chiou S-F, Wang S-K, Hsu C-H, Dai C-F, Chiang MY, Duh C-Y. Durumolides A–E, Anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron. 2008;64:9698–9704. [Google Scholar]
- 11.Cheng S-Y, Wen Z-H, Wang S-K, Chiou S-F, Hsu C-H, Dai C-F, Duh C-Y. Anti-inflammatory cembranolides from the soft coral Lobophytum durum. Bioorg. Med. Chem. 2009;17:3763–3769. doi: 10.1016/j.bmc.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S-Y, Wen Z-H, Chiou S-F, Wang S-K, Hsu C-H, Dai C-F, Chiang MY, Duh C-Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009;72:152–155. doi: 10.1021/np800686k. [DOI] [PubMed] [Google Scholar]
- 13.Rashid MA, Gustafson KR, Boyd MR. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J. Nat. Prod. 2000;63:531–533. doi: 10.1021/np990372p. [DOI] [PubMed] [Google Scholar]
- 14.Kashman Y, Groweiss A. Lobolide: A new epoxy cembranolide from marine origin. Tetrahedron Lett. 1977;13:1159–1160. [Google Scholar]
- 15.Kashman Y, Carmely S, Groweiss A. Further cembranoid derivatives from the Red Sea soft corals Alcyonium flaccidum and Lobophytum crassum. J. Org. Chem. 1981;46:3592–3596. [Google Scholar]
- 16.Kinamoni Z, Groweiss A, Carmely S, Kashman Y. Several new cembranoid diterpenes from three soft corals of the Red Sea. Tetrahedron. 1983;39:1643–1648. [Google Scholar]
- 17.Bowden BF, Coll JC, Tapiolas DM. Studies of Australian soft corals. ХХХШ. New cembranoid diterpenes from a Lobophytum species. Aust. J. Chem. 1983;36:2289–2295. [Google Scholar]
- 18.Bowden BF, Coll JC, Decosta MSL, Desilva ED, Mackay MF, Mahendran M, Willis RH. The structure determination of a new cembranolide diterpene from the soft coral Lobophytum cristigalli (Coelenterata, Octocorallia, Alcyonacea) Aust. J. Chem. 1984;34:545–552. [Google Scholar]
- 19.Bowden BF, Coll JC, Heaton A, Konig G, Bruck MA, Cramer RE, Klein DM, Scheuer PJ. The structures of four isomeric dihydrofuran-containing cembranoid diterpenes from several species of soft coral. J. Nat. Prod. 1987;50:650–659. [Google Scholar]
- 20.Subrahmanyam C, Rao CV, Anjaneyulu V, Satyanarayana P, Rao PVS. New diterpenes from a new species of Lobophytum soft coral of the South Andaman coast. Tetrahedron. 1992;48:3111–3120. [Google Scholar]
- 21.Yamada K, Ryu K, Miyamoto T, Higuchi R. Bioactive terpenoids from octocorallia. 4. Three new cembrane-type diterpenoids from the soft coral Lobophytum schoedei. J. Nat. Prod. 1997;60:798–801. [Google Scholar]
- 22.Li G, Zhang Y, Deng Z, van Ofwegen L, Proksch P, Lin W. Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J. Nat. Prod. 2005;68:649–652. doi: 10.1021/np040197z. [DOI] [PubMed] [Google Scholar]
- 23.Morales JJ, Espina JR, Rodríguez AD. The structure of euniolide, a new cembranoid from the Caribbean gorgonians Eunicea succinea and Eunicea mammosa. Tetrahedron. 1990;46:5889–5894. [Google Scholar]
- 24.Uchio Y, Eguchi S, Kuramoto J, Nakayama M, Hase T. Lobochedleolide and (7Z)-lobochedleolide, new cembranolides from the soft coral Lobophytum hedleyl Whitelegge. Tetrahedron Lett. 1981;22:4089–4092. [Google Scholar]
- 25.Rodríguez AD, Piña IC, Acosta AL, Ramıírez C, Soto JJ. Synthesis of analogues of Eunicea γ-cembranolides containing cyclic ethers via saponification. J. Org. Chem. 2001;66:648–658. doi: 10.1021/jo001025j. [DOI] [PubMed] [Google Scholar]
- 26.Geran RI, Greenberg NH, MacDonald MM, Schumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972;3:51–61. [Google Scholar]
- 27.Hou R-S, Duh C-Y, Chiang MY, Lin C-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995;58:1126–1130. doi: 10.1021/np50121a026. [DOI] [PubMed] [Google Scholar]
- 28.Stevens M, Balzarini J, Tabarrini O, Andrei G, Snoeck R, Cecchetti V, Fravolini A, De Clercq E, Pannecouque C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005;56:847–855. doi: 10.1093/jac/dki328. [DOI] [PubMed] [Google Scholar]
- 29.Wang L-T, Wang S-K, Soong K, Duh C-Y. New cytotoxic cembranolides from soft coral Lobophytum michaelae. Chem. Pharm. Bull. 2007;55:766–770. doi: 10.1248/cpb.55.766. [DOI] [PubMed] [Google Scholar]