Abstract
Fear can be extinguished by repeated exposure to a cue that signals threat. However, extinction does not erase fear, as an extinguished cue presented in a context distinct from that of extinction results in renewed fear of that cue. The hippocampus, which is involved in the formation of contextual representations, is a natural candidate structure for investigations into the neural circuitry underlying fear renewal. Thus far, studies examining the necessity of the hippocampus for fear renewal have produced mixed results. We isolated the conditions under which the hippocampus may be required for renewal. Rats received lesions of the dorsal hippocampus either prior to tone fear conditioning or following extinction. Fear renewal was measured using discrete tone presentations or a long, continuous tone. The topography of fear responding at test was assessed by comparing “early” and “sustained” renewal, where early fear was determined by freezing to the first discrete tone or equivalent initial segment of a continuous tone and sustained fear was determined by freezing averaged across all discrete tones or the entire continuous tone. We found that following pre-training damage of the hippocampus, early renewal remained intact regardless of lesion condition. However, sustained renewal only persisted in discrete, but not continuous, tone-tested animals. A more extensive analysis of the topography of fear responding revealed that the disruption of renewal was generated when the tone duration at test began to violate that used during extinction, suggesting that the hippocampus is sensitive to mismatches in CS-duration. Post-extinction lesions resulted in an overall reduction of fear renewal. This pattern of results is consistent with those observed for contextual fear conditioning, wherein animals may display a resistance to anterograde amnesia despite the presence of a strong retrograde amnesia for the same contextual information. Furthermore, the data support a role for the hippocampus in sustaining renewal when the CS duration at test does not match that used during extinction.
Keywords: fear conditioning, extinction, hippocampus, renewal, timing
Introduction
The ability to switch between incompatible behaviors is critical to survival. Mammals must learn to respond to hostile situations with species-specific defensive responses (Bolles, 1970; Bolles and Fanselow, 1980), but must also learn when these responses should be inhibited. This ability is important from a therapeutic standpoint, as fear inhibition is the basis of cognitive-behavioral therapy for anxiety disorders (Craske et al., 2008). Pavlovian fear conditioning provides an excellent model for investigating the competition between the processes underlying fear expression and inhibition.
In fear conditioning, a neutral stimulus, such as a tone (conditional stimulus; CS), becomes associated with an aversive stimulus, such as a shock (unconditional stimulus; US), so that the tone alone elicits fear responses (conditional response; CR). Thereafter, fear can be extinguished if the CS is presented in the absence of the US; however, this does not erase the original learning but instead, produces a new association that inhibits fear expression (Bouton, 2002; Bouton, 2004; Pavlov, 1927). This inhibition is under contextual control, as evidenced by the renewal of fear when an extinguished CS is presented outside the extinction context (Bouton, 1993; Bouton and Bolles, 1979).
Because renewal tracks mismatches between extinction and test contexts, the hippocampus, which is important for the formation of contextual representations (Fanselow, 2000), is a natural candidate structure for mediating renewal. However, studies investigating this possibility have failed to reach a consensus. Findings implicating the hippocampal formation in the contextualization of fear extinction and its subsequent renewal (e.g. Corcoran et al., 2005; Corcoran and Maren, 2001; Corcoran and Maren, 2004; Ji and Maren, 2005) are discrepant with those in which fear renewal persists despite hippocampal compromise (Frohardt et al., 2000; Wilson et al., 1995).
While these studies share a number of methodological differences, important dissimilarities between CS duration parameters and the time period analyzed at test provide potential sources for the origin of this discrepancy. Notably, Maren and colleagues consistently employ the use of a continuous, eight minute tone-CS at test, and selectively analyze anything from the first minute (Corcoran and Maren, 2001; Corcoran and Maren, 2004), the first four minutes (Hobin et al., 2006; Ji and Maren, 2005), or an average of the entire eight minute tone (Ji and Maren, 2008b). Importantly, in all these studies, this single, continuous CS was dramatically longer in duration that the CS used for both acquisition and extinction (10 sec). Moreover, this uninterrupted tone omits any inter-trial intervals (ITIs), further disconnecting test conditions from acquisition and extinction. In contrast, studies by Bouton and colleagues maintain the same, 60-second CS duration across acquisition, extinction and test (Frohardt et al., 2000; Wilson et al., 1995), and analyze the first of four CS’s at test. These procedural differences between investigators suggest that a hippocampal requirement for fear renewal may be influenced by violations of CS duration at test as well as the CS-period selected for analysis.
Thus, we sought to investigate whether the hippocampus is in fact required for fear renewal and if so, under what conditions. We hypothesized that hippocampal involvement in fear renewal would be under the control of CS duration and the ability to sustain fear responding across a test session. This hypothesis is additionally supported by previous research implicating the hippocampus in the maintenance of fear responding when the CS duration is long (Blanchard and Fial, 1968; Quinn et al., 2008). The idea that extinction is sensitive to temporal change is supported by studies showing that the extinction of a CS is specific to its duration (Drew et al., 2004) and that animals encode the ITI used during extinction and use this information to guide subsequent extinction retrieval and renewal (Todd et al., 2010). Thus, the question remaining is whether or not the hippocampus is required when temporal factors are manipulated between extinction and test.
In investigating the conditions under which the hippocampus is required for renewal, we also examined whether the timing of hippocampal lesions could be a contributing factor (e.g. pre-training, post-extinction, pre-test, etc). We predicted that the effect of hippocampal manipulations on renewal should follow the pattern of results seen in contextual fear conditioning studies (Fanselow, 2010). That is, when context conditioning is followed by damage to the hippocampus, animals display retrograde amnesia for this fear (Anagnostaras et al., 1999; Kim and Fanselow, 1992). Conversely, if the hippocampus is damaged prior to conditioning, animals tend to compensate and overcome anterograde amnesia (Maren et al., 1997; Wiltgen et al., 2006). Thus, we hypothesized that post-extinction lesions would have a stronger impact on fear renewal than pre-training lesions.
Materials and Methods
Subjects
The subjects were 126 naïve, adult male Long-Evans rats (70 in the pre-training lesion experiment, 56 in the post-extinction lesion experiment), initially weighing 270–300 g, purchased from Harlan (Indianapolis, IN). Rats were individually housed and maintained on a 12-hour light/dark cycle with access to food and water ad libitum. Animals were handled daily (one-two minutes per rat) for at least one week prior to the start of surgery and behavioral training. The procedures used in this experiment were in accordance with policy set and approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles.
Surgery
Rats were anesthetized with sodium pentobarbital (i.p., 65 mg/kg) and atropine sulphate (i.p., 0.4 mg/kg). Each rat was then shaved across the head and their eyes were coated with a hydrating ointment. Animals were then mounted into stereotaxic instruments (Kopf Instruments, Tujunga, CA) and the scalp was cleaned (70% ethyl alcohol and Betadine), incised, and retracted. The skull was adjusted so that bregma and lambda were in the same horizontal plane. Four small holes (two per side) were drilled into the skull to allow for a stainless steel injector cannula (33 gauge) aimed at the dorsal hippocampus to be positioned 2.8 mm posterior, 1.6 mm lateral, 3.5 mm ventral to bregma (rostral coordinates) and 4.2 mm posterior, 2.6 lateral, and 3.5mm ventral to bregma (caudal coordinates). Injection cannulae (33 gauge) were attached to a 5 μl microsyringe (Hamilton Instruments) via polyethylene tubing (PE20) and inserted into guide cannulae (28 gauge) attached to the arms of the stereotax. Microsyringes were mounted into a syringe pump (Harvard Apparatus, South Natick, MA) for controlled microinfusions of NMDA (20 mg/ml; Sigma-Aldrich, St. Louis, MO), dissolved in 0.01 M phosphate buffered saline (PBS). Cannulae were lowered and infusions of .4 μl of NMDA/site were made across 4 min (.1 μl/min rate). Cannulae remained in place for an additional two minutes to allow for adequate NMDA diffusion and reduction of backflow. Sham surgeries were identical except that injection cannulae were not lowered and infusions were not made. Following infusions, incisions were closed with stainless steel wound clips and animals were given i.p. injections of the analgesic/anti-inflammatory ketoprofen (2 mg/kg) and placed on heating pads until they recovered from anesthesia. Ketoprofen injections were continued for an additional two days post-surgery. In addition, rats were given the antibiotic trimethoprim sulfa (TMS) in their drinking water, weighed, monitored and handled for one-week following surgery. Rats were allowed a total of 10–14 days of recovery prior to behavioral training.
Apparatus
All behavioral training was performed using two sets of four identical fear conditioning chambers (30 × 25 × 25 cm, Med-Associates, Inc St. Albans, VT), equipped with a Med-Associates VideoFreeze system. Animals received fear conditioning and testing in one context. Fear extinction was either conducted in the same context, or a novel, distinct context. Contexts were differentiated by chamber-shape, illumination, odor, cleaning solution, background noise, and transport. In both contexts, individual boxes were enclosed in sound-attenuating chambers (Med-Associates) and each set of four boxes was contained in an individual, dedicated experimental room, which provided a unique spatial location for each context.
One context was comprised of chambers with aluminum sidewalls and a Plexiglas rear wall with blue dots. The “standard” grid floor pattern consisted of 16 stainless steel rods (4.8 mm thick) spaced 1.6 cm apart (center to center) (Contextual Conditioning System, Med-Associates, Inc.). Pans underlying each box were sprayed with a thin film of Simple GreenR to provide the context with a scent. Chambers were individually lit from above with white house lights and cleaned with 70% isopropyl alcohol in between squads. Individual fans mounted above each chamber were turned on to provide background noise (60dB). The experimental room in which chambers were located was brightly lit with overhead lights. Animals were transported to the context in squads of 4 in their homecages, which were slid onto hanging racks mounted to a portable cart and covered with a white sheet.
In the chambers of the second context, a black plastic insert provided sidewalls that sloped inwards from the floor to form a triangular roof. The rear wall was white opaque plastic and the distinct grid flooring pattern consisted of two planes of “staggered” stainless steel rods (4.8mm thick) spaced 1.6 cm apart (center to center) (Contextual Conditioning System, Med-Associates, Inc). The context was scented and cleaned with a 1% acetic acid solution. The background fan and chamber houselights were turned off. The experimental room in which chambers were housed was lit by dim red houselights. Animals were transported in squads of four using a square black plastic tub divided in four with a black plastic insert. The tub had bedding covering the floor and was carried by hand from the vivarium to experimental chambers to provide a distinct mode of transport. Chambers in both contexts were cleaned with a 10% bleach solution following each day of behavioral testing. Contexts were counterbalanced across groups.
Procedure
Rats were delay conditioned to fear a tone CS (80 dB, 2800 Hz), extinguished of this fear and subsequently tested for fear renewal or extinction memory (controls). In the pre-training lesion experiment, surgery (DH or sham) occurred prior to fear conditioning (see figure 2A). In the post-extinction lesion experiment, surgeries occurred one day after extinction training (see figure 3A).
FIGURE 2.
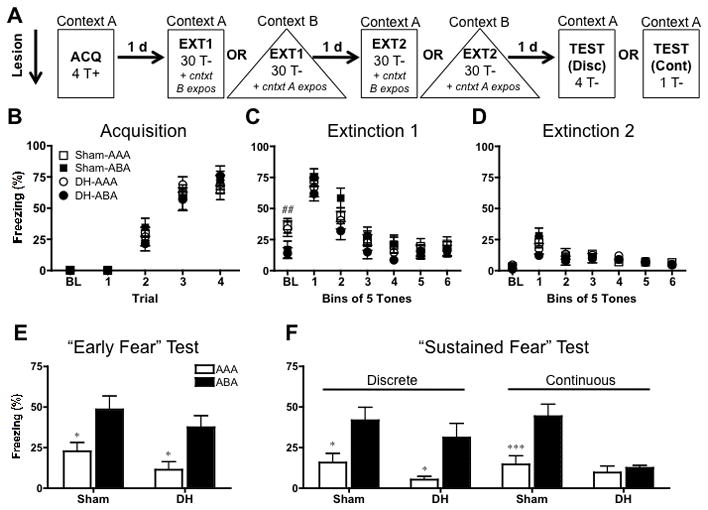
Design and behavioral results for pre-training lesion experiment. A: Rats were given pre-training lesions of the dorsal hippocampus (DH) or sham lesions. Following a ~12 day recovery period, rats were fear conditioned (4 tone-shock trials) in context A and extinguished for 2 days (30 tone alone presentations) in context A or B (AAA and ABA groups, respectively). On each extinction day, animals also received context exposure to the alternate context for an equivalent amount of time. One day later, they were tested for tone fear in context A using either 4 discrete tones (Disc) or 1 continuous tone (Cont). B: Mean (± SEM) freezing during the baseline period (BL) and to each tone presentation comprising a tone-shock trial. All animals acquired fear regardless of whether they received sham surgery (squares) or DH lesions (circles). C: Mean (± SEM) freezing during the baseline period (BL) and during the 6, 5-tone bins that comprised the first extinction session. Animals extinguished in A (open shapes) froze more to the context (BL freezing) compared to animals extinguished in a novel context, B (filled shapes). All animals displayed significant tone fear extinction regardless of group. D: Mean (± SEM) freezing during the baseline period (BL) and to tone bins during the second extinction session. All animals displayed significant tone fear extinction and low levels of baseline fear. E: Mean (± SEM) freezing during the initial 30 seconds of tone testing (collapsed across test type) represents our measure of “Early” fear. Significant early renewal was seen in ABA groups (black bars) when freezing was compared to their respective AAA control group (white bars), regardless of lesion condition. F: Mean (± SEM) freezing averaged across either four discrete tone presentations (left panels) or one continuous tone presentation (right panels) represents our “Sustained” fear measure. DH-lesioned, continuous tone-tested rats failed to show renewal compared to all other groups. Statistically significant renewal (AAA vs. ABA comparison) for each respective surgical condition is signified as follows: *P < .05, **P < .01, ***P < .001. Comparisons between AAA and ABA trained animals collapsed across surgical condition are signified as follows: #P < .05, ##P < .01, ###P < .001.
FIGURE 3.
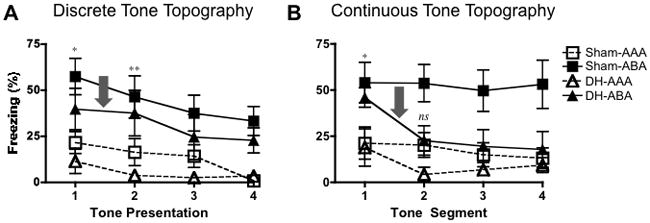
Topography of fear responding during the tone test for the pre-training lesion experiment. A: Mean (± SEM) freezing for animals tested with four discrete tone presentations, (30 sec each, 120 sec total). In line with our Early and Sustained Fear measures (Fig’s 2E and 2F), sham and hippocampally compromised animals tested with discrete tones demonstrate fear renewal across the entire test session. B: Mean (± SEM) freezing for animals tested with one continuous tone presentation. The first 120 sec of the tone is shown broken down into 30 sec segments to allow for direct comparison between discrete and continuous tested animals. Renewal seen in the first tone segment dissipates by the second tone segment for continuous tone tested rats compared to significant renewal maintained from the first to second tone presentation in discrete tested rats (highlighted by grey arrows). This suggests that when a tone at test begins to violate the duration of the discrete tones used during extinction, renewal cannot be supported without a hippocampus. Statistically significant renewal (AAA vs. ABA comparison) for DH lesioned animals is signified as follows: *P < .05, **P < .01, ***P < .001 and ns for when this comparison is non-significant (P > .05).
During fear acquisition, rats were transported to Context A and underwent a three min acclimation period in the experimental chamber. This provided a baseline (“BL”) measure of context fear. Following the BL period, rats received 4 conditioning trials in which 30 s presentations of the tone co-terminated with a 2 s footshock (.9mA). The inter-trial interval (ITI) was 60 s. Following an additional 60 s in the context, rats were transported back to the vivarium. The following day, rats were returned to either Context A (control groups) or placed in a novel Context B (experimental groups) for fear extinction.
During extinction training, a 3 min BL was followed by 30, 30 s tone presentations with a 60 s ITI. In addition to tone-fear extinction, all rats also received context exposure to the alternate context (i.e. the opposite context in which they were extinguished) for 48 minutes (the total time of an extinction session). This served to reduce any confound of high baseline levels of fear at test and also equated the overall amount of time an animal spent in each context. Extinction and context exposure sessions were repeated for a second time the following day.
Animals were tested in Context A for fear renewal or extinction memory either one day following extinction session 2 (pre-training lesions experiment) or 11 days later (post-extinction lesions experiment). Thus, animals were either conditioned in A, extinguished in B and tested in A (“ABA” renewal groups) or conditioned, extinguished and tested in A (i.e. “AAA” control groups). The standard tone test used across the entire study consisted of a three min BL period followed by 4 discrete, 30 s tone presentations (60 s ITI). These discrete tone test parameters mimicked acquisition training with the exception that shock was omitted. The pre-training experiment included an additional set of animals that received a single continuous, 240 s tone presentation at test. This allowed us to compare behavior during a “discrete” tone test to behavior elicited by a “continuous” tone at test. This yielded the following groups in the pre-training experiment: AAA-DH, AAA-sham, ABA-DH, ABA-sham with animals in each group split into either the discrete or continuous tone test. In the post-training lesion experiment the continuous tone test was omitted. Contexts were counterbalanced across groups for all phases of behavioral testing.
Histology
Following behavioral testing, animals were anesthetized and transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were extracted and placed in 4% PFA overnight. The following day they were cryoprotected in a 30% sucrose solution for 72 hours. The brains were then frozen (−20° C) and sectioned on a cryostat (50 μm). Every third section (150 μm) was collected and dry mounted on a microscope slide. Sections were then stained for nissl bodies (cresyl violet), lesions were verified and images were captured using brightfield microscopy (see Figure 1).
FIGURE 1.
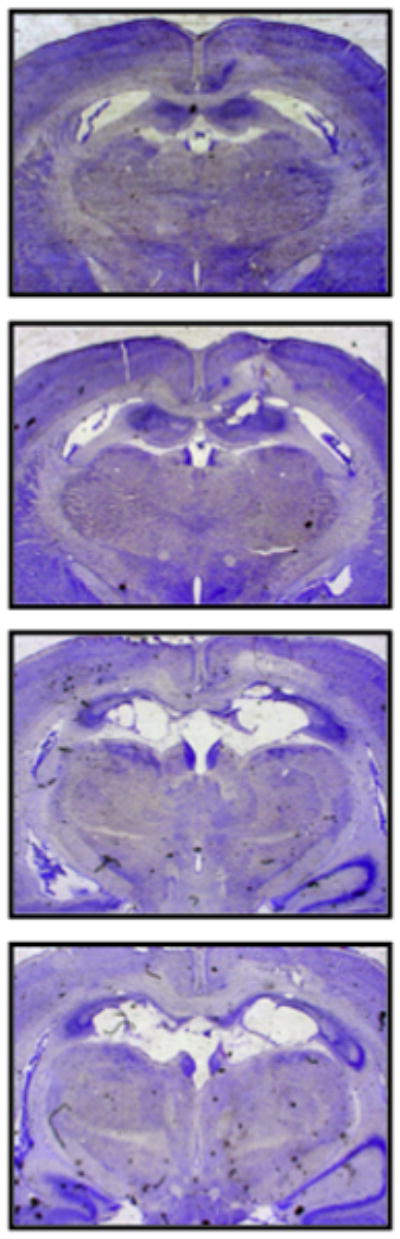
Representative photomicrographs show cresyl violet-stained coronal brain sections following excitotoxic lesions of the dorsal hippocampus. From top to bottom, the sections are 1.60, 2.60, 3.60 and 4.60 mm posterior to bregma.
Data Analysis
Fear was indexed by defensive freezing behavior, as defined by the absence of all movement except for those necessitated by respiration (Fanselow, 1980). Behavior was recorded using an automated near infrared (NIR) video tracking equipment and computer software (VideoFreeze, Med-Associates Inc.). Video was recorded at 30 frames per second and the software calculated the noise (standard deviation) for each pixel in a frame by comparing its grayscale value to previous and subsequent frames. This produced an “activity unit” score for each frame. Based on previous validation with hand scoring (correlation of r >0.9 between automated system and highly trained human observers) freezing was defined as sub-threshold activity (when the motion threshold was held at 50 activity units) for longer than 1 sec. Average freezing was scored for the baseline period in all phases and the first 28 s of each tone used for conditioning (prior to US onset). For presentation purposes, the 30 tone presentations during extinction sessions were blocked into 6 bins of 5 tone presentations and freezing was averaged within each bin. Lastly, freezing during the first discrete 30 s test tone and the initial 30 s of the continuous 240 s test tone were scored to constitute our measure of “Early Fear”. Freezing averaged across the 4 discrete test tones or across the entire 240 sec continuous test tone were scored and constituted our measure of “Sustained Fear”.
Freezing data were statistically analyzed using between-subjects analyses of variance (ANOVAs) and repeated measures (trial) ANOVAs where appropriate. Baseline freezing was analyzed separately as a two-way (context X lesion) ANOVA and provided an indication of contextual fear prior to tone presentations. Test data (“Early” and “Sustained” freezing) was analyzed using two-way ANOVAs (lesion X context). Post-hoc comparisons were performed following significant findings and a Bonferroni correction was applied to control for the number of comparisons made. The level of significance used for all analyses was P < .05.
Results
Excitotoxic lesions of the dorsal hippocampus
A photomicrograph of a representative DH lesion is displayed in Figure 1. The extent of NMDA-induced excitotoxic lesions of the DH was consistent with those previously reported in our laboratory (Quinn et al., 2008; see Wiltgen et al., 2006). Rats with insufficient bilateral damage restricted to the DH were not included. Based on this criterion, 5 animals in the pre-training experiment were excluded. This resulted in the following group sizes for discrete-tone tested animals: DH-AAA (n =10), DH-ABA (n = 8), Sham-AAA (n = 8), Sham-ABA (n = 8), and continuous-tone tested animals: DH-AAA (n = 9), DH-ABA (n = 8), Sham-AAA (n = 7), Sham-ABA, (n = 7). For the post-extinction lesion study, one animal died during surgery and 3 rats were excluded following histological analyses resulting in the following group sizes: DH-AAA (n = 13), DH-ABA (n = 14), Sham-AAA (n = 11), Sham-ABA (n = 14).
Animals with DH lesions acquire and extinguish tone fear
Fear Acquisition
Mean freezing (±SEM) to each 30s tone of 4 fear acquisition trials is displayed in Figure 2B. Rats were split by lesion condition (DH or sham) and again by whether they were extinguished and tested in an “ABA” renewal design or an “AAA” control design, resulting in the following four groups: Sham-AAA, Sham-ABA, DH-AAA and DH-ABA. Data were collapsed across subsequent test type (discrete vs. continuous) until test. All animals displayed < 1% baseline (BL) freezing to the context during the initial 180s acclimation period, suggesting that the surgery in and of itself did not generate inappropriate freezing behavior. In line with previous studies showing that DH damage leaves tone fear intact (Kim and Fanselow, 1992), repeated measures (trial) ANOVA revealed significant tone fear acquisition, (F(3,183) = 132.2; P < .0001), with no main effect of group (F < 1) or trial X group interaction (F < 1).
Fear Extinction
Figures 2C and 2D display the two sessions (1 session/day) of extinction training animals received. Mean freezing (±SEM) during each baseline period (BL) and each bin of 5 extinction tones are displayed. A two-way ANOVA (lesion X context) performed on BL freezing of extinction session 1 found that rats extinguished in a novel context (“ABA” groups) froze significantly less than rats extinguished in the same context as acquisition (“AAA” groups) (F(1,61) = 10.53; P < .01). There was no effect of lesion (F < 1), and no context X lesion interaction (F < 1), demonstrating contextual discrimination occurred in shams and lesioned animals alike. Repeated measures (bin) ANOVA on extinction session 1 (Figure 2C) revealed significant extinction learning across tone bins, (F(5,305) = 101.6; P < 0.0001). As with acquisition, there was no main effect of group (F < 1) or group X bin interaction (F(15,305) = 1.26; P > .05).
A repeated measures (bin) ANOVA performed on the second session of extinction (Figure 2D) similarly found highly significant extinction across tone bins (F(5,305) = 22.21; P < .0001). While there was no main effect of group (F < 1), there was a small group X bin interaction (F(15,305) = 1.84; P < .05). Post-hoc analyses (Bonferroni) revealed the interaction to be driven by an increased level of freezing in Sham-ABA animals contrasted with DH-ABA animals during the first bin (t=3.79; P <.01). However, this difference disappeared by the second bin, as all groups showed similar, low levels of freezing as extinction progressed. By the second session of extinction, any significant differences in baseline were eliminated (F < 1).
“Early” fear renewal remains intact following pre-training DH lesions
“Early” tone fear (freezing during the initial 30 s of the tone) collapsed across test type is displayed in figure 2E. Assessing freezing during the first CS presentation (or equivalent duration for continuous tested animals) allowed us to compare our findings with those of Frohardt et al. (2000). A two-way (context X lesion) ANOVA revealed a significant renewal effect, as ABA-trained renewal groups froze significantly more to the tone than AAA-trained controls (F(1,58) = 16.40; P < .0001). There was no main effect of lesion (F(1,58) = 3.02; P > .05), nor was there a context X lesion interaction (F < 1). Post-hoc analyses (Bonferroni) comparing AAA and ABA groups for each surgical condition found that the renewal effect was present in sham-lesioned animals (t = 2.82; P < .05), as well as DH-lesioned animals (t = 2.91; P < .05). Thus, these data are in line with those observed by Frohardt et al. (2000), and suggest that following pre-training damage to the hippocampus, animals are able to compensate and perform fear renewal, when analysis are restricted to the duration of the initial, extinction-matching CS presentation. Context exposure sessions to the context alternate to that of extinction were administered to reduce levels of baseline freezing to the context prior to test. This ensured that differences in tone fear were not confounded by differences in baseline contextual fear. Indeed, mean baseline percent freezing at test for Sham-AAA, Sham-ABA, DH-AAA, and DH-ABA groups respectively was 2.93%, 1.68%, 3.32%, and 0.79%.
“Sustained” fear renewal to a continuous, but not discrete, tone requires the DH
Following testing for early fear, animals received either three additional discrete 30 s tone presentations with an ITI of 60 s, or the tone remained on in a continuous, uninterrupted fashion for an additional 210 s, resulting in a long, 240 s tone test. Figure 2F displays mean freezing (±SEM) averaged across the four tones (“Discrete” groups, left panels) and across the 240 s uninterrupted tone (“Continuous” groups, right panels). Testing animals with either four discrete CSs or with one longer, continuous CS allowed us to compare the results obtained by Bouton and colleagues (Frohardt et al., 2000) with those obtained by Maren and colleagues (e.g. Ji and Maren, 2005), whom used a discrete compared to a continuous CS test, respectively. In particular, it allowed us to investigate whether the ability to demonstrate fear renewal following pre-training lesions was simply because only “Early” fear was analyzed (Frohardt et al., 2000), or due to the nature of the duration of the tone at test compared to that used during extinction (i.e. discrete vs. continuous). If hippocampus-independent fear renewal was simply an “early” fear effect, then sustained renewal would fall apart in either test condition. However, if hippocampus – independent renewal was due to a mismatch between tone duration at extinction compared to test, then only continuous tone-tested rats should show a deficit in sustained fear renewal. A two-way ANOVA (context X lesion) on discrete-tone tested animals (left panels) revealed a significant renewal effect (F(1,30) = 17.09; P < .0001), with no main effect of lesion type (F(1,30) = 2.83; P > .05) or a lesion X context interaction (F < 1). Post-hoc analyses (Bonferroni) revealed that this renewal effect was significant for both sham-lesioned (t = 2.86; P < .05), and DH-lesioned animals (t = 3.00; P < .05). These results extend our findings from “early” fear responding to “sustained” fear responding for animals tested with discrete tones, demonstrating that renewal can be sustained in the absence of the hippocampus, provided animals are given discrete tones at test. In sharp contrast, sustained fear renewal falls apart when animals are tested with a continuous tone. A two-way (context X lesion) ANOVA revealed a significant renewal effect (F(1,27) = 11.37; P < .01), main effect of lesion (F (1,27) = 14.66; P < .001), and a context X lesion interaction (F (1, 27) = 7.80; P < .01). Post hoc analyses (Bonferroni) revealed that these results were essentially driven by a strong renewal effect in the sham controls (t = 4.17; P < .001), which was lost in animals with DH lesions.
Overall, these results show that in the absence of the hippocampus, animals are able to acquire, extinguish and renew fear provided the tone duration during extinction and test match. Furthermore, they demonstrate that the hippocampus is required for renewal when the CS presented during test is longer in duration than that used during extinction. This suggests that the violation in length of CS duration from extinction to test may comprise an important, hippocampus-dependent aspect of renewal. It is important to note that we did not test the reverse case in which the CS duration at test was shorter than that used during extinction. However, data obtained by Bouton and colleagues (Bouton and Garcia-Gutierrez, 2006), in which renewal is shown to occur when the inter-trial-interval (ITI) of test extends beyond the ITI used during extinction, but not for the reverse (when the test ITI is “contained in” or shorter than that used during extinction), could be taken to show that renewal is sensitive to violations of temporal factors when test durations are not “contained in” those used during extinction.
The DH is required for renewal when tone duration is violated at test
Figures 2 illustrates that animals are able to demonstrate fear renewal following pre-training damage of the hippocampus, unless they are asked to sustain fear responding to a continuous tone. However, these analyses do not differentiate whether animals with hippocampal lesions have deficits in sustaining renewal to a continuous tone because it is longer in total duration compared to discrete-tone tested animals (240 sec and 120 sec, respectively), or because it violates the tone duration used for extinction (30 sec). To answer this question, we re-analyzed the tone fear data to determine the exact topography of fear responding at test. The data is presented in Figure 3. The continuous tone was broken up into 30-second segments, which allowed us to directly compare discrete and continuous tone tested animals and controlled for differences in total tone duration for each test type. The analyses detailed below indicate that a role for the hippocampus in renewal emerges exactly when the test conditions begin to differ from the conditions during extinction.
Figure 3A displays mean freezing (±SEM) during each 30 sec tone presentation for animals tested with four discrete tones, offering a more detailed presentation of the topography of fear responding at test. A repeated measures (tone presentation) ANOVA revealed an overall significant effect of group (F (3, 90) = 8.05; P < 0.001), and tone presentation (F (3, 90) = 8.05; P < 0.0001), with no tone X group interaction (F < 1). Similar to the early and sustained renewal presented in Figure 2, significant fear renewal (ABA animals compared to AAA controls) was exhibited in animals tested with discrete tones, regardless of whether or not they had an intact hippocampus (sham: F (1, 48) = 10.13; P < 0.01; DH: F (1, 48) = 10.32; P < 0.01). This renewal pattern seen across tone presentations in hippocampally-lesioned animals tested with discrete tones was in sharp contrast to the behavioral profile seen in animals given a continuous tone at test. Figure 3B displays the mean freezing (±SEM) for animals tested with a continuous tone. The initial 120 seconds of the 240 sec tone were broken up into 30 sec segments and the first, consecutive four of these segments are presented. While a repeated measures (tone segment) ANOVA revealed a significant effect of group (F (3, 90) = 6.95; P < 0.01), and tone segment (F (3,90) = 4.82; P < 0.01), with no tone X group interaction (F (9, 90) = 1.325; P > 0.05), the significant renewal (ABA compared to AAA) seen across tone segments in sham animals (F (1, 36) = 10.73; P < 0.01) was not exhibited by DH animals (F (1, 54) = 3.712; P > 0.05).
To isolate when during the continuous tone fear renewal fell apart for DH-lesioned animals in contrast to discrete tone tested animals, we performed Bonferroni posttests at each tone presentation or segment. In line with the data displayed for early fear (Figure 2E) and sustained fear (Figure 2G), DH rats were able to demonstrate fear renewal during the first 30 sec segment of the continuous tone (t = 2.69; P < .05) or discrete tone (t = 2.71; P < .05). However, this renewal broke down by the second tone segment for continuous tested animals (t = 1.83; P >.05), while it remained intact during the comparable, second tone presentation for discrete tested animals with DH lesions (t = 3.22; P < .01). This suggests that animals tested with a continuous tone were able to show renewal in a hippocampally-independent manner until the test conditions began to violate those present for extinction (i.e. the tone duration began to extend past 30 sec). This could not be explained by the total time spent in the test session in continuous compared to discrete tested animals (for whom testing included 60 sec ITIs), as Bonferroni analyses on the fourth discrete tone segment also failed to reveal significant renewal in DH-lesioned animals (t <1). These analyses suggest that the hippocampus may be required for fear renewal when the tone parameters used for testing are distinct from those an animal is extinguished on. Thus, the hippocampus may be necessary in cases where inhibitory learning about the CS during extinction must be generalized to new and novel presentations of that CS (i.e. longer durations).
Fear renewal is attenuated following post-extinction DH lesions
Our behavioral results for animals with pre-training damage of the dorsal hippocampus demonstrate that the DH is not necessary for fear acquisition, extinction and renewal, provided the CS duration during test matches that during extinction. However, these data are not enough to determine whether or not the hippocampus might normally be used to mediate renewal. We investigated this possibility by giving rats post-extinction lesions of the hippocampus and subsequently testing for renewal.
Fear acquisition and extinction
Fear acquisition and extinction training proceeded in a manner identical to that used for the pre-training lesion experiment. As before, all groups displayed an average of < 1 % baseline freezing prior to fear acquisition and significant tone fear acquisition across trials (F (3, 144) = 140.0; P < 0.0001), with no main effect of group (F < 1) and no trial X group interaction (F < 1). All animals subsequently showed significant extinction of this fear across tone bins during extinction session 1 (F (5, 240) = 77.38; P < 0.0001) and extinction session 2 (F (5, 220) = 17.45; P < 0.0001) (not shown). A two way ANOVA (context X lesion) revealed that baseline fear prior to extinction session 1 was significantly greater in AAA groups compared to ABA groups (F (1, 48) = 58.33; P < 0.0001), with no effect of subsequent lesion condition or interaction Fs < 1). As in the pre-training data, this increased baseline fear in AAA animals can be attributed to greater contextual fear to context A (the training context) for animals being extinguished in A as opposed to those ABA animals being extinguished in the novel context, B. There were no effects of context, lesion or context X lesion interaction during the baseline period prior to extinction session 2 (Fs < 1).
Following extinction, animals received DH or sham (control) lesions and were subsequently tested for freezing to four discrete tone presentations in context A (see figure 4A for design). We omitted the continuous tone test in this experiment as the pre-training experiment already determined that sustained freezing during a continuous tone requires the hippocampus. Instead, we questioned whether a discrete tone test would be sensitive to retrograde amnesia effects despite being resilient to anterograde amnesia effects.
FIGURE 4.
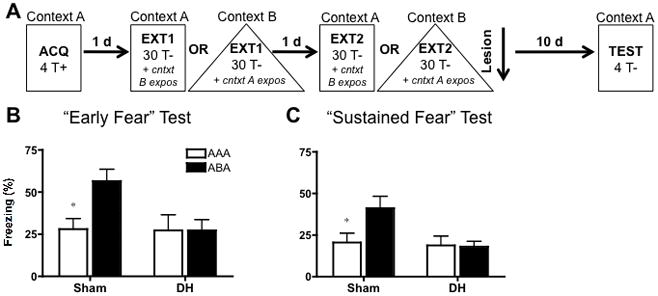
Design and behavioral results for the post-extinction lesion experiment. A: Rats were fear conditioned (4 tone-shock trials) in context A, and extinguished for 2 days (30 tone alone presentations) in context A or B (AAA and ABA groups, respectively). On each extinction day, animals also received context exposure to the alternate context for an equivalent amount of time. One day after extinction, rats were given DH lesions or sham surgery. Following a ~10 day recovery period, rats were tested for tone fear in context A using 4 discrete tones. B: Mean (± SEM) freezing during the first of 4 discrete, 30 sec tones represents our measure of “Early” fear. Significant early renewal (ABA rats (in black) compared to respective AAA rats (in white)) was present in sham but not DH lesioned animals. C: Mean (± SEM) freezing averaged across the 4 discrete tones presented at test represents our “Sustained” fear measure. As with the Early fear data, significant sustained renewal was present in sham but not DH lesioned animals. Statistically significant renewal (AAA vs. ABA comparison) is signified as follows: *P < .05.
“Early” Fear Renewal Test
“Early” fear, or mean freezing (±SEM) to the first of four, discrete, 30 s tones (ITI: 60 s) is displayed in figure 4B. A two way ANOVA (context X lesion), revealed a significant effect of lesion (F (1, 48) = 4.07; P < .05), and a trend for effects of context (F (1, 48) = 3.68; P < .10) and context X lesion interaction (F (1, 48) = 3.67; P = .06). Subsequent post-hoc analyses (Bonferroni) found this lesion effect to be driven by significant fear renewal in sham lesioned animals (t = 2.653; P < .05), but a renewal failure for DH-lesioned animals (t = .002; P > .05). Context exposure sessions to the context alternate to that of extinction (not shown) ensured low levels of BL freezing (< 5%) prior to test
“Sustained” Fear Renewal Test
Figure 4C displays “sustained” or average freezing (±SEM) across the four tone presentations administered at test. Again, there was a significant main effect of lesion (F (1, 48) = 4.07; P < .05), and trends for an effect of context (F (1, 48) = 3.09; P = .085) and lesion X context interaction (F(1, 48) = 3.62; P = .06). Post-hoc analyses (Bonferroni) found that renewal was significant for sham-lesioned animals (t = 2.531; P < .05), but was absent in animals with DH lesions (t = .104; P > .05). Thus, in contrast to behavior following pre-training lesions, animals that received lesions of the dorsal hippocampus subsequent to extinction failed to demonstrate either early or sustained fear renewal when given discrete tone presentations at test. These findings illustrate that the ability to demonstrate fear renewal independent of the hippocampus only occurs if an animal was fear conditioned and extinguished in the absence of the hippocampus but not if hippocampal damage is sustained post-extinction. This failure to observe renewal following post-extinction damage to the DH is consistent with findings from Maren and colleagues (Corcoran and Maren, 2001; Corcoran and Maren, 2004; Ji and Maren, 2005).
Discussion
Resolving the Puzzle
Although the role of the hippocampus in contextual fear has been extensively studied, its exact contribution to fear renewal has been open to question. Given that the hippocampus is thought to encode contextual information and form integrated, gestalt-like contextual representations (Fanselow, 2000), a role for the hippocampus in a context-sensitive effect such as renewal would seem likely. However, to date, research investigating this possibility has generated mixed results. On the one hand, renewal seemed to persist in the face of hippocampal compromise (Frohardt et al., 2000; Wilson et al., 1995). On the other hand, manipulations of the hippocampal formation have been shown to disrupt renewal (Corcoran et al., 2005; Corcoran and Maren, 2001; Corcoran and Maren, 2004; Hobin et al., 2006; Ji and Maren, 2005; Ji and Maren, 2008a; Ji and Maren, 2008b). Collectively, these findings generate a seeming puzzle regarding the necessity of the hippocampus in fear renewal. The present study sought to address this puzzle by tracing hippocampal involvement in fear renewal back to the nature of fear responding at test.
By manipulating the duration of the tone at test, comparing the difference between early fear and sustained fear, and contrasting pre-training with post-extinction lesions of the DH, we were able to extract conditions under which the hippocampus both is and isn’t required for fear renewal. We found that fear renewal during the early portion of testing endured despite pre-training lesions of the DH. This renewal response was able to persist in a sustained manner, provided animals were tested with discrete tone presentations that temporally matched those used during extinction. However, we found that renewal was disrupted if animals were asked to sustain freezing to a long, continuous tone. We discerned that this hippocampal deficit began to emerge as soon as the tone duration at test began to violate that used during extinction, suggesting that the DH may be required when animals must renew responding to a CS that does not temporally match the CS extinguished. Taken collectively, these findings suggest that the sensitivity of fear renewal to pre-training damage of the DH depends on the temporal nature of the tone used at test.
In contrast to the pre-training lesion data, the results obtained following post-extinction lesions are more straightforward. Animals with post-extinction lesions of their DH failed to show significant fear renewal, despite being tested with the pre-training lesion-resilient discrete tone test. This failure occurred irrespective of the topography of fear responding, as animals showed attenuated early and sustained fear renewal. Thus, these post-extinction lesion data suggest that if the DH is intact during fear acquisition and extinction, then it is required for expression of fear renewal at test. These data support findings in which compromising the hippocampus subsequent to extinction training results in attenuated fear renewal at test (Corcoran and Maren, 2001; Corcoran and Maren, 2004; Ji and Maren, 2005). Therefore, the question of whether or not the hippocampus is required for fear renewal is additionally determined by when damage to the hippocampus occurs.
A new framework
The present study sought to elucidate the seemingly controversial findings regarding the role of the hippocampus in fear renewal. However, in doing so, we also discovered an important distinction between pre-training and post-extinction lesions of the DH: namely, animals were able to display fear renewal following pre-training lesions, but failed to do so if lesions were made post-extinction. In other words, fear renewal occurred in the absence of the hippocampus, but not if the hippocampus was allowed to learn during acquisition and extinction. This pattern of results is consistent with literature on contextual fear wherein animals are able to condition and express contextual fear following pre-training hippocampal lesions (Wiltgen et al., 2006), but fail to express contextual fear if lesions are made post-training (Anagnostaras et al., 1999; Kim and Fanselow, 1992). In other words, despite suffering retrograde amnesia for a contextual fear memory, animals will not necessarily display anterograde amnesia for the same contextual information (Fanselow, 2010; Maren et al., 1997). The data presented here, for the first time, extends this pattern of results from contextual fear to fear renewal, as post-extinction lesioned animals show a retrograde amnesia for the contextual information required to drive renewal, but also demonstrate a resistance to anterograde amnesia for the same information if lesions were made pre-training. Thus, the differential effects of pre-training and post-extinction lesions on fear renewal found here can be re-interpreted as an instantiation of retrograde amnesia coupled with a failure to obtain anterograde amnesia. In this sense, the role of the hippocampus – to encode environmental information and provide an integrated contextual representation that can subsequently enter into a number of associative relationships – may be homogeneous across behavioral effects.
By uniting these renewal data with the established data on contextual fear conditioning, a number of interesting implications about fear renewal emerge. For example, in contextual fear conditioning, animals with a damaged hippocampus are able to learn about a context, presumably by recruiting an alternate, compensatory structure (Fanselow, 2010). This compensation is what allows for the resistance to anterograde amnesia processes seen in lesioned animals given adequate training (Wiltgen et al., 2006). Likewise, in fear renewal, contextual encoding during acquisition and extinction by compensatory structures may be sufficient to allow for appropriate fear renewal at test. As in the case of contextual fear, the identity of this compensatory structure remains an open and extremely interesting question.
Hippocampus and Stimulus Mismatch
In addition to the distinction generated by pre-training and post-extinction lesions, the results obtained here also provide insight into the role of the hippocampus as a structure that tracks mismatches between stimuli. Without the hippocampus, rats became very sensitive to a mismatch between the duration of the CS used in extinction training and that presented during testing. This suggests that temporal aspects of the CS were encoded during extinction (Drew et al., 2004). Interestingly, this knowledge was not expressed in intact animals, suggesting that the hippocampus may normally cause animals to ignore a difference in the properties of a stimulus, even though they likely encoded these properties.
In a general sense, this pattern is consistent with the data of Quinn et al. (2009), which manipulated the physical, rather than temporal, properties of the tone between fear acquisition and testing. In that study, intact rats that received tone-shock pairings froze equally to the reinforced tone and a novel white noise. The converse was true of rats trained with the white noise. However, animals with DH lesions only responded to the trained CS. Thus, in both the Quinn et al. (2009) study and the present one, lesions of the hippocampus resulted in increased discriminative performance, suggesting that the hippocampus seems to prevent expression of specific information that has been encoded about a stimulus. Such a result seems to be best accounted for by the pattern completion processes that has been proposed for CA3 (Gold and Kesner, 2005; Kesner et al., 2000; Lee and Kesner, 2004; Nakazawa et al., 2004; Rudy et al., 2004). In both cases, the hippocampus recognizes sufficient similarity of the test and trained stimulus (acquisition training in the Quinn et al., study; extinction training in the present study) to generate, at test, responding appropriate for the trained stimulus.
Given that lesions of the hippocampus are thought to leave classic associative conditioning intact (Rudy and Sutherland, 1989; Squire, 1992), the temporal mismatch detection seen in lesioned animals also suggests that the associative system encodes specific temporal attributes of the training condition. This interpretation is in line with conditioning theories, which emphasize that besides associations; Pavlovian conditioning also results in the encoding of specific temporal information (Barnet et al., 1993; Drew et al., 2004; Leising et al., 2007). Because of its pattern completion function, the hippocampus may obscure contributions that this temporal encoding gives to performance.
The hippocampus appears to play two different roles that influence performance during fear renewal testing. Just as it does for direct conditioning to a context, the hippocampus normally contributes to the encoding and retrieval of contextual information. This is apparent with post-training lesions, however, animals with pre-training lesions can compensate for the loss of the hippocampus (Fanselow, 2010). In addition, the hippocampus causes the animals to treat a longer test stimulus similar to the shorter stimulus used in extinction. The former process relates to the hippocampus’ role in forming integrated memory representations of a context (Fanselow, 2000). The later process may relate to the pattern completion computations performed by CA3 (Gold and Kesner, 2005; Kesner et al., 2000; Lee and Kesner, 2004; Nakazawa et al., 2004; Rudy et al., 2004).
Acknowledgments
This research was supported by National Institute of Mental Health grant RO1MH062122 (M.S.F.) and a University of California, Los Angeles Cota Robles Fellowship (M.Z.). The authors would like to thank Ariel Badger for technical assistance.
References
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19(3):1106–14. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Temporal encoding as a determinant of blocking. J Exp Psychol Anim Behav Process. 1993;19(4):327–41. doi: 10.1037//0097-7403.19.4.327. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Fial RA. Effects of limbic lesions on passive avoidance and reactivity to shock. J Comp Physiol Psychol. 1968;66(3):606–12. doi: 10.1037/h0026512. [DOI] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defence reactions and avoidance learning. Psychological review. 1970;77:32–48. [Google Scholar]
- Bolles RC, Fanselow MS. A Perceptual-Defensive-Recuperative Model of Fear and Pain. Behavioral and Brain Sciences. 1980;3(2):291–301. [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–86. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5(4):368–78. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Garcia-Gutierrez A. Intertrial interval as a contextual stimulus. Behav Processes. 2006;71(2–3):307–17. doi: 10.1016/j.beproc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25(39):8978–87. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21(5):1720–6. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11(5):598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Drew MR, Yang C, Ohyama T, Balsam PD. Temporal specificity of extinction in autoshaping. J Exp Psychol Anim Behav Process. 2004;30(3):163–76. doi: 10.1037/0097-7403.30.3.163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15(4):177–82. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2010;14(1):7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav Neurosci. 2000;114(2):227–40. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15(6):808–14. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16(2):174–82. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12(3):270–6. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 inthe contextual encoding and retrieval of extinguished fear. Learn Mem. 2008a;15(4):244–51. doi: 10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Lesions of the entorhinal cortex or fornix disrupt the context-dependence of fear extinction in rats. Behav Brain Res. 2008b;194(2):201–6. doi: 10.1016/j.bbr.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Wallenstein GV. Testing neural network models of memory with behavioral experiments. Curr Opin Neurobiol. 2000;10(2):260–5. doi: 10.1016/s0959-4388(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14(3):301–10. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Leising KJ, Sawa K, Blaisdell AP. Temporal integration in Pavlovian appetitive conditioning in rats. Learn Behav. 2007;35(1):11–8. doi: 10.3758/bf03196069. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88(2):261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5(5):361–72. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned Reflexes. Anrep GV, translator. Oxford University Press; 1927. [Google Scholar]
- Quinn JJ, Wied HM, Liu D, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate generalization in auditory delay fear conditioning. Eur J Neurosci. 2009;29(8):1692–700. doi: 10.1111/j.1460-9568.2009.06727.x. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, Fanselow MS. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus. 2008;18(7):640–54. doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–85. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav Brain Res. 1989;34(1–2):97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Todd TP, Winterbauer NE, Bouton ME. Interstimulus interval as a discriminative stimulus: evidence of the generality of a novel asymmetry in temporal discrimination learning. Behav Processes. 2010;84(1):412–20. doi: 10.1016/j.beproc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behav Neurosci. 1995;109(5):828–36. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–91. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


