Abstract
Mutations/deletions of the tumor suppressor phosphatase and tensin homolog PTEN, results in PI3K/Akt pathway hyperactivation and potentially alters oncogenic responses to targeted receptor tyrosine kinase inhibitors. We previously showed that hepatocyte growth factor (HGF):c-Met pathway inhibition decreases tumor growth and oncogenic signaling responses in PTEN-null/Met+ gliomas. Here we utilize two tet-on PTENwt-inducible glioma cell lines and xenograft models to examine the influence of PTEN on oncogenic signaling responses to HGF:c-Met pathway inhibitors. Reconstitution of PTEN inhibited Akt by >80% and inhibited cell growth by ~70–75 % in both cell lines in vitro. C-Met inhibition alone inhibited in vitro cell growth by ~80–85 % and the magnitude of growth inhibition was not altered by combining PTEN reconstitution with c-Met inhibition. Combining PTEN reconstitution with Met inhibition arrested a higher percentage of cells in G1/G0 phase of the cell cycle when compared to either PTEN reconstitution or c-Met inhibition alone. Both PTEN reconstitution alone and inhibiting autocrine HGF:c-Met signaling alone, using anti-HGF mAb, robustly inhibited the growth of subcutaneous and intracranial glioma xenografts. Combining anti-HGF therapy with PTEN reconstitution did not significantly alter the magnitude of xenograft growth inhibition. Semi-quantitative immunohistopathological analyses revealed that the inhibition of glioma xenograft angiogenesis and cell proliferation by anti-HGF mAb was greatest in conjunction with PTEN reconstitution. In contrast, xenograft cell apoptosis was greatest in response to anti-HGF therapy alone and PTEN reconstitution abrogated the apoptotic response to anti-HGF therapy. These results provide new insights into how PTEN modulates glioma responses to the inhibition of HGF:c-Met signaling and possibly other receptor tyrosine kinase pathways.
Keywords: hepatocyte growth factor, Akt, xenograft, apoptosis, angiogenesis
INTRODUCTION
Mutations/deletions of tumor suppressors, such as the phosphatase and tensin homolog deleted on chromosome 10q (PTEN), lead to Akt hyperactivation and are commonly found in malignant neoplasms [1]. Receptor tyrosine kinase (RTK) systems such as those involving c-Met, epidermal growth factor receptor (egfr), and platelet derived growth factor receptor (pdgfr) commonly influence PTEN associated oncogenic signaling [2–5]. Amplifications and/or activating mutations in receptor tyrosine kinases lead to hyperactivation of downstream signaling pathways, like the PI3K/Akt pathway, that mediate oncogenic phenotypic responses. Dysfunction of these oncogenic molecular pathways contributes to uncontrolled tumor cell proliferation, survival, invasiveness, and tumor angiogenesis and poor patient prognoses in a variety of solid malignancies [1].
PTEN is a polypeptide with dual lipid and protein phosphatase activities. PTEN dephosphorylates the lipid second messenger phosphatidylinositol 3,4,5 triphosphate (PIP3) to create phosphatidylinositol 3,4 biphosphate (PIP2). Conversion of PIP3 to PIP2 diminishes the downstream effects of PI3K including the extent of Akt activation [6]. Somatic deletions/mutations of PTEN are commonly found in a wide variety of solid tumors and are detected in 40–50% of glioblastomas [7–8]. PTEN expression in PTEN-null glioma cells suppresses glioma growth in vitro and in vivo inhibiting cell migration and invasion, while also inducing apoptosis [9–12]. PTEN is shown to co-regulate RTK mediated gene expression in glioma models and influences the response to targeted kinase inhibitors [13–14].
C-met and its cognate ligand hepatocyte growth factor (HGF) have been implicated in the formation and progression of a variety of solid tumor types [15]. c-Met is a potent activator of the Ras/MapKinase and PI3K/Akt pathways and targeting this receptor tyrosine kinase/ligand system has anti-oncogenic effects in several pre-clinical model systems [16–18]. Preclinical evidence supporting the role of HGF/c-Met in tumorigenesis has lead to the development of a plethora of c-Met pathway inhibitors now entering Phase I/II clinical trials [19]. Previous studies have suggested that the therapeutic efficacy of receptor tyrosine kinase inhibitors is predicted by PTEN activity, with PTEN loss rendering tumors unresponsive [14]. Other evidence suggest that tumor responses to RTK inhibitors are not predicted by the activity of any other single pathway component including PTEN but rather are determined by the integrated activity of multiple pathway components that together determine total oncogenic “flux” [20]. With the emergence of novel therapeutic agents aimed at the HGF/c-Met pathway, the influence of PTEN on the tumor response to HGF.c-Met pathway inhibition requires further attention.
We previously reported that anti-HGF therapy inhibits glioma growth in vitro and in vivo in a HGF+/c-Met+/ PTEN-null glioma model. Here, we investigate whether PTEN reconstitution alters tumor sensitivity to anti-HGF therapeutics utilizing previously characterized glioma cell lines that express wild-type PTEN under the control of a tet-responsive promoter [21]. Specifically, we investigate if PTEN function alters glioma xenograft growth, cell proliferation, angiogenesis, and apoptosis responses to c-Met inhibition.
METHODS
Reagents
SU11274 and PHA665752 are anti- c-Met inhibitors shown to block the catalytic domain of the c-Met receptor. L2G7 is an anti-HGF monoclonal antibody shown to inhibit the direct interaction of HGF and c-Met and 5G8 is the isotype matched control monoclonal antibody.
Tet-on inducible cell lines
The tet-on PTENwt inducible cell line was a kind gift from Dr. Maria Georgescou, Radu et al. developed a tetracycline-inducible methods for expressing PTENwt using a retroviral vector in PTEN-deficient U87 and U251 glioma cell lines. In their system, two retroviral constructs pCXn/TR2 and pCXbR(TO)-PTEN, were introduced simultaneously into U87 or U251 glioma cells by infection. The pCXn/TR2 construct encodes a repressor (TR) of the tetracycline operator that is found in the second construct. The second construct contains the inducible promoter that controls transcription of the desired gene, in this case PTEN. When tetracycline is absent, the repressor prevents PTEN expression from the second retroviral construct. When tetracycline, or its derivative doxycycline, is present, the repressor is inhibited and catalytically active PTEN is expressed.
Immunoblot analysis
Total protein was extracted from glioma xenografts and cells using radioimmunoprecipitation assay (RIPA) buffer (1% Igepal, 0.5% sodium deoxycholate, and 0.1% SDS in PBS) containing fresh 1X protease and 1X phosphatase inhibitors (Calbiochem) at 4°C. Tissue extracts were sonicated on ice and centrifuged at 5,000 RPM at 4°C for 5 minutes. Supernatants were assayed for protein concentrations by Coomassie protein assay (Pierce) according to the manufacturer's recommendations. Aliquots of 40 or 60µg of total protein were combined with Laemmli loading buffer containing β-mercaptoethanol and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) according to the method of Towbin et al. with some modifications [22–23]. For immunoblot analyses, proteins were electrophoretically transferred to nitrocellulose with a semidry transfer apparatus (GE Healthcare) at 50 mA for 60 minutes. Membranes were incubated for 1 h in Odyssey Licor Blocking Buffer at room temperature and then overnight with primary antibodies at 4°C in 5% BSA in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBS/T). Membranes were then washed 3 X with TBS/T, incubated with secondary antibody at 1:10,000 for 1 h in TBS/T, washed 3 X with TBS/T, followed by washing 2 X with TBS. Proteins were detected and quantified using the Odyssey Infrared Imager (LI-COR Biosciences).
Cell viability assay
Cell viability was measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. Cells were plated at 50,000/well in 24-well tissue culture plates and cultured for 24 hours before treatment with specified reagents. Twenty-four to 72 hours after treatment, MTT was added to each well at a final concentration of 150 µg/mL, and the cells were incubated for 2 hours at 37°C. The medium was then removed, and the cell layer was dissolved with dimethyl sulfoxide (DMSO). The formazen reaction product was quantified spectrophotometrically at 570 nm using a Spectra MAX 340pc plate reader (Molecular Devices, Sunnyvale, CA, USA). The results are expressed as the percentage of absorbance measured in control cultures after subtracting the background absorbance from all values.
Flow cytometry analysis
Cell cycle analysis was quantified using Propidium Iodide (BD Biosciences, San Diego, CA) via the manufacturer’s instructions. Briefly, cells were trypsinized, pelleted by centrifugation, and resuspended in Annexin V binding buffer (150 mM NaCl, 18 mM CaCl2, 10mM HEPES, 5 mM KCl, 1mM MgCl2). FITC-conjugated Annexin V (1 µg/ml) and propidium iodide (PI, 50 µg/ml) were added to cells and incubated for 30 min at room temperature in the dark. Non-immune mouse IgG was used as the negative control. Analyses were performed on a FACscan (Becton-Dickinson, Mountain View, CA). Data were analyzed with CellQuest software (Becton-Dickinson).
Tumor xenografts
Glioma xenografts were generated as previously described [24]. Female 6- to 8-week-old mice (National Cancer Institute, Frederick, MD) were anesthetized by i.p. injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). For subcutaneous xenografts, Nu/nu mice received 4 × 106 cells in 0.05 mL of plain media s.c. in the dorsal flank. When tumors reached ~200 mm3, the mice were randomly divided into groups (n = 5 per group) and received the indicated doses of either L2G7, an anti-HGF neutralizing antibody, or isotype matched control mAb (5G8) in 0.1 ml PBS i.p. as previously described [17]. Tumor volumes were estimated by measuring two dimensions [length (a) and width (b)] and calculating volume as V = ab2 / 2[17; 25]. At the end of each experiment, tumors were excised, frozen in liquid nitrogen and protein was extracted for immunoblot analysis.
For intracranial xenografts, Scid/beige mice received 1×105 cells /2 µl by stereotaxic injection into the right caudate/putamen [24]. L2G7 or 5G8 mAb was administered as above. Groups of mice (n = 5) were sacrificed by perfusion fixation at 24 hours after the last injection and the brains removed for histologic studies. Tumor volumes were quantified by measuring tumor cross-sectional areas on H&E-stained cryostat sections using computer-assisted image analysis as previously described [24]. Tumor volumes were estimated based on the formula: vol = (sq. root of maximum cross-sectional area)3 [26].
The Johns Hopkins University Institutional Animal Care and Use Committee approved all animal protocols used in this study.
Immunohistochemistry
Cryostat sections were stained with anti-cleaved caspase-3, anti-MIB-1, or anti-laminin antibodies as previously described [26]. Biotinylated-conjugated secondary antibodies followed by incubation with 3,3'-diaminobenzidine peroxidase substrate was used to detect primary Abs. Anti-MIB-1 stained sections were counterstained with Gill's hematoxylin solution. Anti-cleaved caspase 3 and anti-laminin stained sections were counterstained with methyl green. Proliferation, apoptotic, and microvessel density indices were determined by computer-assisted quantification using ImageJ Software (rsb.info.nih.gov/ij/) essentially as previously reported [17].
Statistical methods
Statistical analysis consisted of one-way ANOVA followed by the Tukey or Dunnet’s multiple-comparison-test using Prism (GraphPad software Inc., San Diego, CA). P < 0.05 was considered significant. All experiments reported here represent at least three independent replications. For the in vivo studies reported in Figures 3 and 4, a representative experiments was chosen to summarize at least three independent replications with a total of n = 15 animals per group). Data are represented as mean values ± standard deviation (SD).
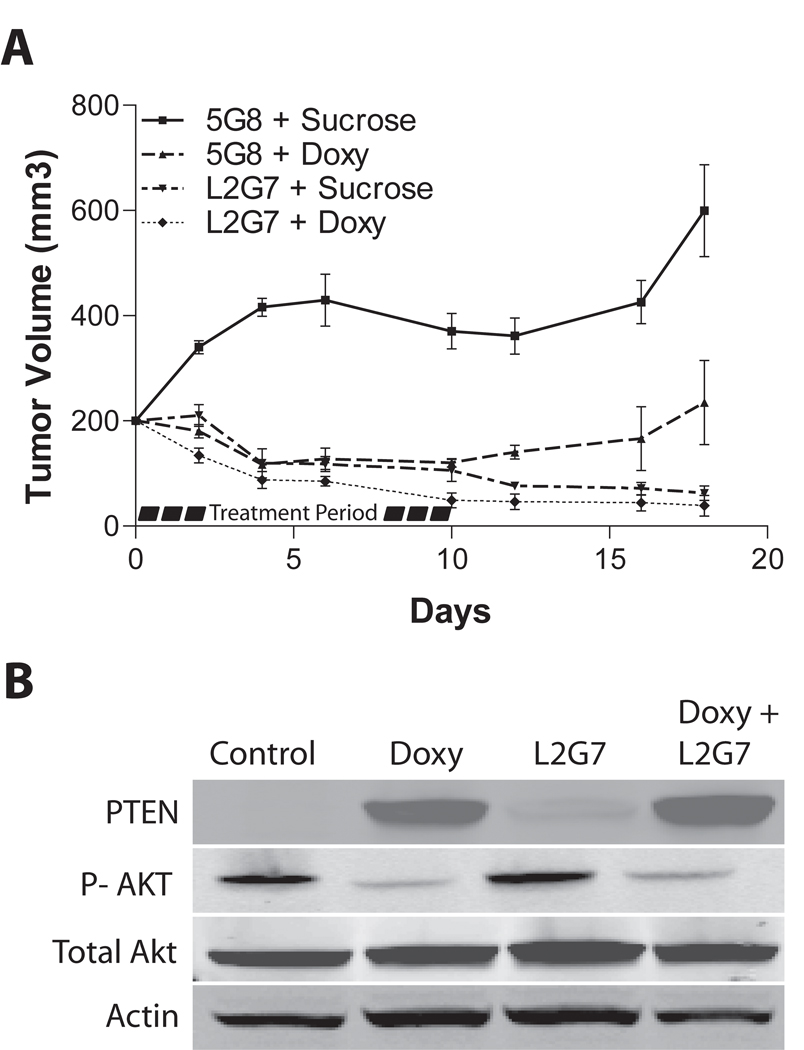
Figure 3. PTEN reconstitution and anti-HGF therapy alter tumor growth responses in subcutaneous glioma xenografts.
Pre-established U87-tetPTEN subcutaneous xenografts (200 mm3) were treated ± doxycyline (2 mg/ml in drinking water) with either anti-HGF L2G7 or control mAb 5G8 (1.25 mg/kg, i.p) on days 0,2,4,6 and 8. Doxycycline was withdrawn on Day 10. Xenografts were measured every alternate day and tumor volumes calculated as described in Materials and Methods. B) Immunoblot analysis of PTEN and phospho-AktSer473 in subcutaneous xenografts sacrificed on Day 6.
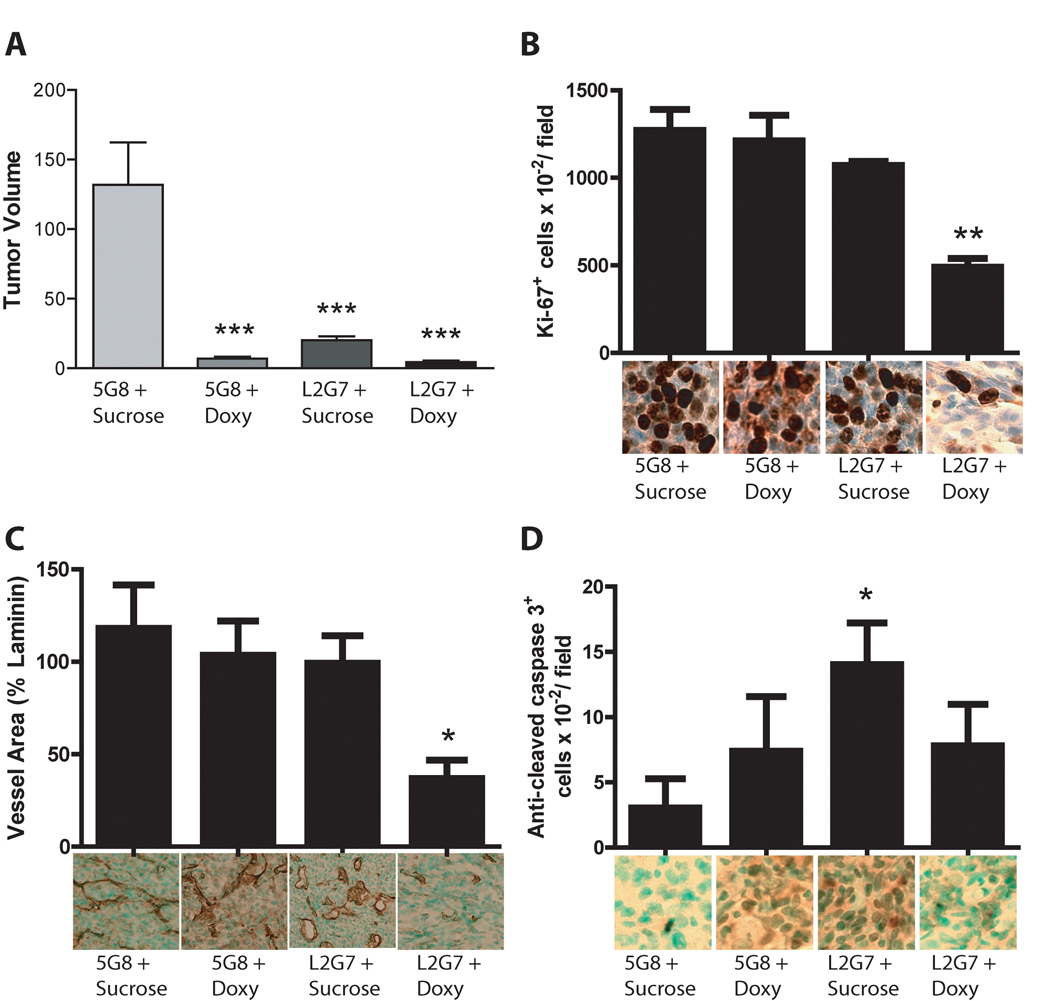
Figure 4. PTEN Reconstitution alters cell responses to anti-HGF therapeutics in orthotopic glioma xenografts.
Animals bearing U87-tetPTEN intracranial xenografts were treated ± doxycycline (2 mg/mL in drinking water) for 6 days with either anti-HGF or control mAb 5G8 (1.25mg/kg i.p.) for 6 days. Intracranial tumor xenograft sections were analyzed for tumor volume (A), cell proliferation (B), angiogenesis (C), and apoptosis (D) as described in Materials and Methods. *** = P < 0.001 compared to controls, * = P < 0.05 compared to controls, L2G7 alone or Doxy alone ** = P < 0.01 compared to control, L2G7 alone or Doxy alone
RESULTS
PTEN Reconstitution and c-Met inhibition decrease Akt activation and glioma cell growth
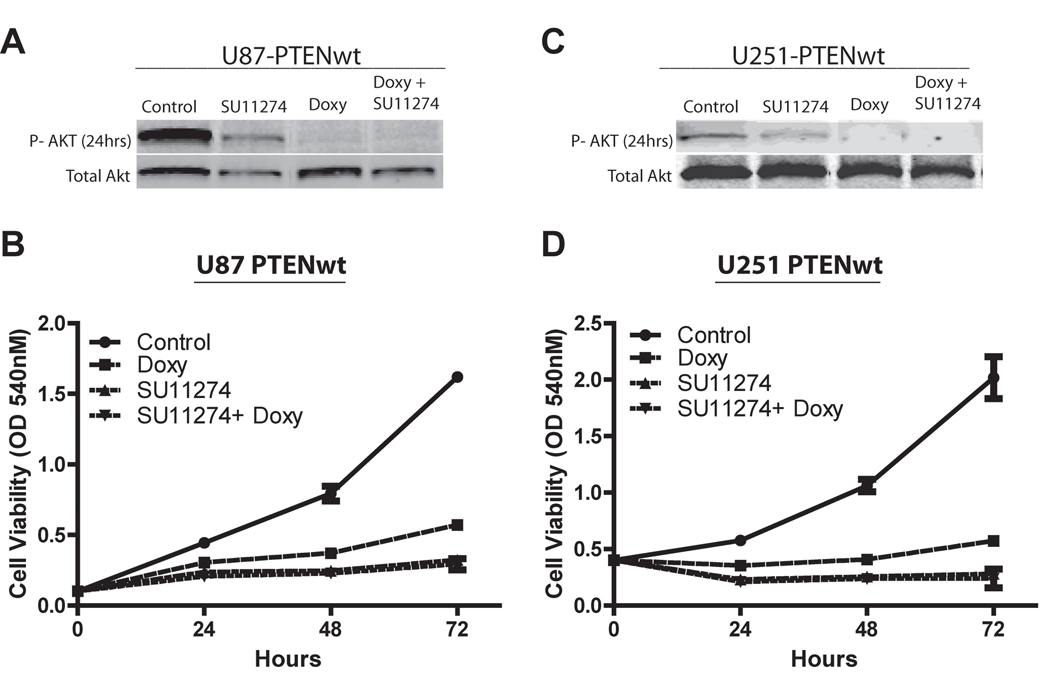
We found that PTEN reconstitution, c-Met pathway inhibition or their combination significantly decreased Akt activation and glioma growth as assessed by immunoblot analysis and cell viability assays, respectively. C-Met inhibition with 10 µM SU11274 decreased Akt activation by ~30% in both U87 and U251 glioma cell lines (P < 0.05), whereas PTEN reconstitution alone and in combination with 10 µM SU11274 decreased Akt activation by 90% in both cell lines (P < 0.05) (Figure 1A and C). Reconstituting PTEN in U87 and U251 glioma cells inhibited cell growth by ~70% and ~75%, respectively, when measured 72 hours after initiating treatment. C-Met inhibition alone or combined with PTEN reconstitution both inhibited cell growth by ~80% in U87 cells and by ~85% in U251 cells (Figure 1B and D). Thus, c-Met inhibition alone had a potent inhibitory effect on cell growth that was not altered by PTEN reconstitution.
Figure 1. PTEN reconstitution and c-Met inhibition decrease Akt activation and glioma cell growth responses.
U87-tetPTEN and U251-tetPTEN glioma cells were acclimated overnight in low serum (0.1% FBS), and then treated with the c-Met inhibitor SU11274 (10uM) ± doxycycline (2ug/mL). A, C) Immunoblot analysis of phospho-AktSer473 and total Akt were performed 24 hours after treatment. Cells were assayed 24, 48 or 72 hours after treatment for cell viability via MTT assay (B,D).
PTEN Reconstitution and c-Met inhibition alter glioma cell cycle progression
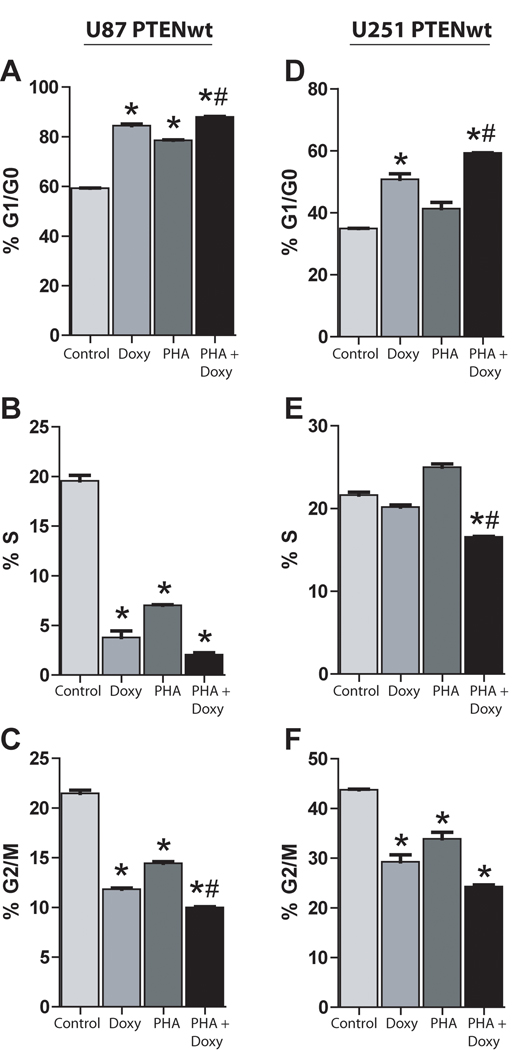
Cell cycle responses were examined to determine if PTEN alters cell proliferation responses to c-Met inhibition. U87 and U251 glioma cells treated with either 100 nM of PHA665752, doxycycline to induce PTEN expression, or their combination were subjected to cell cycle analysis via flow cytometry. PTEN reconstitution alone, Met inhibition alone or the combination each arrested cell cycle progression in the G1/G0 phase and decreased the percentage of cells in the G2/M phase in comparison to controls (P < 0.05) (Figure 2A, C, D and F). However, combining c-Met inhibition and PTEN restoration in both cell lines led to a modest statistically significant increase in G1/G0 cell cycle arrest compared to either c-Met inhibition or PTEN reconstitution alone (P < 0.01) (Figure 2A and D).
Figure 2. PTEN Reconstitution and Met inhibition alter glioma cell cycle progression.
U87-tetPTEN or U251-tetPTEN cells were acclimated overnight in low serum (0.1% FBS) and treated with the c-Met inhibitor PHA665752 (100 nM) ± doxycycline (2ug/mL) for 24 h before cells were fixed and labeled with propidium iodide. Cell cycle analysis was performed via flow cytometry and percentage of cells in the G1/G0 phase (A, D), S phase (B, E) and G2/M phase were determined. * = P < 0.05 compared to controls, # = P < 0.01 compared to PHA alone or Doxy alone
PTEN reconstitution and anti-HGF therapy alter tumor growth responses in subcutaneous glioma xenografts
We examined the effects of PTEN reconstitution, c-Met pathway inhibition, and their combination on the growth characteristics of glioma xenografts. U87-PTENwt cells were implanted subcutaneously in Nu/Nu mice and tumors were allowed to reach a size of ~200 mm3. Animals were then treated +/− doxycycline (2mg/mL in drinking water) with either control mAb (5G8) or neutralizing anti-HGF mAb (L2G7), 1.25mg/kg i.p. every alternate day for 10 days. We have previously shown that L2G7 therapy effectively inhibits c-Met receptor activation (phosphorylation) and downstream signaling in wild-type U87 glioma xenografts [17]. L2G7 alone, PTEN reconstitution alone, and combination L2G7 + PTEN reconstitution markedly inhibited tumor growth (Figure 3A). At day 10, corresponding with the last day of treatment, L2G7 alone or PTEN reconstitution alone inhibited U87 tumor xenograft growth by ~75%. Semiquantitative immunoblot analysis of tumor extracts revealed a substantial reduction in Akt activation (serine 473 phosphorylation) in response to PTEN reconstitution (Figure 3B). Combining L2G7 and PTEN reconstitution also markedly inhibited tumor growth by ~85%, (P < 0.05). There was a trend, which did not reach statistical significance, toward greater tumor growth inhibition in response to PTEN reconstitution + L2G7 when compared to either treatment alone.
PTEN Reconstitution alters glioma responses to anti-HGF therapeutics in orthotopic glioma xenografts
Glioma responses to PTEN reconstitution ± HGF:c-Met pathway inhibition were examined in more detail using an orthotopic intracranial model. U87- PTENwt cells were implanted to the caudate/putamen of Scid/beige mice. Beginning on post-implantation day 15, animals were treated +/− Doxycycline (2mg/ml in drinking water) with either 5G8 or L2G7 (1.25mg/kg i.p.) administered every alternate day for 6 days. Animals were sacrificed and tumor sizes quantified by morphometric histological analysis. PTEN reconstitution alone and L2G7 alone both reduced tumor xenograft size by ~90% and ~80%, respectively (Figure 4A). Furthermore, PTEN reconstitution + L2G7 inhibited tumor growth by ~95% (Figure 4A). The effects of PTEN reconstitution alone, HGF:c-Met pathway inhibition alone, or their combination on tumor growth were similar in orthotopic and subcutaneous U87PTENwt xenografts.
We analyzed orthotopic xenograft histological sections for the effects of PTEN reconstitution, HGF:c-Met pathway inhibition, or both on cell proliferation using anti-Ki67 staining (Figure 4B), angiogenesis using anti-laminin staining (Figure 4C), and apoptosis using anti-cleaved caspase 3 staining (Figure 4D). Neither PTEN reconstitution nor L2G7 monotherapy affected tumor Ki-67 labeling or tumor vascular density when compared to controls. However, PTEN reconstitution + L2G7 inhibited cell proliferation and angiogenesis by ~60% and ~65%, respectively, consistent with cooperativity (P <0.05). There was a trend of 2-fold increase in apoptosis in response to PTEN reconstitution alone that was not statistically significant. L2G7 therapy alone statistically significantly increased tumor cell apoptosis 4-fold (P <0.05). Glioma cell apoptosis in response to PTEN reconstitution + L2G7 was significantly less than that seen in response to L2G7 alone. Thus, while PTEN reconstitution and anti-HGF therapy cooperated to inhibit tumor cell proliferation and angiogenesis, PTEN reconstitution rendered tumor cells less sensitive to developing apoptosis in response to anti-HGF therapy.
DISCUSSION
Hyperactivation of the oncogenic serine/threonine kinase Akt occurs in up to 65% of glioblastoma and is very common in other brain and systemic malignancies [1; 27]. AKT hyperactivity most commonly results from amplifications, mutations, deletions, or hyperactivation of upstream signaling molecules such as the receptor tyrosine kinases (RTKs) EGFR, PDGFR, and c-Met, the intracellular second messengers PI3K and through loss of the tumor suppressor PTEN [1; 6; 7; 28]. Activating Akt mutations occur less commonly and independent of RTK gains and PTEN loss [1; 28]. Considerable effort toward developing targeted therapeutics has focused on inhibiting RTK-Akt signaling due to its importance in regulating tumor cell growth, invasion, survival, and sensitivity to DNA damaging agents [1; 29]. The influence of PTEN on tumor responses to RTK inhibition remains incompletely characterized. We previously reported that inhibiting HGF/c-Met signaling in an HGF+ /Met+/ PTEN-null glioma model robustly inhibits tumor-initiating capacity, tumor xenograft growth, and resistance to cytotoxic therapeutics [2; 17; 24]. Here we show that reconstituting PTEN alters the response of a glioblastoma xenograft model to HGF/c-Met pathway inhibition, namely by enhancing the inhibition of glioma cell proliferation and tumor angiogenesis but diminishing tumor cell apoptosis.
One explanation for the lack of an additive in vivo anti-tumor effect from combining PTEN restoration and c-Met pathway inhibition could be attributed to the high dependency of these cell lines on c-Met and PTEN. In our experiments, we aim to isolate the effects of c-Met and PTEN on the tumor growth response, whereas clinical specimens demonstrate heterogeneity in their drivers of tumor progression. Under conditions where PTEN and c-Met/HGF are predominant drivers of tumor malignancy, additive anti-tumor effects might have been detected.
HGF/c-Met pathway signaling is recognized to support the malignant behavior of subsets of multiple solid malignancies [15]. Understanding how distinct molecular backgrounds influence tumor responses to HGF/c-Met pathway inhibitors is of considerable importance. Based on an elegant Phase II trial in patients with glioblastoma, Mellinghoff et al concluded that PTEN loss renders tumors unresponsive to the inhibition of EGFR and potentially other RTKs [30]. Our current findings using a well characterized isogenic glioblastoma model are consistent with previous findings showing that malignancies can robustly respond to RTK inhibition even in the absence of PTEN [17; 20; 31]. Differences in the RTK pathways examined, c-Met versus EGFR, differences in tumor genetic heterogeneity, or differences in the magnitudes of RTK inhibition might account for the differences in these clinical and pre-clinical results. The sensitivity of PTEN-null U87 xenografts to RTK inhibition suggests that down-regulating PI3-kinase activity can compensate for the absence of the lipid phosphatase PTEN in reducing tumor levels of PIP3 and thereby reduce the overall flux to Akt activation. In prior studies, we showed that expressing the constitutively active RTK EGFRvIII renders Met-dependent tumors insensitive to Met pathway inhibitors [31]. This effect is likely mediated at least in part by the fact that EGFRvIII signaling is parallel and redundant with c-Met signaling and maintains the flux through oncogenic PI3K/Akt and Ras/MAPK relatively independent of c-Met.
Our in vitro findings confirm the results of Li et al showing that HGF:c-Met pathway inhibition and adenoviral-based PTEN restoration additively inhibit proliferation and cell cycle progression in PTEN-null glioblastoma cells[32]. Our current results expand on these findings by examining specific tumor cell responses within the more complex orthotopic in vivo context. We were surprised that PTEN reconstitution and anti-HGF therapy in combination cooperatively inhibited tumor cell proliferation and angiogenesis in the absence of a comparable effect on tumor growth. This is likely explained by the apoptotic response that was greatest in response to anti-HGF therapy alone, ~2-fold greater than the response to the combination. The greater apoptotic response to anti-HGF alone suggests that in the absence of PTEN, c-Met inhibition preferentially inhibits tumor growth via a pro-apoptotic mechanism. This finding is consistent with our prior studies in U87 glioma xenografts and HGF+/Met+ medulloblastoma models [31; 33].
CONCLUSION
Taken together, our current findings reveal potentially important differences in the anti-tumor response to c-Met inhibition depending upon PTEN status. While PTEN status may not necessarily alter the magnitude of tumor growth inhibition to anti-HGF therapy, PTEN status does influence the magnitude of proliferation inhibition relative to apoptosis promotion. These results provide new insights into the molecular basis for different tumor responses to HGF:c-Met pathway inhibition and suggest that the relative benefits of combining HGF/c-Met pathway inhibitors with other anti-proliferative agents or pro-apoptotic agents will be influenced by tumor PTEN status.
Acknowledgments
Funding Sources: This work was supported by NIH grants NS32148 (JL) the United Negro College Fund/Merck Science Initiative (CRG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 2.Bowers DC, Fan S, Walter KA, Abounader R, Williams JA, Rosen EM, et al. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 2000;60:4277–4283. [PubMed] [Google Scholar]
- 3.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 4.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 5.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 6.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 8.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 9.Cheney IW, Johnson DE, Vaillancourt MT, Avanzini J, Morimoto A, Demers GW, et al. Suppression of tumorigenicity of glioblastoma cells by adenovirus-mediated MMAC1/PTEN gene transfer. Cancer Res. 1998;58:2331–2334. [PubMed] [Google Scholar]
- 10.Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–5008. [PubMed] [Google Scholar]
- 11.Furnari FB, Lin H, Huang HS, Cavenee WK. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci U S A. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura M, Gu J, Takino T, Yamada KM. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–449. [PubMed] [Google Scholar]
- 13.Abounader R, Reznik T, Colantuoni C, Martinez-Murillo F, Rosen EM, Laterra J. Regulation of c-Met-dependent gene expression by PTEN. Oncogene. 2004;23:9173–9182. doi: 10.1038/sj.onc.1208146. [DOI] [PubMed] [Google Scholar]
- 14.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 15.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 16.Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- 17.Kim KJ, Wang L, Su YC, Gillespie GY, Salhotra A, Lal B, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 18.Martens T, Schmidt NO, Eckerich C, Fillbrandt R, Merchant M, Schwall R, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res. 2006;12:6144–6152. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Yao W, Newton RC, Scherle PA. Targeting the c-MET signaling pathway for cancer therapy. Expert Opin Investig Drugs. 2008;17:997–1011. doi: 10.1517/13543784.17.7.997. [DOI] [PubMed] [Google Scholar]
- 20.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 21.Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol. 2003;23:6139–6149. doi: 10.1128/MCB.23.17.6139-6149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reznik TE, Sang Y, Ma Y, Abounader R, Rosen EM, Xia S, et al. Transcription-dependent epidermal growth factor receptor activation by hepatocyte growth factor. Mol Cancer Res. 2008;6:139–150. doi: 10.1158/1541-7786.MCR-07-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lal B, Xia S, Abounader R, Laterra J. Targeting the c-Met pathway potentiates glioblastoma responses to gamma-radiation. Clin Cancer Res. 2005;11:4479–4486. doi: 10.1158/1078-0432.CCR-05-0166. [DOI] [PubMed] [Google Scholar]
- 25.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 26.Abounader R, Lal B, Luddy C, Koe G, Davidson B, Rosen EM, et al. In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. Faseb J. 2002;16:108–110. doi: 10.1096/fj.01-0421fje. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 28.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386–393. doi: 10.1158/1535-7163.MCT-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–381. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- 31.Lal B, Goodwin CR, Sang Y, Foss CA, Cornet K, Muzamil S, et al. EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Mol Cancer Ther. 2009;8:1751–1760. doi: 10.1158/1535-7163.MCT-09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Guessous F, DiPierro C, Zhang Y, Mudrick T, Fuller L, et al. Interactions between PTEN and the c-Met pathway in glioblastoma and implications for therapy. Mol Cancer Ther. 2009;8:376–385. doi: 10.1158/1535-7163.MCT-08-0627. [DOI] [PubMed] [Google Scholar]
- 33.Binning MJ, Niazi T, Pedone CA, Lal B, Eberhart CG, Kim KJ, et al. Hepatocyte growth factor and sonic Hedgehog expression in cerebellar neural progenitor cells costimulate medulloblastoma initiation and growth. Cancer Res. 2008;68:7838–7845. doi: 10.1158/0008-5472.CAN-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]