Abstract
OBJECTIVES:
We investigated the effects of chronic (eight weeks) low- to moderate-intensity swimming training on thermal pain sensitivity in streptozotocin-induced diabetic female rats.
METHODS:
Female Wistar rats (n = 51) were divided into the following groups: trained streptozotocin-induced diabetic rats [hyperglycemic trained (HT)], sedentary streptozotocin-induced diabetic rats [hyperglycemic sedentary (HS)], normoglycemic trained rats (NT) and normoglycemic sedentary rats (NS). Diabetes was induced by a single injection of streptozotocin (50 mg/kg, i.p.). One day after the last exercise protocol (60 min/day, five days/week for eight weeks) in the trained groups or after water stress exposure (ten min/twice a week) in the sedentary groups, the rats were subjected to a hot plate test.
RESULTS:
After eight weeks of swimming training, streptozotocin-induced diabetic rats presented a significantly lower body mass (trained: 219.5±29 g, sedentary: 217.8±23 g) compared with the normoglycemic groups (trained: 271±24 g, sedentary: 275.7±32 g). Interestingly, we did not find differences in blood glucose levels (mg/dl) between the trained and sedentary groups of the hyperglycemic or normoglycemic rats (HT: 360.2±66.6, HS: 391.7±66.7, NT: 83.8±14.0, NS: 77.5±10.1). In the hot plate test, the rats from the HT group presented a significantly lower latency than the other rats (HT: 11.7±7.38 s, HS: 7.02±7.38 s, NT: 21.21±7.64 s, NS: 22.82±7.82 s).
CONCLUSION:
Low-to-moderate swimming training for a long duration reduces thermal hyperalgesia during a hot plate test in streptozotocin-induced diabetic female rats.
Keywords: Diabetes mellitus, Diabetic neuropathy, Pain sensitivity, Physical exercise, Swimming training, Hot plate test
INTRODUCTION
According to the International Diabetes Federation,1 diabetes mellitus (DM) is a great challenge for public health. Diabetes is a serious problem that is increasing worldwide, and estimates predict that 380 million people will be affected in 2025. Experimental research on diabetic neuropathy is usually carried out using genetic animal models or chemically induced diabetic animal models to provide information on the underlying mechanisms and to evaluate potential therapies, and both models have enhanced our understanding of the disease.2
Approximately 60 to 70% of people with diabetes present some neuropathy3, and studies have estimated that 50 to 70% of all nontraumatic amputations in the USA are diabetic patients with neuropathy. Diabetic neuropathy may be classified as peripheral, autonomic, proximal, focal, or multifocal.4 Diabetic peripheral neuropathy has been reported to be the most common neuropathy, and diabetic peripheral neuropathy leads to pain and sensitivity loss, which reduces life expectancy and increases health costs.4,6 One of the most elusive symptoms is pain, which is typically characterized by mechanical and thermal hyperalgesia or allodynia.2 Common complaints include a constant burning discomfort, severe hyperesthesia, deep aching pain, stabbing or “electric shock”-like sensations and sensations in the lower limbs.5
Early prevention of diabetic neuropathy is necessary to avoid serious complications that can begin in the asymptomatic period of onset. Exercise is useful for lowering plasma glucose levels both during exercise and following exercise. In addition, exercise has been shown to increase insulin sensitivity. Skeletal muscles are the major site for metabolic fuel consumption in the resting state, and increased muscle activity during vigorous aerobic exercise greatly increases fuel requirements.7 Studies in animal models have indicated a general improvement in the ability of diabetic animals to conduct and sustain chronic physical exercise, especially regarding the metabolism of energy substrates and secreting hormones.8
Gender differences have been evaluated in experimentally induced pain paradigms through diverse methods, including pressure, electrical, ischemic, and thermal chemical stimuli. According to a review published by Fillingim et al.,9 females have a lower pain threshold than males, and evidence has shown that sexual hormones are involved in this process. Although the mechanisms involving the role of estrogen in pain perception are not entirely clear, changes in plasma estrogen levels can influence pain sensitivity by either decreasing or increasing excitability of nerve fibers and brain cells.10 Taken together, these data support the difference between males and females regarding pain sensitivity and suggest that females exhibit greater sensitivity to painful stimulation.9 Data regarding thermal sensitivity in diabetic female rats and the effects of exercise, however, are lacking; thus, the present study examined female rats.
Although previous studies have already examined the effects of physical exercise on pain sensitivity,8 to the best of our knowledge, there is no evidence regarding the effects of physical exercise on pain sensitivity in diabetic female animals. Moreover, exercise training is a nonpharmacological and noninvasive treatment method. In addition, new data are always well received in both scientific and clinical practice. Therefore, this study was undertaken to evaluate the effects of swimming training on the thermal sensitivity of streptozotocin-induced diabetic female rats using a hot plate test.
METHODS
Animals
A total of 51 female Wistar rats (160-250 g) were used in the present study. The care and use of the animals followed the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (National Institutes of Health Publication No. 96-23, revised 1996) and was approved by the Ethics Committee in Research of the Faculdade de Medicina de Marília (protocol number 428/09). The rats were randomly distributed into four groups: normoglycemic sedentary (NS, n = 15), normoglycemic trained (NT, n = 16), hyperglycemic sedentary (HS, n = 9) and hyperglycemic trained (HT, n = 11). The rats were housed in plastic cages (3-4 rats/cage) under a 12-h dark–light cycle at 22°C and 60% air humidity with free access to water and rodent pellet chow. At the end of the experiment, the rats were sacrificed by CO2 exposure.
Streptozotocin-induced diabetes
Diabetes was induced in the hyperglycemic groups by a single intraperitoneal (i.p) injection of streptozotocin (STZ, 50 mg/kg). The STZ was dissolved in a citrate buffer solution (0.1 mol/L citric acid, 0.1 mol/L sodium citrate, pH 4.5). Streptozotocin destroys pancreatic beta cells and induces hyperglycemia. In the normoglycemic groups, we applied a single injection of citrate buffer. Two days after STZ treatment, blood glucose levels were measured to confirm a hyperglycemic state. Blood was obtained from a small nick in the tail and measured with a glucometer (Optium Xceed). The hyperglycemic rats presented blood glucose levels ≥250 mg/100 mL. Plasma glucose levels in the normoglycemic animals remained normal for the duration of the study.11,14
Exercise training protocol
Exercise training was performed in a swimming pool (112 cm×80 cm×50 cm) filled with tap water that was warmed to approximately 27°C, and the exercise intensity was progressively increased. In the first week, the rats swam for ten minutes every day. In the second week, the swimming time was increased each day until the animals could swim for 60 min while wearing a caudal dumbbell weighing 5% of their body weight (overload).15 In each exercise session, eight to ten rats were placed together in the swimming pool to perform the exercise. During the exercise period, the normoglycemic groups were exposed to water stress for ten minutes, twice a week. This swimming protocol has previously been characterized as low to moderate intensity (with a long duration) based on improvements in muscle oxidative capacity.15
Hot plate test
One day after the end of the exercise training protocol period, a hot plate test was conducted as previously described.16 This test measures the time that elapses before the rat demonstrates hind paw licking/shaking and jumping, which indicate pain in response to the applied heat. The hot plate was maintained at 50±1°C, and the animals were placed into a Perspex cylinder on the heated stage. Response latency was measured by recording the time between placement in the cylinder and shaking or licking the paws. The cut-off time was set at 35 seconds to minimize skin injury.
Statistical analysis
We applied the Shapiro-Wilk test to verify the normality of the distributions. A two-way analysis of variance (ANOVA) was used to verify the differences between the normal distributions, and the Kruskal–Wallis test was used to assess differences between nonparametric distributions. The differences were considered significant when the probability of a Type I error was lower than 5% (p<0.05).
RESULTS
STZ-induced diabetic animals presented lower body mass values (HT: 219.8+29 g, HS: 217.8+23 g) compared with the normoglycemic animals (NT: 271+24 g, NS: 275.7+32 g; p = 0.0001).
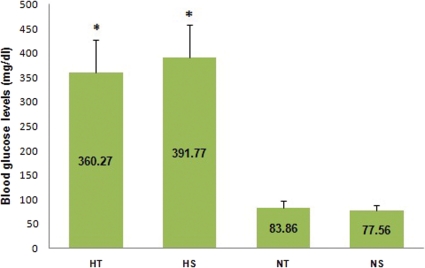
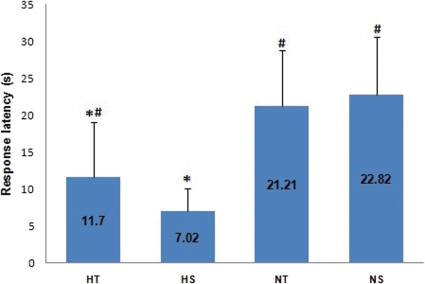
Figure 1 shows that the rats in the STZ-induced diabetic groups (i.e., HS and HT) presented higher blood glucose levels than the normoglycemic rats. Figure 2 shows that the exercise training protocol influenced the response latency in the HT group. Indeed, the response latency in the HT group was higher than the response latency in the HS group.
Figure 1.
Blood glucose levels hot plate of the trained STZ-induced diabetic (HT, n = 11), sedentary STZ-induced diabetic (HS, n = 9), normoglycemic trained (NT, n = 14) and normoglycemic sedentary (NS, n = 16) groups before the hot plate test. *p<0.0001: different from NS and NT.
Figure 2.
Response latency of the trained STZ-induced diabetic (HT, n = 11), sedentary STZ-induced diabetic (HS, n = 9), normoglycemic trained (NT, n = 14) and normoglycemic sedentary (NS, n = 16) groups. The Kruskal Wallis test was used. *p<0.01: different from NS and NT; #p<0.05: different from HS.
DISCUSSION
The aim of the present study was to evaluate the effects of swimming on thermal sensitivity in STZ-induced diabetic female rats. We observed that the HS rats had the lowest latency time of the four groups of rats. Our data suggested that swimming attenuates the hyperalgesia caused by acute hyperglycemia in STZ-induced diabetic rats.
Chronic hyperglycemia is an important factor that can trigger many complications. Previous studies have suggested that attenuation of chronic hyperglycemia may slow or even prevent microvascular and macrovascular complications, such as retinopathy, nephropathy and neuropathies.8 Although the pathogenesis of diabetic neuropathy is multifactorial, increased blood glucose levels are likely the major factor. Moreover, acute hyperglycemia is followed by hyperalgesia, whereas chronic hyperglycemia is followed by hypoalgesia due to impairment of the peripheral neurons.17
Previous studies have evaluated the effects of exercise on diabetic nerve regeneration.18 Selagzi and colleagues19 investigated the effects of swimming training on diabetic peripheral neuropathy development in STZ-induced diabetic male rats. Similar to our findings, they observed that the swimming exercise protocol did not influence blood glucose levels; however, the exercise restored body weight, compound muscle action potential amplitude and potential latency, which are parameters that define motor dysfunction in diabetic animals. In addition, Cunha et al.20 observed that STZ induces mechanical hypernociception, which does not depend on hyperglycemia. Our findings revealed new aspects on how exercise influences diabetic rats. Unlike previous studies, we evaluated female rats and thermal sensitivity rather than motor dysfunction. Furthermore, we found that swimming training improved acute hyperalgesia without influencing blood glucose levels.
Some hypotheses may be raised regarding the observed effects of swimming on thermal sensitivity. Because studies have shown that hypernociception does not depend on a hyperglycemic state,20 we believe that exercise training attenuates hyperalgesia via mechanisms that are not related to a decrease in blood glucose levels. Koltyn et al.21 indicated that the increase in endogenous opioid levels induced by exercise may lead to a feedback inhibition of acute pain in peripheral, spinal and supraspinal neurons. In addition, Stagg and colleagues22 reported that aerobic exercise increased the endogenous opioid concentration in brainstem regions involved in pain regulation and decreased neuropathic pain in a rat neuropathic pain model. It is also plausible that the antioxidant effects of chronic exercise23 attenuated the acute hyperalgesia caused by the hyperglycemic-induced nerve injuries; however, the present study did not measure the antioxidant or pro-oxidant enzyme activities.
The hot plate test was applied 24 h after the last swimming protocol. Therefore, our data could also be the result of an acute effect of the last exercise training because thermal sensation usually increases during exercise.24 Furthermore, we speculated that short-term influences on thermal sensation were due to increases in local skin and brain temperatures. A previous investigation suggested that high brain temperature influences the functions related to the nervous system (alertness, contentment, calmness, and thermal comfort).25 Nonetheless, the literature is unclear on the influence of body temperature on perception.26 Future studies should be performed to investigate the acute effects of exercise during hot plate testing of STZ-induced diabetic animals.
The data from our study indicate that swimming exercise (mild to moderate) is able to reduce thermal hyperalgesia in STZ-induced hyperglycemic female rats. These findings corroborate the results of Bento-Silva et al.,27 which demonstrated that low-intensity swimming exercise decreases the nociception induced by thermal and chemical stimulus after exercise; however, their methodology was different with respect to the intensity, the duration of exercise and the duration of painful stimuli. Moreover, Kuphal et al.28 demonstrated that swimming reduces hyperalgesia induced by intraplantar injection of formalin-induced partial injury in rodent peripheral nerves. Therefore, the difference between our study and Bento-Silva et al. study,27 suggests us to be careful in interpreting our findings.
Unlike our results for the normoglycemic groups (i.e., swimming did not affect the latency during the hot plate test), Mazzardo-Martins et al.29 found that high-intensity swimming reduced acute pain stimulated by intraperitoneal injection of acetic acid (chemical noxious stimulus) in normoglycemic rats. It is believed that the method used to analyze pain sensitivity may influence this result because Koltyn et al.21 indicated that the antinociceptive effect of exercise is more consistently found in studies using electrical or pressure stimulation compared with studies that used temperature to produce pain. Interestingly, the effects of exercise on pain sensitivity are not consistent in the literature, and the controversies seem to be related to different methodologies. Indeed, there are differences in the type, intensity and duration of exercise beyond the pain stimulus protocol.30 Furthermore, the antinociceptive effect of exercise is likely short term, assuming the absence of a difference in the normoglycemic groups, in which the effect was observed 24 h after the last day of physical exercise.
Except for strict control of the blood glucose level, there are few effective ways to influence or delay the natural progression of diabetic neuropathy because of the limitations of current drug therapies. Despite the challenges ahead, the future promises more effective treatments for diabetes and its complications with an aim toward improving patients' quality of life. The present study showed that a swimming exercise protocol influences the thermal nociceptive stimulus in STZ-induced diabetic female rats by attenuating the hyperalgesia caused by the hyperglycemic state. We suggest that exercise provides protection against diabetic neuropathy in rats as a result of multiple mechanisms and provides a means of avoiding the potential deterioration of diabetic neuropathy.
The majority of the data examining the effects of exercise on STZ-induced diabetic animals come from male animals. The reason for this is that the pathogenetic mechanisms in males are not influenced by fluctuations in hormonal activity.31 Interestingly, no study has shown that exercise training can improve acute hyperalgesia in STZ-induced diabetic female rats, and it is still unclear if molecular mechanisms in different genders can be influenced by hormones to improve diabetic neuropathy in exercising rats. The molecular mechanisms underlying the different findings of the present study in female rats require further investigation.
There were several issues in the present study that warranted discussion. First, we did not confirm the involvement of peripheral neurons affected by hyperglycemia. Second, although we observed that exercise attenuated acute hyperalgesia in STZ-induced diabetic female rats, which is a feature of early diabetic neuropathy, we should be careful in extrapolating our data to humans with diabetic neuropathy. Third, we did not measure any parameters to determine whether the rats were effectively trained, such as resting bradycardia or citrate synthase enzyme activity. We controlled training progression by starting with ten minutes of exercise a day for the first week. In the second week, the session times were increased by ten minutes per day until they reached 60 minutes, at which point the rats wore caudal dumbbells weighing 5% of their body weight. Throughout the sessions, the examiner observed the rats to ensure that they were swimming. This is an accepted training protocol in the literature.13,32,34 Finally, because the hot plate test was performed one day after the end of training, the findings could be the result of the acute effects of the last exercise session. Nevertheless, if the animals were tested one week or more after the last exercise session, the effects of two months of exercise training could still be partially restored.
In conclusion, low to moderate swimming training for a long duration in STZ-induced diabetic female rats attenuates thermal hyperalgesia in a hot plate test.
ACKNOWLEDGEMENTS
This research was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
*Study performed at Faculdade de Filosofia e Ciência, UNESP, Marília.
REFERENCES
- 1.International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. World Health Organization. 2006.
- 2.Cunha JM, Funez MI, Cunha FQ, Parada CA, Ferreira SH. Streptozotocin- induced mechanical hypernociception is not dependent on hiperglycemia. Brazilian Journal of Medical and Biological Research. 2009;42:197–206. doi: 10.1590/s0100-879x2009000200008. 10.1590/S0100-879X2009000200008 [DOI] [PubMed] [Google Scholar]
- 3.Pagano L, Proietto M, Biondi R. Diabetic peripheral neuropathy: reflections and drug-rehabilitative treatment. Recenti Progressi Medicine. 2009;100:337–42. [PubMed] [Google Scholar]
- 4.Hung JW, Liou CW, Wang PW, Yeh SH, Lin LW, Lo SK, et al. Effect of 12-week Tai Chi Chuan exercise on peripheral nerve modulation in patients with type 2 Diabetes Mellitus. J Rehabil Med. 2009;41:924–9. doi: 10.2340/16501977-0445. 10.2340/16501977-0445 [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJM, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–86. doi: 10.2337/diacare.27.6.1458. 10.2337/diacare.27.6.1458 [DOI] [PubMed] [Google Scholar]
- 6.Israeili ZH. Advances in the Treatment of Type 2 Diabetes Mellitus. Am J Ther. 2009 Oct 14; doi: 10.1097/MJT.0b013e3181afbf51. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Sridhar B, Haleagrahara N, Bhat R, Kulur AB, Avabratha S, Adhikary P. Increase in the heart rate variability with deep breathing in diabetic patientes after 12-month exercise training. Tohoku J Exp Med. 2010;220:107–113. doi: 10.1620/tjem.220.107. 10.1620/tjem.220.107 [DOI] [PubMed] [Google Scholar]
- 8.Vancini RL, Lira CAB. Aspectos gerais do diabetes mellitus e exercício. Centro de Estudos de Fisiologia do Exercício. Universidade federal de São Paulo. 2004 [Google Scholar]
- 9.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. 10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns BE, Gazerani P. Sex-related differences in pain. Maturitas. 2009;63:292–6. doi: 10.1016/j.maturitas.2009.06.004. 10.1016/j.maturitas.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Coskun O, Ocakci A, Bayraktaroglu, Kanter M. Exercise Training prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Tohoku J Exp Med. 2004;203:145–54. doi: 10.1620/tjem.203.145. 10.1620/tjem.203.145 [DOI] [PubMed] [Google Scholar]
- 12.Farah Vde M, De Angelis K, Joaquim LF, Candido GO, Bernardes N, Fazan R, Jr, et al. Autonomic modulation of arterial pressure and heart rate variability in hypertensive diabetic rats. Clinics. 2007;62:477–82. doi: 10.1590/s1807-59322007000400015. 10.1590/S1807-59322007000400015 [DOI] [PubMed] [Google Scholar]
- 13.Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics. 2009;64:235–44. doi: 10.1590/S1807-59322009000300015. 10.1590/S1807-59322009000300015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Movahedian A, Zolfaghari B, Sajjadi SE, Moknatjou R. Antihyperlipidemic effect of peucedanum pastinacifolium extract in streptozotocin-induced diabetic rats. Clinics. 2010;65:629–33. doi: 10.1590/S1807-59322010000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medeiros A, Oliveira EM, Gianolla R, Casarini DE, Negrão CE, Brum PC. Swimming training increases cardiac vagal activity and induces cardiac hypertrophy in rats. Braz J Med Biol Res. 2004;37:1909–17. doi: 10.1590/s0100-879x2004001200018. 10.1590/S0100-879X2004001200018 [DOI] [PubMed] [Google Scholar]
- 16.Eddy NB, Leimback DJ. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- 17.Gagliardi ART, Neuropatia diabética periférica. J Vasc Bras. 2003;2:67–74. [Google Scholar]
- 18.Balducci S, Iacobellis G, Parisi L, Di Biasi N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20:216–23. doi: 10.1016/j.jdiacomp.2005.07.005. 10.1016/j.jdiacomp.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 19.Malysz T, Ilha J, Nascimento PS, Angelis KD, Schaan BD, Achaval M. Beneficial effects of treadmill training in experimental diabetic nerve regeneration. Clinics. 2010;65:1329–37. doi: 10.1590/S1807-59322010001200017. 10.1590/S1807-59322010001200017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selagzi H, Buyukakilli B, Cimen B, Yilmaz N, Erdogan S. Protective and therapeutic effects of swimming exercise training on diabetic peripheral neuropathy of streptozotocin-induced diabetic rats. J Endocrinol Invest. 2008;31:971–8. doi: 10.1007/BF03345634. [DOI] [PubMed] [Google Scholar]
- 21.Cunha JM, Funez MI, Cunha FQ, Parada CA, Ferreira SH. Streptozotocin-induced mechanical hypernociception is not dependent on hyperglycemia. Braz J Med Biol Res. 2009;42:197–206. doi: 10.1590/s0100-879x2009000200008. 10.1590/S0100-879X2009000200008 [DOI] [PubMed] [Google Scholar]
- 22.Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. 10.2165/00007256-200029020-00002 [DOI] [PubMed] [Google Scholar]
- 23.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–8. doi: 10.1097/ALN.0b013e318210f880. 10.1097/ALN.0b013e318210f880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perse M, Injac R, Strukelj B, Cerar A. Effects of high-fat mixed-lipid diet and exercise on the antioxiidant system in skeletal and cardiac muscles of rats with colon carcinoma. Pharmacol Rep. 2009;61:909–16. doi: 10.1016/s1734-1140(09)70148-3. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom B, Berg K, Vogt Lorentzen F. Human peripheral rewarming during exercise in the cold. J Appl Physiol. 1970;29:191–9. doi: 10.1152/jappl.1970.29.2.191. [DOI] [PubMed] [Google Scholar]
- 26.Salerian AJ, Saleri NG, Salerian JA. Brain temperature may influence mood: a hypothesis. Med Hypotheses. 2008;70:497–500. doi: 10.1016/j.mehy.2007.06.032. 10.1016/j.mehy.2007.06.032 [DOI] [PubMed] [Google Scholar]
- 27.Muller MD, Muller SM, Ryan EJ, Bellar DM, Kim CH, Glickman EL. Pain and thermal sensation in the cold: the effect of interval versus continuous exercise. Eur J Appl Physiol. 2010 Nov 17; doi: 10.1007/s00421-010-1726-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Bento-Silva MT, Santos MAP, Almeida FEC. Exercise of light intensity decrease pain induced for thermal and chemical stimulus in rats. Braz J Biomotric. 2010;4:14–23. [Google Scholar]
- 29.Kuphal KE, Fibuch EE, taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8:989–97. doi: 10.1016/j.jpain.2007.08.001. 10.1016/j.jpain.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 30.Mazzardo-Martins L, Martins DF, Marcon R, Santos UD, Speckhann B, Gadotti VM, et al. High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. J Pain. 2010;11:1384–93. doi: 10.1016/j.jpain.2010.03.015. 10.1016/j.jpain.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Souza JB. Poderia a atividade física induzir analgesia em pacientes com dor crônica. Rev Bras Med Esp. 2009;14:2. [Google Scholar]
- 32.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest. 2003;112:302–7. doi: 10.1172/JCI19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogihara CA, Schoorlemmer GH, Levada AC, Pithon-Curi TC, Curi R, Lopes OU, et al. Exercise changes regional vascular control by commissural NTS in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R291–7. doi: 10.1152/ajpregu.00055.2009. 10.1152/ajpregu.00055.2009 [DOI] [PubMed] [Google Scholar]
- 34.Serra AJ, Santos MH, Bocalini DS, Antônio EL, Levy RF, Santos AA, et al. Exercise training inhibits inflammatory cytokines and more than prevents myocardial dysfunction in rats with sustained beta-adrenergic hyperactivity. J Physiol. 2010;588:2431–42. doi: 10.1113/jphysiol.2010.187310. 10.1113/jphysiol.2010.187310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bocalini DS, Carvalho EV, de Sousa AF, Levy RF, Tucci PJ. Exercise training-induced enhancement in myocardial mechanics is lost after 2 weeks of detraining in rats. Eur J Appl Physiol. 2010;109:909–14. doi: 10.1007/s00421-010-1406-x. 10.1007/s00421-010-1406-x [DOI] [PubMed] [Google Scholar]