Abstract
Background. Respiratory syncytial virus (RSV) is the leading cause of bronchiolitis and viral death in infants. Despite decades of research with traditional or subunit vaccine approaches, there are no approved RSV vaccines. New approaches are therefore urgently needed to develop effective RSV vaccines.
Methods. We developed viruslike particles (VLPs) consisting of an influenza virus matrix (M1) protein core and RSV-F or -G on the surface. We tested the immunogenicity and vaccine efficacy of these VLPs (RSV-F, RSV-G) in a mouse model.
Results. Intramuscular vaccination with RSV-F or RSV-G VLPs elicited IgG2a dominant RSV-specific immunoglobulin G (IgG) antibody responses against RSV-A2 viruses in both serum and lung extract. Mice immunized with VLPs (RSV-F or RSV-G) showed higher viral neutralizing antibodies in vitro and significantly decreased lung virus loads in vivo after live RSV-A2 challenge. RSV-G VLPs showed better protective efficacy than RSV-F VLPs as determined by the levels of lung virus loads and morbidity postchallenge.
Conclusions. This study demonstrates that VLP vaccination provides effective protection against RSV infection. VLPs containing RSV-F and/or RSV-G are potential vaccine candidates against RSV.
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract illness in infants and children worldwide. In the United States, roughly 70 000–120 000 infants are hospitalized annually with RSV related pneumonia or bronchiolitis [1–3]. Effective therapies are not widely available, and there is no licensed vaccine. Approaches to develop subunit vaccines, attenuated viruses, DNA or live vector vaccines have been used but have not resulted in a licensed vaccine.
RSV has 10 genes encoding 11 proteins. Among them, the fusion protein F and glycoprotein G are the major antigens expressed on the virion surface and the most important target of neutralizing and protective antibodies [4]. The sequence of the F protein is highly conserved among RSV isolates, and antibodies to the F protein provide protection against both A and B strains of RSV [5, 6]. In contrast, the sequence of the G protein is variable, and 2 serotypes (A and B strains) showed different antigenicity with strain-specific antibody responses [7, 8].
Viruslike particle (VLP) vaccines are genetically engineered complexes of multiple copies of protein antigens in a particulate viruslike structure that lacks viral genetic material and therefore cannot replicate. Viral proteins presented as VLPs or recombinant vaccine are highly immunogenic and induce protection [3, 4, 9, 10]. The RSV fusion (F) and attachment glycoprotein (G) contain all neutralizing antibody epitopes and several T-cell epitopes [11]. Therefore, it is hypothesized that VLPs containing F or G will induce strong RSV-specific immune responses and immunity. In this study, we developed VLPs consisting of the influenza M1 protein together with the RSV A2 fusion (F) protein or the RSV attachment glycoprotein (G). Immune responses and protection against virus challenge were determined in mice immunized with these VLPs.
METHODS
Cells, Virus, and Antibody
Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900II medium (GIBCO-BRL) at 27°C in flasks at a speed of 140 rpm. The RSV A2 strain was originally provided by Dr Barney Graham (NIH, Bethesda, MD) [12]. HEp-2 cells were obtained from ATCC. Polyclonal goat anti-RSV antibody (Millipore) and mouse anti-RSV fusion protein (Millipore) were used in western blots and virus plaque assay. Secondary antibodies used were HRP conjugated anti-goat antibody (Southern Biotech) and anti-mouse antibody immunoglobulin G (IgG), IgG1, and IgG2a (Southern Biotech).
Preparation of RSV Stock
HEp-2 cells were grown in tissue culture flasks in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). RSV was added, and virus adsorption was carried out in medium without serum for 1 hour at 37°C with 5% CO2. DMEM with 5% FBS was added to the flask and incubated for 2–3 days. RSV-infected cells were removed using a cell scraper and centrifuged at 3000 rpm for 30 minutes to remove supernatants. Infected cell pellets were sonicated and centrifuged at 4°C, and the supernatants were titrated by immunoplaque assay as described below and stored at −80°C.
Construction of rBVs Expressing RSV F, RSV G, and Influenza M1
The RSV A2 F and G genes were polymerase chain reaction (PCR)-amplified using RNA from infected HEp-2 cells as described elsewhere [12]. The RSF-F gene was PCR-amplified from a complementary DNA (cDNA) clone of A2 F by use of primers 5-AAAGAATTCACCATGGAGGAGTTGCTAATCCTCAA-3 and 5-TTACTCGAGTTAGTTACTAAATGCAATATTATT-3 (EcoRI and XhoI underlined) and cloned into pFastBac with EcoRI/XhoI sites, resulting in plasmid pFastBac-F. The RSV-G gene was PCR-amplified from a cDNA clone of A2 G by use of primers 5-AAAGAATTCACCATGTCCAAAAACAAGGACCAAC-3 and 5-TTACTCGAGTACTGGCGTGGTGTGTTG-3 (EcoRI and XhoI underlined) and cloned into pFastBac with EcoRI/XhoI sites, resulting in plasmid pFastBac-G. For influenza M1 gene cloning, A/California/04/2009 virus was inoculated into MDCK cells and total viral RNA was extracted using an RNeasy Mini kit (Qiagen). Reverse transcription (RT) and PCR were performed on extracted viral RNA using the One-Step RT-PCR system (Invitrogen) with gene-specific oligonucleotide primers. The following primer pairs were used for M1: 5-AAAGAATTCACCATGAGTCTTCTAACCGAGGT-3 and 5-TTACTCGAGTTACTCTAGCTCTATGTTGAC-3 (EcoRI and XhoI underlined). Following RT-PCR, a cDNA fragment containing the M1 gene was cloned into the pFastBac vector.
Generation of Recombinant Baculoviruses
Recombinant baculoviruses (rBVs) expressing RSV F, RSV G, or influenza M1 were generated as described in materials and methods. Transfections of DNA containing the above genes were accomplished using cellfectin II (Invitrogen) with SF9 cells as recommended by the manufacturer, followed by transformation of pFastBac containing RSV-F or RSV-G or M1 with white/blue screening. The rBVs were derived by using a Bac-to-Bac expression system (Invitrogen) according to the manufacturer’s instructions.
Production of VLPs
RSV-F VLPs were produced by infecting Sf9 cells with rBVs expressing RSV-F and M1. RSV-G VLPs were produced by infecting Sf9 cells with rBVs expressing RSV-G and M1. Cell culture supernatants were collected on day 2 postinfection with centrifugation at 6000 rpm for 20 minutes at 4°C. VLPs were concentrated with QuixStand (GE) and purified through a 20%–30%–60% discontinuous sucrose gradient at 30 000 rpm for 1 hour at 4°C. The VLP bands between 30% and 60% were collected and then diluted with phosphate-buffered saline (PBS) and pelleted at 28 000 rpm for 40 minutes at 4°C. VLPs were resuspended in PBS overnight at 4°C.
Characterization of VLPs
VLPs were characterized by Western blots and electron microscopy. For Western blot analysis, polyclonal goat anti-RSV antibody was used to probe RSV-G protein; mouse anti-RSV fusion protein was used to probe RSV-F protein. Anti-M1 antibody was used to determine M1 protein content. For electron microscopy and size determinations, negative staining of VLPs was performed followed by transmission electron microscopy (Emory University Core Facility).
RSV Immunoplaque Assay
HEp-2 cells were grown in 12-well plates (Costar) until confluent. Virus stock or lung homogenates from infected mice were serially diluted in DMEM media without FBS. Virus samples were added to the plates and removed after 1 hour incubation at 37°C. Each well received 1 mL of overlay and was incubated 3 days at 37°C. Cells were fixed with ice-cold acetone-methanol (60:40) for 10 minutes. After air drying, anti-F monoclonal antibody and then HRP conjugated anti-mouse IgG antibodies were used. Individual plaques were developed using DAB substrate (Invitrogen).
Immunization, Sample Collection, and Challenge
Female BALB/c mice (Charles River) aged 6–8 weeks were used. Groups of mice (12 mice per group) were intramuscularly immunized twice with 25 μg of VLPs at 4-week intervals. Blood samples were collected by retro-orbital plexus puncture before immunization and at 3 weeks after prime and boost. For virus challenge, naive or vaccinated mice were isofluorane-anesthetized and intranasally infected with 1.5 × 106 plaque-forming units (PFU) in 50 μL of PBS, or mock control samples prepared from uninfected HEp-2 cell monolayers processed in the same way as infected cells. Mice were observed daily to record body weight changes. All animal experiments and husbandry involved in the studies were conducted under the guidelines of the Emory University IACUC.
Antibody Responses
RSV (A2) virus-specific antibodies (IgG, IgG1, and IgG2a) were determined in sera and lung extracts by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtiter plates were coated with 100 μL of RSV virus (3 × 105 PFU) per well in coating buffer at 4°C overnight. The samples were serially diluted and added onto plates after they were washed and blocked with 3% bovine serum albumin (BSA). The plates were then incubated with HRP-conjugated secondary antibodies goat anti-mouse IgG, IgG1, and IgG2a at 37°C for 1.5 hours. The substrate O-phenylenediamine (Zymed) in citrate-phosphate buffer (pH 5.0) containing 0.03% H2O2 (Sigma) was used to develop color. The optical density at 450 nm was read using an ELISA reader (model 680, Bio-Rad).
Antibody Responses Against Cell Surface Proteins
Monolayers of HEp-2 cells in 96-well culture plates were infected with RSV A2 viruses (1.5 × 106 PFU/plate) and incubated for 12 hours. ELISA was performed in parallel with infected cells or uninfected controls. The culture medium was removed, and the cells were fixed with 0.25% glutaraldehyde. PBST containing 0.05% Tween 20 was used to wash the plates. Manual washing was used, and cells were observed to be completely attached at each step. Subsequent procedures were conducted as described above.
Antibody Neutralization
Mouse sera were complement inactivated at 56°C for 30 minutes and then serially diluted. Live RSV A2 was diluted and added. The virus-serum mixtures including a virus-only control were incubated at 37°C for 1 hour and then added to prewashed, confluent monolayers of HEp-2 cells grown in 12-well plates. After 1 hour, the antibody-virus mixture was removed, and the procedure described above was followed. The mean percent plaque reduction by sera from VLP vaccinated mice compared with sera from naive and medium control were determined.
Lung Virus Load
The individual lungs were collected at day 4 postchallenge, and extracts were prepared as homogenates after challenge using frosted glass slides [13]. The homogenates were centrifuged at 1000 rpm for 10 minutes to collect supernatants. The lung virus load was determined in the supernatants by plaque assay as described above.
Statistical Analysis
All parameters were recorded for individual mice within all groups. Statistical comparisons of data were carried out using the analysis of variance and Npar1-way Kruskal–Wallis test of the PC-SAS system. A P value of <.05 was considered significant.
RESULTS
Generation of Constructs
RSV-F and RSV-G genes from RSV strain A2 were amplified by PCR, and the influenza M1 gene was amplified by RT-PCR with primers containing restriction enzyme sites. Genes were cloned into pFastBac vectors, and insertion of RSV-F, RSV-G, and influenza M1 in pFastBac expressing vectors was confirmed by cutting with enzyme sites, EcoRI and XhoI (Figure 1A–C). The nucleotide sequences of the RSV-F, RSV-G, and influenza M1 genes were found to be identical to the previously published sequences (accession numbers FJ614814 for F, AF035006 for G, FJ966085 for M1) by DNA sequencing (Eurofins MWG Operon).
Figure 1.
Constructs of pFastBac vectors and characterization of viruslike particles (VLPs). Respiratory syncytial virus (RSV) fusion F gene (A), attachment glycoprotein G gene (B), and influenza M1 genes (C) are in pFastBac vectors. Genes were amplified by PCR or RT-PCR, as described in Methods. Sizes of F, G, and M1 in vectors were confirmed by cutting with EcoRI and XhoI, and the nucleotide sequences were confirmed by DNA sequencing. VLPs containing M1 and RSV F (D) or RSV G (E) were purified from SF9 cell culture supernatants by sucrose gradient ultracentrifugation and visualized by Western blots. The 20, 5, and 1 μg VLPs were loaded per lane as indicated (D, E).
Production of VLPs (RSV-F, RSV-G)
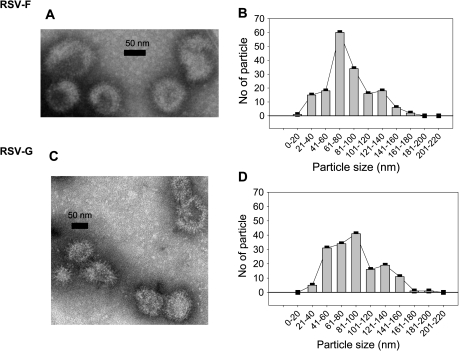
VLPs containing influenza M1 and RSV fusion F or attachment G glycoproteins were produced as described in Methods. The incorporation of F or G and M1 into VLPs was confirmed by western blot using anti-RSV or anti M1 polyclonal antibodies (Figure 1D and E). Both RSV-F or RSV-G VLPs showed spherical shapes with spikes on their surfaces (Figure 2A and C). RSV F VLPs had a peak in size distribution at 80–100 nm, whereas G-VLPs were similar but somewhat more heterogeneous in size (Figure 2B and D). In total, 5–7.5 mg VLPs/liter of cell culture medium were obtained, and their antigenic properties were stable for 1 year at −80°C. Heat-treated VLPs lost immunogenicity and protective capacity.
Figure 2.
Viruslike particles (VLPs) containing M1 and respiratory syncytial virus (RSV) F (A) or RSV G (C) visualized and their size distribution determined (B, D) after negative staining EM (H-7500, Hitachi). Fifty-five (55%) of RSV F VLPs from 170 particles were distributed between 60 and 100 nm (B), and 66% of RSV-G VLPs from 161 particles were distributed between the size 40 and 100 nm (D).
Antibody Responses in Immune Serum
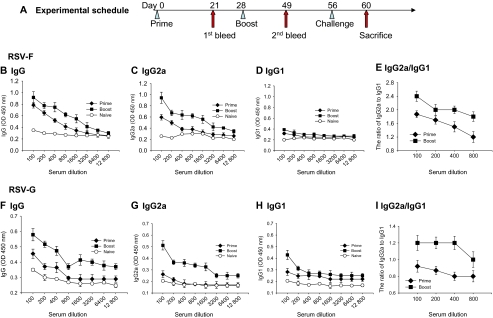
Serum antibody responses were determined in mice immunized with RSV-F or RSV-G VLPs as shown in Figure 3A. The levels of total IgG, IgG2a, and IgG1 antibody responses in the serum specific to RSV after prime and boost were determined. Total IgG and IgG2a responses from the mice immunized with RSV-F VLPs showed significantly higher titers after boost compared with those after prime, indicating the progressive maturation of virus-specific antibodies (Figure 3B and C). Low IgG1 responses were observed both after prime and boost (Figure 3D). In contrast, the levels of IgG2a antibody increased significantly, both after prime and boost, compared with IgG1. The levels of IgG2a were higher after boost than prime at various serum dilutions (Figure 3E). Similar patterns of total IgG, IgG2a, and IgG1 responses to RSV A2 were found in mice immunized with RSV-G VLPs. The levels of IgG and IgG2a antibody responses were lower relative to those observed with RSV-F VLPs. Antibody responses (IgG, IgG2a) were significantly higher in mice after boost compared with primary immunization (Figure 3F and G). As seen in Figure 3, IgG2a-dominant responses were found after the boost but not after prime. These results indicate that both RSV-F and RSV-G VLPs are highly immunogenic and induce IgG2a-dominant responses.
Figure 3.
Experimental schedule and respiratory syncytial virus (RSV)-A2-specific antibody responses to RSV-F or RSV-G viruslike particles (VLPs). A, Groups of mice were intramuscularly immunized twice as indicated with a 4-week interval. At 4 weeks after boost, mice were challenge infected with RSV-A2 virus, and at day 4 after challenge mouse lungs were collected to determine lung virus loads. Enzyme-linked immunosorbent assay (ELISA) plates were coated with whole RSV virus, as indicated in Methods. Serially diluted sera were used after prime and boost. Total immunoglobulin (Ig) G (B), isotypes IgG2a (C), IgG1 (D) responses, and the ratio of IgG2a to IgG1 (E) to RSV-F VLPs, and total IgG (F), isotype IgG2a (G), IgG1 (H), and the ratio of IgG2a to IgG1 (I) to RSV-G VLPs were determined from mice immunized with RSV-F VLPs.
Antibody Responses in Mouse Lung
The lung is the most important location for RSV replication, where disease development occurs. At day 4 after challenge, mouse lungs were harvested and total IgG, IgG1, and IgG2a antibody responses were determined. As shown in Figure 4A and C, significantly higher levels of RSV A2 specific IgG antibodies were found in mice immunized with RSV-F VLPs or RSV-G VLPs. Interestingly, IgG responses specific to RSV-G or RSV-F in immunized mice showed similarly high levels. As seen in Figure 4B and D, significantly higher IgG2a responses were elicited in mice immunized with RSV-F VLPs or RSV-G VLPs compared with IgG1. The ratios of IgG2a to IgG1 from both RSV-G VLPs and RSV-F VLPs were similar at lung extract dilutions of 10, 20, and 40 (Figure 4E). Compared with serum samples, lung extract samples showed a much higher ratio of IgG2a to IgG1 (Figure 3E and I, Figure 4E). These results indicate that both RSV-G VLPs and RSV-F VLPs elicit RSV IgG2a dominant responses in lungs.
Figure 4.
Respiratory syncytial virus (RSV) A2-specific immunoglobulin (Ig) G, IgG2a, and IgG1 responses and the ratio of IgG2a to IgG1 in lung extract samples collected at day 4 postchallenge. Serially diluted lung samples were used to determine IgG (A, C), IgG2a, and IgG1 (B, D) responses and the ratio (E) in mice immunized with RSV-F viruslike particles (VLPs) or immunized with RSV-G VLPs.
Immune Sera Effectively Bind to RSV Infected Cells
Immune responses against cell surface proteins were determined using monolayers of HEp-2 cells infected with RSV A2 or uninfected cells. After 12 hours of incubation, the supernatants were removed and the immune sera were added to the cell surface to determine IgG, IgG1 and IgG2a responses. As seen in Figure 5, sera from mice immunized with RSV-F or RSV-G VLPs from prime and boost showed significantly high levels of total IgG and IgG2a antibody responses against cell surface proteins expressed 12 hours postinfection (Figure 5A, B, D, and E). Total IgG responses against infected or uninfected cell surface proteins were determined in mice immunized with RSV-F VLPs (Figure 5C) or with RSV-G VLPs (Figure 5F), showing background responses in uninfected cells. Interestingly, RSV-G VLP vaccinations showed much higher total IgG and IgG2a responses against cell surface proteins than did RSV-F VLPs. These results indicate that immune sera specifically bind to HEp-2 cell surface expressed proteins.
Figure 5.
Immunoglobulin (Ig) G, IgG1, and IgG2a responses against cell surface expressed respiratory syncytial virus (RSV)-A2 proteins. HEp-2 cells in 96 well culture plates were infected with RSV A2 as described in Methods. Serially diluted mouse sera were added into wells. IgG, IgG2a, and IgG1 responses were determined from mice immunized with RSV-F viruslike particles (VLPs) (A, B) or immunized with RSV-G VLPs (D, E). Total IgG responses were also determined by enzyme-linked immunosorbent assay (ELISA) by using HEp-2 cells infected with RSV A2 or uninfected controls. Sera were obtained at week 2 after prime from mice immunized with RSV-F VLPs (C) or RSV-G VLPs (F).
Neutralizing Antibody Responses
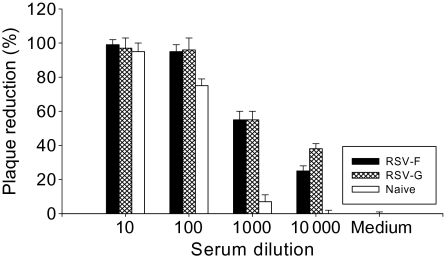
Neutralizing antibody is an important functional component of immune responses induced by vaccination. To determine whether immunization with RSV VLPs induces neutralizing antibodies, an in vitro plaque reduction assay was used. Serially diluted mouse sera at week 3 after boost were complement inactivated, incubated with live RSVA2, and plaque assays were conducted. As shown in Figure 6, RSV-F or RSV-G VLPs showed similar plaque reduction rates. At 1:1000 serum dilutions, either RSV-G or RSV-F VLP vaccination reduced the number of plaques by 55% whereas naive serum reduced plaques by 7%. At the 1:10000 serum dilutions, RSV-G VLPs showed 38% plaque reduction versus 25% reduction by RSV-F VLPs and no reduction by naive sera (Figure 6). Naïve sera showed very high background effects at serum dilution of 1:10 or 1:100, with neutralizing titers >100. Virus neutralizing titers from immunization with RSV-F or RSV-G VLPs were >1000 when the highest serum dilution showing 50% plaque reduction was taken as the neutralizing antibody titer in comparison to the negative medium control showing no plaque reduction. These results indicate that RSV-F and RSV-G VLPs vaccines can induce protective functional antibodies to RSV.
Figure 6.
Serum neutralizing activities. Complement inactivated mouse sera were tested for inhibition of respiratory syncytial virus (RSV)-A2 plaque formation. Serially diluted mouse sera at week 3 after boost were complement inactivated and incubated with live RSV-A2. Virus was diluted and used at 750 PFU/well.
Protection Against Live RSV A2 Virus Challenge Infection
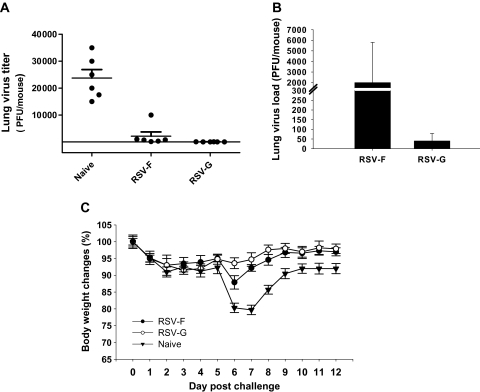
Virus load in lungs and body weight changes following challenge infection are the most important indicator to assess vaccine protective efficacy. Immunized mice were challenge infected with live RSV-A2 virus (1.5 × 106 PFU/mouse) at 4 weeks after boost, and lung virus loads at day 4 postchallenge were determined. Asshown in Figure 7A, significantly decreased lung virus loads were detected in mice immunized with RSV-F (11-fold) or RSV-G VLPs (600-fold) compared with naive mouse controls (P < .01, P < .001). The difference in lung virus loads in mice immunized with RSV-F VLPs and RSV-G VLPs also was compared (Figure 7B). Lower lung virus load was found in mice immunized with RSV-G VLPs than those in mice immunized with RSV-F VLPs (Figure 7B, P < .001). These results indicate that vaccination of mice with RSV-F or RSV-G VLPs effectively can inhibit virus replication in lung. The level of body weight loss postchallenge was measured, and days 6 or 7 postinfection showed the highest body weight loss. Naive control mice showed the highest body weight loss (20%), whereas mice immunized with RSV-F VLPs or RSV-G VLPs showed 13% and 6.4% of body weight loss, respectively, at day 6, and 8% and 5% of body weight loss, respectively, at day 7 postchallenge (Figure 7C). Significant differences in body weight loss were found at day 7 between naive controls and RSV-F (P < .001) or RSV-G VLPs (P < .001), and between RSV-F and RSV-G VLPs (P < .05). The differences correlate with better lung virus clearance, which was observed with RSV-G VLPs compared with RSV-F VLPs. These results indicate that RSV-F and RSV-G VLPs both confer substantial protection of mice from RSV-induced illness.
Figure 7.
Lung virus loads and body weight changes after respiratory syncytial virus (RSV)-A2 challenge. The experiment was repeated twice. A, Lung virus load. Lungs from individual mice in each group (6 mice) were collected on day 4 postchallenge, and lung virus loads (number of plaque-forming units [PFU]) in each mouse were determined. The limit of virus detection is 50 PFU/mL. B, Comparison of lung virus loads between RSV-F and RSV-G viruslike particles (VLPs). The lower scale of the Y axis was used to see differences in lung virus loads between RSV-F and RSV-G VLP immunization. C, Body weight changes. Vaccinated mice (6 mice) were challenged with live RSV-A2 virus. The body weights were monitored daily in which 100% body weight was seen at day 0.
DISCUSSION
In this study, we investigated the protective efficacy of RSV vaccines produced in a viruslike particle form after intramuscular vaccination in a mouse model. We found that VLP vaccination provides effective protection against RSV infection. RSV-F or RSV-G VLPs elicited significant levels of IgG2a-dominant RSV-specific IgG antibody responses and significantly reduced lung viral replication and weight loss upon challenge.
The VLPs we produced targeted the full-length RSV fusion F protein (RSV-F) and attachment G glycoprotein (RSV-G), which possess all neutralizing epitopes as well as several T-cell epitopes [14–17]. We used influenza M1 protein rather than RSV matrix (M) as a core protein in VLPs because matrix proteins are important for virion morphology and RSV is more pleomorphic than influenza virus. Also, some paramyxovirus M proteins are insufficient for VLP formation in the absence of NP and envelope glycoprotein co-expression [18]. Our result indicates that RSV-F or RSV-G VLPs showed spherical particle shapes. The rBV-produced VLP vaccines from serum-free insect cells have advantages over VLPs produced in mammalian cells because mammalian cell-produced VLPs need to be validated to be free of viruses or oncogenic substances originating from the cells and/or serum [19].
It has been reported that an RSV VLPs vaccine containing RSV-G protein induces protection in a mouse model [4]. In this report, a chimeric protein was used in which the ectodomain was from the RSV G protein, but the cytoplasmic and transmembrane domains and M protein were from Newcastle disease virus (NDV). IgG subtype responses from vaccination with these VLPs were not determined. Our study focused on the full length RSV-F or -G proteins in VLPs, and the RSV-G VLPs induced better protection than RSV-F VLPs. RSV-F does not require other viral proteins for fusion activity and can undergo pre- to postfusion triggering upon expression [18, 20]. Possibly some F on the VLP surface may be in a postfusion form, potentially underrepresented on the challenge virus. Alternatively, RSV-F and/or -G may be differentially glycosylated in insect cells used to produce the VLPs, compared with mammalian cells and the underglycosylation of G may enable immune focusing on protectopes (eg, the central region of G). We predict that RSV-F VLPs will provide protection against RSV isolates of A and B subgroups, whereas protection induced by RSV-G VLPs may be more subgroup-specific. In mice and cotton rats, subgroup-heterologous protection is readily achievable with F antigen immunization, and significant but partial subgroup-heterologous protection is typically observed with RSV G antigens [21–24]. Within antigenic subgroups, RSV can be further classified into clades based on sequence analyses of a hypervariable carboxy-terminus region of RSV G [25, 26]. However, the importance of clade-specific Abs to RSV is unclear.
We developed a new ELISA assay to detect IgG, IgG2a, and IgG1 antibodies by using cell surface expressed RSV proteins. Since a correlation of antibody responses with protection was found using cell surface RSV-A2 proteins as binding antigens, detecting antibody using this method may be more reliable and will contributeto RSV vaccine studies. Our RSV-F and RSV-G VLP vaccines induced very high levels of IgG2a isotype responses with very low levels of IgG1 isotype responses. This is encouraging since enhanced disease after RSV challenge is reported to be related to Th2 (IgG1) allergy-like or Th2-associated responses [6, 27, 28]. It has been reported that F and G protein subunit RSV vaccines were weakly immunogenic and caused enhanced pulmonary histopathology [1, 4, 29–31]. In contrast, Venezuelan equine encephalitis virus replicon particles (VRPs), as well as RSV G-expressing Newcastle disease VLPs, were more immunogenic, effective, and did not cause enhanced RSV disease [4, 32]. Since RSV proteins in VLPs are presented in a repetitive, particulate viruslike structure [33], they may be highly immunogenic and induce protective humoral, cellular, and mucosal immune responses [13, 34–36]. To our knowledge this is the first report demonstrating and comparing protection induced by VLPs containing full-length fusion (F) or attachment (G) glycoproteins. RSV-F or RSV-G VLPs successfully inhibit virus replication in the lung and protected mice from infection. We therefore conclude that RSV-F or RSV-G VLPs are promising vaccine candidates.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (1R43AI091230 to F. S. Q., 1R01AI068003 to R. W. C., and 1R01AI087798 to M. L. M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
This research project was supported in part by the Robert P. Apkarian Integrated Electron Microscopy Core of Emory University. We thank James Webster at the Emory University Core facility for assistance with electron microscopy.
References
- 1.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga SM. Fixing a failed vaccine. Nat Med. 2009;15:21–2. doi: 10.1038/nm0109-21. [DOI] [PubMed] [Google Scholar]
- 3.Zhan X, Hurwitz JL, Krishnamurthy S, et al. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–93. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murawski MR, McGinnes LW, Finberg RW, et al. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010;84:1110–23. doi: 10.1128/JVI.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh EE. Humoral, mucosal, and cellular immune response to topical immunization with a subunit respiratory syncytial virus vaccine. J Infect Dis. 1994;170:345–50. doi: 10.1093/infdis/170.2.345. [DOI] [PubMed] [Google Scholar]
- 6.Singh SR, Dennis VA, Carter CL, et al. Immunogenicity and efficacy of recombinant RSV-F vaccine in a mouse model. Vaccine. 2007;25:6211–23. doi: 10.1016/j.vaccine.2007.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev. 2000;13:1–15. doi: 10.1128/cmr.13.1.1-15.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muelenaer PM, Henderson FW, Hemming VG, et al. Group-specific serum antibody responses in children with primary and recurrent respiratory syncytial virus infections. J Infect Dis. 1991;164:15–21. doi: 10.1093/infdis/164.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Takimoto T, Hurwitz JL, Coleclough C, et al. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78:6043–7. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82:2350–7. doi: 10.1128/JVI.02372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson MR, Varga SM. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev Vaccines. 2008;7:1239–55. doi: 10.1586/14760584.7.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore ML, Chi MH, Luongo C, et al. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol. 2009;83:4185–94. doi: 10.1128/JVI.01853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009;83:4489–97. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plotnicky-Gilquin H, Huss T, Aubry JP, et al. Absence of lung immunopathology following respiratory syncytial virus (RSV) challenge in mice immunized with a recombinant RSV G protein fragment. Virology. 1999;258:128–40. doi: 10.1006/viro.1999.9702. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Anderson R. A single amino acid substitution in a recombinant G protein vaccine drastically curtails protective immunity against respiratory syncytial virus (RSV) Vaccine. 2003;21:2500–5. doi: 10.1016/s0264-410x(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Cyr SL, Burt DS, Anderson R. Murine host responses to respiratory syncytial virus (RSV) following intranasal administration of a Protollin-adjuvanted, epitope-enhanced recombinant G protein vaccine. J Clin Virol. 2009;44:287–91. doi: 10.1016/j.jcv.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Murata Y, Lightfoote PM, Falsey AR, Walsh EE. Identification of and human serum reactogenicity to neutralizing epitopes within the central unglycosylated region of the respiratory syncytial virus attachment protein. Clin Vaccine Immunol. 2010;17:695–7. doi: 10.1128/CVI.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiwatpongsakorn S, Epand RF, Collins PL, Epand RM, Peeples ME. Soluble respiratory syncytial virus fusion protein in the fully cleaved, pretriggered state is triggered by exposure to low-molarity buffer. J Virol. 2011;85:3968–77. doi: 10.1128/JVI.01813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesse F, Wagner R. Developments and improvements in the manufacturing of human therapeutics with mammalian cell cultures. Trends Biotechnol. 2000;18:173–80. doi: 10.1016/s0167-7799(99)01420-1. [DOI] [PubMed] [Google Scholar]
- 20.Rawling J, Cano O, Garcin D, Kolakofsky D, Melero JA. Recombinant Sendai viruses expressing fusion proteins with two furin cleavage sites mimic the syncytial and receptor-independent infection properties of respiratory syncytial virus. J Virol. 2011;85:2771–80. doi: 10.1128/JVI.02065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Power UF, Plotnicky H, Blaecke A, Nguyen TN. The immunogenicity, protective efficacy and safety of BBG2Na, a subunit respiratory syncytial virus (RSV) vaccine candidate, against RSV-B. Vaccine. 2003;22:168–76. doi: 10.1016/s0264-410x(03)00570-x. [DOI] [PubMed] [Google Scholar]
- 22.Crowe JE, Jr, Bui PT, Firestone CY, et al. Live subgroup B respiratory syncytial virus vaccines that are attenuated, genetically stable, and immunogenic in rodents and nonhuman primates. J Infect Dis. 1996;173:829–39. doi: 10.1093/infdis/173.4.829. [DOI] [PubMed] [Google Scholar]
- 23.Nicholas JA, Rubino KL, Levely ME, Meyer AL, Collins PL. Cytotoxic T cell activity against the 22-kDa protein of human respiratory syncytial virus (RSV) is associated with a significant reduction in pulmonary RSV replication. Virology. 1991;182:664–72. doi: 10.1016/0042-6822(91)90607-d. [DOI] [PubMed] [Google Scholar]
- 24.Johnson VH, SemLer BL. Defined recombinants of poliovirus and coxsackievirus: sequence-specific deletions and functional substitutions in the 5′-noncoding regions of viral RNAs. Virology. 1988;162:47–57. doi: 10.1016/0042-6822(88)90393-5. [DOI] [PubMed] [Google Scholar]
- 25.Peret TC, Hall CB, Hammond GW, et al. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis. 2000;181:1891–6. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 26.Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79:2221–9. doi: 10.1099/0022-1317-79-9-2221. [DOI] [PubMed] [Google Scholar]
- 27.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–21. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 28.Openshaw PJ. Potential mechanisms causing delayed effects of respiratory syncytial virus infection. Am J Respir Crit Care Med. 2001;163:S10–3. doi: 10.1164/ajrccm.163.supplement_1.2011111. [DOI] [PubMed] [Google Scholar]
- 29.Hancock GE, Heers KM, Smith JD, Scheuer CA, Ibraghimov AR, Pryharski KS. CpG containing oligodeoxynucleotides are potent adjuvants for parenteral vaccination with the fusion (F) protein of respiratory syncytial virus (RSV) Vaccine. 2001;19:4874–82. doi: 10.1016/s0264-410x(01)00228-6. [DOI] [PubMed] [Google Scholar]
- 30.Hancock GE, Heers KM, Smith JD. QS-21 synergizes with recombinant interleukin-12 to create a potent adjuvant formulation for the fusion protein of respiratory syncytial virus. Viral Immunol. 2000;13:503–9. doi: 10.1089/vim.2000.13.503. [DOI] [PubMed] [Google Scholar]
- 31.Murphy BR, Prince GA, Lawrence LA, Croen KD, Collins PL. Detection of respiratory syncytial virus (RSV) infected cells by in situ hybridization in the lungs of cotton rats immunized with formalin-inactivated virus or purified RSV F and G glycoprotein subunit vaccine and challenged with RSV. Virus Res. 1990;16:153–62. doi: 10.1016/0168-1702(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 32.Mok H, Lee S, Utley TJ, et al. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol. 2007;81:13710–22. doi: 10.1128/JVI.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang SM, Song JM, Quan FS, Compans RW. Influenza vaccines based on virus-like particles. Virus Res. 2009;143:140–6. doi: 10.1016/j.virusres.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang SM. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine. 2008;26:3352–61. doi: 10.1016/j.vaccine.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan FS, Vunnava A, Compans RW, Kang SM. Virus-like particle vaccine protects against H1N1 pandemic influenza virus in mice. PLoS One. 2010;5(2):e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]