Abstract
Background. BCG vaccination of infants is thought to provide good protection in all settings. This study investigated whether Malawian infants made weaker responses across a cytokine panel after BCG vaccination, compared with UK infants.
Methods. Diluted whole-blood samples were cultured with Mycobacterium tuberculosis purified protein derivative for 6 days from BCG-vaccinated infants 3 months (n = 40 Malawi, 28 UK) and 12 months (n = 34 Malawi, 26 UK) after vaccination, and also from UK unvaccinated infants (n = 9 at 3 months, n = 10 at 12 months). Forty-two cytokines were measured in supernatants using a multiplex bead array assay. Principal component analysis was used to summarize the overall patterns in cytokine responses.
Results. We found differences in median responses in 27 of the 42 cytokines: 7 higher in the UK and 20 higher in Malawi. The cytokines with higher responses in the UK were all T helper 1 related. The cytokines with higher responses in Malawi included innate proinflammatory cytokines, regulatory cytokines, interleukin 17, T helper 2 cytokines, chemokines, and growth factors. Principal component analysis separated the BCG-vaccinated infants from Malawi from the UK vaccinated infants and from the unvaccinated infants.
Conclusions. Malawian infants make cytokine responses following BCG vaccination, but the cytokine profile is different from that in the UK. The different biosignatures following BCG vaccination in the 2 settings may indicate variability in the protective efficacy of infant BCG vaccination.
Interferon-γ (IFN-γ) has an essential role in protective cell-mediated immunity against Mycobacterium tuberculosis disease, but other cytokines, such as tumor necrosis factor α (TNF-α) and interleukin 12 (IL-12), are also required [1]. Concerted efforts to find new biomarkers for tuberculosis are focusing on tuberculosis in developing countries where new vaccines are most needed [1].
Clinical trials of the BCG vaccine show variable efficacy against pulmonary tuberculosis in adults between populations [2]. Good efficacy was shown in infants against the severe forms of childhood tuberculosis, although none of the trials were conducted in Africa [3]. BCG vaccination induces strong T helper 1 (Th1) responses in Gambian infants [4–6], although the number of multifunctional T cells making IFN-γ, TNF-α, and interleukin 2 (IL-2) did not correlate with protection against disease in South Africa [7, 8].
Population differences in infant immune responses following BCG vaccination were observed in studies comparing the UK and Malawi. Although all BCG-vaccinated infants in the UK made IFN-γ (>62 pg/mL) responses to M. tuberculosis purified protein derivative (PPD) in 6-day whole-blood cultures, only 53% of Malawians made such responses, and the magnitude of the response in those who responded was lower in Malawi [9]. Malawian infants also made low skin test responses and smaller BCG scars than did UK infants [9].
A promising method that may help to identify new biomarkers is the “multiplex” fluorescent bead–based cytokine assay [10], which we have used to show that BCG vaccination induces a complex profile of cytokines in BCG-vaccinated UK infants, including proinflammatory cytokines, T helper 2 (Th2) cytokines, interleukin 17 (IL-17), chemokines, and growth factors [11].
Because Malawian infants produced less IFN-γ to M. tuberculosis PPD following BCG vaccination than UK infants, we investigated whether Malawian infants made weaker responses across a large cytokine panel, compared with UK infants. We aimed to characterize population differences following BCG vaccination, by measuring a panel of 42 cytokines in supernatants from diluted blood cultures stimulated with M. tuberculosis PPD for 6 days. To investigate long-term memory responses, blood specimens were obtained from infants at 3 and 12 months after BCG vaccination.
MATERIALS AND METHODS
Recruitment and Study Design
Infants living in Waltham Forest Primary Care Trust, London, UK and Karonga District, Malawi participating in a large BCG vaccination study were selected for additional cytokine analysis [9]. Infants receiving BCG vaccination at comparable times (between 3 and 13 weeks of age) provided blood samples at 3 and 12 months after vaccination. Infants from the UK were matched as closely as possible on age at vaccination, but on average UK infants were older (median age, 7 weeks; range, 3–13 weeks) than Malawian infants (median age, 5 weeks; range, 3–11 weeks) at the time of vaccination as a result of different vaccination policies in the 2 countries. Unvaccinated control infants were recruited in the UK from the adjacent Redbridge Primary Care Trust. Unvaccinated infants were matched for age to the vaccinated infants. Seventy-seven infants were studied 3 months after vaccination (n = 40 Malawi, n = 28 UK, n = 9 unvaccinated UK), and 70 infants 12 months after vaccination (n = 34 Malawi, n = 26 UK, n = 10 unvaccinated UK). Children of human immunodeficiency virus–positive mothers in Malawi were excluded. The study was approved by the Redbridge and Waltham Forest Health Authority Local Research Ethics Committee, the National Health Sciences Research Council in Malawi, and the Ethics Committee of the London School of Hygiene and Tropical Medicine.
Whole-Blood Assay
Heparinized whole blood was diluted 1 in 10 with Roswell Park Memorial Institute (RPMI) medium containing L-glutamine and cultured on the day of collection with M. tuberculosis PPD (Statens Serum Institut, Copenhagen, RT49, lot 204) at a concentration of 5 μg/mL or medium alone (unstimulated). Cultures were incubated at 37°C with 5% CO2; supernatants were harvested on day 6 and stored at −70°C until assayed in single 25-μL samples by multiplex assay.
Multiplex Assay
The Malawian samples were shipped to London in dry ice and tested simultaneously with UK samples in London. The assay measured 42 cytokine and chemokine concentrations: interleukin 1β (IL-1β), IL-2, interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 8 (IL-8), interleukin 10 (IL-10), interleukin 12p70 (IL-12p70), interleukin 13 (IL-13), interleukin 15 (IL-15), IL-17, interleukin 1α (IL-1α), IFN-γ, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), TNF-α, eotaxin, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), IFN-γ–inducible protein 10 (IP-10), soluble IL-2 receptor α (sIL-2Rα), interferon α2 (IFN-α2), tumor necrosis factor β (TNF-β), interleukin 1 receptor antagonist (IL-1RA), soluble CD40 ligand (sCD40-L), FMS-like tyrosine kinase 3 ligand (Flt3-L), interleukin 7 (IL-7), interleukin 12p40 (IL-12p40), regulated upon activation, normal T cell expressed and secreted (RANTES), macrophage-derived chemokine (MDC), macrophage inflammatory protein 1β (MIP-1β), fractalkine, monocyte chemotactic protein 3 (MCP-3), growth regulated oncogene (GRO), vascular endothelial growth factor (VEGF), platelet derived growth factor AA (PDGF-AA), platelet derived growth factor AB/BB (PDGF-AB/BB), fibroblast growth factor 2 (FGF-2), epidermal growth factor (EGF), transforming growth factor α (TGF-α), interleukin 3 (IL-3), and interleukin 9 (IL-9) using a human “Milliplex” premixed kit according to the manufacturer’s instructions (no. MPXHCYTO60KPMX42, Millipore). Cytokines were measured in single 25-μL samples of unstimulated and M. tuberculosis PPD–stimulated supernatants from diluted whole blood cultured for 6 days. Multiplex plates were read on the Biorad Luminex reader using Bioplex Manager 4.1 software. For each cytokine the standard curve ran from 3.2 to 10 000 pg/mL.
Statistical Analysis
Unstimulated cytokine response values were subtracted from antigen-stimulated results. Multiplex data values <3.2 pg/mL were assigned as 1.6 pg/mL; some values for PDGF-AA, PDGF-AB, and RANTES were lower in value than the unstimulated cytokine response and were set to 1.6 pg/mL. Some values for MCP-1, IL-8, MDC, RANTES, IP-10, and MCP-3 were above the detection limit and were assigned 15,000 pg/mL for MDC and MCP-3, 30000 pg/mL for MCP-1 and IP-10, and 100 000 pg/mL for IL-8 and RANTES, assessed by looking at the highest extrapolated values measured. The magnitude of response could not be analyzed for IL-8 and MCP-1, because many were out of range; samples were not diluted and retested because of cost restraints. Median fold differences between UK and Malawian cytokine responses were calculated, and nonparametric Mann-Whitney tests were used to compare cytokine responses between Malawian and UK infants at each time point. Associations between different cytokines were assessed with Spearman rank correlation coefficients.
Principal component analysis (PCA) summarizes data by reducing their dimensionality, in the example here from 42 cytokines measured to 2 or 3. This is achieved by identifying a few “principal components,” each of which is defined as a weighted sum of the individual cytokine values, that together explain most of the variation in the profile of cytokine responses. The value of each principal component is then calculated for each infant; these values are referred to as “scores” and can be represented graphically to summarize the variation in the data. The principal components were identified through analysis of the log-transformed cytokine data from the UK and Malawi together, using “standardized” log cytokine measurements (mean response subtracted from observed value, then divided by standard deviation) by implementing the PCA on the 42 by 42 correlation matrix that summarizes the correlations among all pairs of cytokines.
RESULTS
Of the 42 cytokines and chemokines tested, there was strong evidence of a difference between responses in UK BCG-vaccinated infants, compared with BCG-vaccinated Malawian infants for 27 cytokines 3 months after BCG vaccination, and for 26 cytokines 12 months after BCG vaccination (Table 1).
Table 1.
Multiplex Results from United Kingdom and Malawian Infants 3 and 12 Months After BCG Vaccination
| Cytokine | 3 months after BCG |
12 months after BCG |
||||||||
| Unvaccinated UK | Vaccinated |
Unvaccinated UK | Vaccinated |
|||||||
| UK | Malawi | Fold differencea | P a | UK | Malawi | Fold differencea | P a | |||
| Proinflammatory | ||||||||||
| IFN-γ | 1.6 | 902 | 76 | 12 | <.001 | 4 | 598 | 44 | 14 | <.001 |
| IL-2 | 1.6 | 10 | 1.6 | 6 | <.001 | 1.6 | 16 | 1.6 | 10 | <.001 |
| TNF-β | 6 | 14 | 4 | 4 | .02 | 1.6 | 9 | 7 | 1 | .29 |
| IL-6 | 200 | 1881 | 954 | 2 | .01 | 423 | 1561 | 1498 | 1 | .24 |
| sIL-2Rα | 4 | 227 | 1400 | 6 | <.001 | 3 | 170 | 847 | 5 | <.001 |
| IFN-α2 | 1.6 | 22 | 108 | 5 | <.001 | 24 | 54 | 104 | 2 | <.001 |
| IL-1α | 13 | 399 | 1173 | 3 | .001 | 29 | 2472 | 1178 | 2 | .03 |
| IL-1RA | 63 | 116 | 222 | 2 | .009 | 115 | 256 | 213 | 1 | .73 |
| TNF-α | 6 | 111 | 139 | 1 | .25 | 13 | 87 | 125 | 1 | .45 |
| IL-1β | 1.6 | 27 | 17 | 1 | .79 | 5 | 17 | 16 | 1 | .85 |
| Th17 | ||||||||||
| IL-17 | 1.6 | 26 | 60 | 2 | .02 | 1.6 | 32 | 53 | 2 | .02 |
| Th2 | ||||||||||
| IL-13 | 1.6 | 47 | 1434 | 30 | <.001 | 1.6 | 14 | 1021 | 74 | <.001 |
| IL-5 | 1.6 | 7 | 75 | 11 | <.001 | 1.6 | 4 | 107 | 31 | <.001 |
| IL-9 | 1.6 | 1.6 | 10 | 6 | <.001 | 1.6 | 1.6 | 10 | 6 | <.001 |
| IL-4 | 1.6 | 1.6 | 4 | 3 | .001 | 1.6 | 1.6 | 1.6 | 1 | .002 |
| T cell regulation | ||||||||||
| IL-10 | 1.6 | 23 | 95 | 4 | .003 | 1.6 | 26 | 55 | 2 | .009 |
| T cell activation | ||||||||||
| IL-12p40 | 11 | 63 | 27.5 | 2 | .03 | 1.6 | 47 | 17 | 3 | .002 |
| IL-12p70 | 1.6 | 1.6 | 6 | 4 | .007 | 1.6 | 3 | 6 | 2 | .001 |
| sCD40-L | 1.6 | 153 | 186 | 1 | .25 | 1.6 | 341 | 194 | 2 | .046 |
| Chemokine | ||||||||||
| MIP-1α | 13 | 623 | 64 | 10 | <.001 | 1.6 | 161 | 38 | 4 | .003 |
| IP-10b | 158 | 12 798 | 3758 | 3 | <.001 | 45 | 30 000 | 3546 | 0 | <.001 |
| MCP-3b | 84 | 860 | 3065 | 4 | <.001 | 85 | 494 | 3004 | 6 | <.001 |
| MDC | 34 | 1415 | 2216 | 2 | .004 | 15 | 358 | 1985 | 6 | <.001 |
| GRO | 148 | 936 | 2171 | 2 | .16 | 126 | 527 | 1673 | 3 | .003 |
| RANTESb | 1.6 | 611 | 1093 | 2 | .21 | 1.6 | 1549 | 1129 | 1 | .99 |
| MIP-1β | 49 | 961 | 567 | 1 | .17 | 62 | 466 | 298 | 2 | .32 |
| Eotaxin | 10 | 29 | 34 | 1 | .31 | 15 | 46 | 36 | 1 | .01 |
| Fractalkine | 25 | 272 | 254 | 1 | .24 | 105 | 255 | 274 | 1 | .38 |
| Growth factor | ||||||||||
| IL-3 | 1.6 | 1.6 | 49 | 31 | <.001 | 1.6 | 1.6 | 38 | 24 | <.001 |
| PDGF-AA | 34 | 108 | 507 | 5 | <.001 | 1.6 | 63 | 337 | 5 | <.001 |
| PDGF-AB/BB | 1.6 | 106 | 381 | 4 | <.001 | 1.6 | 119 | 192 | 2 | .01 |
| TGF-α | 1.6 | 1.6 | 4.5 | 3 | .002 | 1.6 | 3 | 5 | 2 | .33 |
| GM-CSF | 22 | 376 | 717 | 2 | .01 | 22 | 337 | 666 | 2 | .06 |
| G-CSF | 1.6 | 13 | 19.5 | 2 | .049 | 4 | 16 | 14 | 1 | .39 |
| Flt3-L | 1.6 | 17 | 30.5 | 2 | .02 | 1.6 | 26 | 22 | 1 | .91 |
| FGF-2 | 7 | 99 | 111 | 1 | .57 | 20 | 145 | 109 | 1 | .02 |
| VEGF | 1.6 | 106 | 89 | 1 | .55 | 16 | 127 | 107 | 1 | .03 |
| IL-7 | 15 | 89 | 85 | 1 | .95 | 53 | 122 | 95 | 1 | .12 |
| EGF | 1.6 | 16 | 15 | 1 | .61 | 1.6 | 13 | 12 | 1 | .99 |
| Unable to assay | ||||||||||
| IL-15 | 1.6 | 1.6 | 1.6 | 1 | 1.6 | 1.6 | 1.6 | 1 | ||
| IL-8b | 4059 | 15 892 | 100 000 | 6 | 50 394 | 100 000 | 100 000 | 1 | ||
| MCP-1b | 8415 | 9816 | 30 000 | 3 | 30 000 | 30 000 | 30 000 | 1 | ||
Data are median cytokine responses, pg/mL, measured by 42-multiplex assay in supernatants from diluted whole-blood cultures that were stimulated with Mycobacterium tuberculosis purified protein derivative for 6 days unless otherwise indicated. Boldface text represents values for which there is statistical evidence of higher median values compared with median values from the other country. Abbreviations: EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; Flt3-L, FMS-like tyrosine kinase 3 ligand; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; GRO, growth regulated oncogene; IFN-α, interferon α; IFN-γ, interferon-γ; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-1RA, interleukin 1 receptor antagonist; IL-2, interleukin 2; IL-3, interleukin 3; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-7, interleukin 7; IL-8, interleukin 8; IL-9, interleukin 9; IL-10, interleukin 10; IL-12p40, interleukin 12p40; IL-12p70, interleukin 12p70; IL-13, interleukin 13; IL-15, interleukin 15; IL-17, interleukin 17; IP-10 (IFN-γ inducible protein 10); MCP-1, monocyte chemotactic protein 1; MCP-3, monocyte chemotactic protein 3; MDC, macrophage-derived chemokine; MIP-1α, macrophage inflammatory protein 1α; MIP-1β, macrophage inflammatory protein 1β; PDGF-AA, platelet derived growth factor AA; PDGF-AB/BB, platelet derived growth factor AB/BB; RANTES, regulated upon activation, normal T cell expressed and secreted; sCD40-L, soluble CD40 ligand; sIL-2Rα, soluble IL-2 receptor α; TGF-α, transforming growth factor α; Th2, T helper 2; Th17, T helper 17; TNF-α, tumor necrosis factor α; TNF-β, tumor necrosis factor β; VEGF, vascular endothelial growth factor.
Comparisons are between vaccinated UK infants and vaccinated Malawi infants.
Some values out of range. Note that for fold difference, the reference group is the one with the lower response.
Cytokine Responses
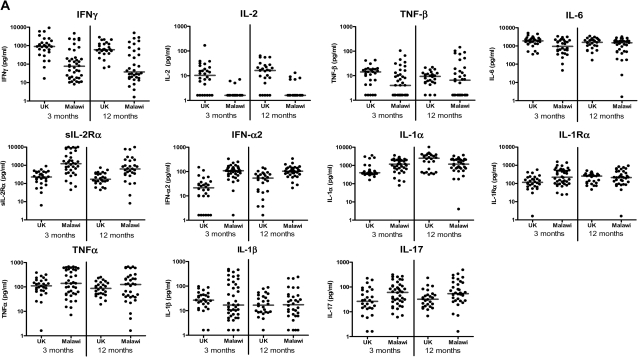
Three months after BCG vaccination, 4 of the 10 proinflammatory cytokines, IFN-γ (P < .001), IL-2 (P < .001), TNF-β (P = .02), and IL-6 (P = .01), had higher median responses in the UK than in Malawi, whereas for 4 cytokines, sIL-2Rα (P < .001), IFN-α2 (P < .001), IL-1α (P = .01), and IL-1RA (P = .009), median responses were higher in Malawian infants. For the remaining 2 proinflammatory cytokines, there was no evidence of a difference in TNF-α (P = .25) or IL-1β (P = .79) between UK and Malawian infants. There was also strong evidence of a higher median response in Malawian infants, compared with UK infants, in the T helper 17 (Th17) proinflammatory cytokine IL-17 (P = .02). Differences were maintained at 12 months for IFN-γ, IL-2, sIL-2Rα, IFN-α2, and IL-17. There was still evidence of a difference in median responses to IL-1α, but with higher responses in the UK (P = .03) (Figure 1; Table 1).
Figure 1.
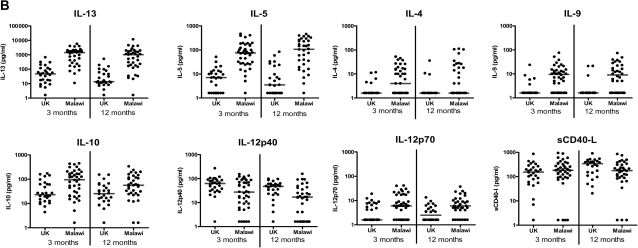
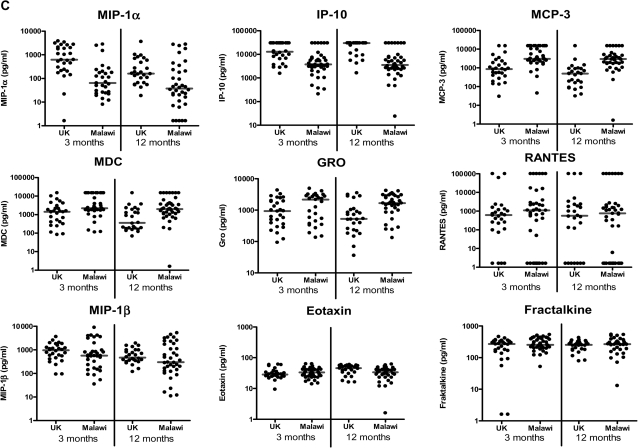
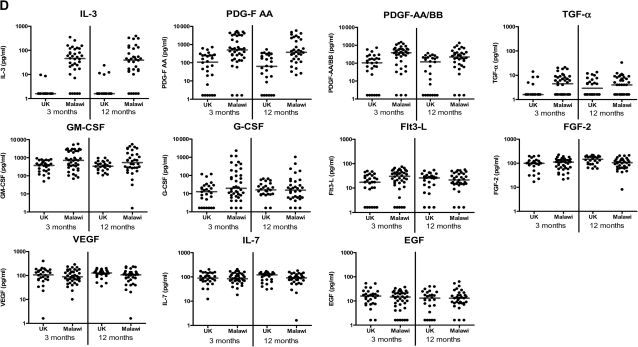
Cytokine responses measured by 42-multiplex assay. A, Proinflammatory cytokines; B, T helper 2, regulatory, and T cell activation cytokines; C, Chemokines; and D, Growth factors in supernatants from diluted whole-blood cultures that were stimulated with Mycobacterium tuberculosis purified protein derivative for 6 days were measured using a 42-multiplex assay 3 months and 12 months after BCG vaccination in UK and Malawian infants (3 months: UK n = 28, Malawi n = 40; 12 months: UK n = 26, Malawi n = 36). Line represents median response. EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; Flt3-L, FMS-like tyrosine kinase 3 ligand; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; GRO, growth regulated oncogene; IFN-α, interferon α; IFN-γ, interferon-γ; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-1RA, interleukin 1receptor antagonist; IL-2, interleukin 2; IL-3, interleukin 3; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-7, interleukin 7; IL-9, interleukin 9; IL-10, interleukin 10; IL-12p40, interleukin 12p40; IL-12p70, interleukin 12p70; IL-13, interleukin 13; IL-17, interleukin 17; IP-10 (IFN-γ inducible protein 10); MCP-3, monocyte chemotactic protein 3; MDC, macrophage-derived chemokine; MIP-1α, macrophage inflammatory protein 1α; MIP-1β, macrophage inflammatory protein 1β; PDGF-AA, platelet derived growth factor AA; PDGF-AB/BB, platelet derived growth factor AB/BB; RANTES, regulated upon activation, normal T cell expressed and secreted; sCD40-L, soluble CD40 ligand; sIL-2Rα, soluble IL-2 receptor α; TGF-α, transforming growth factor α; TNF-α, tumor necrosis factor α; TNF-β, tumor necrosis factor β; VEGF, vascular endothelial growth factor.
For all Th2 cytokines tested, IL-4 (P = .001), IL-5 (P < .001), IL-13 (P < .001), and IL-9 (P < .001), responses were higher in Malawi 3 and 12 months after vaccination than in the UK (Figure 1; Table 1). There was strong evidence of greater T cell regulation in Malawi than in the UK, with higher median IL-10 responses at both 3 and 12 months after BCG vaccination (P = .003 and .009, respectively). Whereas there was evidence of higher median IL-12p40 responses (P = .03, 3 months) in the UK, compared with Malawi, there was evidence of higher IL-12p70 responses in Malawian infants at both 3 months (P = .007) and 12 months (P = .001) after vaccination. There was no evidence of a difference in responses in sCD40-L at 3 months after vaccination (P = .25), whereas there was evidence of higher sCD40-L responses in Malawian infants at 12 months after BCG vaccination (P = .046). (Figure 1; Table 1). IL-15 was not detectable in any infant at any time point.
Chemokine Responses
UK infants had higher median MIP-1α responses (P < .001) and IP-10 responses (P < .001), whereas Malawian infants had higher median MCP-3 responses (P < .001) and MDC responses (P = .004) at both 3 and 12 months after vaccination (P values for 3 month time point). Malawian infants made higher median GRO responses 12 months after vaccination (P = .003), and UK infants made higher median eotaxin responses 12 months after vaccination (P = .01). There was no evidence of a difference in responses between the UK and Malawi in RANTES (P = .21, 3 months), MIP-1β (P = .17, 3 months), or fractalkine (P = .24, 3 months) at either time point (Figure 1; Table 1). Because many of the IL-8 and MCP-1 responses were above the limit of detection of the assay in both groups, it was not possible to compare the magnitude of responses between the UK and Malawi.
Growth Factors
The growth factors IL-3 (P < .001), PDGF-AA (P < .001), PDGF-AB/BB (P < .001), and GM-CSF (P = .01) had higher median responses in Malawi than in the UK at 3 and 12 months after vaccination (P values for 3 month time point). TGF-α, G-CSF, and Flt3-L median responses were higher in Malawian infants at 3 months (P = .002, .049, and .002, respectively) but not at 12 months after BCG vaccination (P = .33, .39, and .91, respectively). There was no evidence of a difference in median FGF-2 (P = .57) or VEGF (P = .55) responses between the UK and Malawi 3 months after BCG vaccination, but responses were higher in UK infants at 12 months (P = .02 and .03, respectively). There was no evidence of a difference in median IL-7 or EGF responses at either time point (P = .95 and .61, respectively, at 3 months) (Figure 1; Table 1).
Comparison of Responses at 3 and 12 Months
There was no evidence of a difference in the median response of any of the measured cytokines between 3 and 12 months after BCG vaccination in Malawian infants, although in general, responses were slightly lower at 12 than at 3 months. In the UK, responses were higher in IL-12p40, MIP-1α, MCP-3, and MIP-1β 3 months after vaccination, compared with 12 months after vaccination. At 12 months, responses were higher in IFN-α2, IL-1α, IL-1RA, sCD40L, IP-10, eotaxin, FGF2, and TGF-α (Supplementary Table 1).
Timing of BCG Vaccination
The infants were vaccinated over a wide age range, and the median age of vaccination was younger in Malawi than in the UK. After stratification into earlier (3–7 weeks) and later (8–13 weeks) vaccination, the strong evidence of differences between the 2 countries persisted (Supplementary Table 2).
Correlations Between Cytokines and Chemokines
There was a positive association between IFN-γ and many of the other cytokines. In Malawi, 28 of the 38 cytokines (IL-15, IL-8, and MCP-1 not included) had strong correlations with IFN-γ (correlation coefficients of ≥0.6), whereas in the UK only 10 of 38 had strong correlations (Table 2). In Malawi, IFN-γ positively correlated with many proinflammatory cytokines, such as TNF-β, IL-6, sIL-2Rα, IL-1α, IL-1β, TNF-α, and IL-17, but also with the Th2 cytokine IL-13 and the regulatory cytokine IL-10. There was a different pattern of correlation in the UK and Malawi, with production of such cytokines as IL-17 and IL-10 positively correlating strongly with that of IFN-γ in Malawians but not in the UK. At 12 months after BCG vaccination, associations between IFN-γ and other cytokines were similar to those at 3 months (Supplementary Table 3).
Table 2.
Spearman Rank Correlation Coefficients (ρ) of Cytokines and Chemokines With Interferon γ (IFN-γ), and Principal Component Analysis as Measured by 42 Multiplex Assay in Supernatants Stimulated with Mycobacterium tuberculosis Purified Protein Derivative for 6 Days, in UK and Malawian Infants 3 Months After BCG Vaccination
| IFN-γ Spearman rank correlation coefficients |
Contributions of principal component analysis |
||||
| Cytokine | UK | Malawi | Component 1 | Component 2 | Component 3 |
| Proinflammatory | |||||
| IFN-γ | 1.0 | 1.0 | 0.11 | −0.26 | −0.03 |
| IL-2 | 0.4 | 0.3 | 0.10 | −0.24 | 0.10 |
| TNF-β | 0.6 | 0.8 | 0.19 | −0.23 | −0.02 |
| IL-6 | 0.6 | 0.8 | 0.21 | −0.15 | −0.01 |
| sIL-2Rα | 0.9 | 0.8 | 0.19 | 0.19 | −0.24 |
| IFN-α2 | 0.2 | 0.2 | 0.02 | 0.19 | 0.16 |
| IL-1α | 0.2 | 0.8 | 0.17 | 0.15 | 0.18 |
| IL-1RA | 0.3 | 0.6 | 0.18 | 0.08 | 0.11 |
| IL-1β | 0.7 | 0.8 | 0.18 | 0.00 | −0.34 |
| TNF-α | 0.8 | 0.9 | 0.25 | −0.02 | −0.14 |
| Th17 | |||||
| IL-17 | 0.1 | 0.7 | 0.09 | 0.07 | −0.30 |
| T cell activation | |||||
| IL-12p40 | 0.7 | 0.7 | 0.22 | 0.04 | −0.03 |
| IL-12p70 | 0.4 | 0.7 | 0.19 | 0.00 | 0.05 |
| sCD40-L | 0.5 | 0.8 | 0.20 | 0.05 | 0.07 |
| T cell regulation | |||||
| IL-10 | 0.3 | 0.7 | 0.09 | 0.22 | −0.10 |
| Th2 | |||||
| IL-13 | 0.4 | 0.8 | 0.11 | 0.34 | −0.03 |
| IL-5 | 0.4 | 0.3 | 0.03 | 0.31 | 0.12 |
| IL-9 | 0.2 | 0.5 | 0.05 | 0.22 | 0.10 |
| IL-4 | 0.0 | 0.5 | 0.07 | 0.21 | 0.05 |
| Chemokine | |||||
| MIP-1α | 0.6 | 0.4 | 0.17 | −0.20 | −0.13 |
| IP-10 | 0.0 | 0.8 | −0.05 | −0.07 | −0.05 |
| MCP-3 | 0.3 | 0.7 | 0.12 | 0.09 | 0.17 |
| MDC | 0.3 | 0.7 | 0.12 | −0.04 | 0.16 |
| RANTES | 0.0 | 0.8 | 0.12 | −0.11 | 0.20 |
| MIP-1β | 0.4 | 0.6 | 0.21 | −0.05 | −0.12 |
| GRO | 0.3 | 0.6 | 0.19 | 0.12 | −0.09 |
| Eotaxin | 0.3 | 0.7 | 0.21 | 0.03 | 0.22 |
| Fractalkine | 0.6 | 0.7 | 0.22 | −0.04 | 0.11 |
| Growth factor | |||||
| IL-3 | −0.2 | 0.5 | 0.04 | 0.32 | 0.13 |
| PDGF-AA | 0.1 | 0.7 | 0.15 | 0.10 | −0.26 |
| PDGF-AB/BB | 0.0 | 0.2 | 0.06 | 0.12 | 0.18 |
| TGF-α | 0.0 | 0.3 | 0.05 | 0.04 | 0.17 |
| GM-CSF | 0.8 | 0.9 | 0.23 | 0.12 | −0.18 |
| G-CSF | 0.2 | 0.7 | 0.19 | 0.05 | −0.27 |
| Flt3-L | 0.3 | 0.7 | 0.19 | 0.00 | 0.05 |
| IL-7 | 0.5 | 0.8 | 0.24 | −0.09 | 0.16 |
| FGF-2 | 0.3 | 0.8 | 0.20 | −0.02 | 0.18 |
| EGF | 0.2 | 0.1 | 0.11 | −0.14 | 0.23 |
| VEGF | 0.6 | 0.8 | 0.22 | −0.12 | 0.18 |
Boldface values represent “major” positive (≥0.10) and negative (≤-0.10) contributions to the principal component analysis. Abbreviations: EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; Flt3-L, FMS-like tyrosine kinase 3 ligand; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; GRO, growth regulated oncogene; IFN-α, interferon α; IFN-γ, interferon-γ; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-1RA, interleukin 1receptor antagonist; IL-2, interleukin 2; IL-3, interleukin 3; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-7, interleukin 7; IL-9, interleukin 9; IL-10, interleukin 10; IL-12p40, interleukin 12p40; IL-12p70, interleukin 12p70; IL-13, interleukin 13; IL-17, interleukin 17; IP-10 (IFN-γ inducible protein 10); MCP-3, monocyte chemotactic protein 3; MDC, macrophage-derived chemokine; MIP-1α, macrophage inflammatory protein 1α; MIP-1β, macrophage inflammatory protein 1β; PDGF-AA, platelet derived growth factor AA; PDGF-AB/BB, platelet derived growth factor AB/BB; RANTES, regulated upon activation, normal T cell expressed and secreted; sCD40-L, soluble CD40 ligand; sIL-2Rα, soluble IL-2 receptor α; TGF-α, transforming growth factor α; Th2, T helper 2; Th17, T helper 17; TNF-α, tumor necrosis factor α; TNF-β, tumor necrosis factor β; VEGF, vascular endothelial growth factor.
Principal Component Analysis
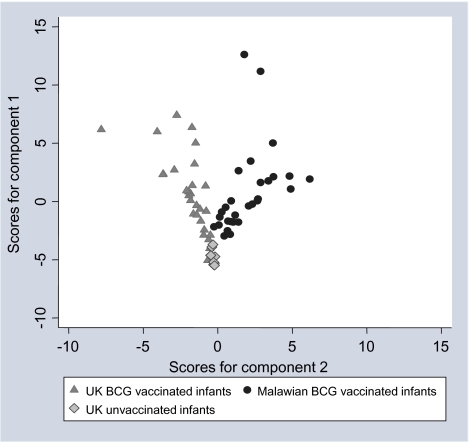
PCA of UK BCG-vaccinated and Malawian BCG-vaccinated infants 3 months after BCG vaccination showed that 55% of the variation could be explained with the first 3 components. Using the first 3 components shows that all 39 cytokines except IP-10 (IL-15, IL-8, and MCP-1 not included) are essential to explain the variation among the vaccinated infants in the UK and Malawi, because it was only for IP-10 that its weight in all of the first 3 principal components was so small that it could be equated approximately to zero (Table 2). Using the first 2 components to explain the variation within the 39 cytokines included, the UK unvaccinated, UK vaccinated, and Malawian vaccinated infants clearly separated into 3 groups, and the variation among individuals who were vaccinated was much more simply summarized (Figure 2). At 12 months after BCG vaccination, PCA showed similar results, with the first 3 components showing that all 39 cytokines were essential to explain the variation among the vaccinated infants (data not shown).
Figure 2.
Score values for components 1 and 2 from the principal component analysis of multiplex responses. Score values are shown for UK BCG-vaccinated infants (triangles) and Malawian BCG-vaccinated infants (circles) 3 months after vaccination and unvaccinated UK infants (diamonds) for components 1 and 2 from the principal component analysis of the 42-multiplex data.
DISCUSSION
BCG vaccination provides variable protection against pulmonary tuberculosis, protecting UK adolescents but not Malawian adults [2]. It is thought that BCG vaccination offers similar protection in all settings in infants, although none of the clinical trials were conducted in Africa [12]. Given our earlier finding that IFN-γ production in response to M. tuberculosis PPD was lower in BCG-vaccinated Malawian infants, compared with UK vaccinated infants [9], we investigated a more comprehensive infant cytokine profile expecting that cytokine responses in Malawian infants would be lower overall than in UK infants. We now show that the immune responses induced by BCG vaccination differ in both profile and magnitude between the 2 settings. There was evidence of differences in median responses in 27 of the 42 cytokines and chemokines tested between the UK and Malawi, 7 of which were higher in the UK and 20 of which were higher in Malawi.
The 7 cytokines and chemokines that were higher in the UK than in Malawi 3 months after BCG vaccination are all Th1 related: IFN-γ, IL-2, TNF-β, IL-6, IL-12p40, MIP-1α, and IP-10, which have been shown to be involved in immunity to tuberculosis. TNF-β, IL-12p40, and MIP-1α have been shown to be involved in granuloma formation [13–15], and IL-6 and IP-10 are produced in greater amounts in tuberculosis patients than in controls, although their role is uncertain [16–18].
The 20 cytokines and chemokines produced in greater concentrations in Malawian infants, compared with UK infants, include innate proinflammatory cytokines, regulatory cytokines, IL-17, Th2 cytokines, chemokines and 7 growth factors. Many of these cytokines, such as the T cell–derived Th2 cytokines and the regulatory cytokine IL-10, are not considered to be protective in immunity to tuberculosis, because they inhibit IFN-γ responses, although they may be important in regulating pathology [19]. The full role of IL-17 is not known; however, it is not thought to aid development of protective immune responses against tuberculosis when produced during a primary immune response [20, 21]. The higher IL-17 responses seen in Malawi following BCG vaccination may represent suboptimal responses.
The greatest fold difference in cytokine responses between the UK and Malawi was in IL-13 at both time points. Malawian infants made higher cytokine responses in all the Th2 cytokines tested and in IL-9 than did UK infants. IL-9 was until recently considered to be a Th2 cytokine, but a distinct Th9 subset producing IL-9 has now been described [22]. A recent study in BCG-vaccinated Gambian infants using a whole-blood assay also showed that PPD induced IL-13 secretion [23].
The Malawian infants also produced higher IL-10 and sIL-2Rα than did UK infants 3 months after vaccination, providing evidence of increased T cell regulation in Malawian infants, compared with UK infants. Interleukin 2Rα production may reflect increased T cell activation or T cell regulation [24].
Overproduction of Th2, T regulatory cytokines, and IL-9 may impair Th1 immune responses and impede induction of anti-mycobacterial immunity [25, 26]. Notably, however, in both countries, it was the individuals who produced high Th2 responses who made high IFN-γ responses (also seen in adolescents [G. Black et al, in preparation]), suggesting that some individuals make stronger cytokine responses in general following vaccination.
Higher concentrations of IL-4 and IL-13 together with lower concentrations of IFN-γ in Malawian infants, compared with UK infants, could result in alternative macrophage activation, which may negatively influence protective immunity against tuberculosis [27]. Alternative activation of macrophages results in a different macrophage phenotype including increased endocytosis and macrophage fusion, decreased autophagy, and altered phagocytic receptor repertoire and cytokine and chemokine secretion (including reduced IL-1β, IL-12, TNF-α, and IP-10 and increased IL-1RA) [27].
Malawian BCG-vaccinated infants had higher innate proinflammatory cytokine responses for IFN-α2, IL-1α, and IL-12p70, compared with UK vaccinees. It remains unclear whether IFN-α is protective or detrimental in M. tuberculosis immunity; it may lead to the development of dendritic cells with altered phenotype, “immunoprivileged macrophage-like cells” that synthesize TNF-α and IL-10 but not IL-12 [28–30].
Malawian infants made higher responses for 7 growth factors, which could indicate that Malawian infants’ immune systems are more active in the development and differentiation of new cell types, compared with the immune systems of UK infants, following BCG vaccination. Adolescents in Malawi have also been found to have a lower proportion of naive T cells and a greater proportion of memory T cells than do UK adolescents, perhaps in part due to the higher burden of such infections as cytomegalovirus in Malawi [31]. The higher concentrations of IFN-α in the Malawian infants, compared with UK infants, following BCG vaccination could in turn result in increased inhibition of telomerase activity [32, 33], which may contribute to increased telomere erosion, expansion of T cells, and altered memory T cell responses.
Growth factors have been suggested for use as adjuvants in new vaccines in the hope that they would boost Th1 responses [34, 35]. This study suggests that even in the presence of high concentrations of growth factors, Th1 responses are low in Malawian infants, and suggests that such vaccine strategies may not be beneficial in Malawian infants with a Th2-polarized immune profile. Future tuberculosis vaccine strategies could be tailored to inhibit, before vaccination, cytokines that suppress the protective cytokine induction, or to strengthen induction of protective cytokines while not inducing those that inhibit protection.
This study identified that 27 cytokines and chemokines were differentially expressed in the UK and Malawi following BCG vaccination. Measuring such secreted analytes at a single time point is not ideal, because the optimal time for measurement will vary, but the advantage of measuring so many products simultaneously outweighs any potential loss of sensitivity. It is not yet clear how much cytokine or which combination of cytokines are required for protection, and perhaps the absolute concentrations of cytokines and chemokines are not as important as the overall pattern of cytokines, for example, the ratio of Th1 to Th2 or Th1 to regulatory T cells, that could determine the level of protection BCG vaccination provides. Larger studies are needed to assess the protection BCG offers to infants while simultaneously looking at the cytokines and chemokines induced by vaccination [36], in order to identify the pattern of cytokine expression required for successful induction and maintenance of protection.
Given the large population differences observed in the immune responses to BCG vaccination in the UK and Malawi, it is probable that BCG vaccination does not offer equal protection to infants across such different settings. The infants studied here were vaccinated between 3 and 13 weeks of age, rather than at birth. Despite the recommended policy that BCG be given at or shortly after birth, many infants in developing countries are vaccinated late. A local survey revealed that only 22% of infants were vaccinated in the first week of life [37]. It is unknown when Malawian infants are first exposed to environmental mycobacteria. IFN-γ responses to M. tuberculosis PPD were higher in infants vaccinated at birth than in those with delayed vaccination, although the confidence intervals were wide and the differences were not statistically significant (A. Ben-Smith et al, in preparation), whereas Gambian infants vaccinated at 4.5 months had lower IFN-γ, IL-6, and IL-17 responses, compared with those vaccinated at birth [23]. Stratified analysis of infants in this study showed that there was no evidence of a difference in cytokine responses in those vaccinated between 3 and 7 weeks of age and those who were vaccinated between 8 and 13 weeks of age, although the study power for these comparisons was low. We plan to compare the cytokine profiles in Malawian infants vaccinated at birth or with delayed vaccination and to assess the cytokine profile to nonspecific stimuli, as well as mycobacterial antigens, to assess whether Malawian infants vaccinated at birth have a similarly skewed cytokine profile. It was not possible to perform intracellular flow cytometry on the samples from these infants, because of the small blood volumes available, but studies in the UK have indicated that IFN-γ is produced by both CD4 T cells, most of which do not produce other cytokines, and natural killer cells (M. K. Lalor, unpublished data; [38]).
The results from this study have given a new perspective on the variable protection provided by BCG in adults. Previously, evidence pointed toward prior exposure to environmental mycobacteria resulting in BCG vaccination failing to provide protection in such countries as Malawi. Here we have shown that immunologically naive infants produce an entirely different cytokine profile following BCG vaccination in the UK and Malawi. We propose that the pattern of cytokines that Malawian infants produce following vaccination, and after contact with infectious diseases, is already predetermined in utero, at birth, or within the first few months of life. This may be due to maternal factors, genetic factors, epigenetic factors, nutritional factors, and/or environmental factors, such as burdens of infectious diseases within the population as a whole. Additional studies to examine cytokine profiles of mothers and their infants prior to and after vaccination are required. Such population-specific differences in immune profiles may have wider implications for both immunity against tuberculosis and the protective efficacy of other vaccines.
Supplementary Data
Supplementary Data are available at The Journal of Infectious Diseases online.
Funding
This work was funded by the Wellcome Trust (063558/Z/01/B) and by the Bill & Melinda Gates Foundation through the Grand Challenges in Global Health Initiative (37772). N. M. was funded by a Wellcome Trust fellowship (WT083495MA). The funders played no role in the design, analysis, or preparation of this manuscript.
Supplementary Material
Acknowledgments
We thank Dr Christine Sloczynska at Waltham Forest Primary Care Trust and Dr Makki Hameed at Redbridge Primary Care Trust and Shakuntala Patel for their help with the UK infant study, and all the mothers and babies who participated in the study.
The study was designed by M. K. L., S. F., P. G.-S., R. E. W., A. B.-S., M. J. N., H. M., A. C. C., S. G. S., P. E. F., and H. M. D., and all authors contributed to the management of the study. Recruitment and sample collection were coordinated by R. B. and H. M. Data management was performed by K. B. Laboratory assays were performed by M. K. L. Data analysis was performed by M. K. L. and S. F. The article was written by M. K. L. taking into account the comments and suggestions from the authors. All authors had the opportunity to comment on the analysis and interpretation and approved the final version for publication.
References
- 1.Kaufmann SH, Parida SK. Tuberculosis in Africa: learning from pathogenesis for biomarker identification. Cell Host Microbe. 2008;4:219–28. doi: 10.1016/j.chom.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–8. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 4.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–55. [PubMed] [Google Scholar]
- 5.Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31:1531–5. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Finan C, Ota MO, Marchant A, Newport MJ. Natural variation in immune responses to neonatal Mycobacterium bovis bacillus Calmette-Guerin (BCG) vaccination in a cohort of Gambian infants. PLoS One. 2008;3:e3485. doi: 10.1371/journal.pone.0003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares AP, Scriba TJ, Joseph S, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–77. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalor MK, Ben-Smith A, Gorak-Stolinska P, et al. Population differences in immune responses to bacille Calmette-Guerin vaccination in infancy. J Infect Dis. 2009;199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djoba Siawaya JF, Roberts T, Babb C, et al. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS One. 2008;3:e2535. doi: 10.1371/journal.pone.0002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalor MK, Smith SG, Floyd S, et al. Complex cytokine profiles induced by BCG vaccination in UK infants. Vaccine. 2009;28:1635–41. doi: 10.1016/j.vaccine.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colditz GA, Berkey CS, Mosteller F, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 13.Jacobs M, Brown N, Allie N, Ryffel B. Fatal Mycobacterium bovis BCG infection in TNF-LT-alpha-deficient mice. Clin Immunol. 2000;94:192–9. doi: 10.1006/clim.2000.4835. [DOI] [PubMed] [Google Scholar]
- 14.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(suppl 3):S189–93. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 16.Hussain R, Kaleem A, Shahid F, et al. Cytokine profiles using whole-blood assays can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. J Immunol Methods. 2002;264:95–108. doi: 10.1016/s0022-1759(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 17.Djoba Siawaya JF, Beyers N, van Helden P, Walzl G. Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis. Clin Exp Immunol. 2009;156:66–77. doi: 10.1111/j.1365-2249.2009.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzurri A, Sow OY, Amedei A, et al. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Rook GA, Dheda K, Zumla A. Immune systems in developed and developing countries; implications for the design of vaccines that will work where BCG does not. Tuberculosis (Edinb) 2006;86:152–62. doi: 10.1016/j.tube.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Goldsack L, Kirman JR. Half-truths and selective memory: interferon gamma, CD4+ T cells and protective memory against tuberculosis. Tuberculosis (Edinb) 2007;87:465–73. doi: 10.1016/j.tube.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Romano M, D'Souza S, Adnet PY, et al. Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine. 2006;24:3353–64. doi: 10.1016/j.vaccine.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 22.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 23.Burl S, Adetifa UJ, Cox M, et al. Delaying bacillus Calmette-Guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J Immunol. 2010;185:2620–8. doi: 10.4049/jimmunol.1000552. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LA, Jay G, Nelson DL. The released interleukin 2 receptor binds interleukin 2 efficiently. J Immunol. 1986;137:3841–4. [PubMed] [Google Scholar]
- 25.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7:327–37. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 26.Wu B, Huang C, Kato-Maeda M, et al. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin Immunol. 2008;126:202–10. doi: 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 28.Mariotti S, Teloni R, Iona E, et al. Mycobacterium tuberculosis diverts alpha interferon-induced monocyte differentiation from dendritic cells into immunoprivileged macrophage-like host cells. Infect Immun. 2004;72:4385–92. doi: 10.1128/IAI.72.8.4385-4392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchant A, Amedei A, Azzurri A, et al. Polarization of PPD-specific T-cell response of patients with tuberculosis from Th0 to Th1 profile after successful antimycobacterial therapy or in vitro conditioning with interferon-alpha or interleukin-12. Am J Respir Cell Mol Biol. 2001;24:187–94. doi: 10.1165/ajrcmb.24.2.4274. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, O'Donnell MA, Luo Y. Dose-dependent synergy of Th1-stimulating cytokines on bacille Calmette-Guerin-induced interferon-gamma production by human mononuclear cells. Clin Exp Immunol. 2007;149:178–85. doi: 10.1111/j.1365-2249.2007.03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Smith A, Gorak-Stolinska P, Floyd S, et al. Differences between naive and memory T cell phenotype in Malawian and UK adolescents: a role for cytomegalovirus? BMC Infect Dis. 2008;8:139. doi: 10.1186/1471-2334-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed JR, Vukmanovic-Stejic M, Fletcher JM, et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 2004;199:1433–43. doi: 10.1084/jem.20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbar AN, Vukmanovic-Stejic M. Telomerase in T lymphocytes: use it and lose it? J Immunol. 2007;178:6689–94. doi: 10.4049/jimmunol.178.11.6689. [DOI] [PubMed] [Google Scholar]
- 34.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–87. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triccas JA, Shklovskaya E, Spratt J, et al. Effects of DNA- and Mycobacterium bovis BCG-based delivery of the Flt3 ligand on protective immunity to Mycobacterium tuberculosis. Infect Immun. 2007;75:5368–75. doi: 10.1128/IAI.00322-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanekom WA. The immune response to BCG vaccination of newborns. Ann N Y Acad Sci. 2005;1062:69–78. doi: 10.1196/annals.1358.010. [DOI] [PubMed] [Google Scholar]
- 37.Jahn A, Floyd S, Mwinuka V, et al. Ascertainment of childhood vaccination histories in northern Malawi. Trop Med Int Health. 2008;13:129–38. doi: 10.1111/j.1365-3156.2007.01982.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith SG, Lalor MK, Gorak-Stolinska P, et al. Mycobacterium tuberculosis PPD-induced immune biomarkers measurable in vitro following BCG vaccination of UK adolescents by multiplex bead array and intracellular cytokine staining. BMC Immunol. 2010;11:35. doi: 10.1186/1471-2172-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.