Abstract
Bone morphogenetic protein (BMP) receptors signal by phosphorylating Smad1, which then associates with Smad4; this complex moves into the nucleus and activates transcription. Here we report the existence of a natural inhibitor of this process, Smad6, a longer version of the previously reported JV15-1. In Xenopus embryos and in mammalian cells, Smad6 specifically blocks signaling by the BMP/Smad1 pathway. Smad6 inhibits BMP/Smad1 signaling without interfering with receptor-mediated phosphorylation of Smad1. Smad6 specifically competes with Smad4 for binding to receptor-activated Smad1, yielding an apparently inactive Smad1–Smad6 complex. Therefore, Smad6 selectively antagonizes BMP-activated Smad1 by acting as a Smad4 decoy.
Keywords: Smad proteins, BMP receptors, TGFβ, Xenopus, antagonist, mammalian cells
Members of the transforming growth factor β (TGFβ) family of cytokines, which also includes activins and the bone morphogenetic proteins (BMPs), initiate signaling from the cell surface by interacting with two distinct serine/threonine kinase receptors (Massagué and Weis-Garcia 1996; ten Dijke et al. 1996). Ligand binding induces the formation of a complex in which the type II receptor phosphorylates and activates the type I receptor; this protein then propagates the signal by phosphorylating a family of signal transducers, the Smad proteins (Massagué et al. 1997). To date, eight vertebrate Smad proteins, Smad1–Smad-7 and Smad-9, are cloned (Massagué et al. 1997; Topper et al. 1997; Watanabe et al. 1997). The members of this family have conserved amino- and carboxy-terminal domains, hereafter referred to as the N-domain and the C-domain. The C-domain has effector function, as it participates in homomeric and heteromeric interactions (Hata et al. 1997; Shi et al. 1997; Wu et al. 1997) and acts as a transcriptional activator (Chen et al. 1996; Liu et al. 1996).
Although Smads display common structural features, recent studies have revealed that their functions in the signaling pathway are diverse and can be divided into three classes: (1) receptor-regulated Smad proteins, which act as direct substrates of the receptors; (2) Smads that act as functional partners of the receptor-regulated Smads; and (3) antagonistic Smads or anti-Smads. Smad1, Smad2, Smad3, Smad5, and Smad9 belong to the first class: Smad 1 and presumably its closely related members Smad5 and Smad9 mediate BMP signaling (Graff et al. 1996; Hoodless et al. 1996; Lechleider et al. 1996; Liu et al. 1996; Thomsen 1996; Yingling et al. 1996; Kretzschmar et al. 1997; Suzuki et al. 1997; Watanabe et al. 1997) whereas Smad2 and Smad3 mediate TGFβ/activin signaling (Baker and Harland 1996; Eppert et al. 1996; Graff et al. 1996; Macias-Silva et al. 1996; Zhang et al. 1996). These Smads are directly phosphorylated on serine residues at their carboxy-terminal ends (SSXS motif) by the specific type I receptor (Macias-Silva et al. 1996; Kretzschmar et al. 1997), and their phosphorylation leads to formation of a heteromeric complex with the second Smad class, which includes Smad4 in vertebrates (Lagna et al. 1996; Zhang et al. 1997). Unlike receptor-regulated Smads, which are specific for each pathway, the tumor suppressor Smad4/DPC4 (Hahn et al. 1996) acts as a shared partner for both BMP-specific and TGFβ/activin-specific Smads (Lagna et al. 1996; Zhang et al. 1997) and plays an essential role as a transcriptional activator in the nucleus (Liu et al. 1997).
Recently, a third class of Smads has been reported, whose members act as antagonists of these signaling pathways (Hayashi et al. 1997; Imamura et al. 1997; Nakao et al. 1997; Topper et al. 1997; Tsuneizumi et al. 1997). When overexpressed, these Smads can interact with various type I receptors and nonselectively inhibit signaling by various TGFβ superfamily members, leading to the notion that antagonistic Smads act as general blockers of type I receptors (Hayashi et al. 1997; Imamura et al. 1997; Nakao et al. 1997). In contrast to this model, the results presented here show that both in Xenopus and in mammalian cells, Smad6 efficiently blocks Smad1 signaling, without inhibiting Smad2 signaling. Consistently, we observe a direct, BMP-dependent interaction of Smad6 with Smad1, but not with Smad2 or Smad4. The Smad1–Smad6 interaction can prevent the formation of the Smad1–Smad4 complex, which is required for BMP signaling. Although Smad6 can associate with type I receptors, it does so indiscriminately and inhibits Smad1 signaling without inhibiting receptor-mediated phosphorylation. Thus, we conclude that Smad6 inhibits BMP/Smad1 signaling in vivo by acting as a selective Smad4 decoy.
Results
Smad6 structure
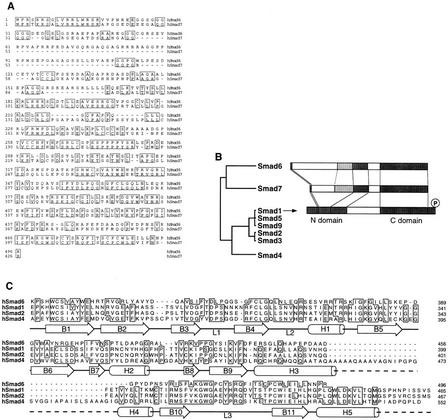
Smad6 (originally known as JV15-1) was previously reported to be a 235-amino-acid protein encoded by various human EST clones (Riggins et al. 1997). By screening a human T-cell cDNA library with a probe corresponding to the Smad6 C-domain, we isolated a cDNA encoding a 496-amino-acid protein (Fig. 1A) with a typical full-length Smad domain structure (Fig. 1B). The screening of a Xenopus library yielded partial cDNA clones encoding products that were >67% identical to the last 276 amino acids of the human Smad6 sequence. Smad6 is more closely related to Smad7 (40% identity; Fig. 1A) than to other Smads (17%–19% identity; Fig. 1B). Smad6 lacks the carboxy-terminal SSXS motif that serves as a receptor phosphorylation site in receptor-regulated Smads (Macias-Silva et al. 1996; Kretzschmar et al. 1997) and lacks an additional portion at the carboxyl terminus that is highly conserved in other Smads (Fig. 1C).
Figure 1.
Structure of Smad6. (A) Alignment of the predicted amino acid sequence of human Smad6 and Smad7. Identical residues are boxed. (B) Smad homology tree and schematic comparison of the structures of Smad6, Smad7, and Smad1. The amino and carboxy-terminal homologous regions (N- and C-domain) are darkly shaded. A region conserved only between Smad6 and Smad7 is lightly shaded. (C) Alignment of C-domain sequences of Smad6 and other Smads, indicating the secondary structure elements of the Smad4 C-domain (Shi et al. 1997). In the crystal structure of Smad4 this region forms an α-helix (α-helix 5) that associates with α-helices 3 and 4 in a three-helix bundle. This structure contributes the formation of a Smad4 homotrimer by interacting with loops 1 and 2 of the adjacent monomer. The region corresponding to loops 1 and 2 in Smad6 is also very divergent from Smad4 and the receptor-regulated Smads. However, most of the components that constitute the β-sandwich core structure of the Smad4 C-domain are conserved in Smad6.
Smad6 RNA injection causes formation of ectopic dorsal axes in Xenopus embryos
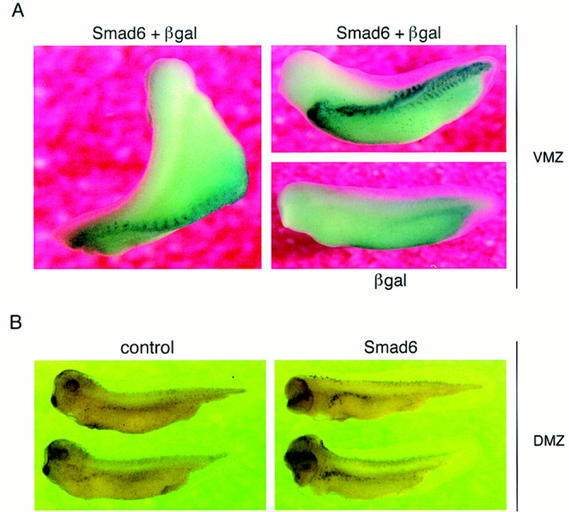
Whole-mount in situ hybridization of Xenopus embryos at gastrula, neurula, and tadpole stages with a Xenopus Smad6 probe revealed a generalized pattern of Smad6 expression in all stages (data not shown). To begin to address the function of Smad6 in vivo, RNA encoding human Smad6 was coinjected with β-galactosidase RNA, used as lineage tracer, into the ventral vegetal blastomeres of eight cell stage embryos. Embryos injected with these transcripts, develop a secondary dorsal axis in >90% of the cases (n = 30; Fig. 2A). Injection of greater amounts of Smad6 induced secondary axes that were proportionally more complete (Fig. 2A, top right). Furthermore, β-gal staining of the injected embryos revealed that the progeny of the injected blastomere directly contributed to the ectopic axis, suggesting an organizer type of activity mediated by Smad6 (Fig. 2A). As with other Smad C-domains (Baker and Harland 1996; Liu et al. 1996), constructs encoding the isolated C-domain of Smad6 (human or Xenopus) were more potent than full-length Smad6 at generating this phenotype (data not shown).
Figure 2.
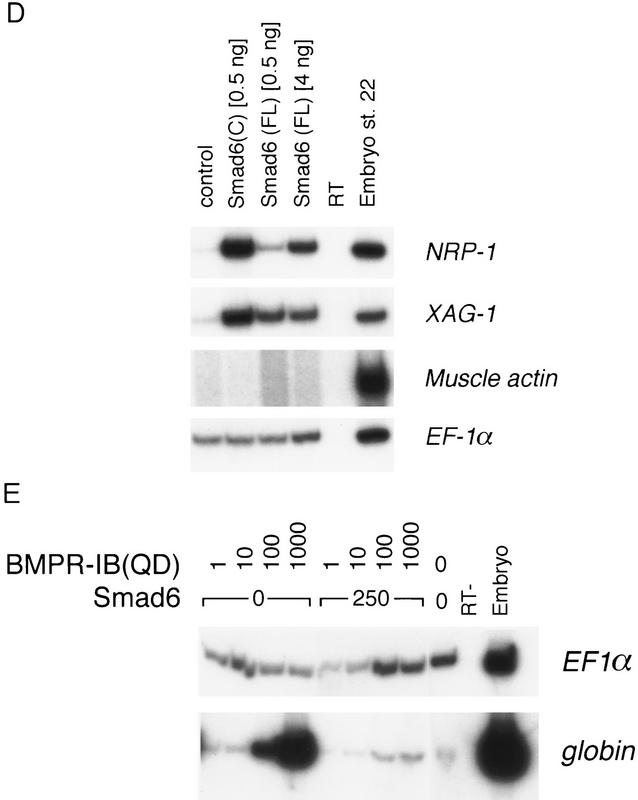
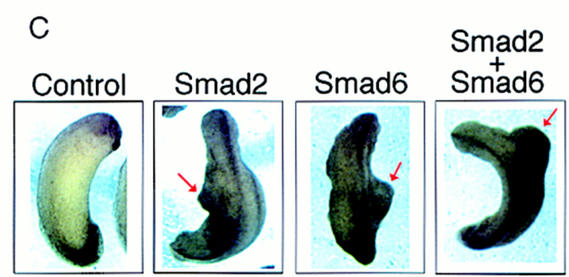
Smad6 induces secondary axes and neuralizes ectoderm in Xenopus. (A) Smad6 overexpression induces the formation of a secondary axis. Smad6 RNA (0.5–4 ng) was coinjected with nuclear β-galactosidase (β-gal)RNA (100 pg) into the ventral vegetal blastomeres of eight cell stage embryos. Unlike control embryos injected with β-gal alone (bottom right), Smad6 coinjected embryos develop an ectopic dorsal axis (left and top right). β-Galactosidase staining of the injected embryos reveals that the progeny of the injected blastomere directly contribute to the ectopic axis. Dorsal at left; anterior at top. Lateral at right; anterior at left. (B) Smad6 does not affect formation of the primary dorsal axis. Smad6 RNA (1–4 ng) was injected into the dorsal marginal zone of four cell stage embryos. Compared to control embryos (left panel), Smad6-injected tadpoles display enlarged heads and cyclopia, but have normal body axes (right). (C) Secondary axis formation by the activin signaling molecule Smad2 is enhanced by coinjection of Smad6. Smad2 RNA (1 ng), Smad6 RNA (1 ng), or both RNAs (1 ng each) were injected into ventral vegetal blastomeres of eight cell stage embryos. Coinjection of Smad2 and Smad6 produces secondary axes (red arrows) that are more complete than those obtained by injection of either Smad alone. Anterior is at top. (D) Smad6 neuralizes ectodermal explants. Smad6 and Smad6 C-domain RNAs were injected at the indicated amount in the animal pole of two-cell stage embryos. At the blastula stage, animal caps were dissected and cultured in saline solution. At the tailbud stages (stage 22), total RNA was harvested and analyzed by RT–PCR for the presence of the indicated transcripts. Full-length human Smad6 (0.5 and 4 ng) induces NRP-1, a pan–neural marker, and XAG-1, a marker of cement gland. Cement gland is induced efficiently even at the lower dose; induction of neural tissue requires a higher dose of Smad6. The isolated C-domain RNA is a more potent inducer of both XAG-1 and NRP-1 than full-length Smad6. Neither construct induced muscle actin, a marker of dorsal (paraxial) mesoderm. EF-1α, ubiquitously expressed, is a loading control. RNA from whole embryos (Embryo) provides the positive control. The RT lane is identical to the embryo lane, except that reverse transcriptase was omitted. (E) Smad6 interferes with blood induction by a constitutively active BMP receptor. BMPR-IB(QD) RNA (1–1000 pg) was injected either alone or together with Smad6 RNA (250 pg) in the animal pole. BMPR-IB(QD)-injected ectodermal explants show induction of globin at stage 30, and this response is blocked by coexpressed Smad6.
Control injections of Smad6 in the dorsal marginal zone produced embryos with enhanced head structures but with a normal primary axis, suggesting the inability of Smad6 to interfere with dorsal mesoderm formation (Fig. 2B). The injection of RNAs encoding activin (Thomsen et al. 1990), inhibitors of the BMP pathway, such as Noggin (Smith and Harland 1992), Chordin (Sasai et al. 1994), Follistatin (Hemmati-Brivanlou et al. 1994; Sasai et al. 1995), or the dominant-negative type I BMP receptor (tBMPR-I) (Graff et al. 1994; Suzuki et al. 1994), can give rise to a similar secondary axis phenotype. Overexpression of Smad2 has the same effect [Fig. 2C (Baker and Harland 1996; Graff et al. 1996)]. In this assay, coinjection of Smad2 and Smad6 produces secondary axes that are more frequent and extend more anteriorly than those obtained by injection of either Smad alone at the chosen concentrations (Fig. 2C), suggesting an additive effect. The results obtained with Smad6 are therefore consistent with either activation of the activin pathway or inhibition of BMP signaling.
Smad6 inhibits BMP signaling and neuralizes ectodermal explants
To differentiate between the two possibilities described above, we took advantage of the ectodermal explant (animal cap) assay. When removed at blastula stages and cultured in saline solution, animal caps develop as epidermis. This is the result of endogenous BMP signaling that induces and maintains the epidermal fate within the explants. Any interference with this signaling pathway unveils the “default” fate of the ectoderm and the cells switch their fate to neural (Wilson and Hemmati-Brivanlou 1997). Animal caps incubated with activin protein, on the other hand, develop as mesoderm (Asashima et al. 1990; Smith et al. 1990; Thomsen et al. 1990; van den Eijnden-Van Raaij et al. 1990). Therefore, the animal cap assay offers an easy way to distinguish between an effect of Smad6 that is due to the activation of the activin pathway versus an interference with the BMP pathway.
Embryos were injected with either control RNA or transcripts encoding full-length Smad6 or its C-domain in the animal pole. Animal caps were removed at blastula stage and allowed to develop until controls reached tailbud stages, at which time they were assayed by reverse transcription (RT)–PCR for cell fate choices. Figure 2D shows that overexpression of Smad6 or Smad6 C-domain (amino acids 272–496) leads to the induction of NRP-1, a pan-neural marker, and XAG-1, a marker of cement gland (an organ associated with neural tissue in Xenopus). Interestingly, low levels of full-length Smad6 (0.5 ng) induced mostly cement gland, whereas a higher dose (4 ng) also induced neural tissue efficiently. A similar dose-dependent effect has also been observed for Noggin and tBMPR-IA and is thought to reflect a gradual inhibition of the morphogen-like action of the BMPs (Wilson et al. 1997). Importantly, no expression of the axial mesodermal marker muscle actin was detected (Fig. 2D), indicating that induction of neural tissue is direct. Furthermore, coinjection of Smad6 inhibited the induction of the ventral mesoderm marker globin by the constitutively active BMP type I receptor BMPR-IB(QD) (Fig. 2E; Kretzschmar et al. 1997). These results strongly suggest that Smad6 acts as an antagonist of BMP signaling rather than as a mediator of activin signaling.
Smad6 is an inhibitor of BMP/Smad1 signaling
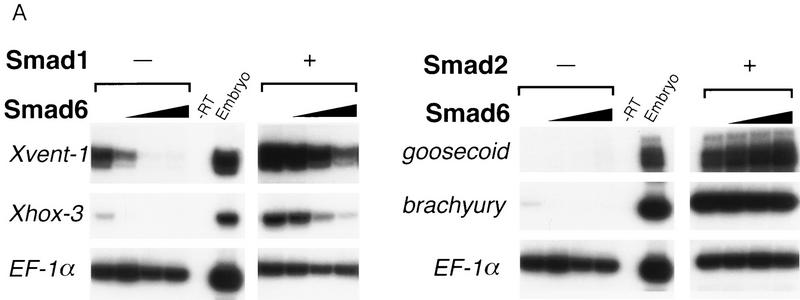
To determine whether Smad6 might be an inhibitor of BMP signals mediated by Smad1, we tested the effects of the corresponding transcripts, alone or in combination, on the induction of ventral mesoderm markers in the animal cap assay. Overexpression of Smad6 alone inhibited the basal level of expression of Xvent-1 and Xhox-3 (Fig. 3A). Induction of these two markers is known to require endogenous BMP signals mediated by Smad1 (Lagna et al. 1996; Liu et al. 1996; Thomsen 1996). Furthermore, Smad6 potently inhibited the overinduction of these markers by coinjected Smad1 (Fig. 3A). Smad6 inhibited Smad1 signaling specifically, as it did not interfere with the induction of a general mesoderm marker (brachyury) or a dorsal mesoderm marker (goosecoid) by the activin mediator Smad2 (Fig. 3A; Baker and Harland 1996; Graff et al. 1996). Consistently, Smad6 was unable to inhibit the induction of muscle actin in animal caps incubated with activin (data not shown).
Figure 3.
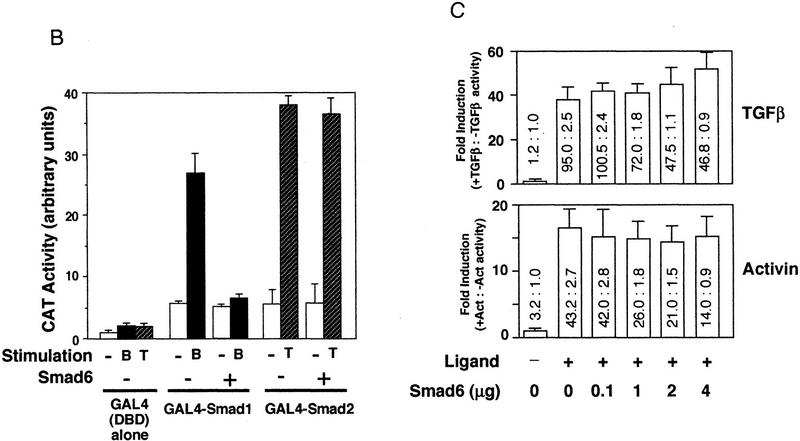
Smad6 specifically inhibits the BMP/Smad1 signaling pathway. (A) Smad6 blocks induction of ventral mesoderm by Smad1 but not induction of dorsal mesoderm by Smad2. An increasing amount of Smad6 RNA (0.5–4 ng) was coinjected with or without a fixed amount of Smad1 or Smad2 RNA (2 ng). Animal caps from injected embryos were collected at gastrula stage (stage 11.5) and subjected to RT–PCR. Xvent-1 and Xhox-3 are markers of ventral mesoderm; goosecoid is a dorsal mesoderm marker and brachyury is a pan–mesodermal marker. (B) BMP-dependent transcriptional activation of GAL4–Smad1 fusion protein is blocked by Smad6. R-1B/L17 cells were transfected with the reporter gene (G1E1BCAT, 1 μg), appropriate receptors (TβR-I for TGFβ or BMPR–IB and BMPR–II for BMP2), and either a vector containing the GAL4 DNA-binding domain (DBD) alone or fusion constructs of DBD with Smad1 or Smad2 in the presence or absence of Smad6 (2 μg). Cells were incubated with or without 5 nm BMP2 (B) or 100 pm TGFβ (T) for 18 hr. CAT activity is expressed as the mean ± s.d. of three independent experiments. (C) TGFβ or activin-induced transcriptional activation of the 3TP promoter is not inhibited by Smad6. R-1B/L17 cells were transfected with the reporter gene (p3TP–lux) and type I receptors for TGFβ (TβR-l) or activin (ActR-IB) and were treated with or without 100 pm TGFβ (top) or 2 nm activin (bottom) for 18 hr. The ratios of stimulated to unstimulated levels of luciferase activity are indicated numerically, and their quotients plotted in the bar graph. Data are the mean ± s.d. of triplicate values. Notice that although Smad6 transfection decreased the basal as well as the agonist-induced levels of luciferase activity, it did not decrease the relative induction by TGFβ or activin.
To investigate the action of Smad6 in a mammalian cell system, we determined the effect of Smad6 on transcriptional activation by Smad1 and Smad2. In R-1B/L17 mink lung epithelial cells, full-length Smad1 or Smad2 fused to the DNA-binding domain of GAL4 activates transcription of a GAL4 reporter gene in response to BMP2/4 or TGFβ signals, respectively (Fig. 3B; Liu et al. 1996). This activity requires the presence of Smad4 in the cell (Liu et al. 1997). Cotransfection of Smad6 completely abolished the activation of GAL4–Smad1 by BMP2 but did not affect the activation of GAL4–Smad2 by TGFβ (Fig. 3B). Moreover, a GAL4–Smad6 C-domain fusion protein was unable to activate transcription in this assay (data not shown). We also tested the activation of the p3TP–lux reporter, which contains TGFβ/activin responsive elements from the PAI-1 promoter. Transient transfection of p3TP–lux into L17/R1B cells, together with the type I receptor TβR-I or ActR-IB, generated a strong induction of p3TP–lux activity in response to TGFβ or activin (Fig. 3C). Doses of Smad6 vector well above those that inhibited BMP signaling in this cell line (see Fig. 3B) did not diminish the induction of 3TP–lux by TGFβ or activin. Therefore, both in Xenopus embryos and in mammalian cells, Smad6 selectively inhibited BMP and Smad1 signaling.
Nonselective interaction with type I receptors
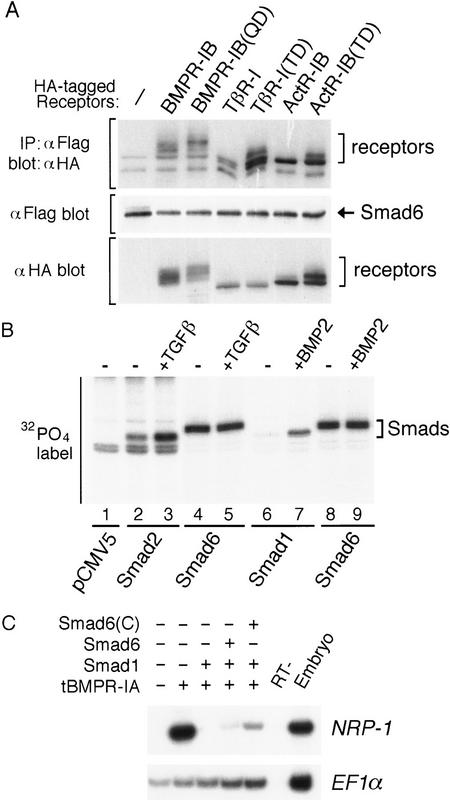
To determine whether Smad6 might selectively interfere with the BMP/Smad1 signaling pathway at the level of the BMP receptors, we cotransfected Smad6 constructs with wild-type or constitutively active forms of various type I receptors into COS cells, and analyzed the interaction of Smad6 with these receptors. When overexpressed in mammalian cells, both the full-length Smad6 (Fig. 4A) and the Smad6 C-domain (data not shown) can interact with the BMP type I receptor BMPR-IB, and the interaction is somewhat stronger with the hyperactive mutant BMPR-IB(QD). Smad6 also bound to the kinase-deficient BMPR-IB receptor [BMPR-IB(KR), data not shown]. However, an interaction was detected also with the TGFβ type I receptor TβR-I and the activin type I receptor ActR–IB and their respective activated forms, TβR-I(TD) (Wieser et al. 1995) and ActR–IB(TD) (Attisano et al. 1996) (Fig. 4A). Smad6 is a phosphoprotein, but in contrast to Smad1 and Smad2 (Eppert et al. 1996; Hoodless et al. 1996; Macias-Silva et al. 1996; Kretzschmar et al. 1997) its phosphorylation level was not increased in response to TGFβ or BMP (Fig. 4B). Thus, Smad6 appears to interact with type I receptors nonselectively.
Figure 4.
Smad6 is a phosphoprotein which binds to type I receptors nonspecifically and inhibits downstream of BMP receptors. (A) Smad6 nonspecifically interacts with type I receptors of the TGFβ superfamily. COS cells were transfected with Flag-tagged Smad6 and HA-tagged wild-type or constitutively active type I receptor (QD and TD) for BMPs (BMPR-IB), TGFβ (TβR-I), or activins (ActR–IB). Cell lysates were subjected to anti-Flag immunoprecipitation using a monoclonal antibody followed by immunoblotting using anti-HA polyclonal antibody (toppanel). Immunoprecipitates of cells transfected with receptor alone did not contain specific proteins (data not shown). Similar levels of Smad6 and receptor expression were confirmed by analyzing aliquots of total cell lysate by SDS-PAGE followed by immunoblotting (middle and bottom panels). (B) The level of phosphorylation of Smad6 is unchanged by BMP2 or TGFβ stimulation. R-1B/L17 cells were transiently transfected with an empty vector (pCMV5) or with the Flag-tagged Smads indicated at the bottom. Smad2- or Smad6-transfected cells (lanes 2–5) were cotransfected with TβR-I; Smad1- or Smad6-transfected cells (lanes 6–9) were cotransfected with BMPR-IB and BMPR-II. Cells were labeled with [32P]phosphate, stimulated with (+) or without (−) 100 pm TGFβ or 5 nm BMP2 for 20 min. Flag-tagged Smads were purified by immunoprecipitation with anti-Flag M2 antibody and analyzed by SDS-PAGE and autoradiography. (C) Smad6 inhibits receptor-independent Smad1 signaling. Expression of dominant-negative BMP type I receptor (tBMPR-IA, 1 ng of RNA) induces neural tissue (NRP-1 marker) in Xenopus animal caps. Smad1 (1 ng of RNA) prevents neuralization when coexpressed with tBMPR-IA, but its activity is inhibited by Smad6 and Smad6(C) (2 ng of RNA). EF1α is the loading control.
Smad6 inhibits signaling downstream of BMP receptors
Expression of truncated BMP type I receptor constructs induces expression of neural markers in Xenopus ectodermal explants by inhibiting the antineuralizing activity of endogenous BMP (Graff et al. 1994; Suzuki et al. 1994; Xu et al. 1995). This dominant-negative effect can be reversed by overexpression of Smad1 (Graff et al. 1996; Thomsen 1996). It is thought that under these conditions, BMP signaling is restored in a BMP receptor-independent manner through the basal activity of the overexpressed Smad1 (Graff et al. 1996; Thomsen 1996). We tested whether Smad6 would inhibit Smad1 signaling under these conditions. An ability of Smad6 to inhibit receptor-independent Smad1 signaling would suggest that Smad6 acts downstream of BMP receptors. Figure 4C shows that coexpression of Smad6 or Smad6(C) blocked the antineuralizing effect of Smad1. Thus, although Smad6 interacts nonspecifically with type I receptors, it appears to inhibit the BMP/Smad1 pathway at a level downstream of the receptor.
Smad6 specifically associates with Smad1 in response to BMP
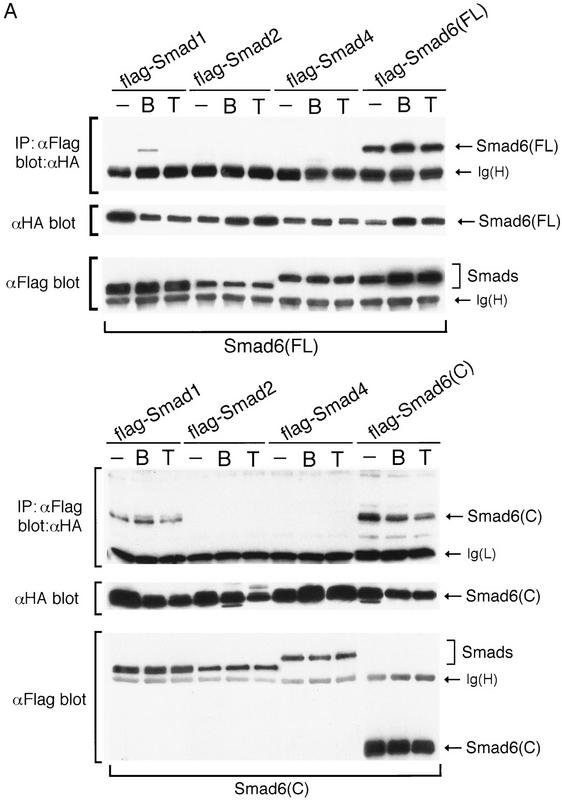
Because Smad6 inhibits downstream of the receptor, we searched for evidence of a specific mechanism at the level of Smad–Smad interactions. To this end, Smad constructs tagged at the amino terminus with the flag epitope were cotransfected with Smad6 tagged at the amino terminus with HA, and interactions between these proteins were investigated in the presence or absence of agonists. Figure 5A (top panel) shows that Smad6 associated with itself in a ligand-independent fashion. In addition, Smad6 associated with Smad1 in response to BMP2 but not in response to TGFβ, and finally, Smad6 did not interact with Smad2 or Smad4 in response to either BMP2 or TGFβ.
Figure 5.
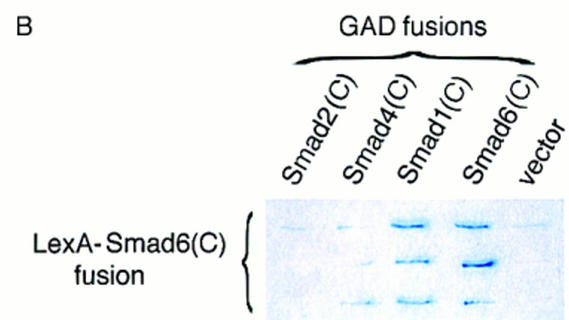
Smad6 interact with itself and Smad1, but not with Smad2 or Smad4. (A) Specific interaction between Smad1 and Smad6. COS cells were transiently transfected with Flag-tagged Smads as indicated on the top, and HA-tagged Smad6 (toppanel) or Smad6 C-domain (bottom panel). Cells were treated with 5 nm BMP2 or 100 pm TGFβ for 1 hr before harvest. Cell lysates were subjected to immunoprecipitation with anti-Flag antibody and then immunoblotting using the anti-HA monoclonal antibody 12CA5. Expression of Smads was measured by anti-Flag or anti-HA immunoprecipitation of aliquots of cell lysates, followed by anti-Flag or anti-HA immunoblotting (bottompanel). [Ig(H) and Ig(L)] Immunoglobulin heavy and light chain bands, respectively. (B) The interaction between Smad6 C-domain and Smad1 C-domain is specific and direct. The C-domains of Smad1, Smad2, Smad4, and Smad6 fused to the GAL4 activation domain (GAD) were tested for interaction with Smad6 C-domain fused to the LexA DNA-binding domain in yeast. Interaction was monitored by the β-galactosidase assay, which allowed us to score for association by the presence of blue color.
In parallel assays, the Smad6 C-domain associated constitutively with Smad1, and this interaction was somewhat increased in response to BMP2 but not in response to TGFβ. However, no association was observed between the Smad6 C-domain and either Smad2 or Smad4 (Fig. 5A, bottom panel). The specificity of the Smad6–Smad1 association and the direct nature of this interaction were demonstrated by use of a yeast two-hybrid system. Using this approach, we have previously shown that the homo- and hetero-oligomeric interactions of Smad2 and Smad4 are mediated by their C-domains (Hata et al. 1997). A LexA–Smad6 C-domain fusion protein was used as bait. Although the C-domain of Smad6 was able to interact with another Smad6 C-domain or with the Smad1 C-domain, it did not interact with the C-domains of Smad2 or Smad4 (Fig. 5B). This suggests that Smad6 and Smad1 can interact directly and specifically with each other in a BMP-dependent fashion.
Smad6 competitively inhibits formation of the Smad1–Smad4 complex
The properties of the Smad6–Smad1 interaction described above are very similar to those of the Smad4–Smad1 interaction. In both cases, the interaction between the full-length proteins is dependent on BMP stimulation (Lagna et al. 1996) and the interaction by the C-domains is constitutive (Hata et al. 1997; A. Hata and J. Massagué, unpubl.). A major difference is that Smad4 can interact with different receptor-activated Smads (Lagna et al. 1996; Zhang et al. 1997), whereas Smad6 interacts with receptor-activated Smad1 but not receptor-activated Smad2. These observations not only suggested that Smad1 is the specific target of Smad6 but also raised the possibility that Smad6 might act by competing with Smad4 for receptor-activated Smad1.
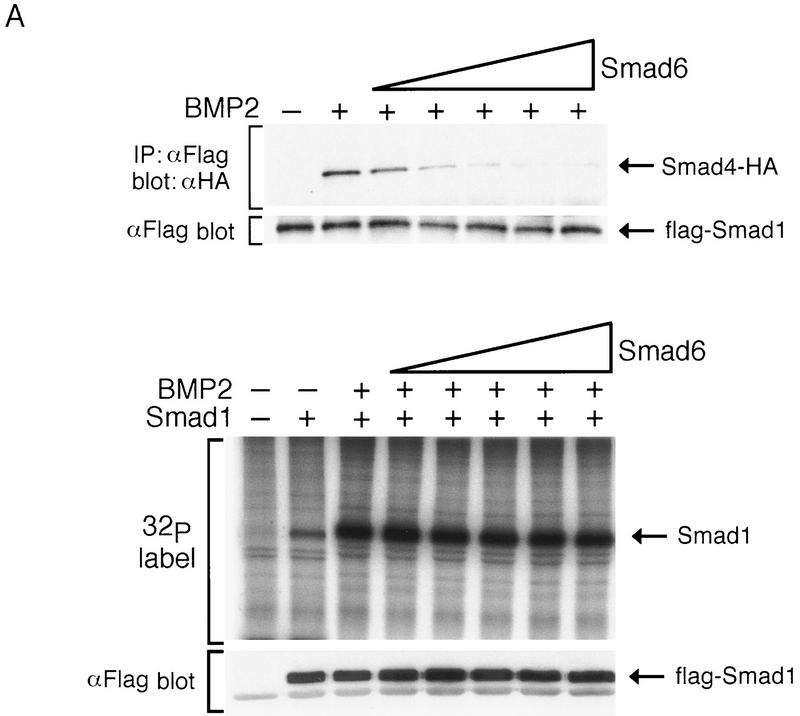
To test this hypothesis, we determined the effect of Smad6 on Smad1–Smad4 association. Smad6 overexpression completely inhibited the formation of a Smad1–Smad4 complex in response to BMP2 (Fig. 6A, top panel). Importantly, concentrations of Smad6 that completely inhibited formation of the Smad1–Smad4 complex in response to BMP2 did not inhibit the BMP2-induced phosphorylation of Smad1 (Fig. 6A, bottom panel). We did observe an inhibition of Smad1 phosphorylation by Smad6, but only when the Smad6–Smad1 concentration ratio was 10-fold higher than the highest ratio used in Figure 6A (data not shown).
Figure 6.
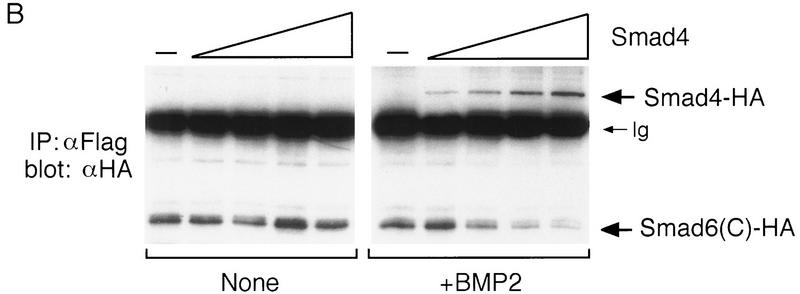
Smad6 inhibits the formation of the Smad1–Smad4 complex. (A) Smad6 inhibits the BMP-dependent complex formation between Smad1 and Smad4 but does not inhibit the BMP-dependent phosphorylation of Smad1. COS cells were transiently transfected with BMPR-IB/BMPR-II (0.1 μg) (BMP2 + lanes), Flag-tagged Smad1 (1 μg), and increasing amount of Smad6 (1, 2.5, 5, 7.5, and 10 μg). One-half of the cells was treated with 5 nm BMP2 for 30 min and harvested. Cell lysates were subjected to immunoprecipitation with anti-Flag M2 antibody followed by immunoblotting with anti-HA polyclonal antibody (Y-11) (top panel, IP; αFlag; blot, αHA). Expression of Smad1 was monitored by analyzing aliquots of total cell lysate by SDS-PAGE followed by immunoblotting with anti-Flag antibody (top panel, αFlag blot). The remaining half of the cells was labeled with [32P]phosphate, stimulated with 5 nm BMP2 for 20 min, and harvested. Cell lysates were subjected to immunoprecipitation with anti-Flag M2 antibody and analyzed by SDS-PAGE and autoradiography (bottom panel, 32P label). Expression of Smad1 was monitored by immunoprecipitation followed by immunoblotting using anti-Flag M2 antibody (bottom panel, αFlag blot). (B) Smad4 disrupts the formation of a complex between Smad1 and Smad6(C). COS cells were transiently transfected with Flag-tagged Smad1 (2 μg), HA-tagged Smad6 C-domain (2 μg), and increasing amounts of HA-tagged Smad4 (1, 2, 4, 8 μg). Cells in the right panel were treated with 5 nm BMP2 for 1 hr before harvest. Cell lysates were subjected to immunoprecipitation with anti-Flag M2 antibody followed by immunoblotting using anti-HA monoclonal antibody (12CA5). The migration of Smad6 C-domain and Smad4 proteins is indicated at right. Increasing doses of Smad4 do not affect Smad1–Smad6(C) complex formation in the absence of BMP2 stimulation; however, in the presence of BMP2, formation of the Smad1–Smad4 complex inhibits Smad1–Smad6(C) complex formation. (C) Smad4 rescues the Smad6-induced inhibition of BMP-dependent Smad1 activation. R-1B/L17 cells were transfected with the reporter gene (GAL4–lux, 1 μg), BMPR-IB (1 μg), BMPR-II (0.1 μg), and either a vector containing the GAL4 DNA binding domain (DBD) alone or a GAL4(DBD)–Smad1 fusion construct. Smad6 (2 μg) and/or Smad4 (4 μg) were cotransfected where indicated. Cells were incubated with (solid bars) or without (open bars) 5 nmr for 18 hr. Luciferase activity is expressed as the mean ± s.d. of two independent experiments.
To determine more directly whether Smad6 and Smad4 compete for receptor-activated Smad1, we cotransfected HA epitope-tagged versions of full-length Smad4 and the Smad6 C-domain together with flag epitope-tagged Smad1. In the absence of BMP stimulation, the constitutive association of Smad1 with Smad6 C-domain described above was not inhibited by Smad4 (Fig. 6B, left). However, upon stimulation with BMP2, increasing concentrations of Smad4 progressively associated with Smad1 displacing the Smad6 C-domain from the complex (Fig. 6B, right). In accordance with this observation, inhibition of BMP2-induced GAL4–Smad1 activation by Smad6 was rescued by coexpression of Smad4 (Fig. 6C). Collectively, these results suggest that Smad6 acts not by inhibiting receptor activation of Smad1 but by competitively inhibiting the association of Smad4 with receptor-activated Smad1.
The inhibitory activity of Smad 6 segregates with its ability to interact with Smad1
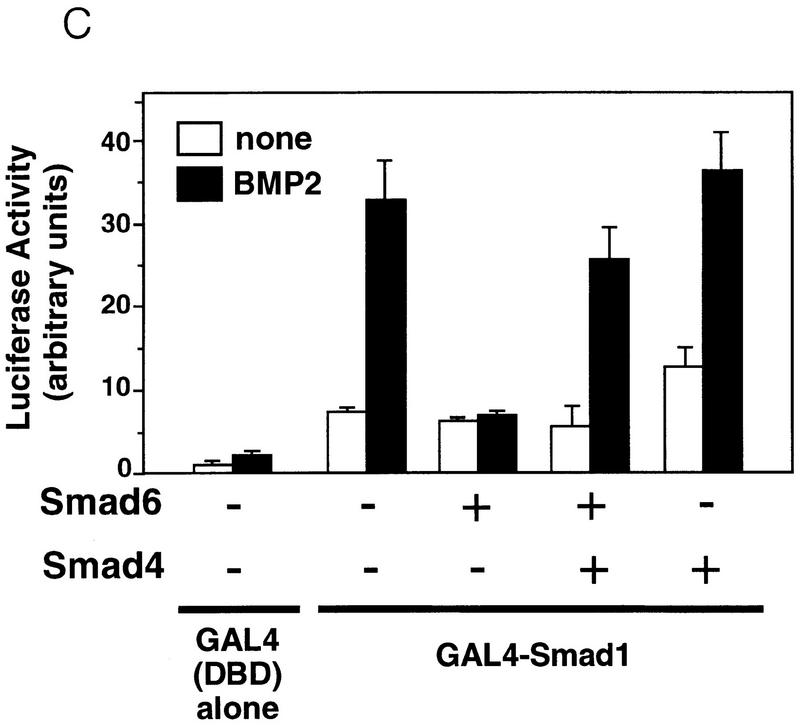
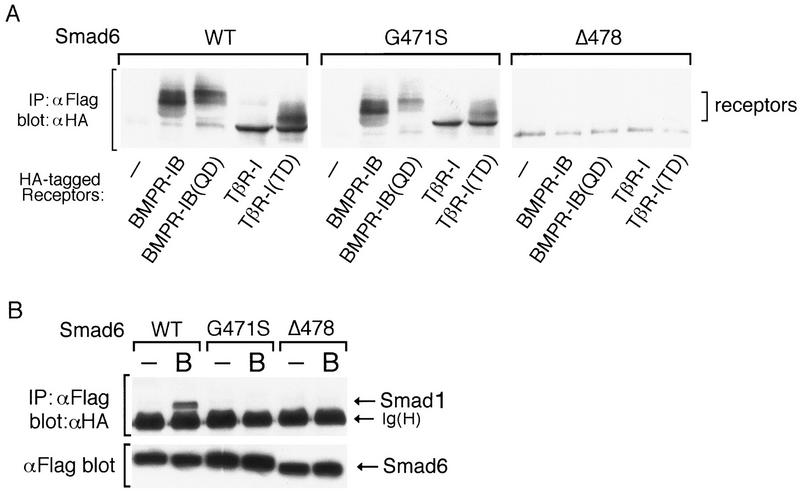
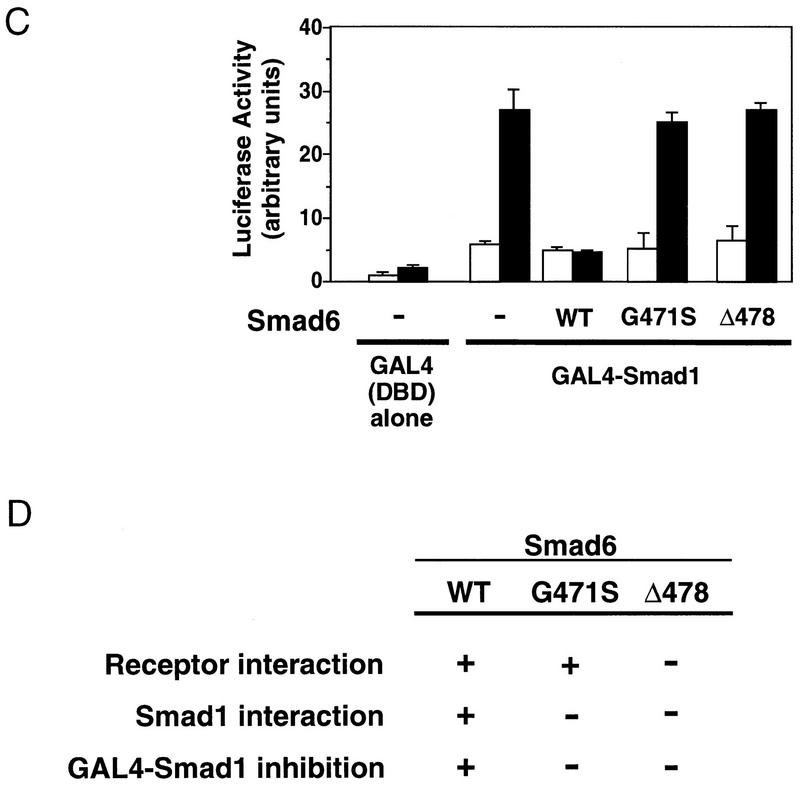
To test further the mechanism of Smad6 action, we generated two Smad6 mutants, G471S and Δ478. Both mutations are located in the L3 loop, a region implicated in heteromeric Smad–Smad interactions and exposed to the surface in the Smad4 crystal structure (Shi et al. 1997). The G471S mutant corresponds to a point mutation found in Drosophila Mad (Newfeld et al. 1996), whereas the Δ478 mutant is deleted of amino acids 478–496 at the carboxyl terminus and is equivalent to a cancer mutation in Smad4/DPC4 (Hahn et al. 1996). The Δ478 mutant protein was unable to interact either with type I receptors for the TGFβ superfamily (Fig. 7A) or with Smad1 (Fig. 7B) and lost the ability to inhibit BMP-dependent GAL4–Smad1 activation (Fig. 7C). Importantly, the G471S mutant protein could still associate with type I receptors (Fig. 7A) but lacked the ability to interact with Smad1 (Fig. 7B) or to inhibit signaling (Fig. 7C). The failure of the G471S mutant to inhibit Smad1 responses while retaining its ability to bind receptors strongly supports a mechanism in which the inhibitory action of Smad6 is triggered not by a block at the receptor level but by a physical interaction with Smad1 that prevents formation of the Smad1–Smad4 heterocomplex.
Figure 7.
The inhibitory activity of Smad 6 segregates with its ability to interact with Smad1. (A) Smad6(G471S) interacts with the type I receptors of TGFβ family. Flag-tagged wild-type Smad6 (WT) or two different mutants (G471S and Δ478) were cotransfected into COS cells with HA-tagged wild-type or constitutively active type I receptor (QD and TD) for BMPs (BMPR-IB) or TGFβ (TβR-I). Cell lysates were subjected to anti-Flag immunoprecipitation using a monoclonal antibody followed by immunoblotting using anti-HA polyclonal antibody. Similar levels of receptor and Smad6 expression were confirmed (data not shown). (B) Smad6 mutants fail to interact with Smad1 on BMP stimulation. Flag-tagged wild-type Smad6(WT) or mutants (G471S and Δ478) were cotransfected into COS cells with HA-tagged Smad1. Cell lysates were subjected to anti-Flag immunoprecipitation using a monoclonal antibody followed by immunoblotting using anti-HA polyclonal antibody (top panel). Similar levels of receptor and Smad6 expression were confirmed by anti-Flag Western blot (bottom panel). (C) Smad4 prevents Smad6 from inhibiting Smad1. R1B/L17 cells were transfected with the reporter gene (GAL4–lux, 1 μg), BMPR-IB (1 μg), BMPR-II (0.1 μg) and either a vector containing the GAL4 DNA-binding domain (DBD) alone or a GAL4(DBD)–Smad1 fusion construct. Smad6 (2 μg) and/or Smad4 (4 μg) were cotransfected where indicated. Cells were incubated with (solid bar) or without (open bar) 5 nm BMP2 for 18 hr. Luciferase activity is expressed as the mean ± s.d. of two independent experiments. (D) Summary table of the activities of Smad6 mutants.
Discussion
Smad6 as a selective antagonist of the BMP/Smad1 signaling pathway
Smad6 is a divergent member of the Smad family—its closest relative being Smad7. The human Smad6 cDNA clones isolated in the present study encode a protein with an overall domain structure typical of Smads. Most of the sequence elements that form the core β-sandwich in the crystal structure of the Smad4 C-domain (Shi et al. 1997) are discernible in the Smad6 C-domain. However, Smad6 lacks the carboxy-terminal SSXS receptor phosphorylation sequence found in receptor-regulated Smads (Macias-Silva et al. 1996; Kretzschmar et al. 1997), as well as a carboxy-terminal region, which participates in a three-α-helix bundle in Smad4 that mediates homotrimeric interactions (Shi et al. 1997). Thus , the Smad6 C-domain may have an overall fold similar to that of Smad4 but may establish homo-oligomeric interactions through unique nonconserved regions.
The present results show that Smad6 can selectively antagonize the BMP/Smad1 signaling pathway. During development and in the adult, BMPs control many aspects of tissue formation and homeostasis (for review, see Hogan 1996), and regulation of BMP activity is of critical importance. In the extracellular space, BMP activity is inhibited by BMP-sequestering proteins including Noggin, Chordin, and Follistatin. The present study reveals that Smad6 is a member of a novel class of BMP pathway inhibitors operating intracellularly. Evidence for this is provided by experiments with Xenopus embryos. In the ectoderm of these vertebrates, BMP4 suppresses the differentiation of neural tissue and induces epidermal fate (Wilson and Hemmati-Brivanlou 1995). Interference with BMP signaling in ectodermal explants, by use of dominant-negative BMP ligands or receptors, results in the appearance of neural fate. In the ventral side of the ectoderm, where BMP signals remain active, the cells adopt an epidermal fate (for review, see Wilson and Hemmati-Brivanlou 1997). BMP signaling is also involved in patterning of the mesoderm, where it induces ventral fate; interfering with BMP signaling in the ventral side reveals the default dorsal fate of the mesoderm (for review, see Graff 1997). In this study we show that Smad6 can induce cement gland and neural tissues in ectodermal explants in a cell-autonomous and dose-dependent manner, without inducing mesoderm. Furthermore, Smad6 also inhibits induction of a ventral mesoderm marker by a BMP receptor. Similar effects were observed recently with Dad, a Drosophila homolog of Smad6 (Tsuneizumi et al. 1997).
Smad6 does not interfere with formation of the primary axis, a process that requires signaling via the activin receptor (Hemmati-Brivanlou and Melton 1992). When directly challenged by Smad6 in a Xenopus animal cap assay, Smad1, but not Smad2, action was inhibited by Smad6. Furthermore, Smad6 antagonized BMP signaling in a GAL4 transcriptional assay in lung epithelial cells, but it did not antagonize TGFβ or activin in this assay or in a 3TP–lux reporter assay. Thus, Smad6 inhibits BMP/Smad1 signaling selectively, without inhibiting Smad2 signaling in Xenopus embryos or TGFβ and activin effects in mammalian cells. Smad6 may complement intracellularly the BMP inhibitory functions of Noggin, Chordin, or Follistatin and may play a key role in cell-autonomous determination of cell fate.
Smad6 as a Smad4 decoy: specific competition for receptor-activated Smad1
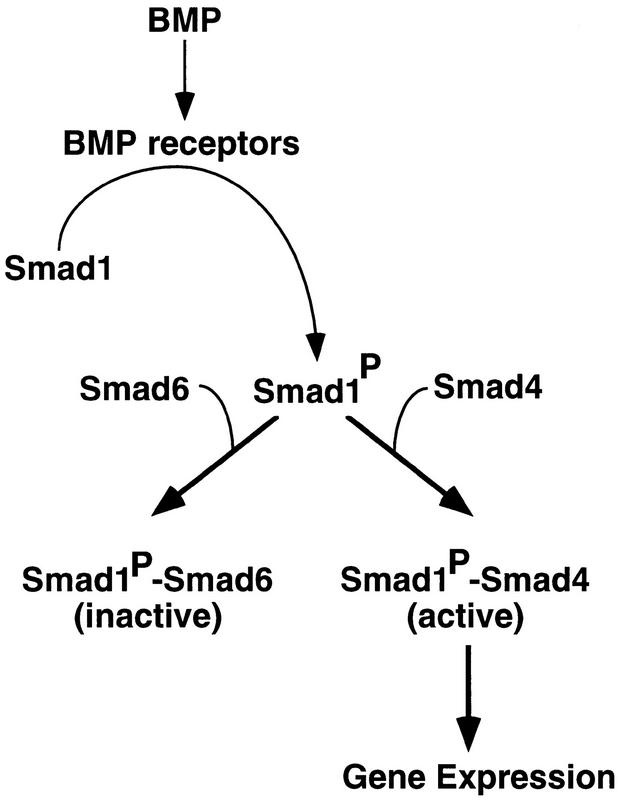
What mechanism accounts for the selective inhibition of the BMP/Smad1 pathway by Smad6? Our study suggests that Smad6 acts by inhibiting the formation of a Smad1–Smad4 complex upon BMP receptor activation. Formation of this complex, which requires receptor-mediated phosphorylation of Smad1, is essential for Smad1 signaling (Lagna et al. 1996; Kretzschmar et al. 1997). Under our conditions, Smad6 inhibits the formation of this complex not by inhibiting Smad1 phosphorylation but by competing with Smad4 for receptor-activated Smad1 (Fig. 8). Upon BMP receptor activation, a Smad1–Smad6 complex is formed at the expense of Smad1–Smad4 complex formation. As with the Smad1–Smad4 interaction (Hata et al. 1997), a Smad1–Smad6 interaction directly mediated by the C-domains of these proteins is detected in the yeast two-hybrid system. Thus, Smad4 and Smad6 may compete for overlapping binding sites in Smad1.
Figure 8.
Proposed mechanism of action of Smad6. Upon phosphorylation by the BMP type I receptor, Smad1 can interact with either Smad4 or Smad6. Although the Smad1–Smad6 complex is inactive, the Smad1–Smad4 complex triggers the expression of BMP responsive genes. The ratio between Smad4 and Smad6 in the cell can modulate the strength of the BMP signal.
The selectivity of the Smad1–Smad6 interaction observed here is consistent with the specificity of the Smad6 anti-BMP effects and the ineffectiveness of Smad6 as an inhibitor of Smad2 signaling. Under our assay conditions, Smad6 does not interact with Smad2 in response to TGFβ in mammalian cells, or in a yeast two-hybrid system. It was recently proposed that Smad7 inhibits TGFβ signaling by binding to TGFβ receptors and inhibiting receptor-mediated Smad2 phosphorylation (Hayashi et al. 1997; Nakao et al. 1997). We observed that when overexpressed, Smad6 can interact indiscriminately with wild type, kinase-defective or activated mutant forms of type I receptors for BMP, TGFβ, or activin. However, levels of Smad6 that are sufficient to block the Smad1–Smad4 interaction and signaling in mammalian cells do not inhibit BMP-induced Smad1 phosphorylation. Furthermore, a Smad6 mutant (G471S) that binds to the type I receptors but does not bind to Smad1 no longer inhibits BMP/Smad1 signaling. Thus, the interaction between Smad6 and type I receptors cannot account for the ability of Smad6 to specifically inhibit Smad1 signaling.
Recently, it was reported by Topper et al. (1997) and Imamura et al. (1997) that Smad6 can inhibit TGFβ signaling. However, the former study used the product of a Smad6 clone identical to JV15-1 which, according to our unpublished observations, is expressed at very high levels (∼100-fold higher) when compared to full-length Smad6. At such levels, Smad6 may interfere with TGFβ receptor function. Like Imamura et al. (1997), we observed a reduction of Smad1 phosphorylation by Smad6 but only at concentration ratios of Smad6 to Smad1 10-fold higher than those required to fully inhibit Smad1–Smad4 association and signaling. The anti-receptor mechanism proposed by others might play a role in conditions of high Smad6 overexpression. The mechanism proposed here is more consistent with the ability of Smad6 to selectively inhibit BMP/Smad1 signaling in Xenopus and mammalian cells.
By preventing the Smad1–Smad4 interaction, Smad6 would inhibit those BMP responses that depend on it. It is possible that Smad6 binding might direct the activated Smad1 to a different set of intracellular targets. However, we have no evidence that the Smad1–Smad6 complex has a separate signaling function. The effects that we have observed can therefore be attributed to the ability of Smad6 to function as a Smad4 decoy, generating inactive Smad1–Smad6 complexes in competition with Smad4 (Fig. 8). Smad6 may set a threshold that Smad4 must surpass to achieve signal propagation via Smad1. Because Smad6 does not associate with Smad2, the threshold imposed by Smad6 would not interfere with the TGFβ signaling pathway. This and other Smad pathways may be controlled by separate antagonists perhaps related to Smad6.
Materials and methods
Cloning of human and Xenopus Smad6
The C-domain of human Smad6 (amino acids 272–496) was obtained by PCR amplification from a human liver cDNA library. Primers for the PCR were designed on the basis of the reported sequence for JV15-1 (Riggins et al. 1997). Using this partial human Smad6 fragment as a probe, cDNA libraries made from human Jurkat cells (Clontech) and Xenopus tadpole head (Hemmati-Brivanlou et al. 1991) were screened. The human library yielded 12 independent clones that were isolated and sequenced. Except for some clones containing the Smad6 sequence fused to unrelated fragments, all of the cDNAs encoded a 496-amino-acid protein (GenBank accession no. AF035528). Two Xenopus cDNAs were found to encode mostly overlapping sequences corresponding to the last 276 amino acids of human Smad6 (GenBank accession no. AF035529).
Construction of expression vectors
Human Smad6(FL)/CS2, human Smad6(C)/CS2, and Xenopus Smad6(C)/CS2 were generated by subcloning the entire open reading frame or the C-domain regions of human and Xenopus Smad6 (amino acids 272–496 of human Smad6) into a modified CS2 vector (Baker and Harland 1996; Turner and Weintraub 1994). Human Flag-Smad6(FL or C)/CS2 and HA–Smad6(FL or C)/CS2 were constructed from Smad6(FL or C)/CS2 by introducing the Flag or HA epitope tags at the amino terminus by PCR. The construction of Flag–Smad1/pCMV5, Flag–Smad2/CS2, Flag–Smad4/CS2, Smad4–HA/pCMV5, pGAL4–Smad1, and pGAL4–Smad2 is described elsewhere (Lagna et al. 1996; Liu et al. 1996; Hata et al. 1997). Smad6 (Δ478 and G471S) was generated by a PCR-based method.
Cell lines and transfections
COS cells and R-1B/L17 cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% FCS and minimal essential media containing 10% FCS, respectively. For transient transfection, cells were seeded at 70%–80% confluency and were transfected with DEAE–dextran as described previously (Attisano et al. 1996).
In vivo phosphate labeling
For [32P]phosphate labeling, transiently transfected COS cells were washed and preincubated with phosphate-free media. The cells were then incubated with media containing 1 mCi/ml of [32P]phosphate for 2 hr at 37°C. Twenty minutes before the end of labeling, 100 pm TGF-β or 5 nm BMP2 (a gift from V. Rosen, Genetics Institute, Cambridge, MA) was added. Immunoprecipitations and immunoblotting analyses were performed as described (Lagna et al. 1996).
Yeast two-hybrid system
The LexA–Smad6(C) (amino acids 272–496) fusion construct and GAL4 activation domain (GAD)–Smads fusions were created in pBTM 116 (Hata et al. 1997) and pGAD424 (Clontech), respectively. Interactions were tested by measuring β-galactosidase activity.
Transcriptional assay
R-1B/L17 cells were transiently transfected with 1 μg of reporter plasmid (G1E1BCAT, GAL4–lux, or p3TP–lux), 0.1 μg of BMPR–IB/BMPR–II,TβR-I, or ActR–IB, 0.5 μg of the GAL4 derivatives (for GAL4 assay), and different amounts of Smad6/CS2. Cells were treated with or without 100 pm TGFβ, 5 nm BMP2, or 2 nm activin for 18 hr, and CAT or luciferase assay was performed as described before (Kretzschmar et al. 1997).
Xenopus injections and animal cap assay
RNAs used for injections were synthesized in vitro in the presence of cap analog using the mMessage mMachine kit (Ambion) and SP6 RNA polymerase. The DNA templates were obtained linearizing the corresponding pCS2 constructs with AscI. For the induction of secondary axis, RNAs encoding Smads were coinjected with nuclear β–galactosidase RNA as described previously (Smith and Harland 1991). For the animal cap assay, RNA (10 nl, 0.5–4 ng) was introduced in the animal pole of two-cell embryos. Animal caps were explanted at blastula stage and cultured to the indicated stage. RT–PCR was performed as described (Lagna et al. 1996).
Acknowledgments
We thank P. Wilson and D.C. Weinstein for critical reading of the manuscript, D. Wotton for help with the yeast two-hybrid system and J. Marden for technical assistance. We also thank the Genetics Institute for the BMP2 protein. National Institutes of Health grants to Memorial Sloan-Kettering Cancer Center (J.M. and A.H.-B.; HD32105-01) supported this work. A.H. is a research associate and J.M. an investigator of the Howard Hughes Medical Institute. A.H.-B. acknowledges support from the Searle, McKnight and Merck Foundations.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL j-massague@ski.mskcc.org; FAX (212) 717-3298. E-MAIL brvnlou@rockvax.rockefeller.edu; FAX (212) 327-8656.
References
- Asashima M, Nakano H, Shimada K, Kinoshita K, Ishii K, Shibai H, Ueno N. Mesodermal induction in early amphibian embryos by activin A (erythroid differentiation factor) Wilhelm Roux’s Arch Dev Biol. 1990;198:330–335. doi: 10.1007/BF00383771. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Montalvo E, Massagué J. Activation of signaling by the activin receptor complex. Mol Cell Biol. 1996;16:1066–1073. doi: 10.1128/mcb.16.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Harland RM. A novel mesoderm inducer, mMadr-2, functions in the activin signal transduction pathway. Genes & Dev. 1996;10:1880–1889. doi: 10.1101/gad.10.15.1880. [DOI] [PubMed] [Google Scholar]
- Chen X, Rubock MJ, Whitman M. A transcriptional partner of MAD proteins in TGF-β signalling. Nature. 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui L-C, Bapat B, Gallinger S, Andrulis IL, Thomsen GH, Wrana JL, Attisano L. MADR2 maps to 18q21 and encodes a TGFβ-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- Graff JM. Embryonic patterning: to BMP or not to BMP, that is the question. Cell. 1997;89:171–174. doi: 10.1016/s0092-8674(00)80196-8. [DOI] [PubMed] [Google Scholar]
- Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP Receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Graff JM, Bansal A, Melton DA. Xenopus Mad proteins transduce distinct subsets of signals for the TGFβ superfamily. Cell. 1996;85:479–487. doi: 10.1016/s0092-8674(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Hahn SA, Schutte M, Hoque ATMS, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Hata A, Lo RS, Wotton D, Lagna G, Massagué J. Mutations increasing autoinhibition inactivate tumour suppressors Smad2 and Smad4. Nature. 1997;388:82–87. doi: 10.1038/40424. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu Y, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992;359:609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, de la Torre JR, Holt C, Harland RM. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991;111:715–724. doi: 10.1242/dev.111.3.715. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin is expressed in the Spemann organizer and displays direct neuralizing activity. . Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes & Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O’Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signalling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Liu F, Hata A, Doody J, Massagué J. The TGF-β family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes & Dev. 1997;11:984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4 and SMAD proteins in TGFβ signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- Lechleider RJ, de Caestecker MP, Dehejia A, Polymeropoulos MH, Roberts AB. Serine, phosphorylation, chromosomal localization and TGFβ signal transduction by human bsp-1. J Biol Chem. 1996;271:17617–17620. doi: 10.1074/jbc.271.30.17617. [DOI] [PubMed] [Google Scholar]
- Liu F, Hata A, Baker J, Doody J, Cárcamo J, Harland R, Massagué J. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature. 1996;381:620–623. doi: 10.1038/381620a0. [DOI] [PubMed] [Google Scholar]
- Liu F, Puponnot C, Massagué J. Dual role of the Smad4/DPC4 tumor suppressor in TGFβ-inducible transcriptional complexes. Genes & Dev. 1997;11:3157–3167. doi: 10.1101/gad.11.23.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL. MADR2 is a substrate of the TGFβ receptor and phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- Massagué J, Hata A, Liu F. TGF-β signalling through the Smad pathway. Trends Cell Biol. 1997;7:187–192. doi: 10.1016/S0962-8924(97)01036-2. [DOI] [PubMed] [Google Scholar]
- Massagué J, Weis-Garcia F. Serine/threonine kinase receptors: Mediators of TGF-β family signals. In: Pawson T, Parker P, editors. Cancer Surveys. London, UK: Imperial Cancer Research Fund; 1996. pp. 41–64. [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin N-E, Heldin C-H, ten Dijke P. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Newfeld SJ, Chartoff EH, Graff JM, Melton DM, Gelbart WM. Mothers against dpp encodes a conserved cytoplasmic protein required in DPP/TGFβ responsive cells. Development. 1996;122:2099–2108. doi: 10.1242/dev.122.7.2099. [DOI] [PubMed] [Google Scholar]
- Riggins GJ, Kinzler KW, Vogelstein B, Thiagalingam S. Frequency of Smad gene mutations in human cancers. Cancer Res. 1997;57:2578–2580. [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Shi Y, Hata A, Lo RS, Massagué J, Pavletich NP. A structural basis for mutational inactivation of the tumour suppressor Smad4. Nature. 1997;388:87–93. doi: 10.1038/40431. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- ————— Expression cloning of noggin, a new dorsalizing factor localized to the Spemann Organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BMJ, Van Nimmen K, Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990;345:729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Chang C, Yingling JM, Wang X-F, Hemmati-Brivanlou A. Smad5 induces ventral fates in Xenopus embryo. Dev Biol. 1997;184:402–405. doi: 10.1006/dbio.1997.8548. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, Heldin CH. Signaling via hetero-oligomeric complexes of type I and type II serine/threonine kinase receptors. Curr Opin Cell Biol. 1996;8:139–145. doi: 10.1016/s0955-0674(96)80058-5. [DOI] [PubMed] [Google Scholar]
- Thomsen G. Xenopus mothers against decapentaplegic is an embryonic ventralizing agent that acts downstream of the BMP-2/4 receptor. Development. 1996;122:2359–2366. doi: 10.1242/dev.122.8.2359. [DOI] [PubMed] [Google Scholar]
- Thomsen G, Woolf T, Whitman M, Sokol S, Vaughan J, Vale W, Melton DA. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Topper JN, Cai J, Qiu Y, Anderson KR, Xu Y, Deeds J, Feeley R, Gimeno C, Woolf E, Tayber O, Mays G, Sampson BA, Schoen F, Gimbrone M, Falb D. Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci. 1997;94:9314–9319. doi: 10.1073/pnas.94.17.9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg TB, Christian JL, Tabata T. Daughters against dpp modulates dpp organizing activity in drosophila wing development. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes & Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- van den Eijnden-Van Raaij AJM, van Zoelent EJJ, van Nimmmen K, Coster CH, Snoek GT, Durston AJ, Huylebrock D. Activin-like factor from Xenopus laevis cell line responsible for mesoderm induction. Nature. 1990;345:732–734. doi: 10.1038/345732a0. [DOI] [PubMed] [Google Scholar]
- Watanabe TK, Suzuki M, Omori Y, Hishigaki H, Horie M, Kanemoto N, Fujiwara T, Nakamura Y, Takahashi E. Cloning and characterization of a novel member of the human Mad gene family (MADH6) Genomics. 1997;42:446–451. doi: 10.1006/geno.1997.4753. [DOI] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- ————— Vertebrate neural induction: Inducers, inhibitors, and a new synthesis. Neuron. 1997;18:699–710. doi: 10.1016/s0896-6273(00)80311-6. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. Concentration-dependent patterning of the Xenopus extoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–3184. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- Wu R-Y, Zhang Y, Feng X-H, Derynck R. Heteromeric and homomeric interacions correlate with signaling activity and functional cooperativity of Smad3 and Smad4/DPC4. Mol Cell Biol. 1997;17:2521–2528. doi: 10.1128/mcb.17.5.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Kim J, Taira M, Zhan S, Sredni D, Kung HF. A dominant negative bone morphogenetic protein 4 receptor causes neuralization in Xenopus ectoderm. Biochem Biophys Res Commun. 1995;212:212–219. doi: 10.1006/bbrc.1995.1958. [DOI] [PubMed] [Google Scholar]
- Yingling JM, Das P, Savage C, Zhang C, Padgett RW, Wang X-F. Mammalian Dwarfins are phosphorylated in response to TGF-β and are implicated in control of cell growth. Proc Natl Acad Sci. 1996;93:8940–8944. doi: 10.1073/pnas.93.17.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng X-H, Wu R-Y, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGFβ response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Musci T, Derynck R. The tumor suppressor Smad4/DPC4 as a central mediator of Smad function. Curr Biol. 1997;7:270–276. doi: 10.1016/s0960-9822(06)00123-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman L, De Jesus-Escobar J, Harland R. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]