Abstract
Chronic ingestion of high concentrations of hexavalent chromium [Cr(VI)] in drinking water induces intestinal tumors in mice. To investigate the mode of action (MOA) underlying these tumors, a 90-day drinking water study was conducted using similar exposure conditions as in a previous cancer bioassay, as well as lower (heretofore unexamined) drinking water concentrations. Tissue samples were collected in mice exposed for 7 or 90 days and subjected to histopathological, biochemical, toxicogenomic, and toxicokinetic analyses. Described herein are the results of toxicokinetic, biochemical, and pathological findings. Following 90 days of exposure to 0.3–520 mg/l of sodium dichromate dihydrate (SDD), total chromium concentrations in the duodenum were significantly elevated at ≥ 14 mg/l. At these concentrations, significant decreases in the reduced-to-oxidized glutathione ratio (GSH/GSSG) were observed. Beginning at 60 mg/l, intestinal lesions were observed including villous cytoplasmic vacuolization. Atrophy, apoptosis, and crypt hyperplasia were evident at ≥ 170 mg/l. Protein carbonyls were elevated at concentrations ≥ 4 mg/l SDD, whereas oxidative DNA damage, as assessed by 8-hydroxydeoxyguanosine, was not increased in any treatment group. Significant decreases in the GSH/GSSG ratio and similar histopathological lesions as observed in the duodenum were also observed in the jejunum following 90 days of exposure. Cytokine levels (e.g., interleukin-1β) were generally depressed or unaltered at the termination of the study. Overall, the data suggest that Cr(VI) in drinking water can induce oxidative stress, villous cytotoxicity, and crypt hyperplasia in the mouse intestine and may underlie the MOA of intestinal carcinogenesis in mice.
Keywords: risk assessment, carcinogenesis, hexavalent chromium, Cr(VI), mode of action, MOA
Until recently, there was inadequate information to assess the oral carcinogenicity of hexavalent chromium [Cr(VI)] (International Agency for Research on Cancer, 1990; U.S. EPA, 1991, 1998); however, a 2-year cancer bioassay conducted by the National Toxicology Program (NTP) reported that administration of Cr(VI) in drinking water (in the form of sodium dichromate dihydrate [SDD]) induced tumors in the small intestines of mice at ≥ 57 mg/l SDD (≥ 20 mg/l Cr(VI)) and in the oral cavities of rats at ≥ 172 mg/l SDD (≥ 60 mg/l Cr(VI)) (NTP, 2008; Stout et al., 2009). Because low levels of Cr(VI) are prevalent in groundwater in certain geographical areas (Oze et al., 2007), wide-spread impact to some drinking water supplies has occurred. For example, approximately one third of California drinking water supplies contain low levels of Cr(VI)—mostly ranging from 0.001 to 0.005 mg/l (California Department of Health Services, 2009); thus, the effects of Cr(VI) at low concentrations is of interest. Notably, the concentrations inducing cancer in rodents are orders of magnitude greater than typical drinking water exposures (Thompson et al., 2011).
In the NTP 2-year cancer bioassay (NTP, 2008), diffuse intestinal epithelial hyperplasia was observed in the small intestine of mice at all concentrations examined (NTP, 2008) and at ≥ 62.5 mg/l SDD in an earlier 90-day study (NTP, 2007). In stark contrast to mice, neither diffuse hyperplasia nor tumors were reported in the rat small intestine at any of the drinking water concentrations tested (NTP, 2007, 2008). The non-neoplastic intestinal lesions in the mouse were characterized by the NTP investigators as “regenerative hyperplasia secondary to previous epithelial cell injury” (NTP, 2008) and suggest that the tumors may have been caused by prolonged tissue injury and proliferative pressure on crypt cells (Thompson et al., 2011). Despite evidence that chromium can be genotoxic and mutagenic (McCarroll et al., 2010; Nickens et al., 2010; O'Brien et al., 2003), there are limited data to evaluate the mode of action (MOA) of Cr(VI) in the small intestine. Thus, the MOA for small intestinal tumors at high concentration exposures is uncertain, raising questions as to whether observations of cancer at high doses in rodents are of relevance for humans exposed at levels that are typically orders of magnitude lower.
Using the NTP Cr(VI) study data (NTP, 2007, 2008) and the MOA framework in the U.S. EPA Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005), we hypothesized a plausible MOA for the mouse intestinal tumors that includes the following key events: saturation of the reductive capacity of the upper gastrointestinal (GI) tract, absorption of Cr(VI) into the intestinal epithelium, oxidative stress and inflammation, cell proliferation, direct and/or indirect DNA modification, and mutagenesis (Thompson et al., 2011). Although available data generally support the plausibility of these key events, the following key data gaps were identified: (1) species-specific measures of reductive capacity of the upper GI tract, (2) measures of intestinal oxidative stress and inflammation, (3) a molecular basis for the cause of cell proliferation, (4) measures of target tissue DNA modification (e.g., Cr-mediated damage, oxidative damage, epigenetic changes—all of which have been shown to occur in other tissues and by other routes of exposure), and (5) whether Cr(VI) exposure results in measurable mutations (e.g., p53 and ras) in target tissues within 90 days of exposure. These data gaps are being addressed through a series of Cr(VI) MOA studies.
As part of this project, a 90-day drinking water study was designed to acquire the biochemical, genomic, and species-specific toxicokinetic data needed to extrapolate among species and from the high doses that caused tumors in rodents to environmentally relevant exposures in humans. The study design is similar to that of the 90-day NTP study (NTP, 2007); however, the drinking water concentrations employed were based on those in the 2-year bioassay (NTP, 2008) and included two additional lower drinking water concentrations. In addition, the present study includes analyses of biochemical and genomic changes in the target tissues after 7 and 90 days of exposure. Herein, we report the results of the in-life portion of the 90-day mouse study as well as descriptions of the histopathological findings, biochemical analyses related to oxidative status, and the chromium content in target tissues. In addition to informing the MOA, these data will be used to provide phenotypic anchoring for the toxicogenomic results once completed. Toxicogenomic, in vivo mutagenicity, and toxicokinetic modeling results, as well as findings from a companion 90-day drinking water study in rats, will be published separately upon completion of those studies.
MATERIALS AND METHODS
Test substance.
SDD (99.95% pure; CAS 7789-12-0) was obtained from Sigma-Aldrich Inc. (Milwaukee, WI) and was stored at room temperature and protected from light. The dose formulations were prepared at concentrations of 0.3, 4, 14, 60, 170, and 520 mg/l SDD in tap water, which is equivalent to 0.1, 1.4, 4.9, 20.9, 59.3, and 181 mg/l Cr(VI) (The Cr(VI) concentration is equivalent to approximately 35% of the SDD concentration.). On the first, third, fifth, and seventh (final) batch preparations, samples were collected and shipped to Brooks Rand Laboratories (Seattle, WA) for analysis of Cr(VI) content. The first batch was also analyzed for total chromium. Samples were prepared and analyzed in accordance with EPA Method SW-7196A and modified (Standard Operating Procedures, SOP BR-0085) to confirm the Cr(VI) concentrations of the administered drinking water. In Method 7196A, Cr(VI) is complexed with diphenylcarbazide in an acidic solution and measured colorimetrically at an absorbance of 540 nm. Batches found to differ from the target concentration by ± 10% were not used. Prior to use, dose formulations of SDD were stored in sealed Nalgene carboys at room temperature protected from light. SDD has been shown to be stable for 42 days in dosed water formulations at a concentration of 41.8 mg/l when stored under these conditions (NTP, 2008).
Animals and husbandry.
The in-life studies were conducted at Southern Research Institute (Birmingham, AL), the same research facility at which both the NTP 13-week study and the NTP 2-year bioassay (NTP, 2007, 2008) were conducted. The water bottles, sipper tubes, cage size and type, number of animals per cage, bedding, environmental conditions, and food were the same as in the NTP studies. Female B6C3F1 mice were obtained from Charles River (Raleigh, NC). The mice were approximately 4–5 weeks of age when they arrived and were allowed to acclimate for approximately 2 weeks. At the start of the study, the mice were approximately 6–7 weeks of age and weighed between 13.3 and 22.9 g. Irradiated NTP-2000 Wafers (Zeigler Bros., Gardners, PA) were provided ad libitum during the pre-study and study periods. Water (dosed or control) was supplied in amber glass water bottles. Teflon-lined lids with stainless steel, double-balled sipper tubes were used. Water bottles were changed twice weekly, or as needed. Before the start of the study, samples of tap water from the animal facility were analyzed, and no known contaminants were present that would be expected to interfere with or affect the outcome of the study.
The animals were group-housed (5 per cage) in solid bottom polycarbonate cages on a stainless steel rack in a room maintained at a temperature of 68.4°F–78.0°F and a relative humidity of 24.7–76.5%. Excursions outside the desired temperature (69°F–75°F) and humidity (35–65%) ranges were brief in duration and did not adversely affect the health of the animals or outcome of the study. Fluorescent lighting provided illumination approximately 12 h per day. Irradiated hardwood bedding chips (Sani Chips; P.J. Murphy Forest Products Corp., Montville, NJ) were used as bedding material. No known contaminants were present in the bedding that would have been expected to interfere with or affect the outcome of the study. Cage size and animal care conformed to the guidelines of the Guide for the Care and Use of Laboratory Animals, the U.S. Department of Agriculture through the Animal Welfare Act (Public Law 99-198) and to the applicable SOPs of Southern Research.
Study design.
Because male and female mice responded similarly to Cr(VI) in terms of effects in the alimentary canal, only female mice were used in this study (The design of this study was informed by the Toxicology Excellence for Risk Assessment Expert Panel overseeing the Cr(VI) MOA Research Program, who recommended that multiple sexes were not necessary for this study. The panel report is available at http://www.tera.org/Peer/Chromium/Chromium.htm.). Mice were assigned to their respective dose groups using a computerized randomization procedure designed to yield comparable group mean body weights. The body weights required for randomization were determined the week before the treatment began. After randomization, mice were assigned to treatment groups as indicated in Table 1 and were provided with food and drinking water ad libitum until study termination at days 8 or 91 (Table 1). Water and food consumption were measured weekly for each cage of animals throughout the study, and values were reported as an average consumption (milliliters/animal per day or grams/animal per day, respectively). Each animal was weighed the week before treatment began (for randomization), on day 1, weekly thereafter, and prior to scheduled euthanasia. All animals were observed at least twice daily during the pre-study and study periods for signs of mortality and moribundity. Each animal was removed from its cage and examined for clinical signs of toxicity on day 1 and weekly thereafter.
TABLE 1.
Summary of Study Designa
| Number of animals | ||||||||
| SDD (mg/l) | Toxicology and histopathology |
Biochemical evaluations |
Gene expression analyses |
Mutation analyses | Toxicokinetic analyses | |||
| Day 8 | Day 91 | Day 8 | Day 91 | Day 8 | Day 91 | Day 91/92 | Day 92 | |
| 0 | 5 F | 10 F | 10 F | 20 F | 10 F | 10 F | 10 F | 5 F |
| 0.3 | 5 F | 10 F | 10 F | 20 F | 10 F | 10 F | 10 F | 5 F |
| 4 | 5 F | 10 F | 10 F | 20 F | 10 F | 10 F | 10 F | 5 F |
| 14 | 5 F | 10 F | 10 F | 20 F | 10 F | 10 F | 10 F | 5 F |
| 60 | 5 F | 10 F | 10 F | 20 F | 10 F | 10 F | 10 F | 5 F |
| 170 | 5 F | 10 F | 10 F | 20 F | 10 F | 10 F | 10 F | 5 F |
| 520 | 5 F | 10 F | 10 F | 20 F | 10 F | 10 F | 10 F | 5 F |
Note. F, female mice.
Some animals were treated for 90 or 91 days and sacrificed thereafter to accommodate study size.
Pathology and histopathology.
Mice in the groups designated for pathologic examination (i.e., those in the toxicology and histopathology groups) were euthanized by CO2 asphyxiation and subjected to a complete gross examination at the time of necropsy. The postmortem examination of each mouse included, but was not limited to, examination of the external surfaces of the body, all orifices of the body, and the cranial, thoracic, abdominal, and pelvic cavities and their contents. Based on findings of the NTP (2008) bioassay , the oral cavity, duodenum, jejunum, and any gross lesions were collected from each mouse and saved in 10% neutral-buffered formalin for histopathologic evaluation. Fixed tissues were trimmed, processed, and sectioned to approximately 5 μm. The tissue sections were mounted on glass slides and stained with hematoxylin and eosin for microscopic examination. All slides were submitted to a veterinary pathologist for evaluation and diagnosis. Tissues were diagnosed and categorized using standardized nomenclature with lesions ranked for severity for comparison among groups. Half of the mice designated for macroscopic and microscopic pathology evaluation were also used for collection of samples for evaluation of iron status. One bone marrow smear (stained with Prussian blue) was prepared from 5 mice per group.
GSH and GSSG analyses.
GSH and GSSG were measured in plasma, as well as in oral epithelial scrapings and in duodenal and jejunal epithelial scrapings from 5 animals per group on days 8 and 91 and in ileal epithelial scrapings from 5 animals per group on day 91. For collection of blood samples, each mouse was anesthetized with ketamine/xylazine (87 mg ketamine/kg; 13.4 mg xylazine/kg) injected intraperitoneally or with CO2/O2 by inhalation, and blood samples were collected from the retro-orbital sinus into tubes containing heparin as anticoagulant. Samples were gently mixed by inversion and placed on ice. Samples were centrifuged at approximately 4°C for 5 min for separation of plasma. Plasma was collected and mixed in a 1:1 ratio with 2× redox quenching buffer (RQB), to yield final concentrations of 20mM HCl, 5mM diethylenetriamine pentaacetic acid, and 1mM 1,10-phenanthroline. The 2× RQB also contained 5% ultrapure grade trichloroacetic acid. Samples were snap frozen and stored at −80°C until analysis. Immediately following blood collection, each mouse was euthanized using CO2. Samples of oral, duodenal, jejunal (proximal 6 cm), and ileal (proximal 6 cm; day 91 only) epithelia were collected, immediately placed into tubes containing 0.5 ml 2× RQB on ice, and incubated for approximately 10–15 min to allow penetration of the buffer into the tissues. The samples were then snap frozen in liquid nitrogen and stored at −80°C until analysis. GSH and GSSG were determined fluorometrically using the o-phthalaldehyde procedure as previously described (Senft et al., 2000). The calculation for redox potential (ΔE) is described in detail elsewhere (Dalton et al., 2004) and is as follows: ΔE = −240 mV − (61.5 mV/2) × log([GSH]2/[GSSG]).
Cytokine and chemokine analyses.
One sample of oral cavity and one sample of duodenum from each animal were snap frozen and stored at −80°C until homogenization. Homogenization buffer solution was prepared by dissolving phenylmethanesulfonylfluoride (PMSF) in 2-propanol. The solution was vortexed ∼30 s and set aside. Tris was weighed and transferred to a beaker and deionized (DI) water was added. The pH was adjusted to 7.5 with 1 N NaOH while stirring. NaCl, Tween 20, and 1 ml of the PMSF/2-propanol solution were added to the Tris/DI water solution. Protease Inhibitor Cocktail I [containing 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), EDTA, bestatin, E-64, leupeptin, and aprotinin] was reconstituted by the addition of 10 ml of DI water, vortexed ∼30 s, and added to the Tris/DI water/NaCl/Tween 20/PMSF solution. The final solution was stirred ∼7 min to ensure uniform mixture. The final concentrations of the constituents in the homogenization buffer were 20 mmol/l Tris, 150 mmol/l NaCl, 1% 2-propanol, 0.174 mg/ml PMSF, 0.05% Tween 20, 2mM AEBSF, 1mM EDTA, 130μM bestatin, 14μM E-64, 1μM leupeptin, and 0.3μM aprotinin. Tissues were then homogenized in 0.3 ml using an Omni THQ homogenizer (Omni International, Kennesaw, GA) and a disposable hard tissue tip. After homogenization, samples were snap frozen and stored at −80°C until assayed. For serum collection, each mouse was anesthetized with CO2/O2, and blood samples were collected from the retro-orbital sinus into serum separator tubes containing no anticoagulant. The contents of the tubes were centrifuged to separate serum. The tissue and sera samples were analyzed for 22 cytokines/chemokines via a Milliplex Mouse Cytokine kit (Millipore, Billerica, MA) on a Luminex 200 (Austin, TX). The cytokines/chemokines included are listed in Supplementary Table S1. Cytokine levels were normalized to protein content.
Protein carbonyl analyses.
Duodenal tissues were homogenized in a PBS buffer containing a cocktail of protease inhibitors (Millipore) and 0.005% final volume of butylated hydroxytoluene (Sigma, St Louis, MO) to prevent oxidation. Homogenization was completed using 0.5 or 1 ml (depending on tissue weight) of the buffer described above and an Omni THQ homogenizer and a disposable hard tissue tip. After homogenization, an ammonium sulfate precipitation was completed to remove any residual nucleic acids from the protein. The protein pellet was resuspended in the same buffer and a Micro-BCA assay (Pierce, Rockford, IL) was completed to determine the protein concentration. Samples were then stored at −80°C. Protein carbonyls were measured via OxiSelect Protein Carbonyl ELISA Kit (Cell Biolabs, San Diego, CA) per the manufacturer’s instructions.
8-hydroxydeoxyguanosine analyses.
DNA was extracted from the oral cavity and duodenum tissue samples using Gentra PureGene Tissue Kits (Qiagen, Valencia, CA). To each vial of tissue sample, 300 μl of cell lysis buffer (included in the Qiagen kit) was added, and the tissues were homogenized using an Omni THQ homogenizer and a disposable hard tissue tip. DNA was then extracted following the manufacturer’s instructions. Genomic DNA samples were rehydrated with 50 μl of DNA hydration solution (included in the Qiagen kit), and concentrations were determined using the PicoGreen kit (Invitrogen, Carlsbad, CA) and a Bio-Rad Versafluor fluorimeter (Hercules, CA). 8-Hydroxydeoxyguanosine (8-OHdG) was measured via OxiSelect Oxidative DNA Damage ELISA Kit (Cell Biolabs) per the manufacturer’s instructions.
Iron status.
Iron status was evaluated in half of the mice designated for macroscopic and microscopic pathological evaluation. On day 91, five mice per group were anesthetized using CO2/O2, and blood samples (∼0.4 ml) were collected from the retro-orbital sinus into tubes containing no anticoagulant. The contents of the tubes were centrifuged to separate serum. One aliquot of serum was used for measurement of serum iron and another was snap frozen and stored at approximately −80°C. Serum iron was measured using the Cobas c501 Clinical Chemistry Analyzer (Version 04-02; Roche Diagnostics, Indianapolis, IN). Blood ferritin and transferrin were measured by commercial Ferritin (Mouse) ELISA and Transferrin (Mouse) ELISA kits purchased from ALPCO (Salem, NH).
Measurement of total chromium in GI tissues.
Samples of the duodenum, jejunum, ileum, glandular stomach, and oral cavity were collected for evaluation of total chromium (Cr) content from mice exposed for 90 days. Five mice per group were anesthetized using CO2, and tissues were removed, flushed of contents, snap frozen, and stored at approximately −80°C. Samples were shipped frozen to Brooks Rand Laboratories where samples were thawed lightly homogenized and approximately 100 mg of tissue was digested in nitric acid in a controlled microwave digestion program. Samples were then brought to a final volume of 8 ml with DI water. Analysis was performed using EPA Draft Method 1638 (modified) using inductively coupled plasma-mass spectrometry (ICP-MS) with Dynamic Reaction Cell (DRC) technology. Digested sample were analyzed utilizing internal standardization with rhodium (Rh). This method incorporates ionization of the sample in an inductively coupled RF plasma, with detection of the resulting ions by mass spectrometer on the basis of their mass-to-charge ratio. The limit of detection was 0.02 μg Cr/g tissue.
Additional analyses relevant to the MOA study.
Additional blood and tissue samples were collected for gene expression analyses, mutation analyses, analysis of chromium levels in genomic DNA, and toxicokinetic analyses (Table 1). The results from these studies will be described in future publications when the analyses are completed. The histopathological and biochemical findings described in the present publication are expected to provide a phenotypic anchor to the gene expression analyses.
Data management and statistical evaluation.
Provantis (Version 7; Instem Life Sciences Systems Ltd., Staffordshire, UK) was used for the direct online capture of most in-life and pathology data. In addition, Provantis interfaced with the Cobas c501 Clinical Chemistry Analyzer (Version 04-02; Roche Diagnostics) for capture of serum iron data. Environmental monitoring of animal rooms (i.e., temperature/humidity and light/dark cycles) was performed using the Edstrom Watchdog System (Version 5.13; Edstrom Industries Inc., Waterford, WI). The remainder of the data was collected manually.
For consistency with NTP practices, biochemical and clinical endpoints were first tested for dose-related trends using Jonckheere's test (Jonckheere, 1954). Biochemical data sets with a significant trend were then analyzed by Williams' (parametric) or Shirley's tests (nonparametric) (Shirley, 1977), whereas Dunnett's (parametric) or Dunn's (nonparametric) tests were run if there was not a monotone trend. Water consumption and bodyweight data were analyzed by one-way ANOVA followed by Dunnett’s tests. Food intake was found to be non-normally distributed and was analyzed by Kruskall-Wallis test, followed by Wilcoxon-Mann-Whitney test with Bonferroni adjustment. Statistical packages used included R (http://www.R-project.org), Prism 5 for Mac (GraphPad Software, San Diego, CA, www.graphpad.com) and Provantis. Microscopic lesion data were analyzed by Fisher’s exact test (one-sided) using Prism 5 for Mac.
RESULTS
Gross and Microscopic Findings
General health.
Administration of Cr(VI) as SDD in drinking water for 90 days had no effect on the survival of the mice nor were there any clinical signs of treatment-related toxicity in this study. There was a significant decrease in bodyweight at 520 mg/l SDD, which was first evident on day 15 and continued throughout the study. The maximum differences between mean body weights of mice in the 170 and 520 mg/l SDD dose groups versus those in the vehicle control group were < 5% and < 10%, respectively. Therefore, the observed deficits in bodyweight gain were deemed to be minimal (170 mg/l) or mild (520 mg/l) and were considered to be of no toxicological or biological significance. Data on water consumption suggest the weight difference reported herein might be related to changes in water intake. A summary of bodyweight and water consumption data is presented in Supplementary materials (Supplementary Fig. S1 and Table S2). SDD had no effect on food consumption at any dose level (data not shown), whereas water consumption in the 170 and 520 mg/l SDD dose groups were significantly lower than in mice in the vehicle control group. Based on the bodyweight and water consumption values, average daily ingested SDD and Cr(VI) levels over the duration of the study were estimated (Supplementary Table S2). The reductions in bodyweight were consistent with observations in the NTP (2007) study, where no change was reported at 62 mg/l SDD but small decreases were observed at doses ≥ 125 mg/l SDD. Similarly, the NTP (2007) reported reductions in water intake at doses ≥ 125 mg/l SDD.
Pathology on day 8.
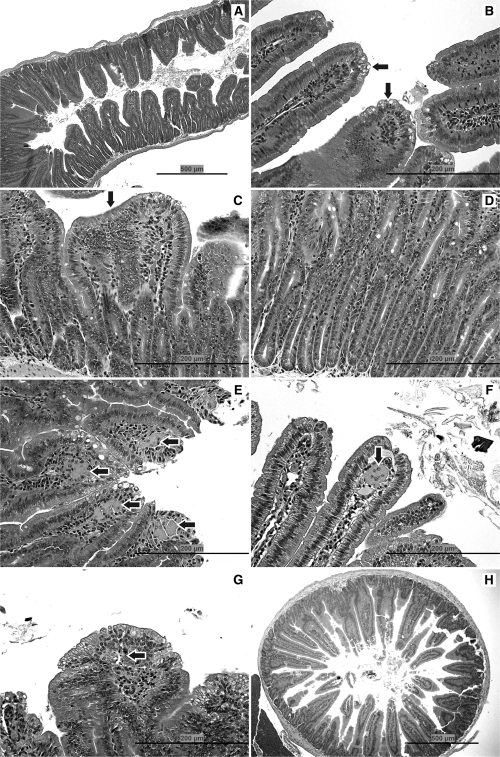
No treatment-related gross lesions were observed in the oral cavity or small intestine on day 8 nor were any microscopic lesions observed in the oral cavity. In the duodenum, cytoplasmic vacuolization of the villous epithelium was observed at ≥ 170 mg/l SDD. At 520 mg/l SDD, villous atrophy and crypt cell hyperplasia were also evident in the duodenum (Fig. 1A). In the jejunum, cytoplasmic vacuolization of the villous epithelium was observed at ≥ 170 mg/l SDD (Fig. 1B); however, the effects were milder and less prevalent than in the duodenum (Table 2). There were no apparent adverse effects in the small intestine at ≤ 60 mg/l SDD.
FIG. 1.
Pathology. (A) Duodenum of mouse exposed to 520 mg/l SDD for 8 days: with villous atrophy, blunting and fusion, and crypt epithelial hyperplasia. (B) Jejunum of mouse exposed to 170 mg/l SDD for 8 days: with cytoplasmic vacuolization of the villous epithelium (arrows). (C) Duodenum of mouse exposed to 520 mg/l SDD for 90 days: with villous atrophy, blunting and fusion (arrow), and crypt epithelial hyperplasia. (D) Duodenum of mouse exposed to 520 mg/l SDD for 90 days: with crypt epithelial hyperplasia. (E) Duodenum of mouse exposed to 520 mg/l SDD for 90 days: with histiocytic cellular infiltration of the villous lamina propria (arrows) and cytoplasmic vacuolization of the villous epithelium. (F) Jejunum of mouse exposed to 170 mg/l SDD for 90 days: with a multinucleate syncytium in the villous lamina propria (arrow). (G) Duodenum of mouse exposed to 520 mg/l SDD for 90 days: with apoptosis in the lamina propria (arrow) and cytoplasmic vacuolization of the villous epithelium. (H) Duodenum of a control mouse on day 91.
TABLE 2.
Summary of Histopathology in the Mouse Duodenum and Jejunum
| Pathology | SDD (mg/l) |
||||||
| 0 | 0.3 | 4 | 14 | 60 | 170 | 520 | |
| Day 8 | |||||||
| Duodenum | |||||||
| Villous cytoplasmic vacuolization | — | — | — | — | — | + (3/5) | + (5/5)a |
| Villous atrophy | — | — | — | — | — | — | + (3/5) |
| Crypt cell hyperplasia | — | — | — | — | — | — | + (3/5) |
| Jejunum | — | — | — | — | |||
| Villous cytoplasmic vacuolization | — | — | — | — | — | + (1/5) | + (1/5) |
| Villous atrophy | — | — | — | — | — | — | — |
| Crypt cell hyperplasia | — | — | — | — | — | — | — |
| Day 91 | |||||||
| Duodenum | |||||||
| Villous cytoplasmic vacuolization | — | — | — | — | + (5/10)a | + (10/10)a | + (7/10)a |
| Villous atrophy | — | — | — | — | — | + (1/10) | + (2/10)a |
| ++ (3/10)a | |||||||
| Apoptosis | — | — | — | — | — | + (3/10) | + (4/10)a |
| Crypt cell hyperplasia | — | — | — | — | — | + (9/10)a | + (4/10)a |
| ++ (5/10)a | |||||||
| Histiocytic infiltration in the villous lamina propria | — | — | — | — | + (1/10) | + (8/10)a | + (5/10)a |
| ++ (2/10)a | ++ (5/10)a | ||||||
| Multinucleated syncytia in the villous lamina propria | — | — | — | — | — | p (2/10) | p (4/10) |
| Jejunum | |||||||
| Villous cytoplasmic vacuolization | — | — | — | — | + (4/10)a | + (8/10)a | + (5/10)a |
| Villous atrophy | — | — | — | — | — | + (3/10) | + (1/10)a |
| ++ (3/10)a | |||||||
| Apoptosis | — | — | — | — | — | — | + (2/10) |
| Crypt cell hyperplasia | — | — | — | — | — | + (5/10) a | + (3/10)a |
| ++ (4/10)a | |||||||
| Histiocytic infiltration in the villous lamina propria | — | — | — | — | — | + (8/10)a | + (9/10)a++ |
| ++ (1/10)a | (1/10)a | ||||||
| Multinucleated syncytia in the villous lamina propria | — | — | — | — | — | — | p (1/10) |
Note. +, minimal; ++, mild; p, present; —, 0/5 or 0/10. Histopathological grading—villous cytoplasmic vacuolization: minimal = cytoplasmic vacuolization of < 25% of the villous epithelium, mild = cytoplasmic vacuolization of ≥ 25% and < 50% of the villous epithelium; villous atrophy: minimal = < 25% reduction of the villous length, mild = ≥ 25 and < 50% reduction of villous length; apoptosis: minimal = < 5 apoptotic cells per ×400 field, mild = ≥ 5 and < 10 apoptotic cells per ×400 field; crypt cell hyperplasia: minimal = > 1 and ≤ 2 times the normal crypt depth, mild = > 2 and ≤ 3 times the normal crypt depth; histiocytic infiltration in the villous lamina propria: minimal = a few macrophages in fewer than half of the villi, mild = macrophages in the villi which were readily discernible at ×10 and present in ≥ 50% and < 75% of the villi.
Significantly different (p ≤ 0.05) from control by Fisher’s exact test (note: + and ++ were combined for statistical tests).
Pathology on day 91.
Consistent with findings from a previous 13-week drinking water study (NTP, 2007), no treatment-related gross lesions were observed after 90 days of exposure to SDD. There were no microscopic lesions observed in the oral cavity. In the duodenum, 50% of the mice exhibited cytoplasmic vacuolization of the villous epithelium at a concentration of 60 mg/l SDD (Table 2). At 170 mg/l SDD, all of the mice exhibited villous cytoplasmic vacuolization in the duodenum and 90% exhibited of crypt cell hyperplasia. There were also signs of villous atrophy (1/10) and apoptosis (3/10) at 170 mg/l SDD. At 520 mg/l SDD, 70% of the mice exhibited villous cytoplasmic vacuolization, 90% exhibited crypt cell hyperplasia, 50% exhibited villous atrophy, and 40% exhibited apoptosis. Histiocytic cellular infiltration into the villous lamina propria was observed in the duodenum of all mice at ≥ 170 mg/l SDD. Multinucleated syncytia (fused cells) in the villous lamina propria were present in 40% of the mice exposed to 520 mg/l SDD. Histopathological finding in the duodenum is summarized in Figure 1C–E, 1G and 1H).
In the jejunum, 40% of the mice exhibited cytoplasmic vacuolization of the villous epithelium at 60 mg/l SDD (Table 2). At 170 mg/l SDD, 80% of the mice exhibited villous cytoplasmic vacuolization, 50% exhibited crypt cell hyperplasia, and 30% exhibited villous atrophy. At 520 mg/l SDD, 50% of the mice exhibited villous cytoplasmic vacuolization, 70% exhibited crypt cell hyperplasia, 40% exhibited villous atrophy, and 2/10 mice exhibited apoptosis. Histiocytic cellular infiltration into the villous lamina propria was also observed in the jejunum of 90% and 100% of mice exposed to 170 and 520 mg/l SDD, respectively. Finally, multinucleated syncytia in the villous lamina propria were present in the jejunum of one mouse exposed to 520 mg/l SDD (Fig. 1F).
Biochemical Evaluations
GSH and GSSG on day 8.
In cells, the abundant levels of low-molecular weight reducing agents such as GSH, cysteine, and ascorbate are thought to be important for reducing Cr(VI) to Cr(III) (Zhitkovich, 2005). Glutathione is estimated to be present in many cells at 1–10mM (Schafer and Buettner, 2001) and is therefore expected to play a major role in Cr(VI) reduction. Because the ratio of GSH/GSSG is a key indicator of cellular redox status (Meister and Anderson, 1983; Moriarty-Craige and Jones, 2004; Schafer and Buettner, 2001), the GSH/GSSG ratio was examined in several portions of the alimentary canal. Table 3 shows the effects of Cr(VI) on the levels of GSH and GSSG in the oral, duodenal, and jejunal epithelia, as well as in the plasma on day 8.
TABLE 3.
Redox Values in Epithelia and Plasma after 7 Days of Exposure to SDD
| SDD (mg/l) | GSH (nmol/mg) | GSSG (nmol/mg) | GSH/GSSG | ΔE (mV)a | ||||
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | |
| Oral mucosa | ||||||||
| 0 | 43.23 | 2.47 | 4.40 | 0.22 | 9.92 | 0.73 | −206.97 | 1.62 |
| 0.3 | 45.12 | 5.07 | 5.12 | 0.52 | 8.82 | 0.60 | −205.68 | 2.35 |
| 4 | 44.75 | 1.84 | 4.53 | 0.25 | 9.97 | 0.56 | −207.60 | 1.12 |
| 14 | 47.02 | 5.41 | 5.37 | 0.62 | 8.77 | 0.38 | −206.24 | 1.87 |
| 60 | 43.17 | 5.13 | 4.90 | 0.56 | 8.79 | 0.15 | −205.06 | 2.06 |
| 170 | 46.34 | 5.63 | 5.67 | 0.80 | 8.26b | 0.19 | −205.29 | 1.35 |
| 520 | 46.38 | 4.57 | 5.88b | 0.46 | 7.88c | 0.32 | −204.78 | 1.59 |
| Duodenum | ||||||||
| 0 | 16.77 | 1.26 | 0.24 | 0.03 | 71.33 | 5.15 | −220.58 | 1.36 |
| 0.3 | 17.57 | 1.25 | 0.30 | 0.03 | 59.99 | 6.85 | −218.77 | 2.03 |
| 4 | 16.99 | 0.73 | 0.30 | 0.01 | 57.33 | 0.49 | −218.11 | 0.56 |
| 14 | 16.75 | 0.94 | 0.31 | 0.01 | 53.41 | 2.69 | −216.86 | 1.44 |
| 60 | 17.61 | 1.08 | 0.36b | 0.01 | 48.47b | 2.00 | −216.24 | 1.36 |
| 170 | 18.92 | 1.49 | 0.43c | 0.01 | 44.28c | 2.72 | −215.89 b | 1.77 |
| 520 | 19.98 | 1.32 | 0.48c | 0.02 | 41.23c | 1.86 | −215.75b | 1.37 |
| Jejunum | ||||||||
| 0 | 15.30 | 0.74 | 0.34 | 0.02 | 45.29 | 2.11 | −213.49 | 1.08 |
| 0.3 | 14.14 | 0.95 | 0.37 | 0.01 | 38.43 | 1.68 | −210.20 | 1.39 |
| 4 | 13.63 | 0.48 | 0.33 | 0.02 | 41.12 | 1.66 | −210.70 | 0.58 |
| 14 | 12.09 | 0.36 | 0.36 | 0.02 | 34.51d | 2.30 | −206.68 | 1.05 |
| 60 | 14.25 | 0.99 | 0.32 | 0.01 | 45.51 | 5.25 | −212.28 | 2.29 |
| 170 | 11.47c | 0.23 | 0.29 | 0.01 | 40.37 | 1.86 | −208.15c | 0.71 |
| 520 | 12.79b | 0.45 | 0.33 | 0.01 | 38.72 | 1.54 | −209.05b | 0.98 |
| Plasma | GSH (μM) | GSSG (μM) | ||||||
| 0 | 25.45 | 0.74 | 3.59 | 0.35 | 7.34 | 0.65 | −137.40 | 1.26 |
| 0.3 | 24.86 | 0.55 | 4.18 | 0.27 | 6.05 | 0.46 | −134.59 | 1.17 |
| 4 | 27.69 | 1.53 | 4.20 | 0.33 | 6.84 | 0.92 | −137.35 | 2.17 |
| 14 | 26.91 | 1.45 | 4.04 | 0.32 | 6.92 | 0.83 | −137.10 | 2.44 |
| 60 | 32.45c | 1.37 | 4.18 | 0.23 | 7.93 | 0.81 | −141.62 | 1.80 |
| 170 | 30.41b | 1.09 | 4.85b | 0.23 | 6.35 | 0.45 | −137.90 | 1.46 |
| 520 | 31.46b | 1.82 | 5.07c | 0.23 | 6.28 | 0.53 | −138.12 | 1.84 |
See “MATERIALS AND METHODS” for calculation.
Statistically significant from control by Shirley’s tests (p < 0.05).
Statistically significant from control by Shirley’s tests (p < 0.01).
Statistically significant from control by Dunn’s tests (p < 0.01).
In the oral epithelium, there was a significant (p ≤ 0.05) increase in GSSG relative to control at 520 mg/l SDD and a significant decrease in the GSH/GSSG ratio at ≥ 170 mg/l SDD. In the duodenum, there was a significant (p ≤ 0.05) increase in GSSG and a significant decrease in the GSH/GSSG ratio, both starting at 60 mg/l SDD. The ΔE was significantly (p ≤ 0.05) increased relative to control at ≥ 170 mg/l SDD, meaning a decrease in redox potential. In the jejunum, there was a significant decrease in GSH and an increase in ΔE relative to control at ≥ 170 mg/l SDD. Figure 2 depicts the changes in the GSH/GSSG ratio in the duodenum and jejunum after 7 days of exposure to SDD. In the plasma, there were significant increases in both GSH and GSSG starting at 60 and 170 mg/l SDD, respectively; however, there were no statistically significant changes in the GSH/GSSG ratio or redox potential (Table 3).
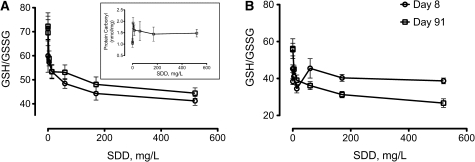
FIG. 2.
Measures of GSH/GSSG ratio in the duodenum (A) and jejunum (B) after 7 and 90 days of exposure to SDD. Data plotted are mean and SEM (n = 5 animals), which are also reported in Tables 3 and 4. Inset: mean protein carbonyl content in duodenum at day 91.
Overall, the data at day 8 indicate an apparent oxidative stress response in the duodenum and, to a lesser extent, the jejunum. The changes in the plasma indicate a potential systemic oxidative stress response at the higher drinking water concentrations.
GSH and GSSG on day 91.
Measures of GSH, GSSG, and redox potential after 90 days of exposure to Cr(VI) in drinking water are shown in Table 4. Changes in the oral mucosa were generally sporadic and did not demonstrate a clear dose-response trend. As examples, GSH levels were significantly (p < 0.05) elevated at 60 mg/l SDD only, and GSSG levels were significantly (p < 0.01) elevated at 14 and 60 mg/l SDD but not at higher concentrations. The GSH/GSSG ratio was significantly decreased (p < 0.05) at a single concentration, 14 mg/l SDD. Overall, there were no consistent changes in glutathione parameters in the oral mucosa following 90 days of exposure.
TABLE 4.
Redox Values in Epithelia and Plasma after 90 Days of Exposure to SDD
| SDD (mg/l) | GSH (nmol/mg) |
GSSG (nmol/mg) |
GSH/GSSG |
ΔE (mV)a |
||||
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | |
| Oral mucosa | ||||||||
| 0 | 39.12 | 2.02 | 3.91 | 0.11 | 10.05 | 0.61 | −205.86 | 1.48 |
| 0.3 | 40.35 | 1.03 | 4.25 | 0.11 | 9.52 | 0.30 | −205.69 | 0.69 |
| 4 | 43.93 | 2.77 | 4.49 | 0.16 | 9.92 | 0.98 | −207.08 | 2.00 |
| 14 | 43.08 | 1.17 | 5.38b | 0.22 | 8.04c | 0.26 | −204.30 | 0.58 |
| 60 | 46.01c | 1.87 | 5.42b | 0.38 | 8.62 | 0.58 | −205.98 | 1.21 |
| 170 | 40.54 | 1.42 | 4.68 | 0.26 | 8.74 | 0.44 | −204.55 | 0.97 |
| 520 | 37.71 | 1.19 | 4.13 | 0.16 | 9.15 | 0.28 | −204.25 | 0.66 |
| Duodenum | ||||||||
| 0 | 18.20 | 1.00 | 0.25 | 0.02 | 72.29 | 5.58 | −221.94 | 1.52 |
| 0.3 | 20.06 | 1.17 | 0.29 | 0.02 | 69.58 | 5.41 | −222.69 | 1.80 |
| 4 | 20.14 | 0.57 | 0.35 | 0.02 | 58.89 | 3.10 | −220.69 | 0.74 |
| 14 | 21.43d | 0.85 | 0.40e | 0.01 | 53.36d | 2.96 | −220.18 | 1.14 |
| 60 | 21.96d | 1.08 | 0.42e | 0.03 | 53.12d | 3.06 | −220.40 | 1.20 |
| 170 | 23.92e | 0.96 | 0.51e | 0.04 | 48.10e | 3.08 | −220.23 | 0.93 |
| 520 | 23.04e | 1.39 | 0.52e | 0.04 | 44.44e | 2.19 | −218.65 | 1.14 |
| Jejunum | ||||||||
| 0 | 17.95 | 0.55 | 0.33 | 0.02 | 56.21 | 5.28 | −218.35 | 1.67 |
| 0.3 | 18.00 | 1.19 | 0.33 | 0.03 | 55.67 | 3.41 | −218.32 | 1.20 |
| 4 | 16.81 | 0.53 | 0.37 | 0.01 | 45.08 | 2.15 | −214.72 | 0.98 |
| 14 | 15.97 | 0.71 | 0.42d | 0.04 | 39.09d | 2.81 | −212.04d | 1.03 |
| 60 | 14.55d | 0.51 | 0.41d | 0.02 | 36.15d | 2.15 | −209.80d | 1.14 |
| 170 | 12.89e | 0.80 | 0.41d | 0.02 | 31.28e | 1.73 | −206.18e | 1.55 |
| 520 | 12.46e | 0.94 | 0.47e | 0.02 | 26.70e | 2.30 | −203.47e | 2.04 |
| Ileum | ||||||||
| 0 | 15.85 | 0.88 | 0.33 | 0.03 | 49.67 | 3.15 | −215.13 | 0.89 |
| 0.3 | 16.98 | 0.97 | 0.35 | 0.02 | 48.52 | 2.37 | −215.76 | 1.34 |
| 4 | 16.78 | 1.22 | 0.37 | 0.06 | 47.00 | 3.61 | −215.02 | 0.84 |
| 14 | 16.66 | 1.02 | 0.32 | 0.02 | 51.85 | 2.99 | −216.37 | 1.34 |
| 60 | 16.40 | 0.70 | 0.37 | 0.04 | 45.85 | 2.58 | −214.56 | 0.42 |
| 170 | 16.17 | 1.48 | 0.34 | 0.03 | 48.03 | 2.59 | −214.82 | 1.49 |
| 520 | 17.18 | 1.35 | 0.32 | 0.03 | 55.30 | 2.57 | −217.61 | 0.93 |
| Plasmaf | GSH (μM) | GSSG (μM) | ||||||
| 0 | 35.23 | 2.03 | 5.98 | 0.47 | 6.05 | 0.61 | −139.06 | 1.83 |
| 0.3 | 39.63 | 2.98 | 6.73 | 0.17 | 5.91 | 0.45 | −140.35 | 2.02 |
| 4 | 37.96 | 3.31 | 8.77 | 0.55 | 4.46 | 0.60 | −135.55 | 3.22 |
| 14 | 44.21 | 2.94 | 11.84e | 1.41 | 4.04 | 0.69 | −136.13 | 3.03 |
| 60 | 50.13e | 1.81 | 10.02e | 0.40 | 5.05 | 0.33 | −141.55 | 1.29 |
| 170 | 53.07e | 2.34 | 16.49e | 1.35 | 3.31e | 0.39 | −136.51 | 1.96 |
| 520 | 54.32e | 3.43 | 18.47e | 0.93 | 3.00e | 0.32 | −135.41 | 2.21 |
See “MATERIALS AND METHODS” for calculation.
Statistically significant from control by Dunn’s tests (p < 0.01).
Statistically significant from control by Dunn’s tests (p < 0.05).
Statistically significant from control by Shirley’s tests (p < 0.05).
Statistically significant from control by Shirley’s tests (p < 0.01).
n = 4 for plasma GSH, GSH/GSSG, and ΔE.
In the duodenum, statistically significant (p ≤ 0.05) alterations in GSH, GSSG, and the GSH/GSSG ratio were observed at ≥ 14 mg/l SDD (Table 4). Like the duodenum, significant (p ≤ 0.05) increases in GSSG and decreases in the GSH/GSSG ratio were observed at ≥ 14 mg/l SDD. Unlike the duodenum, however, GSH levels were significantly (p ≤ 0.05) decreased, beginning at 60 mg/l SDD. Notably, there were no significant changes in any parameter in the more distal ileum. Figure 2 depicts the changes in the GSH/GSSG ratio in the duodenum and jejunum after 90 days of exposure to SDD. Interestingly, the effects of SDD on the GSH/GSSG ratio in the duodenum did not appear to worsen with prolonged exposure (Fig. 2A). In contrast, the GSH/GSSG ratio in the jejunum, which was unaffected after 7 days of exposure (Fig. 2B), was significantly decreased after 90 days of exposure at concentrations ≥ 14 mg/l SDD.
In the plasma, significant (p ≤ 0.05) increases in GSSG and GSH were observed starting at 14 and 60 mg/l SDD, respectively, and significant (p ≤ 0.01) decreases in the GSH/GSSG ratio were observed at ≥ 170 mg/l SDD (Table 4). Overall, the plasma data indicate that following 90 days of exposure to Cr(VI) in drinking water, there may be systemic changes consistent with an oxidative stress response albeit without any apparent effect on the plasma redox potential.
Protein carbonyl on day 91.
To further examine the effects of SDD exposure in the duodenum, protein oxidation was measured at day 91. A statistically significant increase in protein carbonyls was observed at 4 mg/l SDD. The mean values were generally elevated in the higher treatment groups; however, the values were not statistically different from control animals (Fig. 2 inset).
8-OHdG on day 91.
In order to examine whether the oxidative responses in the duodenum also resulted in oxidative DNA damage, 8-OHdG content was analyzed after 90 days of exposure. Relative to control, there were no statistically significant increases in 8-OHdG in the duodenum (or oral mucosa) at any concentration (Supplementary Fig. S3).
Cytokine and chemokine analyses on day 91.
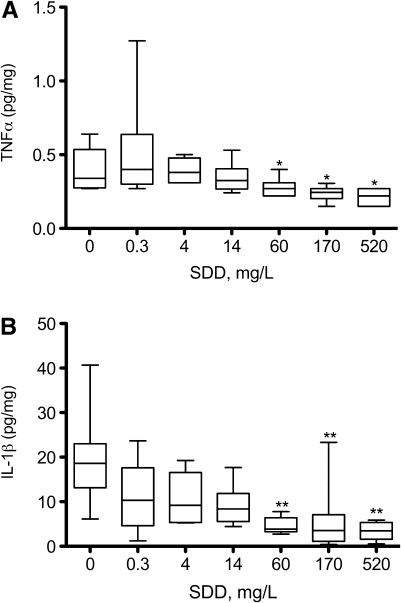
Considering the oxidative and pathological effects (particularly histiocytic infiltration) observed in the duodenum, it is conceivable that Cr(VI) might induce inflammatory responses either systemically or within the target tissues. To assess this, a multiplex of 22 cytokines and chemokines were measured in the plasma, duodenum, and oral mucosa on day 91. For all three tissues, the data were non-normal and exhibited heterogeneous variance. In the serum, few cytokines exhibited significant changes from untreated animals. In the oral mucosa, significant differences from control animals were generally limited to the highest treatment dose. In the duodenum, several cytokines were significantly altered—generally beginning at 60 mg/l SDD. Interestingly, the cytokines changes in the duodenum were inversely related to SDD concentration. The variability in the data generally obscured visual dose-response effects; however, results for TNFα and IL-1β exhibited clear dose-dependent trends (Fig. 3).
FIG. 3.
Measures of TNFα (A) and IL-1β (B) in the duodenum after 90 days of exposure to SDD. Data were normalized to protein content. Whiskers represent the 10th and 90th percentiles. *p < 0.05, **p < 0.01 compared with control group by Shirley’s test.
Clinical Chemistry
Cr(VI) exposure was previously shown to induce mild microcytic hypochromic anemia in both rats and mice (NTP, 2008). Therefore, iron status was assessed in the serum and bone marrow following 90 days of exposure to Cr(VI). There were no clearly discernible effects of Cr(VI) on iron stores in the bone marrow or circulating iron, ferritin, or transferrin (Supplementary Table S3).
Tissue Concentrations of Total Chromium
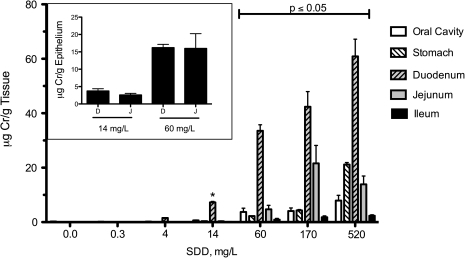
Total chromium levels were measured in the oral cavity, glandular stomach, and small intestine following 90 days of exposure to Cr(VI) in drinking water (Fig. 4). Significant (p ≤ 0.05) increases in chromium were observed at ≥ 60 mg/l SDD in the oral cavity, glandular stomach, jejunum, and ileum. In the duodenum, significant (p ≤ 0.05) increases in total chromium were observed at ≥ 14 mg/l SDD. Notably, the concentrations of total chromium observed in the duodenum starting at 60 mg/l SDD were substantially greater than the levels measured in any of the other tissues at all SDD concentrations. It should be noted that the total chromium level in the jejunum, which is 2–3 times longer than the duodenum, represent an average over the entire length of the tissue. As shown in the Figure 4 inset, analysis of total chromium in scraped epithelial cells from the duodenum and proximal jejunum of animals exposed to 14 or 60 mg/l SDD were similar; this is likely the case in all treatment groups. Thus, the lower chromium levels measured in the entire jejunum suggest that chromium levels in the distal portion of the jejunum are low and more similar to the levels measured in the ileum.
FIG. 4.
Measures of total chromium (Cr) in the oral mucosa, glandular stomach, and small intestine following 90 days of exposure to SDD in drinking water. Note that all values in control and 0.3 mg/l SDD groups are estimates and were either below the method detection limit (MDL) or above the MDL but below the method reporting limit (MRL). The MDL and MRL for the intestinal samples were 0.02 and 0.15 μg/g, respectively, whereas the MDL and MRL for oral cavity tissue are about 10-fold higher. Data plotted are mean and SEM. All tissues exhibited significant (p < 0.001) trend for increased Cr levels. Bars under the capped line were all significantly increased relative to their respective control tissues by Shirley’s test (p ≤ 0.05). *Significantly different from control by Shirley’s test (p < 0.05). The inset shows a comparison of Cr in scraped epithelial cells from the duodenum (D) and proximal portion of the jejunum (J) in the 14 and 60 mg/l SDD treatment groups. Data represent mean and SEM.
DISCUSSION
This study was designed to investigate the MOA for the intestinal tumors observed in mice following chronic exposure to Cr(VI) in drinking water. As part of a larger set of Cr(VI) MOA studies outlined in Table 1, the results presented herein are also intended to serve as a phenotypic anchor to toxicogenomic studies being conducted as part of the project. Although the focus herein is on the mouse small intestine, the oral mucosa was also studied for future comparisons with a 90-day rat study. Rats were previously shown to develop oral cavity tumors (but not intestinal tumors) following oral exposure to Cr(VI) (NTP, 2008). Consistent with the previous NTP studies (NTP, 2007, 2008), there were no histopathological lesions in the mouse oral mucosa. In addition, at day 91, there were no apparent treatment-related changes in GSH, GSSG, or 8-OHdG levels in the oral mucosa. Overall, these findings suggest that exposure to Cr(VI) in drinking water is unlikely to result in sustained oxidative stress or cytotoxicity in the mouse oral cavity.
In the small intestine, significant decreases in the GSH/GSSG ratio occurred in the duodenum at day 8 starting at 60 mg/l SDD and significant changes in redox potential (ΔE) occurred at ≥ 170 mg/l SDD. Histopathological lesions observed included cytoplasmic vacuolization (≥ 170 mg/l SDD), atrophy (520 mg/l SDD), and crypt hyperplasia (520 mg/l SDD). Notably, these results suggest that exposure to high concentrations of Cr(VI) induced a fairly rapid onset of compensatory cell proliferation. In the jejunum, there was a significant decrease in GSH levels and increase in ΔE at ≥ 170 mg/l SDD; however, there were no significant changes in the GSH/GSSG ratio.
After 90 days of exposure to Cr(VI), similar histopathological lesions were observed in the duodenum as at day 8 but occurred at lower concentrations: cytoplasmic vacuolization occurred at ≥ 60 mg/l SDD, whereas atrophy, apoptosis, and crypt hyperplasia occurred at ≥ 170 mg/l SDD. The incidence and severity of microscopic lesions increased at 170 and 520 mg/l SDD. Statistically significant changes in GSH, GSSG, and the GSH/GSSG ratio were observed at ≥ 14 mg/l SDD, and these effects were somewhat more pronounced at 170 and 520 mg/l SDD. These data suggest that biochemical changes in GSH and GSSG are sensitive indicators of redox status, and these changes serve as more sensitive indicators of exposure and/or toxicity than histopathology. Despite clear dose-dependent changes in oxidative status, protein carbonyl levels (measures of protein oxidation) were only modestly elevated and generally exhibited an all-or-nothing response (Fig. 2). Review of literature where protein carbonyl content has been measured generally indicates such phenomena; for example, treatment of cells with 0.1–1mM peroxide was shown to result in only modest increases in protein carbonyl content (see Wang et al., 2003).
Oxidative DNA damage (as measured by 8-OHdG) was not significantly increased in any treatment group relative to control mice. This finding is consistent with a study that reported no increases in 8-OHdG (using 32P post labeling) in mucosal scrapings from the duodenum and stomach of mice exposed to 14 or 57 mg/l SDD in drinking water for 9 months (De Flora et al., 2008). In contrast, previous studies involving intratracheal instillation of SDD for 3 days resulted in an increase in 8-OHdG in the rodent lung (Izzotti et al., 1998). The basis for the 8-OHdG differences observed in the lung and intestine is not well understood at this time and requires further investigation. Nonetheless, the lack of increase in 8-OHdG is not novel, as studies with peroxisome proliferators have shown that oxidative stress can be induced without detectible increases in DNA damage (including 8-OHdG), and that such exposure can lead to elevated expression of genes related to DNA repair (Rusyn et al., 2004). Thus, it is conceivable that 8-OHdG lesions were repaired or that the oxidative stress was not sufficient to induce measurable increases in 8-OHdG. The toxicogenomics data from the mice in this study (Table 1) may ultimately provide insights as to whether exposure to Cr(VI) resulted in changes in genes related to repair of oxidative DNA damage.
Although there was histiocytic infiltration in the small intestine, cytokines and chemokines were not elevated systemically or locally in the duodenum. Interestingly, the changes in cytokines and chemokines that were observed in the duodenum were all inversely related to the SDD concentration. Data in Figure 3 suggest that Cr(VI) reduced the duodenal levels of TNFα and IL-1β in a dose-dependent manner, both of which are regulated by NF-κB (Rahman and MacNee, 2000; Roberts et al., 2009). Decreases in TNFα and IL-1β are also apparent at the transcript level (Kopec et al., Thompson, Kim, Forgacs, Harris, O'Brien, Proctor, Burkhalter, Haws, and Zacharewski, in preparation). Previous studies have shown that treatment of cells with Cr(VI) ameliorates TNFα-induced NF-κB–driven luciferase activity in vitro—potentially due to redox-mediated protein thiol modification (Shumilla et al., 1998, 1999). Thus, it is possible that chromium affects either TNFα or NF-κB activity and may disrupt inflammatory signaling. Alternatively, the variability and trend toward decreased cytokine levels in the duodenum may reflect the complex temporal nature of inflammatory signaling and/or an overall decrease in protein expression levels due to toxicity.
The histological lesions and changes in biochemical parameters in the duodenum are consistent with the tissue chromium levels. As shown in Table 5, significant increases in chromium were detected in the duodenum at ≥ 14 mg/l SDD, which coincided with significant decreases in the GSH/GSSG ratio. At 60 mg/l SDD, histological lesions in the form of cytoplasmic vacuolization were observed. At ≥ 170 mg/l SDD, atrophy and apoptosis were observed in the intestinal villous, and hyperplasia was observed in the intestinal crypt. These findings show a clear progression of effects in the mouse duodenum following 90 days of exposure to Cr(VI) that includes changes in redox status (≥ 14 mg/l SDD), oxidative stress and cytoplasmic vacuolization (≥ 60 mg/l SDD), and atrophy, apoptosis, and regenerative crypt cell hyperplasia (≥ 170 mg/l SDD). A similar pattern is likely for the jejunum; however, the available data do not allow for direct assessment of this notion because, unlike the duodenum, only the most proximal portion of the jejunum was used for biochemical analyses, whereas the entire jejunum was used for chromium analysis.
TABLE 5.
Summary of Significant Biochemical and Pathological Findings at Day 91 in the Mouse Duodenum
| Endpoint | SDD, mg/l |
|||||
| 0.3 | 4 | 14 | 60 | 170 | 520 | |
| Tissue Cr | — | — | ↑ | ↑ | ↑ | ↑ |
| GSH/GSSG | — | — | ↓ | ↓ | ↓ | ↓ |
| Vacuolization | — | — | — | ↑ | ↑ | ↑ |
| Atrophy/apoptosis | — | — | — | — | ↑ | ↑ |
| Crypt cell proliferation | — | — | — | — | ↑ | ↑ |
In the present study, GSSG levels increased in both the duodenum and the jejunum after 90 days of exposure to Cr(VI). This could result from general Cr(VI)-induced oxidative stress as antioxidants such as GSH are diminished in concentration or possibly due to intracellular reduction of Cr(VI) to Cr(V). The latter can lead to hydrogen peroxide formation, with subsequent catabolism by glutathione peroxidase that simultaneously converts GSH to GSSG (see Thompson et al., 2011). In contrast to GSSG, GSH levels exhibited different responses in intestinal segments: a dose-dependent increase in the duodenum and a dose-dependent decrease in the jejunum. GSH levels typically decrease when reactive compounds bind to GSH and increase after activation of electrophile response elements upstream of genes involved in GSH biosynthesis (Franco and Cidlowski, 2009). Thus, the increase in GSH observed in the duodenum may represent an oxidative stress response that includes enhanced GSH synthesis. Indeed, preliminary toxicogenomic analyses indicate that the glutamate-cysteine ligase catalytic subunit (Gclc) mRNA, which codes for the rate-limiting enzyme in GSH synthesis, is elevated at several SDD concentrations (Kopec et al., in preparation). Moreover, the glutathione peroxidase (Gpx1) mRNA, which codes for the enzyme that catalyzes the GSH-dependent detoxification of peroxide, is elevated at ≥ 170 mg/l SDD (Kopec et al., in preparation). Elevations in Gclc at concentrations where chromium was not detected in the duodenal tissue may represent responses to extracellular thiol status as studies suggest that intestinal cells regulate the extracellular redox environment, and redox sensitive cell surface proteins can influence intracellular gene expression (Dahm and Jones, 2000; Go et al., 2009; Mannery et al., 2010; Moriarty-Craige and Jones, 2004). A more thorough analysis of toxicogenomic data collected from other animals in this study may provide additional insight into the regulation of GSH and oxidative stress in the intestinal segments and oral mucosae of both mice and rats.
The dosimetry data in the GI tract reported herein indicate that chromium levels are highest in the duodenum (and proximal jejunum). Notably, none of the other tissues exhibited chromium levels as high as those observed in the duodenum at SDD concentrations that were reported to be carcinogenic in the NTP (2008) 2-year bioassay. Interestingly, chromium levels in the glandular stomach were much lower than in the proximal small intestine. The higher chromium level in the proximal small intestine relative to the stomach is likely related to the structure and function of the small intestine, which is designed for nutrient absorption. These dosimetry data may explain the absence of gastric tumors in mice in the NTP (2008) study.
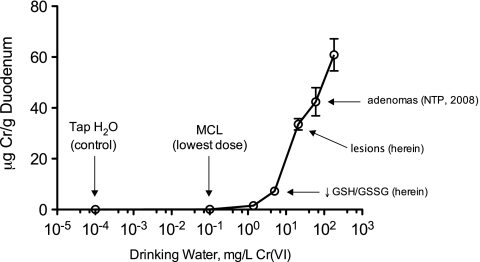
Figure 5 shows the duodenal chromium tissue levels as a function of the concentration of Cr(VI) in SDD-treated drinking water. The effects reported herein occurred at tissue levels much greater than levels which result from consumption of chromium in the municipal tap water, which was 10−4 mg/l Cr(VI) for the control animals. Moreover, SDD concentrations ≥ 60 mg/l were bright yellow (see Supplementary Fig. S3) and concentrations ≥ 170 mg/l were associated with reduced water intake (Supplementary Table S2), presumably due to unpalatability. The 0.3 mg/l SDD group in this study (0.1 mg/l Cr(VI)) is equivalent to the current federal drinking water standard for total chromium, and at this exposure, no appreciable increases in tissue chromium levels or adverse effects were observed. Notably, the chromium concentrations in the duodenum at day 91 in the three highest treatment groups are estimated to approach or exceed 1mM—a tissue dose that is clearly toxic in in vitro assays. These findings suggest that the doses that caused cancer in mice in the NTP study are associated with cytotoxicity in target tissues. Furthermore, the increases in plasma GSH and GSSG levels observed at the highest concentrations in this study might reflect systemic oxidative stress and/or toxicity. In this regard, it was previously suggested that the bodyweight decreases observed in the NTP (2008) 2-year bioassay (similar to those in the present study) indicate that the highest SDD concentration may have exceeded the maximum tolerated dose (Stern, 2010).
FIG. 5.
Duodenal chromium levels after 90 days of exposure plotted in terms of Cr(VI) (not SDD). The tap water used to treat control animals contains trace levels of Cr(VI), measured in this study at approximately 0.098 μg/l total Cr (including Cr(VI)). The lowest treatment concentration in the study is equivalent to the current MCL, 100 μg/l total Cr. Tissue levels at the MCL are nearly 1000-fold lower than those that resulted in intestinal tumors in concurrent control animals in the 2-year NTP bioassay. Data plotted are mean and SEM.
Additional studies currently underway with tissues collected from the mice treated in this study, including cytogenetic analyses, measurement of chromium in genomic DNA, and in vivo mutations, should provide additional measures of DNA damage. Moreover, the toxicogenomic data for tissues from this study should provide an additional and perhaps more sensitive means to assess the dose-response for key events in the MOA including oxidative responses. Finally, identical studies are also underway in rats for the purpose of interspecies comparisons.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported by the Cr(VI) Panel of the American Chemistry Council.
Supplementary Material
Acknowledgments
The authors thank Drs Michael Dourson, Kirk Kitchin, David Gaylor, and Xianglin Shi for review of an earlier version of this manuscript. The authors also thank Andrew Tachovsky, Buddy Burkhalter, and Elizabeth Kuriakose for assistance with statistical analyses; Dr Rich May for his assistance with the ELISA assays; and Dr Michael Carakostas for insights regarding clinical findings. We are also grateful for the contributions provided by the Toxicology Excellence for Risk Assessment Expert Panel overseeing the Cr(VI) Research Program (a panel report is available at http://www.tera.org/Peer/Chromium/Chromium.htm).
References
- California Department of Health Services (CDHS) Chromium-6 in Drinking Water: An Overview of Sampling Results. 2009. Available at: http://www.cdph.ca.gov/certlic/drinkingwater/pages/chromium6sampling.aspx. Accessed July 7, 2011. [Google Scholar]
- Dahm LJ, Jones DP. Rat jejunum controls luminal thiol-disulfide redox. J. Nutr. 2000;130:2739–2745. doi: 10.1093/jn/130.11.2739. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- De Flora S, D'Agostini F, Balansky R, Micale R, Baluce B, Izzotti A. Lack of genotoxic effects in hematopoietic and gastrointestinal cells of mice receiving chromium(VI) with the drinking water. Mutat. Res. 2008;659:60–67. doi: 10.1016/j.mrrev.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- Go YM, Craige SE, Orr M, Gernert KM, Jones DP. Gene and protein responses of human monocytes to extracellular cysteine redox potential. Toxicol. Sci. 2009;112:354–362. doi: 10.1093/toxsci/kfp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Chromium, nickel and welding. IARC Monogr. Eval. Carcinog. Risks Hum. 1990;49:1–648. [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Bagnasco M, Camoirano A, Orlando M, De Flora S. DNA fragmentation, DNA-protein crosslinks, postlabeled nucleotidic modifications, and 8-hydroxy-2'-deoxyguanosine in the lung but not in the liver of rats receiving intratracheal instillations of chromium(VI). Chemoprevention by oral N-acetylcysteine. Mutat. Res. 1998;400:233–244. doi: 10.1016/s0027-5107(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- Mannery YO, Ziegler TR, Hao L, Shyntum Y, Jones DP. Characterization of apical and basal thiol-disulfide redox regulation in human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G523–G530. doi: 10.1152/ajpgi.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll N, Keshava N, Chen J, Akerman G, Kligerman A, Rinde E. An evaluation of the mode of action framework for mutagenic carcinogens case study II: chromium (VI) Environ. Mol. Mutagen. 2010;51:89–111. doi: 10.1002/em.20525. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu. Rev. Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- Nickens KP, Patierno SR, Ceryak S. Chromium genotoxicity: A double-edged sword. Chem. Biol. Interact. 2010;188:276–288. doi: 10.1016/j.cbi.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP. NTP technical report on the toxicity studies of sodium dichromate dihydrate (CAS No. 7789-12-0) administered in drinking water to male and female F344/N rats and B6C3F1 mice and male BALB/c and am3-C57BL/6 mice. 2007 NTP Toxicity Report Series Number 72, NIH Publication No. 07-5964. [PubMed] [Google Scholar]
- NTP. NTP technical report on the toxicology and carcinogenesis studies of sodium dichromate dihydrate (CAS No. 7789-12-0) in F344/N rats and B6C3F1 mice (drinking water studies) 2008 NTP TR 546. NIH Publication No. 08-5887. [PubMed] [Google Scholar]
- O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Oze C, Bird DK, Fendorf S. Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6544–6549. doi: 10.1073/pnas.0701085104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EM, Doorn JA, Slikker W. Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 2009;112:4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Asakura S, Pachkowski B, Bradford BU, Denissenko MF, Peters JM, Holland SM, Reddy JK, Cunningham ML, Swenberg JA. Expression of base excision DNA repair genes is a sensitive biomarker for in vivo detection of chemical-induced chronic oxidative stress: identification of the molecular source of radicals responsible for DNA damage by peroxisome proliferators. Cancer Res. 2004;64:1050–1057. doi: 10.1158/0008-5472.can-03-3027. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Senft AP, Dalton TP, Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000;280:80–86. doi: 10.1006/abio.2000.4498. [DOI] [PubMed] [Google Scholar]
- Shirley E. A non-parametric equivalent of Williams' test for contrasting increasing dose levels of a treatment. Biometrics. 1977;33:386–389. [PubMed] [Google Scholar]
- Shumilla JA, Broderick RJ, Wang Y, Barchowsky A. Chromium(VI) inhibits the transcriptional activity of nuclear factor-kappaB by decreasing the interaction of p65 with cAMP-responsive element-binding protein-binding protein. J. Biol. Chem. 1999;274:36207–36212. doi: 10.1074/jbc.274.51.36207. [DOI] [PubMed] [Google Scholar]
- Shumilla JA, Wetterhahn KE, Barchowsky A. Inhibition of NF-kappa B binding to DNA by chromium, cadmium, mercury, zinc, and arsenite in vitro: evidence of a thiol mechanism. Arch. Biochem. Biophys. 1998;349:356–362. doi: 10.1006/abbi.1997.0470. [DOI] [PubMed] [Google Scholar]
- Stern AH. A quantitative assessment of the carcinogenicity of hexavalent chromium by the oral route and its relevance to human exposure. Environ. Res. 2010;110:798–807. doi: 10.1016/j.envres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Stout MD, Herbert RA, Kissling GE, Collins BJ, Travlos GS, Witt KL, Melnick RL, Abdo KM, Malarkey DE, Hooth MJ. Hexavalent chromium is carcinogenic to F344/N rats and B6C3F1 mice after chronic oral exposure. Environ. Health Perspect. 2009;117:716–722. doi: 10.1289/ehp.0800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Haws LC, Harris MA, Gatto NM, Proctor DM. Application of the U.S. EPA mode of action Framework for purposes of guiding future research: a case study involving the oral carcinogenicity of hexavalent chromium. Toxicol. Sci. 2011;119:20–40. doi: 10.1093/toxsci/kfq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. National primary drinking water regulations-synthetic organic chemicals and inorganic chemicals; monitoring for unregulated contaminants; national primary drinking water regulations implementation; national secondary drinking water regulations. Final rule. Fed. Regist. 1991;56:3526–3597. [Google Scholar]
- U.S. EPA. Toxicological Review of Hexavalent Chromium in Support of Summary Information on the Integrated Risk Information System (IRIS) Washinton, D.C: U.S. Environmental Protection Agency; 1998. [Google Scholar]
- U.S. EPA. Guidelines for Carcinogen Risk Assessment, EPA/630/P-03/001F. Washington, D.C: Risk Assessment Forum: U.S. Environmental Protection Agency; 2005. [Google Scholar]
- Wang L, Nishida H, Ogawa Y, Konishi T. Prevention of oxidative injury in PC12 cells by a traditional Chinese medicine, Shengmai San, as a model of an antioxidant-based composite formula. Biol. Pharm. Bull. 2003;26:1000–1004. doi: 10.1248/bpb.26.1000. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem. Res. Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.