Abstract
Background. Glomerulomegaly, the abnormal enlargement of glomeruli, has been related to an increased risk of glomerulosclerosis, but the degree of enlargement that constitutes glomerulomegaly has not been defined.
Methods. The principal stereological methods for estimating glomerular volume are [1] the disector/Cavalieri method that is considered the ‘gold standard’ for measuring individual glomerular volume (IVglom) and [2] the disector/fractionator technique that estimates average glomerular volume (Vglom) together with total glomerular number (Nglom) for the entire kidney. The two methods produce different estimates with Vglom consistently exceeding IVglom. This study compares glomerular volumes obtained by the two methods in autopsy kidneys of 39 African American and 34 US white adult males, and correlates the values with Nglom, body mass index (BMI), hypertension, glomerulosclerosis and race, factors known or thought to influence glomerular volume.
Results. For the smallest glomeruli, Vglom was 25% larger than IVglom with the difference increasing to over 50% for kidneys with the largest glomeruli. Both Vglom and IVglom showed significant inverse correlations with Nglom and significant direct correlations with BMI and hypertension. African Americans had larger IVglom and Vglom than whites, but only IVglom was significant. The 90th percentile for IVglom was 6.81 μm3 × 106 and 13.10 μm3 × 106 for Vglom, but larger glomerular size did not separate hypertensive from non-hypertensive subjects nor did it show any significant relationship to glomerulosclerosis. While Vglom overestimated glomerular size compared with IVglom, both measurements demonstrated similar relationships to factors influencing glomerular volume.
Conclusions. With neither method could glomerulomegaly, the abnormal enlargement of glomerular size predisposing to glomerulosclerosis, be determined.
Keywords: body mass index, glomerular number, glomerulomegaly, hypertension
Introduction
Abnormally large glomeruli are thought to be susceptible to glomerulosclerosis [1,2]. The term ‘glomerulomegaly’ has been applied to such glomerular enlargement, and individuals and populations at increased risk of progressive glomerulosclerosis are reported to have kidneys characterized by glomerulomegaly [3–10]. Such circumstances include the congenital disorder of oligomeganephronia and the acquired condition of obesity [3,8,9,11]. It is also suggested that glomerulomegaly may reflect underlying structural differences in the kidney that place susceptible racial groups at increased risk for chronic kidney disease (CKD) [4–7].
The association of glomerulomegaly with CKD is supported by experimental evidence that indicates that mesangial ‘stretch’ and podocyte depletion resulting from excessive glomerular enlargement lead to glomerulosclerosis [12,13]. Nevertheless, the definition of glomerulomegaly remains vague, and the size of a glomerulus that constitutes glomerulomegaly has not been established.
Adult glomerular size is influenced by several factors. The dominant factor appears to be glomerular number, which is determined during fetal development, and has a strong positive relationship with birth weight [14,15]. The second factor is obesity, and the third is elevated blood pressure which apparently increases glomerular size independently of obesity or glomerular number [16,17]. The fourth factor may be glomerulosclerosis [1]. When glomeruli are lost in CKD due to glomerulosclerosis, the remaining non-sclerosed glomeruli are thought to undergo compensatory hypertrophy. Compensatory glomerular hypertrophy is hypothesized to increase the rate of progression of CKD as perfusion is shifted onto an increasingly smaller number of enlarged glomeruli [1].
Estimates of glomerular volume vary considerably depending upon the method of measurement. The Cavalieri stereological method in which the cross-sectional areas of glomeruli are measured in serial sections cut completely through a glomerulus is considered the ‘gold standard’ and provides estimates of individual glomerular volume (IVglom) [18–20]. The disector method uses two microscopes tandemly coordinated to visualize microscopic fields of the same area of tissue in sections a known distance apart. When used to sample glomeruli for measurement, it is termed the disector/Cavalieri method [10]. Another commonly used method, the disector/fractionator stereological procedure, provides estimates of both nephron number (Nglom) and mean glomerular volume (Vglom) [18–20].
We have published several reports of human Vglom using the disector/fractionator method [14,17,21] as well as reports of human IVglom using the disector/Cavalieri method [16,22,23]. In these reports, the average IVglom per kidney differs from Vglom by ~ 40% with average IVglom being the smaller volume. In this current study, we use a subset of the larger group of kidneys analyzed by the disector/fractionator method that have also had IVglom measured. We consider how different methods of sampling and volume estimation might influence glomerular size estimates and therefore the definition of glomerulomegaly. We address whether the different methods alter the relationships between glomerular volume and Nglom, body mass index (BMI), hypertension and glomerulosclerosis. We additionally consider race as a predictor of glomerular size.
Materials and methods
Specimen and subject data collection
This research used human autopsy tissue. The study was approved by the Institutional Review Board of the University of Mississippi Medical Center as well as the Human Research Ethics Committee of Monash University. Permission for autopsy was obtained from next of kin.
The right kidneys were collected at autopsy at the University of Mississippi Medical Center (Jackson, MS, USA) from persons without known renal disease between the years 1998 and 2005. If both kidneys were grossly normal and equal or approximately equal in size, the right kidney was perfusion-fixed with 10% buffered formalin. The right renal artery or arteries were dissected and cut from beside the aorta. The freshly dissected kidneys were perfused through the renal arteries using a 14G intravenous catheter (18G for small arteries) from a reservoir 125 cm above the workbench at an estimated pressure of 90 mmHg. The kidneys were perfused until clear formaldehyde flowed from the renal veins.
Kidneys showing arteriolonephrosclerosis were included in the study if the subcapsular cortex was slightly granular. In order not to have glomerular number unduly reduced by severe renal scarring and advanced glomerulosclerosis, the kidneys were not collected if there were coarse pits or angular or depressed cortical scars. The kidneys from patients with diabetes were analyzed if they showed arteriolonephrosclerosis, but because of invariably advanced glomerular loss, the kidneys were not analyzed if there was a diffuse diabetic glomerulosclerosis with Kimmelstiel–Wilson nodules.
Excluded were cases of rheumatic or congenital heart disease (bicuspid aortic valves), hereditary hypertrophic cardiomyopathy, dilated cardiomyopathy and cor pulmonale, because normal cardiac function may not have been present, and elevated heart weights as a diagnostic feature of hypertension would be increased independent of systemic blood pressure.
Seventy-three adult kidneys, all from males, were analyzed for Nglom and Vglom and also had IVglom measured. The study cohort consisted of 39 African Americans and 34 whites 18–69 years of age. The causes of death were coronary artery disease (CAD), 34%; cerebrovascular disease (CVD), 8%; pulmonary embolus, 12%; neoplastic, hematologic or infectious disease, 8%; pulmonary disease, 7%; accident, 12%; homicide, 11%; neurological disease not CVD, 3%; and unknown, 5%.
Blood pressures without medication were obtained from University of Mississippi Medical Center records on 26 African Americans and 18 whites. Blood pressures from terminal hospital admissions were not used unless patients were diagnosed as hypertensive and blood pressures were elevated. For patients classified as non-hypertensive, blood pressures were obtained within 3 years of the date of death. Mean arterial blood pressure (MAP) was calculated from an average of at least three blood pressure determinations as diastolic blood pressure plus one-third of the difference between diastolic and systolic blood pressure.
The patients were categorized on the basis of a history of hypertension, consistently elevated blood pressure (≥ 140/90 mmHg), the presence of cardiomegaly, and the severity of renal arteriolosclerosis as follows: Group 1, non-hypertensive: no history of hypertension, blood pressures not elevated and MAP ≤ 106 mmHg; Group 2, probably non-hypertensive: no history of hypertension but no available blood pressure record and three out of four of the following: left ventricular wall thickness ≤ 1.5 cm, heart weight ≤ 520 g (≤ 75% of non-hypertensive, Group 1 subjects), and/or a heart to body weight ratio ≤ 5.24 g/kg (≤ 75% of non-hypertensive, Group 1 subjects); Group 3, probably hypertensive: history of hypertension but no available blood pressure record and left ventricular wall thickness > 1.5 cm, heart weight ≥ 450 g (≥ 25% of hypertensive, Group 4 subjects), and/or heart to body weight ratio ≥ 5.4 g/kg (≥ 25% of hypertensive, Group 4 subjects); and Group 4, hypertensive: history of hypertension, repeatedly elevated blood pressures and MAP ≥ 107 mmHg. For analysis, Group 1 and 2 were considered non-hypertensive, and Group 3 and 4 hypertensive. On this basis, 23 African Americans and 15 whites were classified as hypertensive, and 16 African Americans and 19 whites as non-hypertensive.
Measurement of Nglom and Vglom
Perfused kidneys were bisected and immersed in 10% formalin. After 10 days, both halves of the kidney were cut into slices 4 mm thick, and every fourth slice of both halves was sampled for stereology beginning with the first slice selected as a random number from 1 to 4. The selected slices were sent to Monash University where they were processed for embedding in glycolmethacrylate for the stereological estimation of Nglom and mean glomerular tuft volume (Vglom) using the physical disector/fractionator combination [18–20]. Glycolmethacrylate was chosen as the embedding medium in preference to paraffin to minimize tissue shrinkage. For each subject, the proportional difference between Vglom and average IVglom was calculated as [(Vglom − IVglom) ÷ Vglom] and the percentage difference as the proportional difference × 100%.
Estimation of IVglom
From the tissue slices remaining after sampling for Vglom and Nglom, a single block (10 × 10 × 1 mm) containing full thickness cortex from the medulla to the capsule from the mid-hilar region of each kidney was processed to glycolmethacrylate. The tissue block was serially sectioned at 10 μm using a Leica DM2165 Supercut rotary microtome, with every second section collected for analysis. Sections were stained with periodic acid–Schiff (PAS) and projected onto a table top grid (1 cm × 1 cm) using an Olympus BH-2 microscope with a projection arm at a final magnification of × 312.
The renal cortex was divided into three evenly spaced zones: outer (superficial glomeruli), mid and inner (juxtamedullary glomeruli) cortex. Outer cortical glomeruli were within three to four glomerular diameters of the capsule, while juxtamedullary glomeruli were within three to four glomerular diameters of the corticomedullary junction. The volume of 10 glomeruli in each zone (30 per subject) was measured using the disector/Cavalieri principle. To sample glomeruli with the disector, those glomeruli not present in the first section of the series were selected for measurement in the order that they first appeared in the serial sections. The area of each of the glomerular tuft profiles from the selected glomeruli was determined by point counting (using a 1-cm square grid). IVglom was obtained using the Cavalieri principle by multiplying the sum of the tuft areas for each individual glomerulus by section thickness and then by 2, as every second section was used. On average, 11 glomerular profiles (ranging from 8 to 21) were measured for each glomerulus. Only non-sclerotic glomeruli were measured.
Measurement of glomerulosclerosis
Representative kidney blocks from the upper pole and the mid-portion of the kidney not sampled for stereology were paraffin-embedded. These blocks were cut perpendicular to the cortical surface and contained the full thickness of the cortex and the underlying medulla. Sections were cut 4 µm in thickness and stained with H&E or PAS–hematoxylin. The percentage of sclerotic glomeruli was estimated by counting sclerosed and non-sclerosed glomeruli using PAS–hematoxylin-stained sections. Counts were made in non-overlapping × 100 microscopic fields moving from the subcapsular surface to inner cortex with at least 400 glomeruli being counted per subject.
Statistical methods
The data were collected into Microsoft Excel™ and analyzed with Stata (College Station, TX, USA) software. Linear or multivariate linear regression was used to evaluate the influence of age, race, BMI, hypertension and Nglom, and the interactions between race and hypertension and age and race (predictors) of Vglom and IVglom (outcomes). Differences between groups were analyzed using a t-test if data passed normality and equal variance tests and by a Mann–Whitney rank-sum test if they did not. Kolmogorov–Smirnov tests were applied to continuous variables to determine whether compared data were normally distributed. A skewness and kurtosis test was used to evaluate the normal distribution of datasets not being compared with each other. Discrete variables were compared by chi-square or Fisher exact tests. For all statistical procedures, a P-value < 0.05 was considered significant.
Results
Characteristics of subjects
Table 1 lists the clinical characteristics of subjects whose glomerular volumes were measured as Vglom by the disector/fractionator method and as IVglom by the disector/Cavalieri method. All subjects were male. There were somewhat more African Americans than whites and more hypertensive than non-hypertensive subjects. For both races, hypertensive subjects were older than non-hypertensives (both P < 0.001), and hypertensive whites were older than hypertensive African Americans (P = 0.03). There were proportionately more African Americans than whites who were hypertensive, and hypertensive African Americans tended to have higher MAP than hypertensive whites, but these racial differences were not significant (racial proportion of hypertension, P = 0.30; racial difference in MAP for hypertensive subjects, P = 0.09).
Table 1.
Clinical–pathological features of the study subjects
| African American |
White |
|||||
|---|---|---|---|---|---|---|
| Non-hypertension | Hypertension | P | Non-hypertension | Hypertension | P | |
| n = 16 | n = 23 | n = 19 | n = 15 | |||
| Age (years) | 33 (23–39) | 46 (39–51) | 0.001 | 32 (29–42) | 54 (40–67)* | 0.001 |
| Height (m) | 1.81 ± 0.10 | 1.80 ± 0.07 | 0.68 | 1.75 ± 0.08 | 1.80 ± 0.02 | 0.17 |
| BMI (kg/m2) | 29 ± 5 | 29 ± 7 | 0.80 | 31 ± 8 | 28 ± 9 | 0.26 |
| Heart weight (g) | 440 (379–505) | 590 (500–650) | 0.001 | 450 (375–560) | 530 (450–700) | 0.07 |
| Heart/body weight (g/kg) | 4.5 (4.3–5.1) | 5.8 (5.5–6.8) | 0.001 | 4.8 (4.1–5.3) | 6.3 (5.3–6.9) | 0.01 |
| Nglom | 1 063 829 ± 362 963 | 904 752 ± 343 951 | 0.17 | 948 400 (805 875–1 215 726) | 710 171 (564 164–905 102) | 0.046 |
| Vglom (μm3 × 106) | 7.64 ± 2.56 | 9.75 ± 3.30 | 0.04 | 7.33 ± 3.23 | 8.33 ± 2.34 | 0.32 |
| IVglom (μm3 × 106) | 4.47 ± 1.33 | 5.51 ± 1.35 | 0.02 | 4.02 ± 1.41 | 4.57 ± 1.51 | 0.28 |
| MAP mmHg | 93 ± 7 | 120 ± 9 | 0.001 | 95 (93–99) | 113 (111–114) | 0.01 |
| n = 11 | n = 15 | n = 13 | n = 5 | |||
| GS (%) | 0.64 (0–1.59) | 3.14 (1.29–5.86) | 0.001 | 0.99 (0.78–1.64) | 4.74 (1.83–6.67) | 0.01 |
| Birth weight (kg) | 3.64 ± 0.59 | 3.36 ± 0.16 | 0.27 | 3.31 ± 0.47 | 3.29 ± 0.08 | 0.95 |
| n = 11 | n = 14 | n = 14 | n = 2 | |||
Significant differences between races are indicated by an asterisk (*). Values are mean ± standard deviation for normally distributed data and median with 25th and 75th percentile for non-normally distributed data.
MAP, mean arterial blood pressure; GS, glomerulosclerosis.
*Age for hypertensive whites is greater than for hypertensive African Americans, P = 0.026.
There were no significant racial differences or differences between non-hypertensive and hypertensive subjects in height, BMI or birth weight. Hypertensive whites had significantly fewer glomeruli than non-hypertensive whites. Nglom for hypertensive African Americans was ~ 160 000 less than for non-hypertensives, but the difference was not significant (P = 0.17). Vglom measured by the disector/fractionator method and the average of IVglom measured by the disector/Cavalieri technique were significantly greater in hypertensive compared with non-hypertensive African Americans. Both measurements of average glomerular volume were larger among hypertensive compared with non-hypertensive whites but not significantly. For both races, hypertensive subjects had larger hearts, more glomerulosclerosis and higher MAP than non-hypertensives.
Comparison of Vglom and average IVglom
For all subjects, Vglom was 41 ± 16% greater than average IVglom with no significant difference by hypertension status or race (non-hypertensive by race, P = 0.67; hypertensive by race, P = 0.48). The difference between Vglom and IVglom increased as glomerular volume increased (Table 2). For smaller glomerular volumes, Vglom was 19–26% larger than average IVglom, but at the largest glomerular volumes, the difference was 55–60%.
Table 2.
Vglom, average IVglom and proportional differences between the values by centile rank of glomerular volume
| Centile | Vglom | IVglom | Difference |
|---|---|---|---|
| (m3 × 106) | (m3 × 106) | (95% CI) | |
| 10% | 4.69 | 2.89 | 0.19 (0.04–0.25) |
| 20% | 5.98 | 3.19 | 0.26 (0.21–0.33) |
| 30% | 6.70 | 3.83 | 0.33 (0.26–0.41) |
| 40% | 7.15 | 4.12 | 0.41 (0.33–0.44) |
| 50% | 7.68 | 4.57 | 0.43 (0.41–0.48) |
| 60% | 8.54 | 5.03 | 0.47 (0.43–0.51) |
| 70% | 8.97 | 5.38 | 0.51 (0.47–0.55) |
| 80% | 10.95 | 5.85 | 0.55 (0.51–0.59) |
| 90% | 13.10 | 6.81 | 0.60 (0.56–0.64) |
Definition of glomerulomegaly and associations with body mass index, Nglom, hypertension and glomerulosclerosis
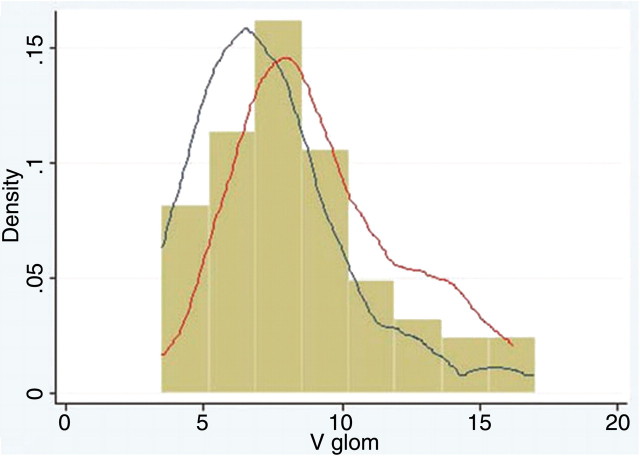
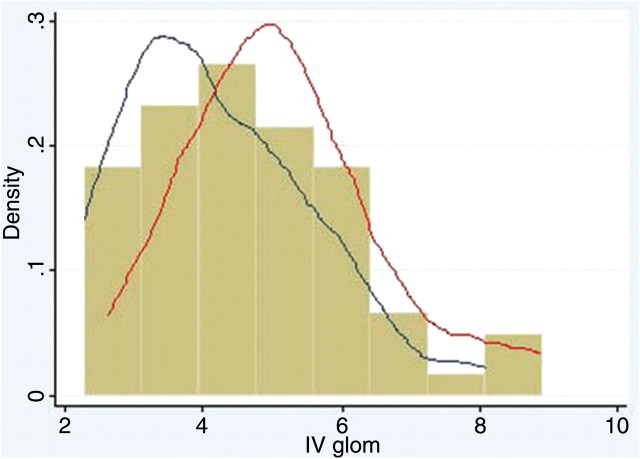
For all subjects, mean Vglom was 8.37 ± 3.06 μm3 × 106, and mean IVglom was 4.70 ± 1.49 μm3 × 106. Histograms of the distributions of Vglom and IVglom are shown in Figures 1 and 2. The median and centile ranks of Vglom and IVglom are shown in Table 2 with the 70th and 90th centile being 8.97 and 13.10 μm3 × 106 for Vglom and 5.38 and 6.81 μm3 × 106 for IVglom.
Fig. 1.
Histogram of Vglom (m3 × 106) for African American and white subjects with kernel density plots of non-hypertensive (blue line) and hypertensive subjects (red line). The volume estimates are skewed towards larger glomerular size.
Fig. 2.
Histogram of IVglom (m3 × 106) for African American and white subjects with kernel density plots of non-hypertensive (blue line) and hypertensive subjects (red line). The volume estimates are more closely clustered around the median than in Figure 1 but are still skewed towards larger glomerular size.
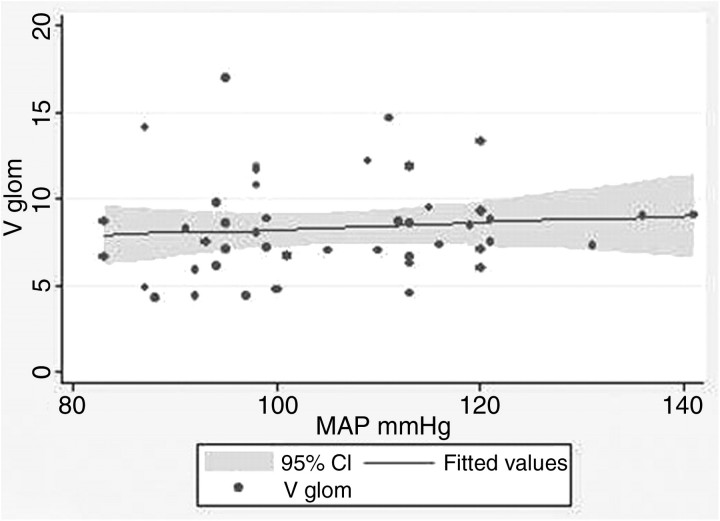
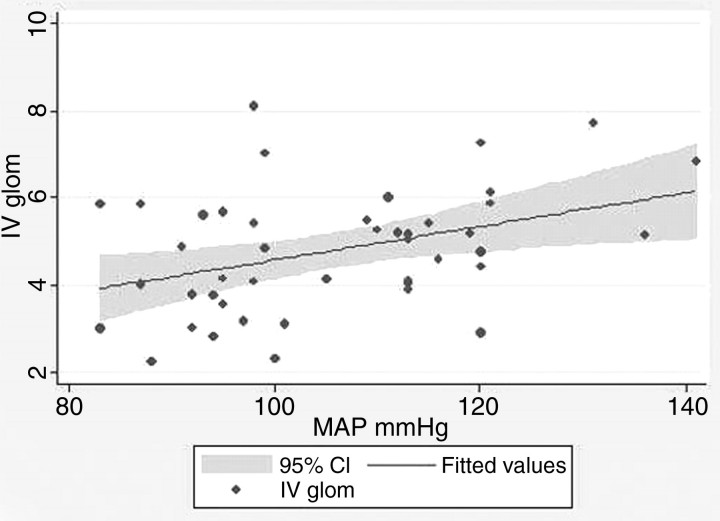
In the relationships between MAP with Vglom and IVglom, there was considerable variation around regression lines, and there was not a level of glomerular volume that separated hypertension from non-hypertension (Figures 3 and 4). Nevertheless, in linear regression, every 3.99-mmHg increase in MAP above 86.8 mmHg predicted a 1.0-μm3 × 106 increase in IVglom (adjusted r2 = 0.130, P = 0.01, α = 0.05:0.743). The correlation between MAP and Vglom was not significant among the 73 subjects in this study (adjusted r2 = − 0.014, P = 0.52, α = 0.05:0.093), but we have 152 subjects in our entire study population who have Nglom and Vglom estimates and a determination of MAP [23]. For these subjects, every 1.0-μm3 × 106 increase in Vglom predicted a 2.01-mmHg increase in MAP above 87.9 mmHg (adjusted r2 = 0.098, P = 0.001, α = 0.05:0.983), a relationship very similar to that obtained with IVglom.
Fig. 3.
Vglom (m3 × 106) plotted against MAP. There is no significant relationship between the two variables.
Fig. 4.
IVglom (m3 × 106) plotted against MAP. There is a significant direct relationship between the two variables (adjusted r2 = 0.130, P = 0.01). A MAP = 107 mmHg is hypertensive, and there is no level of IVglom that separates hypertensive from non-hypertensive subjects.
Multivariate regression showed that BMI, Nglom and hypertension contributed significantly to both estimates of glomerular volume. Nglom made the greatest contribution to Vglom with BMI and hypertension each having lesser and nearly equal influences (Table 3). For IVglom, BMI, Nglom and hypertension made somewhat equal contributions to glomerular size.
Table 3.
Multivariate analysis showing the contributions of BMI, Nglom, hypertension and glomerulosclerosis (GS) to Vglom and IVglom
| Vglom (m3 × 106) |
IVglom (m3 × 106) |
|||||
|---|---|---|---|---|---|---|
| Coefficient | P | SS marginal | Coefficient | P | SS marginal | |
| BMI (kg/m2) | 0.096 | 0.02 | 41.6 (17.4%) | 0.048 | 0.02 | 10.4 (21.5%) |
| Nglom | − 4.42 × 10 − 6 | 0.001 | 150.0 (62.8%) | − 1.52 × 10 − 6 | 0.01 | 16.5 (34.1%) |
| Hypertension | 1.71 | 0.01 | 31.6 (13.2%) | 1.039 | 0.01 | 16.1 (33.3%) |
| GS | − 0.115 | 0.13 | 15.8 (6.6%) | − 0.068 | 0.08 | 5.4 (11.2%) |
| Model adjusted | Model adjusted | |||||
| r2 = 0.282,P < 0.001 | r2 = 0.216,P = 0.001 | |||||
Hypertension is assigned a value of 1 and non-hypertension a value of 0.
There was no significant relationship between either measurement of glomerular volume and glomerulosclerosis. Although the relationships approached significance for both Vglom and IVglom, the coefficients were negative indicating a reduction rather than an increase in glomerular volume as glomerulosclerosis increased. The histopathology of all glomerulosclerosis encountered in these cases was characterized by glomerular obsolescence. Focal segmental glomerulosclerosis and glomerular solidification that might have indicated hyperperfusion injury were not seen.
Associations of Vglom and IVglom with race and hypertension
The average IVglom but not Vglom of African Americans was significantly larger than whites (IVglom, African Americans 5.08 ± 1.42 μm3 × 106, whites 4.27 ± 1.46 μm3 × 106, P = 0.02; Vglom, African Americans 8.89 ± 3.16 μm3 × 106, whites 7.78 ± 2.87 μm3 × 106, P = 0.12). With Vglom and IVglom as dependent variables and hypertension and race and the race–hypertension interactions as independent variables, hypertension in multivariate regression showed similar weak but not significant direct relationships to both glomerular volume measurements (Table 4). Neither race nor the racial interaction with hypertension contributed significantly to Vglom or IVglom. When Vglom and IVglom are analyzed with race, age and the race–age interaction as independent variables, a direct relationship between IVglom and race and an inverse association between IVglom and the age–race interaction approached but did not achieve significance (Table 4).
Table 4.
Relationships of hypertension, race and the hypertension–race interaction, and age, race and the age–race interaction with Vglom and IVglom
| Vglom (m3 × 106) | IVglom (m3 × 106) | |
|---|---|---|
| Hypertension | 3.230 (P = 0.14) | 1.525 (P = 0.14) |
| Race | − 0.306 (P = 0.76) | − 0.449 (P = 0.35) |
| Interaction | − 1.116 (P = 0.43) | − 0.487 (P = 0.46) |
| Model adjusted r2 | 0.069 (P = 0.047) | 0.120 (P = 0.01) |
| Age (years) | 0.120 (P = 0.21) | 0.082 (P = 0.28) |
| Race | 0.606 (P = 0.91) | 1.262 (P = 0.07) |
| Interaction | − 0.046 (P = 0.42) | − 0.050 (P = 0.07) |
| Model adjusted r2 | 0.066 (P = 0.12) | 0.084 (P = 0.03) |
Hypertension is assigned a value of 1 and non-hypertension a value of 0. African American race is assigned a value of 1 and white race a value of 0.
Discussion
This study showed that estimates of mean glomerular volume of a kidney as determined by the disector/fractionator technique and expressed as Vglom, or obtained as the arithmetic mean of a sample of glomeruli measured by the Cavalieri principle and expressed as IVglom, were considerably different and that the difference increased with increasing glomerular volume. Nevertheless, despite the differences, Vglom and IVglom had similar relationships to factors significantly associated with changes in glomerular volume. These factors were an inverse correlation with glomerular number and direct correlations with BMI and hypertension.
With respect to glomerulosclerosis, we found no evidence that adult glomerular loss leads to enlargement of the remaining glomeruli. Our experimental design excluded the collection of kidneys if they demonstrated any more than moderate arteriolonephrosclerosis with the average amount of glomerulosclerosis in the kidneys of hypertensive subjects being < 5%. Within those parameters, the correlation between glomerulosclerosis and glomerular volume approached significance, but the regression coefficients for both Vglom and IVglom were negative, indicating that glomerular size tended to decrease as glomerulosclerosis increased. The study showed no morphologic evidence of hyperperfusion injury, but rather, the pattern of glomerulosclerosis seen in all kidneys with any degree of arteriolonephrosclerosis was glomerular obsolescence which has been attributed to decreased rather than increased glomerular perfusion [24]. The inverse relationship between both Vglom and IVglom and glomerulosclerosis could be the result of the retraction of glomerular tufts that is seen early in the development of obsolescence [24].
With regard to race, African Americans had somewhat larger glomeruli than whites, and among all subjects, the racial difference between IVglom, although not Vglom, was significant. This difference may be related to a larger number of African Americans having hypertension at a younger age than whites, but with the number of subjects in the study, the interactions of age and hypertension with race showed no significant effect on glomerular volume by either method of measurement that could be related to race.
It should be expected that measurements of glomerular size would vary depending on the method of sampling and measurement and that with different methods the variation might be considerable. In the present study, the two methods used to estimate glomerular volume have numerous differences [18–20]. In short, with the disector/Cavalieri principle the cross-sectional areas (A) of glomeruli sampled with the disector are measured and summed in serial sections cut completely through a glomerulus using the equation V = ΣAn × t × f, where n is the number of sections, t is section thickness and f is the inverse of the section sampling fraction (i.e. every second section). Because each glomerular profile is independently measured, this technique makes no assumptions about the shape of the glomerulus, and within a kidney, each glomerulus is measured separately from every other glomerulus. A shortcoming with the disector/Cavalieri method is that the requirement for serial sectioning makes it very time-consuming and therefore allows volumes to be estimated for only a small number of glomeruli.
The disector/fractionator method determines glomerular volume by an indirect method. After several sampling and subsampling steps, a known and three-dimensionally representative fraction of the kidney cortex is embedded and then serially sectioned [18–20]. A known fraction (one-tenth) of the sections and an adjacent ‘lookup’ section are collected. The disector method is used to estimate Nglom, and at the same time, grid points overlying glomerular profiles and cortex are counted [10,18]. Vglom is calculated by dividing the volume density of glomeruli in cortex (VVglom,cort) by the numerical density of glomeruli in cortex (Nvglom,cort). The average volume thereby obtained provides a single mean for the kidney but provides no information about the variation in glomerular volumes within a kidney.
The two methods have notable differences in the manner in which glomeruli are sampled. These differences include sample location, sample distribution and sample size: (i) sample location: for IVglom, glomeruli were sampled from the mid-hilar region only, whereas for Vglom, glomeruli were sampled throughout the cortex; (ii) sample distribution: for IVglom, 10 glomeruli were measured from each of the outer, mid and inner cortex. These thirds were based on cortical thickness in sections and are not equivalent to volumetric thirds. As a consequence, inner glomeruli have been oversampled compared with outer glomeruli which were undersampled. In regard to sample distribution, we have not found that there is any significant difference in glomerular volume in the different cortical zones of the human kidney [16]; (iii) sample size: for IVglom, 30 glomeruli were measured per kidney. For Vglom, a mean value is obtained following point counting on hundreds of glomeruli per kidney.
Measurements by any method will be affected by several factors that include section thickness and the formulae used to calculate volume. It is well known that overprojection occurs with thicker sections [25]. Our estimates of Vglom were obtained using 20-μm sections, whereas IVglom was estimated using 10-μm sections. If a sphere is sectioned at a very thin thickness, the radius of each circular disc of the sphere would be the ideal measurement. If each section was cut at 10 or 20 μm, each circular plane would overproject to the longest radius of the thicker discs producing a somewhat larger estimate of spherical volume.
The phenomenon of overprojection is known as the Holmes effect [25,26]. A correction factor can be applied to account for section thickness using the formula Kt (Vv) = 1 ÷ 1/[1 + 3t/d/2]. In a sphere 250 μm in diameter, the correction factor for a 10-μm section would be 0.94, and for a 20-μm section 0.89, a 5% difference. The correction factor is largest when structures are small with corrections being most significant when the diameter of a measured structure is less than 12 times the thickness of the section [25]. This is clearly the opposite of what we are seeing between Vglom and IVglom in which differences increase as glomeruli become larger.
Nevertheless, the fact that section thickness is important can be seen by comparing the study of Nglom and Vglom by Keller et al. [27] in hypertensive and non-hypertensive Europeans with our current results. Using the disector/fractionator method with sections cut at 3 μm, they found the Vglom of 10 hypertensive subjects to be 6.65 m3 × 106, a volume 22% smaller than the Vglom but 30% larger than the IVglom obtained in the present study of our hypertensive whites. Unlike spheres, glomeruli do not have discrete boundaries but rather have ill-defined outlines of varying opacity. Section thickness appears to contribute to Vglom and possibly IVglom estimates in a manner that is not entirely accounted for by formulae for the Holmes effect [25]. Because Vglom is calculated from the volume density (VVglom,cort) of hundreds of glomerular profiles compared with 30 for IVglom, the overprojection is likely to have a greater effect on Vglom estimates.
Since Vglom is a derived value in the disector/fractionator method, it could be overestimated if there were a systematic undercounting of glomeruli. For quality control, we recount the numerical density of glomeruli (Nvglom,cort) in every 10th kidney. The coefficient of variation is 8% with the count as likely to be overestimated as underestimated. Compared with the study of Keller et al. [27], our hypertensive white subjects had Nglom determinations that were very similar to the hypertensive Europeans making Nglom an unlikely factor in the differences in Vglom found in the two studies.
The findings emphasize that when the literature citing glomerular volumes is examined, results may not be comparable, and the details regarding technical methods must be considered. The findings also indicate that when applied to a group of kidneys for comparative purpose, one method is not superior to the other if that method is uniformly applied to the study set.
In an attempt to arrive at a definition of glomerulomegaly, the mean IVglom was found to be 4.70 ± 1.49 μm3 × 106 and the mean Vglom 8.37 ± 1.49 μm3 × 106 with the 90th percentile being 6.81 and 13.10 μm3 × 106, respectively. While we were able to delineate a deviation of glomerular volume from the average and could demonstrate a direct correlation between glomerular volume and blood pressure, there was considerable variation in glomerular volume around the average, and a size that separated hypertension from non-hypertension could not be identified.
While this study cannot establish a glomerular volume that is harmful to the kidney, it does show that hypertension, obesity and lower glomerular number are all associated with larger glomeruli. Obesity and hypertension have been closely linked epidemiologically [28]. While the relationship between glomerular number and hypertension has not been demonstrated with any clarity, it seems intuitive that there should be a lower level of glomerular number and, hence, an upper level of glomerular size that makes the kidney susceptible to injury. As studies in this area of research proceed, investigators should be aware of the differences in glomerular volume estimates that the applied methods produce.
Acknowledgments
This research was funded by grants from the National Institutes of Health (NIH 1 RO1 DK065970-01), the NIH Center of Excellence in Minority Health (5P20M000534-02), the National Health and Medical Research Council of Australia (NHMRC) and the American Heart Association (Southeastern Affiliate).
Conflict of interest statement. None declared.
References
- 1.Fogo A, Ichikawa I. Evidence for a pathogenic link between glomerular hypertrophy and sclerosis. Am J Kidney Dis. 1991;17:666–669. doi: 10.1016/s0272-6386(12)80347-7. [DOI] [PubMed] [Google Scholar]
- 2.Fogo A, Hawkins EP, Berry PL, et al. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerulosclerosis. Kidney Int. 1990;38:115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- 3.Broyer M, Soto B, Gagnadoux M-F, et al. Oligomeganephronic renal hypoplasia. Adv Nephrol. 1997;26:47–63. [PubMed] [Google Scholar]
- 4.Pesce CM, Schmidt K, Fogo A, et al. Glomerular size and the incidence of renal disease in African Americans and Caucasians. J Nephrol. 1994;7:355–358. [Google Scholar]
- 5.Schmidt K, Pesce C, Liu QL, et al. Large glomerular size in Pima Indians: lack of change with diabetic nephropathy. J Am Soc Nephrol. 1992;3:229–235. doi: 10.1681/ASN.V32229. [DOI] [PubMed] [Google Scholar]
- 6.Abdi R, Slakey D, Kittur D, et al. Heterogeneity of glomerular size in normal donor kidneys. Impact of race. Am J Kidney Dis. 1998;32:43–46. doi: 10.1053/ajkd.1998.v32.pm9669422. [DOI] [PubMed] [Google Scholar]
- 7.Bertram JF, Young RJ, Seymour AE, et al. Glomerulomegaly in Australian Aborigines. Nephrology. 1998;4:S46–S53. [Google Scholar]
- 8.Adelman RD, Restaino IG, Alon US, et al. Proteinuria and focal segmental glomerulosclerosis in severely obese adolescents. J Pediatr. 2001;138:481–485. doi: 10.1067/mpd.2001.113006. [DOI] [PubMed] [Google Scholar]
- 9.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 10.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the dissector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 11.Praga M, Hernandez E, Morales E, et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 12.Cortes P, Riser B, Narins RG. Glomerular hypertension and progressive renal disease: the interplay of mesangial stretch, cytokine formation and extracellular matrix synthesis. Contrib Nephrol. 1996;188:229–233. doi: 10.1159/000425098. [DOI] [PubMed] [Google Scholar]
- 13.Macconi D, Sangalli F, Bonomelli m, et al. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol. 2009;174:797–807. doi: 10.2353/ajpath.2009.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughson M, Farris AB, Douglas-Denton RN, et al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 15.Merlet-Benichou C, Gilbert T, Vilar J, et al. Nephron number: variability is the rule. Lab Invest. 1999;79:515–527. [PubMed] [Google Scholar]
- 16.Samuel T, Hoy WE, Douglas-Denton R, et al. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005;16:3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- 17.Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 18.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–172. doi: 10.1016/s0074-7696(08)62497-3. [DOI] [PubMed] [Google Scholar]
- 19.Nyengaard JR. Stereologic methods and their application in kidney research. Estimating total glomerular number in human kidneys with a physical dissector/fractionator combination. Image Anal Stereol. 2000;19:105–108. [Google Scholar]
- 20.Johnson KJ, Wreford NG, Hoy WE, et al. Estimating total glomerular number in human kidneys with a dissector/fractionator combination. Image Anal Stereol. 2000;19:105–108. [Google Scholar]
- 21.McNamara BJ, Diouf B, Hughson MD, et al. Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant. 2008;23:2576–2585. doi: 10.1093/ndt/gfn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimanyi MA, Hoy WE, Douglas-Denton RN, et al. Nephron number and individual glomerular volumes in male Caucasians and African American subjects. Nephrol Dial Transplant. 2009;24:2428–2433. doi: 10.1093/ndt/gfp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara BJ, Diouf B, Hughson MD, et al. Associations between age, body size, and nephron number with individual glomerular volumes in urban West African males. Nephrol Dial Transplant. 2009;24:1500–1506. doi: 10.1093/ndt/gfn636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughson MD. End-stage renal disease. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. 6th edn. Philadelphia, PA: Williams & Wilkins; 2006. p. 1000. [Google Scholar]
- 25.Slomianka L. School of Anatomy and Human Biology—The University of Western Australia; Blue Histology—a brief introduction to stereology. www.lab.anhb.uaw.edu.au. [Google Scholar]
- 26.Aherne WA, Dunnill MS. London: Edward Arnold; 1982. Morphometry; p. 205. [Google Scholar]
- 27.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 28.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]