Abstract
Background. Urinary neutrophil gelatinase-associated lipocalin (uNGAL) is expressed by kidney tubules that are acutely damaged, but few studies have investigated the association of neutrophil gelatinase-associated lipocalin (NGAL) with different forms of chronic kidney disease (CKD). HIV-associated nephropathy (HIVAN) is a progressive form of CKD characterized by collapsing focal segmental glomerulosclerosis and microcytic tubular dilatation that typically leads to end-stage renal disease (ESRD).
Methods. Previously, we reported that microcystic tubular dilatations specifically expressed NGAL RNA, implying that the detection of uNGAL protein could mark advanced HIVAN. To test this idea, we performed a comparative study of diverse proteinuric glomerulopathies in 25 patients who were HIV positive.
Results. Eighteen patients had HIVAN and seven had other glomerulopathies (four membranoproliferative glomerulonephritis, one membranous glomerulonephritis, one amyloid and one malarial GN). HIVAN and non-HIVAN patients did not differ with respect to age, ethnicity, serum creatinine, estimated GFR, proteinuria or the prevalence of hypocomplementemia (6 versus 29%, P = 0.18), but HIVAN patients were less likely to have HCV infections. HIVAN patients expressed 4-fold higher levels of uNGAL than the patients with other glomerulopathies [387 ± 338 versus 94 ± 101 μg/g urine creatinine (uCr), P = 0.02]. A cutpoint of 121.5 μg uNGAL/g uCr demonstrated 94% sensitivity and 71% specificity for the diagnosis of HIVAN, with an area under the receiver operator characteristic curve of 0.88.
Conclusion. In summary, while HIVAN disease is currently diagnosed only by kidney biopsy, uNGAL can distinguish HIVAN from other proteinuric glomerulopathies in the HIV-infected patient, likely because of its specific expression from characteristic microcysts.
Keywords: biomarker, HIV-associated nephropathy, progressive chronic kidney disease, tubular injury, urinary neutrophil gelatinase-associated lipocalin
Introduction
HIV-associated nephropathy (HIVAN) remains the most common and aggressive cause of kidney disease among HIV-positive patients, particularly in those of African descent [1–4]. HIVAN is characterized by heavy proteinuria and a rapid loss of kidney function and is the third leading cause of end-stage renal disease (ESRD) among black patients between the ages of 20 and 64 years [5, 6]. Renal biopsy is currently the only method of diagnosing HIVAN, yet renal biopsy is impractical in many countries where HIVAN is prevalent, and the procedure is not without risks [1, 7–13]. Here, we investigate a potential noninvasive surrogate marker of HIVAN in proteinuric HIV-infected patients.
Urinary neutrophil gelatinase-associated lipocalin (uNGAL) is secreted from the tubular portion of the nephron as a full-length protein just 3 h after the onset of an acute stimulus that damages the nephron [14, 15]. Subsequently, uNGAL resolves over a number of days when the stimulus is withdrawn [14, 16]. Hence, while the association of uNGAL and acute damage is clear, it has not been appreciated whether uNGAL is also expressed by the chronically damaged kidney. In fact, we found that uNGAL was expressed only at low levels in most types of chronic kidney disease (CKD) with the notable exception of HIVAN where tubular microcysts expressed the protein [16–22]. These data implied that among different forms of CKD, uNGAL may be a unique marker of HIVAN.
Methodology
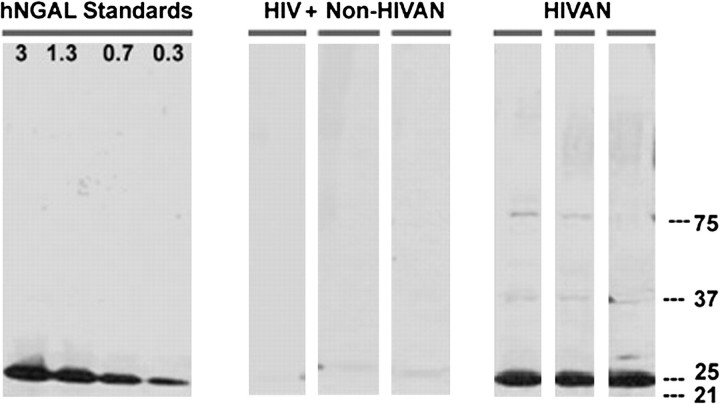
Twenty-five HIV-positive patients who underwent renal biopsy and donated urine at the time of the biopsy were compared with age-, race- and gender-matched HIV-negative control subjects. Renal biopsies were processed at CUMC for light microscopy, immunofluorescence and electron microscopy. Urine samples were stored at −80°C until uNGAL (10 μL) was quantified by immunoblots with non-reducing 4%–15% gradient polyacrylamide gels (Bio-Rad Laboratories) using standards (0.3–3 ng) of human recombinant neutrophil gelatinase-associated lipocalin (NGAL) protein (Figure 1) [16]. Immunoblots were used to detect and quantify the monomeric form of NGAL, which is necessary since some CKD patients express complexes of NGAL linked to other proteins, whereas only the monomeric form has been validated epidemiologically in association with renal disease (Nickolas and Barasch, unpublished data). Urinary liver fatty acid-binding protein (uL-FABP) was measured using a sandwich-type ELISA kit (CMIC Co., Ltd). Urinary N-acetyl-β-d-glucosaminidase (uNAG) was measured using a colorimetric assay kit (Roche Applied Sciences). Investigators were blinded while performing the assays.
Fig. 1.
Immunoblots showing monomeric uNGAL expression in HIV patients. Three HIVAN patients and three HIV-positive patients with other glomerulonephritis’ representative of our cohort are shown. Human recombinant NGAL standards (ng) are shown.
The data are represented both as a concentration and as the absolute values corrected for urinary creatinine. Both measurements are reported for the following reason: a normalized ratio can be misleading in cases of acute renal failure because of rapid decline in the creatinine excretion which would amplify the urinary biomarker signal [23]. However, in patients with CKD, the rate of change of GFR is much slower such that production and excretion of creatinine approximate one another until ESRD is reached. Hence, we report both the absolute biomarker level normalized to urinary creatinine as well as its urinary concentration. Statistical analysis was performed with Stata 10.1.
Results
Our cohort consisted of 18 patients with HIVAN, 4 patients with membranoproliferative glomerulonephritis, 1 with membranous glomerulopathy, 1 with malarial glomerulonephritis and 1 with renal AA amyloidosis. Baseline characteristics of our cohort are presented in Table 1.
Table 1.
Baseline characteristics of the cohorta
| Characteristic | HIV-negative controls | Non-HIVAN | HIVAN | P-valueb |
| N | 24 | 7 | 18 | |
| Age (years) | 43.7 ± 11.1 | 44.8 ± 12.3 | 43.5 ± 10.4 | 0.8 |
| Black (%) | 88 | 86 | 89 | 0.8 |
| BUN (mg/dL) | 11.1 ± 3.8 | 40.5 ± 25.7 | 43.6 ± 24.2 | 0.8 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 2.8 ± 1.6 | 4.1 ± 2.6 | 0.2 |
| eGFR (MDRD) mL/min | 110.8 ± 18 | 46.6 ± 35.8 | 31.5 ± 26.7 | 0.3 |
| Urine protein (g/24 h) | N/A | 7.9 ± 8 | 9.4 ± 7.8 | 0.7 |
| Urine protein (g/g of creatinine) | N/A | 5.5 ± 4.1 | 10.6 ± 7.7 | 0.14 |
| Serum albumin (g/dL) | 4.1 ± 0.5 | 2.5 ± 0.9 | 2.4 ± 0.8 | 0.7 |
| Serum cholesterol (mg/dL) | N/A | 218 ± 99 | 211 ± 99 | 0.9 |
| uNGAL (ng/mL) | 20.1 ± 21.0 | 38.6 ± 22.7 | 191.7 ± 186.9 | 0.04 |
| uNGAL (μg/g of creatinine) | 30.6 ± 39.2 | 94 ± 100.7 | 387.2 ± 339.7 | 0.04 |
| uL-FABP (ng/mL) | 13.9 ± 27.8 | 59.2 ± 27.3 | 169.5 ± 105.5 | 0.01 |
| uL-FABP (μg/g of creatinine) | 14.8 ± 27.7 | 148.9 ± 153.2 | 343.0 ± 186.5 | 0.02 |
| uNAG (mmol/L) | N/A | 5.18 ± 4.19 | 7.1 ± 8.6 | 0.59 |
| uNAG (mmol/g of creatinine) | N/A | 9 ± 7 | 12 ± 8 | 0.41 |
N/A, not applicable.
P-values are for a comparison of HIV patients with and without HIVAN.
Patients with HIVAN and non-HIVAN glomerulopathies were comparable with respect to age, renal function [BUN, serum creatinine (sCr) and estimated GFR (eGFR)], nutritional status (serum albumin and cholesterol), proteinuria and complement levels. In addition, CD4 counts, HCV coinfection and use of highly active antiretroviral therapy did not differ between the groups (Table 1).
Compared to HIV-positive patients with non-HIVAN glomerulopathies, patients with HIVAN had significantly elevated levels of uNGAL as well as uL-FABP, a second indicator of tubular injury. However, there was no difference in uNAG levels between the two groups of patients (Table 1). We also found significantly higher levels of uNGAL and uL-FABP in HIVAN patients compared to our HIV-negative controls.
A significant correlation between uNGAL (μg/g) and uL-FABP (μg/g) but not between these two biomarkers and uNAG (μg/g) was present in our cohort of 25 HIV-positive patients (see table 2). Neither uNGAL nor uL-FABP correlated with sCr, eGFR (MDRD) BUN or proteinuria (see table 2). uNGAL levels were not influenced by the use of HAART (not on HAART 246 ± 285 versus on HAART 407 ± 309, P = 0.45), hypocomplementemia (108 ± 98 versus 297 ± 322, P = 0.28) or HCV infection (159 ± 128 versus 236 ± 312, P = 0.65) and did not show any correlation with CD4 counts (−0.004, P = 0.99). Among HIVAN patients, the percentage of collapsed glomeruli correlated with the sCr and 24-h proteinuria (r = 0.49, P = 0.01; r = 0.48, P = 0.03, respectively) but not with either uNGAL (r = 0.29, P = 0.15) or with uL-FABP (r = 0.1, P = 0.64).
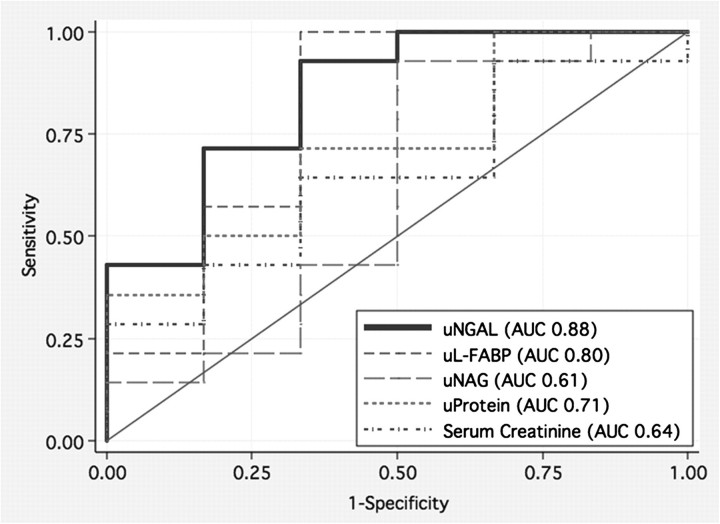
The receiver operator characteristic (ROC) curve for uNGAL (μg/g of uCr) had an area under the curve (AUC) of 0.88 (Figure 2). The concentration of uNGAL with the highest Youden index was 121.5 μg/g uCr, which had a sensitivity of 94% and specificity of 71% (Table 3) for the detection of HIVAN. At a cutpoint of 121.5 μg/g uCr, 88% of cases were correctly classified with a positive likelihood ratio of 3.3 and a positive predictive value of 89%. The ROC curve for uL-FABP (μg/g uCr) had an AUC of 0.80, and the threshold of 116 μg/g uCr had similar sensitivity and specificity (χ2= 1.74, P = 0.2; Figure 2 and Table 3). By contrast, the discriminatory ability to distinguish HIVAN from other glomerulopathies was significantly worse for uNAG and the more traditional markers such as sCr and proteinuria (Figure 2 and Table 4).
Fig. 2.
ROC curves demonstrating the utility of uNGAL to diagnose HIVAN among HIV-positive patients with proteinuria compared to other markers.
Table 3.
Urine NGAL and urine L-FABP test ROC curve characteristicsa
| uNGAL (μg/g) | uL-FABP (μg/g) | ||||||||
| 73.8 | 121.5 | 144.4 | 182 | 320 | 55.8 | 116.2 | 312.5 | 589.8 | |
| Sensitivity (%) | 100 | 94.4 | 77.8 | 66.7 | 50 | 100 | 94.4 | 55.6 | 22 |
| Specificity (%) | 57.1 | 71.4 | 85.7 | 85.7 | 86 | 28.6 | 71.4 | 85.7 | 100 |
| Youden index | 0.57 | 0.66 | 0.63 | 0.52 | 0.44 | 0.29 | 0.66 | 0.41 | 0.22 |
| Correctly classified (%) | 88 | 88 | 80 | 72 | 60 | 80 | 88 | 64 | 44 |
| Positive likelihood ratio | 2.3 | 3.3 | 5.4 | 4.7 | 3.6 | 1.4 | 3.3 | 3.9 | NA |
| Negative likelihood ratio | NA | 0.08 | 0.3 | 0.4 | 0.6 | 0 | 0.08 | 0.5 | 0.78 |
| Positive predictive value (%) | 86 | 89 | 93 | 92 | 90 | 78 | 89 | 91 | 100 |
| Negative predictive value (%) | 100 | 82 | 60 | 50 | 40 | 100 | 82 | 43 | 33 |
NA, not applicable.
Table 4.
Comparison of the ROC curves for different markers of renal injurya
| Noninvasive marker | AUC | 95% confidence interval |
| uNGAL (μg/g of creatinine) | 0.88 | 0.71–1.00 |
| uL-FABP (μg/g of creatinine) | 0.80 | 0.57–1.00 |
| uNAG (mmol/g of creatinine) | 0.61 | 0.33–0.90 |
| Urinary protein (g/24 h) | 0.71 | 0.46–0.97 |
| Serum creatinine (mg/dL) | 0.64 | 0.37–0.91 |
χ2 = 14.57, P = 0.0057.
Discussion
Renal epithelium is a distinct compartment for HIV-1 infection and replication. The kidney responds by glomerular collapse, microcystic tubular dilatation and epithelial simplification [6, 24, 25]. In a mouse model of HIVAN, NGAL messenger RNA was 100-fold higher than in littermate controls, due to its specific expression by medullary tubules that had undergone microcystic dilitation [6]. Consistently, uNGAL was present in HIV-positive children and in patients with HIVAN [22, 26]. Thus, uNGAL appears to be robustly expressed in HIVAN more than in other forms of CKD.
The ROC curve for uNGAL indicated excellent diagnostic utility to detect HIVAN (Figure 2). Similarly, while the ROC curve was not as impressive for uL-FABP, it was not significantly different from uNGAL. Both markers are expressed by the tubular portion of the nephron upon HIV-related cystic changes in the collecting ducts or the thick ascending limb of Henle (uNGAL) [22] or upon tubulointerstitial damage of the proximal nephron (uL-FABP) [27–30]. These data stand in contrast to measures of glomerular function, such as eGFR and proteinuria which were less useful predictors of HIVAN (Figure 1; Table 4). Proteinuria versus eGFR and proteinuria versus collapsed glomeruli correlated, but neither proteinuria nor eGFR could distinguish HIVAN from other forms of proteinuric renal disease. Conversely, uNGAL concentrations failed to correlate with GFR. In summary, it appears that two markers of tubular damage more specifically detect disease progression than markers of glomerular filtration, but we do not have an explanation for the failure of uNAG, which is released following structural damage to the tubular cells, including subclinical injury related to tenofovir, to predict HIVAN [28, 31].
We conclude that HIVAN can be identified by two genes expressed in response to tubular rather than glomerular damage. uNGAL provides the best noninvasive clinical marker of HIVAN. Because our study was limited by the absence of long-term follow-up as well as the limited number of HIV-positive patients with non-HIVAN diagnoses, our data currently suggest two hypotheses for future testing: that uNGAL will be useful in parts of the world where HIVAN is prevalent but renal biopsy is not practical, and second that uNGAL predicts progression in cystic-HIV CKD but not other forms of CKD.
Acknowledgments
Funding. Supported by grants from the US National Institute of Diabetes and Digestive and Kidney Diseases (DK-55388 and DK-58872) and the March of Dimes for Birth Defects.
Additional funding to J.B. was provided by the Glomerular Center of Columbia University. D.A.S was funded by the Doris Duke Charitable Foundation. Columbia has licensed NGAL for use in kidney disease to Abbott Labs and to Biosite/Alere Corporation.
Conflict of interest statement. None declared.
References
- 1.Atta MG. Diagnosis and natural history of HIV-associated nephropathy. Adv Chronic Kidney Dis. 2010;17:52–58. doi: 10.1053/j.ackd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Nebuloni M, Barbiano di Belgiojoso G, Genderini A, et al. Glomerular lesions in HIV-positive patients: a 20-year biopsy experience from Northern Italy. Clin Nephrol. 2009;72:38–45. doi: 10.5414/cnp72038. [DOI] [PubMed] [Google Scholar]
- 3.Han TM, Naicker S, Ramdial PK, et al. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int. 2006;69:2243–2250. doi: 10.1038/sj.ki.5000339. [DOI] [PubMed] [Google Scholar]
- 4.Lucas GM, Lau B, Atta MG, et al. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197:1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winston JA, Burns GC, Klotman PE. The human immunodeficiency virus (HIV) epidemic and HIV-associated nephropathy. Semin Nephrol. 1998;18:373–377. [PubMed] [Google Scholar]
- 6.D'Agati V, Suh JI, Carbone L, et al. Pathology of HIV-associated nephropathy: a detailed morphologic and comparative study. Kidney Int. 1989;35:1358–1370. doi: 10.1038/ki.1989.135. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt CM, Klotman PE. HIV-1 and HIV-associated nephropathy 25 years later. Clin J Am Soc Nephrol. 2007;2(Suppl 1):S20–S24. doi: 10.2215/CJN.03561006. [DOI] [PubMed] [Google Scholar]
- 8.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 9.Winston JA, Klotman ME, Klotman PE. HIV-associated nephropathy is a late, not early, manifestation of HIV-1 infection. Kidney Int. 1999;55:1036–1040. doi: 10.1046/j.1523-1755.1999.0550031036.x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40:1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SD, Kimmel PL. Renal biopsy is necessary for the diagnosis of HIV-associated renal diseases. Nat Clin Pract Nephrol. 2009;5:22–23. doi: 10.1038/ncpneph0990. [DOI] [PubMed] [Google Scholar]
- 12.Ross MJ, Klotman PE. HIV-associated nephropathy. AIDS. 2004;18:1089–1099. doi: 10.1097/00002030-200405210-00002. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JT, Anderson HL, Jr, Markowitz GS, et al. Hepatitis C virus-associated glomerular disease in patients with human immunodeficiency virus coinfection. J Am Soc Nephrol. 1999;10:1566–1574. doi: 10.1681/ASN.V1071566. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Ott KM, Mori K, Li JY, et al. Dual Action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 15.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickolas TL, O'Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Babazono T, Murata H, et al. Clinical significance of urinary liver-type fatty acid-binding protein in patients with diabetic nephropathy. Diabetes Care. 2005;28:2038–2039. doi: 10.2337/diacare.28.8.2038. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo A, Sugaya T, Hikawa A, et al. Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: a multicenter trial. J Lab Clin Med. 2005;145:125–133. doi: 10.1016/j.lab.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju W, Eichinger F, Bitzer M, et al. Renal gene and protein expression signatures for prediction of kidney disease progression. Am J Pathol. 2009;174:2073–2085. doi: 10.2353/ajpath.2009.080888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paragas N, Nickolas TL, Wyatt C, et al. Urinary NGAL marks cystic disease in HIV-associated nephropathy. J Am Soc Nephrol. 2009;20:1687–1692. doi: 10.1681/ASN.2009010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–494. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marras D, Bruggeman LA, Gao F, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8:522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- 25.Rao TK, Filippone EJ, Nicastri AD, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 26.Soler-Garcia AA, Johnson D, Hathout Y, et al. Iron-related proteins: candidate urine biomarkers in childhood HIV-associated renal diseases. Clin J Am Soc Nephrol. 2009;4:763–771. doi: 10.2215/CJN.0200608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama T, Kamijo-Ikemori A, Sugaya T, et al. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol. 2009;174:2096–2106. doi: 10.2353/ajpath.2009.080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamijo A, Sugaya T, Hikawa A, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165:1243–1255. doi: 10.1016/S0002-9440(10)63384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding H, He Y, Li K, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol. 2007;123:227–234. doi: 10.1016/j.clim.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Hall AM, Edwards SG, Lapsley M, et al. Subclinical tubular injury in HIV-infected individuals on antiretroviral therapy: a cross-sectional analysis. Am J Kidney Dis. 2009;54:1034–1042. doi: 10.1053/j.ajkd.2009.07.012. [DOI] [PubMed] [Google Scholar]