We found that Numb directly binds to p120. Numb depletion impaired E-cadherin internalization. aPKC phosphorylated Numb and inhibited its association with p120. In the Numb-depleted cells, the phosphomimetic Numb mutant failed to restore E-cadherin internalization. We propose the mode of action of Numb for intercellular adhesion downstream of aPKC.
Abstract
Cadherin trafficking controls tissue morphogenesis and cell polarity. The endocytic adaptor Numb participates in apicobasal polarity by acting on intercellular adhesions in epithelial cells. However, it remains largely unknown how Numb controls cadherin-based adhesion. Here, we found that Numb directly interacted with p120 catenin (p120), which is known to interact with E-cadherin and prevent its internalization. Numb accumulated at intercellular adhesion sites and the apical membrane in epithelial cells. Depletion of Numb impaired E-cadherin internalization, whereas depletion of p120 accelerated internalization. Expression of the Numb-binding fragment of p120 inhibited E-cadherin internalization in a dominant-negative fashion, indicating that Numb interacts with the E-cadherin/p120 complex and promotes E-cadherin endocytosis. Impairment of Numb induced mislocalization of E-cadherin from the lateral membrane to the apical membrane. Atypical protein kinase C (aPKC), a member of the PAR complex, phosphorylated Numb and inhibited its association with p120 and α-adaptin. Depletion or inhibition of aPKC accelerated E-cadherin internalization. Wild-type Numb restored E-cadherin internalization in the Numb-depleted cells, whereas a phosphomimetic mutant or a mutant with defective α-adaptin-binding ability did not restore the internalization. Thus, we propose that aPKC phosphorylates Numb to prevent its binding to p120 and α-adaptin, thereby attenuating E-cadherin endocytosis to maintain apicobasal polarity.
INTRODUCTION
The dynamic rearrangement of cell–cell adhesion plays a critical role in various physiological processes, including tissue development, wound healing, synaptogenesis, epithelial–mesenchymal transitions, and tumor metastasis. Cadherin mediates cell–cell adhesion by Ca2+-dependent homophilic interactions and establishes adherens junctions. E-cadherin is the major cell–cell adhesion molecule in most epithelial tissues and is important for cell–cell adhesion and epithelial cell polarity. In epithelial cells, E-cadherin localizes to lateral cell–cell contact sites but is excluded from the apical membrane. E-cadherin binds to β-catenin, which is linked to the actin cytoskeleton through α-catenin, and to p120 catenin (p120), which regulates E-cadherin stability and trafficking (Peifer and Yap, 2003; Bryant and Mostov, 2008; Nelson, 2009; Reynolds, 2010).
Recent studies have indicated that E-cadherin trafficking has a pivotal role in remodeling E-cadherin-mediated cell–cell adhesions (Yap et al., 2007; Delva and Kowalczyk, 2009; Ulrich and Heisenberg, 2009). Cell surface E-cadherin undergoes endocytosis and recycling back to the plasma membrane via early and recycling endosomes (Bryant and Stow, 2004; Yeaman et al., 2004). The adhesive strength of the cell–cell contacts partly depends on the amount of E-cadherin at the cell surface (Steinberg and Takeichi, 1994; Yap et al., 2007). Thus, E-cadherin trafficking appears to regulate cadherin-based adhesive activity by balancing the relative amounts of surface-exposed and sequestered intracellular E-cadherin. E-cadherin and VE-cadherin undergo endocytosis though clathrin-dependent and clathrin-independent pathways (Le et al., 1999; Akhtar and Hotchin, 2001; Paterson et al., 2003; Palacios et al., 2005; Xiao et al., 2005; Bryant and Mostov, 2007; Toyoshima et al., 2007). The adaptor protein complex 2 (AP-2) associates with E-cadherin through its dileucine motif and induces E-cadherin endocytosis in a clathrin-dependent manner (Miyashita and Ozawa, 2007; Ishiyama et al., 2010; Reynolds, 2010). The protein p120 regulates E-cadherin trafficking and expression on the cell surface partly by inhibiting the entry of E-cadherin into the endocytic pathways (Davis et al., 2003; Miyashita and Ozawa, 2007; Reynolds, 2010). Further studies have indicated that p120 associates with the membrane-proximal region of E-cadherin and masks its dileucine motif, thereby preventing internalization (Miyashita and Ozawa, 2007). However, the regulatory mechanisms for how E-cadherin is internalized remain largely unknown.
Numb was originally identified as the cell-fate determinant of nervous system development in Drosophila (Betschinger and Knoblich, 2004; Roegiers and Jan, 2004). In sensory organ precursor cells, Numb localizes asymmetrically, segregating into one daughter cell during cell division. Numb binds to Notch and inhibits Notch signaling through its endocytosis, thereby regulating cell fate (Berdnik et al., 2002). Numb comprises an N-terminal phosphotyrosine binding (PTB) domain and a proline-rich region (PRR) spanning the C-terminal side (Figure 1B). The PTB domain conventionally mediates association with the NPxY motif in transmembrane proteins. Numb interacts with endocytic adaptors such as α-adaptin and Eps15 that respectively bind to the DPF and NPF motifs at the C terminus of Numb (Salcini et al., 1997; Santolini et al., 2000). Numb selectively promotes clathrin-dependent endocytosis of transmembrane proteins, including Notch (Traub, 2003; Sorkin, 2004), by linking the adaptors with them.
FIGURE 1:
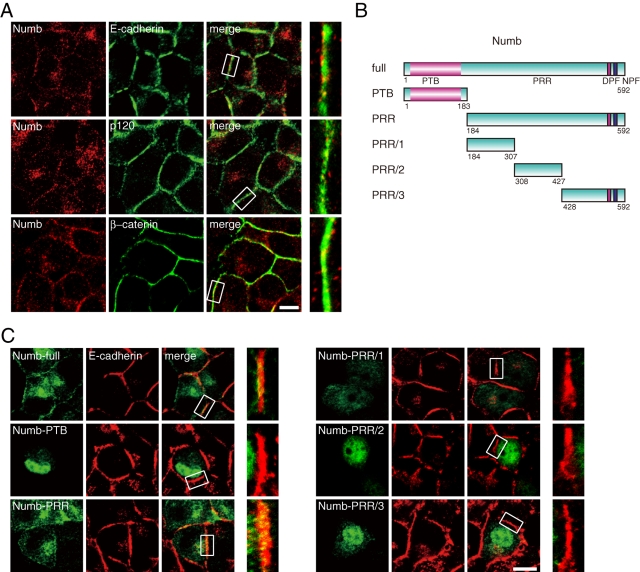
Numb partially overlaps with E-cadherin components at cell–cell contacts. (A) Epithelial MCF7 cells were stained with Numb and cadherin components. The insets in the left panels are magnified in the right panels. Bar, 10 μm. (B) Schematic representation of Numb. The domain organization of Numb and its fragments is represented. PTB, phosphotyrosine-binding; PRR, proline-rich region; DPF, α-adaptin-binding motif; NPF, Eps15-binding motif. (C) GFP-fused Numb was expressed in MCF7 cells and stained with E-cadherin. The insets in the left panels are magnified in the right panels. Bar, 10 μm. All results are representative of more than three experiments.
We previously found that Numb interacts with the L1 adhesion molecule and promotes the polarized endocytosis of L1 at axonal growth cones (Nishimura et al., 2003). Numb associates with integrin-βs (Calderwood et al., 2003), localizes to clathrin-coated structures, and promotes integrin endocytosis for directional cell migration with the PAR complex (Nishimura and Kaibuchi, 2007). The PAR complex, which is composed of PAR-3, PAR-6, and atypical protein kinase C (aPKC), functions in various cell polarization events, including front–rear polarity in migrating cells and apicobasal polarity in epithelial cells (Suzuki and Ohno, 2006; Goldstein and Macara, 2007). PAR-3 interacts with Numb and induces aPKC-mediated phosphorylation of Numb (Nishimura and Kaibuchi, 2007). This phosphorylation prevents the association of Numb with integrin-βs and α-adaptin, which inhibits integrin endocytosis.
It has been shown that Numb forms a complex containing E-cadherin/N-cadherin, β-catenin, and α-catenin and is required for proper formation of adherens junctions in neuroepithelial cells (Rasin et al., 2007). Further studies revealed that Numb is involved in the lateral localization of E-cadherin in epithelial cells and in HGF-induced cell scattering (Wang et al., 2009; Lau and McGlade, 2011). However, it remains unclear whether Numb controls E-cadherin endocytosis, and if so, how the process is regulated.
In light of these observations, we explored the role of Numb in E-cadherin trafficking. We found that Numb primarily interacts with p120 and participates in the endocytosis of E-cadherin with p120 in epithelial cells in an α-adaptin-dependent and clathrin-dependent manner. We also discovered that aPKC phosphorylates Numb, preventing its association with p120, E-cadherin, and α-adaptin, which results in the inhibition of E-cadherin endocytosis. Characterizing the functions of Numb as a cargo-selective adaptor provides the basis for further understanding of the polarized distribution of E-cadherin and the dynamic remodeling of cell–cell adhesion in epithelial cells.
RESULTS
Numb partially overlaps with the E-cadherin complex at cell–cell contacts
To explore the role of Numb in the epithelium, we first examined the distribution of Numb with immunofluorescence analysis using epithelial MCF7 cells. Endogenous Numb showed punctate staining throughout the cytoplasm and accumulation at the cell–cell contacts (Figure 1A). When the cells were costained for E-cadherin and catenins (p120 and β-catenin), Numb partially overlapped with these molecules at intercellular adhesion sites (Figure 1A). Higher-magnification images demonstrated that Numb appeared punctated even at cell–cell contacts. The punctate staining of Numb appears to represent localization to early and recycling endosomes (Smith et al., 2004). Moreover, consistent with an electromicroscopic study of Numb localization (Santolini et al., 2000), punctate staining arose from both basolateral membrane and vesicles containing E-cadherin (see below). We next identified the region of Numb responsible for its localization to the adhesion sites by using GFP-fused Numb fragments (Figure 1B). GFP-Numb-full-length localized to sites of cell–cell contact in a manner similar to endogenous Numb (Figure 1C). Under this condition, GFP-Numb-PRR, including the entire PRR region, accumulated at contact sites where the cadherin complex was enriched, in contrast to Numb fragments with additional deletions (PRR/1, PRR/2, PRR/3) and GFP-Numb-PTB (Figure 1C). These data indicate that Numb localizes to intercellular adhesion sites through its PRR region. Stable targeting of Numb to cell–cell contacts did not simply reflect the binding ability of Numb fragments to E-cadherin or p120 (Figure 2B) but might involve unidentified molecules or a structural conformation of Numb. Of note, GFP-Numb-PTB and GFP-Numb-PRR/3 accumulated in the nucleus. This nuclear accumulation might indicate cell-proliferative and tumor-suppressive roles of Numb (Pece et al., 2004; Colaluca et al., 2008; Yan et al., 2008). Clathrin light chain (CLC) and α-adaptin, a member of AP-2, also slightly accumulated at cell–cell contacts and partially overlapped with Numb (Supplemental Figure 1A). Furthermore, when we expressed the Numb mutants DLA, NLA, or DLA/NLA, which are defective in binding to Eps15, α-adaptin, or both, respectively (Salcini et al., 1997; Santolini et al., 2000), they showed a similar accumulation at cell–cell contacts as wild-type Numb (Supplemental Figure 1B), suggesting that Numb localizes to intercellular adhesion sites independently of the endocytic proteins.
FIGURE 2:
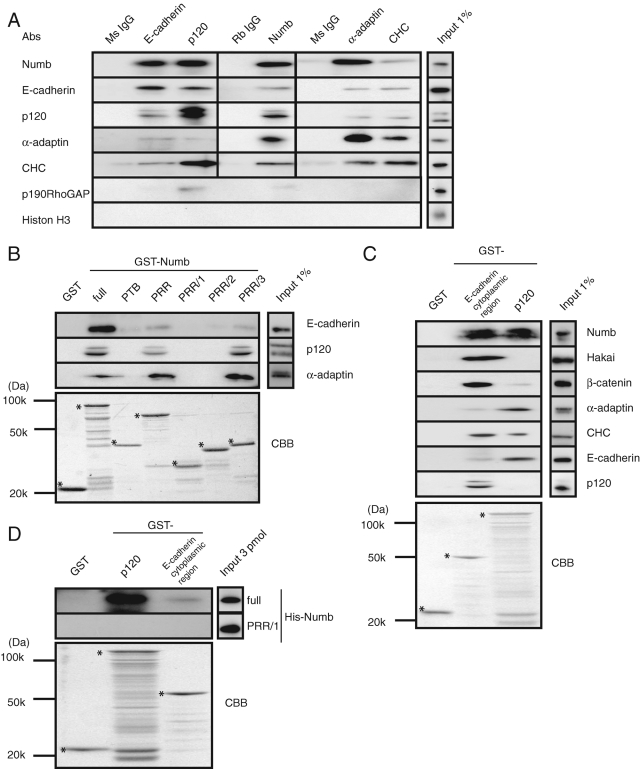
Numb directly binds to E-cadherin and p120. (A) MCF7 cells were lysed, and the supernatants were incubated with the indicated antibodies. The immunocomplexes were subjected to immunoblot analysis with the indicated antibodies. CHC, clathrin-heavy-chain. (B) Beads coated with purified proteins were incubated with the MCF7 cell lysate, followed by immunoblot analysis with the indicated antibodies. The GST fusion proteins were visualized by Coomassie Brilliant Blue (CBB) staining. The asterisks indicate each intact band. α-Adaptin was used as a positive control. (C) Purified GST-fused p120 or E-cadherin cytoplasmic region was incubated with the MCF7 lysate, followed by immunoblot analysis with the indicated antibodies. The GST fusion proteins were visualized by CBB staining. The asterisks indicate each intact band. (D) Purified His-fused Numb proteins were mixed with the beads coated with purified GST-p120 and GST-E-cadherin cytoplasmic region. All results are representative of more than three experiments.
Numb directly binds to p120
Accumulating evidence suggests a functional interaction between Numb and cadherins (Kuo et al., 2006; Rasin et al., 2007; Wang et al., 2009). We therefore examined whether Numb interacts with the cadherin/catenin complex and endocytic proteins in MCF7 cells. When E-cadherin or p120 was immunoprecipitated from MCF7 cells, Numb and the endocytic proteins (clathrin heavy chain [CHC] and α-adaptin) were coimmunoprecipitated (Figure 2A). Similarly, E-cadherin and p120 were detected in the immunoprecipitates of Numb. Furthermore, Numb, E-cadherin, and p120 were coimmunoprecipitated with the endocytic proteins (Figure 2A). p190RhoGAP, which interacts with p120 (Wildenberg et al., 2006), was coimmunoprecipitated primarily with p120 and somewhat with Numb, whereas Histone H3 as a negative control was not detected in the precipitates of either the cadherin complex or endocytic proteins.
To identify the interacting region between Numb and E-cadherin or p120, we performed a pulldown assay using purified GST-Numb-full-length or fragments containing the carboxyl PRR/3 region to precipitate E-cadherin or p120 from the lysate of MCF7 cells (Figure 2B). E-cadherin preferentially bound to full-length Numb, whereas p120 and α-adaptin (used as a positive control) interacted with full-length Numb, PRR, and PRR/3 to a similar extent. Of note, because E-cadherin was not efficiently precipitated with Numb PRR and PRR/3 (in comparison to full-length Numb), the PTB domain might partly contribute to association with E-cadherin (Figure 2B; Wang et al., 2009). Conversely, the GST-E-cadherin cytoplasmic region precipitated Numb, Hakai, β-catenin, α-adaptin, CHC, and p120 (Figure 2C). GST-p120 associated with Numb, β-catenin, α-adaptin, CHC, and E-cadherin (Figure 2C). Considered together with the immunoprecipitation data (Figure 2A), these results suggest that Numb can form a complex with E-cadherin, catenins, and the endocytic proteins.
To determine whether the interaction of E-cadherin and p120 with Numb is direct, we performed an in vitro binding assay using purified proteins. This assay revealed that full-length Numb bound to GST-p120 and loosely bound to the GST-E-cadherin cytoplasmic region (Figure 2D), indicating a direct interaction between Numb and p120.
Depletion of Numb, AP-2, clathrin, and p120 in MCF7 cells
On the basis of the evidence that Numb is involved in the endocytosis of several transmembrane proteins (Berdnik et al., 2002; Nishimura et al., 2003; Nishimura and Kaibuchi, 2007), we next exploited an RNA interference (RNAi) approach to address the roles of Numb in E-cadherin endocytosis in MCF7 cells. Two different small interfering RNAs (siRNAs) targeting either Numb or p120 were prepared, and their effectiveness was confirmed with immunoblot analysis (Supplemental Figure 2, A–D). Numb and p120 siRNA transfection specifically and effectively decreased respective expression to ∼5–20% of the levels in the control scramble-transfected cells without noticeably affecting the expression of the transferrin receptor (Trf-R) (Supplemental Figure 2, A–D). E-cadherin expression in the p120-depleted cells was suppressed to ∼30–50% of the levels in control scramble-transfected cells, which is consistent with previous reports (Davis et al., 2003). Moreover, siRNAs targeting α-adaptin and CHC were also used as controls to block clathrin-dependent endocytosis.
Depletion of Numb, AP-2, and clathrin reduces E-cadherin endocytosis
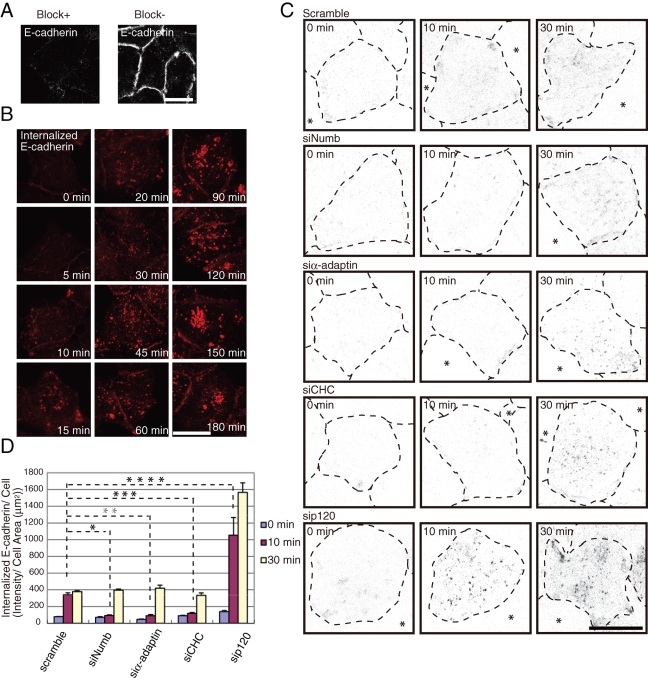
Previous studies have shown that Numb regulates the endocytosis of several receptors, including integrin-βs, as an adaptor protein, whereas p120 stabilizes E-cadherin at the cell surface. On the basis of these studies, we hypothesized that Numb controls E-cadherin endocytosis by associating with p120. We first visualized the internalized E-cadherin with an antibody against the E-cadherin extracellular region (HECD1) in MCF7 cells, according to a previously described method (Paterson et al., 2003). Blocking with excess nonfluorescent antibody almost completely eliminated the immunofluorescence of E-cadherin at the cell surface, which allowed us to detect only the internalized E-cadherin after permeabilization (Figure 3A). Internalized E-cadherin was detected in the cytoplasm after 10 min and increased in a time-dependent manner (Figure 3B). Incubation for more than 30 min led to a small amount of detectable fluorescence at sites of cell–cell contacts, probably due to the recycling of E-cadherin back to the surface. Internalized E-cadherin ultimately accumulated in the central region of the cells (Figure 3B). To elucidate the role of Numb in the internalization of E-cadherin, an internalization assay was performed with the depletion of Numb, p120, α-adaptin, or CHC. Internalization of E-cadherin was delayed in the Numb-, α-adaptin-, and CHC-depleted cells at 10 min after the incubation, whereas internalization was accelerated by the depletion of p120, as expected (Davis et al., 2003). The level of internalized E-cadherin showed no remarkable difference between the Numb-, α-adaptin-, or CHC-depleted cells and the control cells after 30 min of incubation (Figure 3, C and D). These data suggest that Numb, AP-2, and clathrin are involved in E-cadherin internalization and that other pathways, such as clathrin-independent or phagocytic internalization, also contribute to E-cadherin internalization.
FIGURE 3:
Numb is required for effective E-cadherin internalization. (A) Blocking of the antibodies remaining at the cell surface. See Materials and Methods for details. Bar, 10 μm. (B) Internalization of antibody-labeled E-cadherin in MCF7 cells. See Materials and Methods for details. Bar, 5 μm. (C) Internalization of E-cadherin in MCF7 cells transfected with the indicated siRNA. Inverted images are shown here (internalized E-cadherin is evident as black dots). The broken lines represent the cell margins, and the asterisks indicate cell-free space. The images represent the projection views of several confocal sections from the apical to basal membrane. Bar, 10 μm. (D) Quantification of (C) as described in Materials and Methods. All results are representative of more than three experiments. *, **, ***, **** = P < 0.001.
Numb, AP-2, and clathrin are required for E-cadherin endocytosis
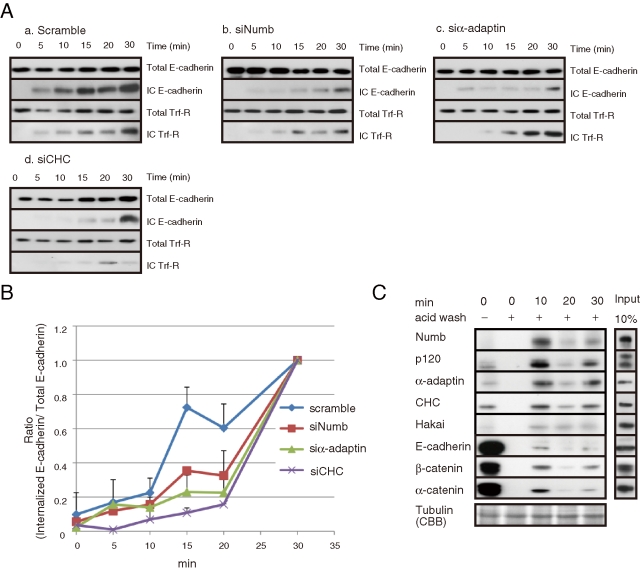
We further examined the effects of Numb depletion on E-cadherin endocytosis biochemically, with an endocytosis assay (see Materials and Methods). Treatment of cells with a glutathione buffer reduced the biotin remaining at the cell surface to an undetectable level (Supplemental Figure 3A), as described previously (Le et al., 1999). Internalized E-cadherin was detectable 5 min after initiation and gradually increased in a time-dependent manner (Supplemental Figure 3, B and C). The transient decrease in internalized E-cadherin at 20 min might represent the beginning of degradation processes (Supplemental Figure 3C; Lu et al., 2003; Palacios et al., 2005). As a control, we used Trf-R, which undergoes clathrin-dependent endocytosis (Hinrichsen et al., 2003; Motley et al., 2003). Trf-R underwent internalization in a time-dependent manner that was similar to the observed rate of E-cadherin internalization (Supplemental Figure 3B). In the Numb-, α-adaptin-, or CHC-depleted cells, the time-dependent increase in intracellular E-cadherin was delayed as compared with the control cells (Figure 4A). The difference was most evident at 15 min after initiation (Figure 4B). Trf-R internalization was also delayed in the α-adaptin- or CHC-depleted cells, whereas Numb knockdown had no apparent effect on Trf-R internalization (Figure 4A), implying that Numb is specifically involved in E-cadherin endocytosis.
FIGURE 4:
Numb is responsible for E-cadherin endocytosis. (A) Endocytosis of E-cadherin was attenuated in the Numb- or endocytic protein-depleted MCF7 cells. (B) Quantification of internalized E-cadherin. The ratio of internalized to total E-cadherin after a 30-min incubation was set to 1. (C) Precipitation of internalized E-cadherin after acid wash to strip the antibody remaining at the cell surface. Numb and other endocytic proteins were coprecipitated with internalized E-cadherin. All results are representative of more than three experiments.
Numb forms a complex with p120 and internalized E-cadherin
We attempted to precipitate internalized E-cadherin with an antibody against the extracellular domain of E-cadherin (see Materials and Methods for details). When surface E-cadherin was precipitated, β-catenin and α-catenin were predominantly coprecipitated, and Numb and endocytic molecules were detected at low levels (Figure 4C). Removal of the antibody from the cell surface with acid buffer prevented the precipitation of cell-surface E-cadherin (Figure 4C), allowing us to precipitate only the internalized E-cadherin. When the internalized E-cadherin was precipitated, we detected an increased amount of Numb; endocytic proteins, including α-adaptin and CHC; and p120 in the immunoprecipitates (Figure 4C). Our immunocytological results also showed that Numb staining partially overlapped with surface E-cadherin and internalized E-cadherin (Supplemental Figure 4, A and B). These data suggest that internalized E-cadherin forms a complex with Numb and that the binding of Numb to the cadherin/catenin complex initiates its endocytosis.
Numb is essential for proper cell adhesion and basolateral localization of E-cadherin and p120 in polarized MDCKII cells
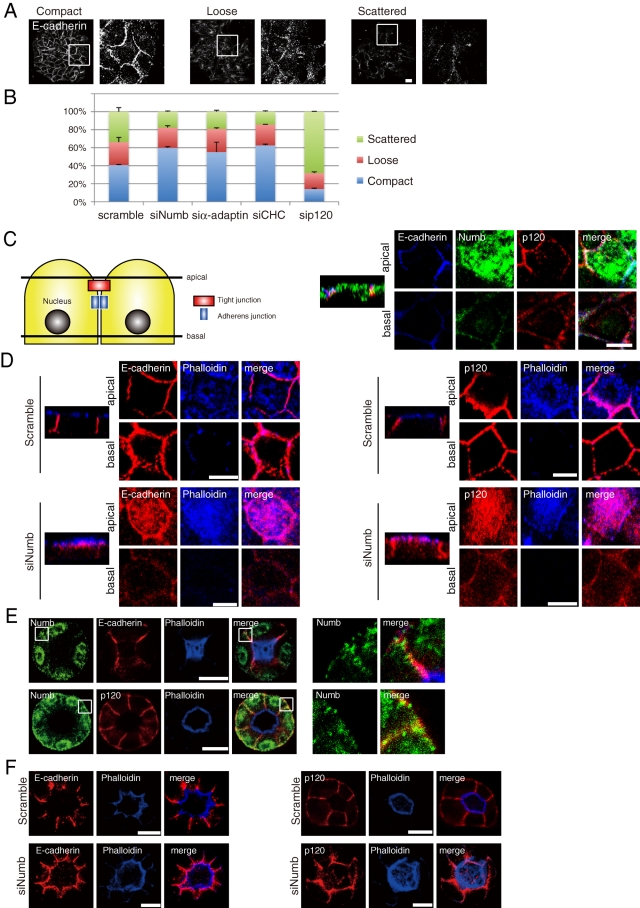
To address the role of Numb in intercellular adhesion and epithelial cell polarity, we examined the effects of Numb depletion on E-cadherin-mediated cell–cell adhesion by categorizing colonies of MCF7 cells based on E-cadherin immunofluorescence (Shtutman et al., 2006; Gross et al., 2009). The colonies were categorized as compact, loose, or scattered depending on the strength of cell adhesion (Figure 5A). In the control scramble-transfected cells, ∼40% of the colonies were categorized as compact. However, the depletion of Numb, α-adaptin, or CHC increased the population of compact colonies (Figure 5B). In contrast, when the cells were transfected with siRNA targeting p120, the cells showed a scattered appearance (Figure 5B). These results suggest that inhibition of E-cadherin endocytosis strengthens intercellular adhesion and that p120 is required for the maintenance of cell–cell contacts.
FIGURE 5:
Numb is essential for proper cell adhesion and basolateral localization of E-cadherin and p120 in polarized MDCKII cells. (A) Representation of various MCF7 colonies. The colonies were classified as compact (>90% of cells in the colony have cell–cell contacts), loose (50–90% cell contacts), or scattered (<50% cell contacts). Bar, 40 μm. (B) Quantification of the colony classification in MCF7 cells transfected with indicated siRNA. Knockdown of Numb or endocytic proteins increased the fraction of tightly associated colonies. (C) The model epithelial cell shows that “apical” corresponds to a x/y section image (x/y focal plane) taken at the subapical region of the monolayer of cells at 4 μm below the apical surface, and “basal” corresponds to the basolateral region (a section 4 μm above the basal surface). The corresponding x/z focal plane image is shown to the left of each x/y image set. Apical is at the top, whereas basal is at the bottom. The same convention is used throughout. Polarized MDCKII cells were stained with Numb, E-cadherin, and p120. Bar, 10 μm. (D) MDCKII cells transfected with the indicated siRNA were stained with E-cadherin and phalloidin. Bar, 10 μm. (E) MDCK 3D cysts were stained on day 3 with Numb, E-cadherin, p120, and phalloidin. Phalloidin was used as an apical marker. Bar, 10 μm. (F) MDCK 3D cysts transfected with indicated siRNA were stained on day 3. Bar, 10 μm. All results are representative of more than three experiments.
We further investigated the role of Numb in polarized MDCKII cells. Consistent with a previous report (Wang et al., 2009), Numb accumulated at the lateral membrane where E-cadherin and p120 localized and at the apical membrane region (Figure 5C and Supplemental Figure 5). The effectiveness of siRNA targeting Numb in MDCKII cells was confirmed with immunoblot analysis (Supplemental Figure 2, E and F). In Numb-depleted cells, E-cadherin and p120 mainly localized to the apical membrane region (Figure 5D), but the locations of ZO-1 and Na+/K+-ATPase, a tight junction and a basolateral protein, respectively, were almost unchanged (Supplemental Figure 6, A and C). These results imply that Numb depletion induces a change in the localization of E-cadherin and p120 from the lateral membrane to the apical membrane region.
We then examined whether Numb controls the localization of E-cadherin and p120 in MDCK 3D cysts (Debnath et al., 2003; Mostov and Martin-Belmonte, 2006). Numb localized to the basolateral membrane where E-cadherin and p120 also accumulated (Figure 5E), whereas actin filaments accumulated at the apical membrane (Debnath et al., 2003). In the cysts of the Numb-depleted MDCKII cells, the localization of E-cadherin and p120 was dramatically shifted to the apical side near the actin filaments (Figure 5F), but ZO-1 was apparently unaffected (Supplemental Figure 6B). These results suggest that the apical accumulation of E-cadherin and p120 results from the impairment of Numb-mediated endocytosis and/or exocytosis.
Numb controls E-cadherin endocytosis by interacting with the C-terminal region of p120
To further understand the functional relationship between Numb and p120, we constructed various p120 fragments and tested their association with Numb in MCF7 cells (Figure 6, A and B). The full-length p120 and the p120 fragments containing both ARM repeats and their N-terminally proximal region (p120-2, p120-5) associated with E-cadherin, whereas other p120 fragments (p120-1, p120-3, p120-4, and p120-6) failed to associate with E-cadherin (Figure 6B), as described previously (Ireton et al., 2002). Under these conditions, Numb interacted with the full-length p120 and p120 fragments containing the C-terminus (p120-2, p120-4, p120-6) (Figure 6B), indicating that Numb interacts with the C-terminal region of p120, which is distinct from the E-cadherin-binding region.
FIGURE 6:
Numb controls E-cadherin endocytosis by interacting with the C-terminal region of p120. (A) Schematic representation of p120. The domain organization of p120 and its fragments is represented. (B) Immunoprecipitation of p120 fragments from the transfected MCF7 cells. The asterisks indicate each intact band. (C) Immunostaining of GFP-p120 mutants in polarized MDCKII cells. The insets in the panels are magnified in the right panels. Bar, 10 μm. (D) Immunoprecipitation of Numb from MCF7 cells expressing indicated GFP fusions. The asterisks indicate each GFP fusions. (E) Overexpression and rescue experiments with p120 fragments and mutants in E-cadherin endocytosis. MCF7 cells were transfected with the indicated GFP-p120 mutants, siRNA, and sr-p120 constructs. The ratio of total intensity of internalized to Cell Area (μm2) of scramble + GFP-GST was set to 1. *, P < 0.05; **, P < 0.01. Expression level of each construct was biochemically measured and displayed in Supplemental Figure 7. All results are representative of more than three 3 experiments.
We next examined the localization of p120 mutants in polarized MDCKII cells. GFP-p120-full-length localized to the cell–cell contacts where E-cadherin localized. GFP-p120-5 localized not only to cell–cell contacts but also to the apical side where E-cadherin localized (Figure 6C). Expression of GFP-p120-5 induced the apical accumulation of E-cadherin (in ∼80% of the cells) but had no appreciable effects on ZO-1 distribution (Figure 6C). The expression of p120-5 might inhibit the linkages between E-cadherin and Numb by replacing endogenous p120, resulting in the compromised accumulation of E-cadherin at the apical membrane. These data suggest that the C-terminal region of p120 is required for the proper localization of p120 and E-cadherin. Consistent with our data, it has been reported that the C-terminal region of p120 is involved in E-cadherin trafficking (Liu et al., 2007).
To test the physiological importance of the interaction of p120 with Numb, we examined the effects of various fragments of p120 on E-cadherin internalization. Immunoblot analysis showed that there was only slight variation in the expression level between constructs (Supplemental Figure 7A). Under these conditions, overexpression of full-length p120 and p120-5 showed minimal effects, whereas overexpression of p120-6, which prevented the interaction between p120 and Numb (Figure 6D), decreased the level of internalized E-cadherin (Figure 6E), suggesting that p120-6 acts as the dominant negative fragment. These results indicate that the interaction between p120 and Numb is required for E-cadherin internalization.
We also performed rescue experiments with the p120 mutants. The expression of the siRNA-resistant (sr)-p120-full-length, but not that of p120-6, restored E-cadherin internalization in p120-depleted cells to control levels. Of note, the sr-GFP-p120 fragment lacking the Numb-binding region (p120-5) rescued the effects of p120 depletion more efficiently than sr-p120-full-length (Figure 6E). This may be due to the lacking of the interaction of p120-5 with Numb.
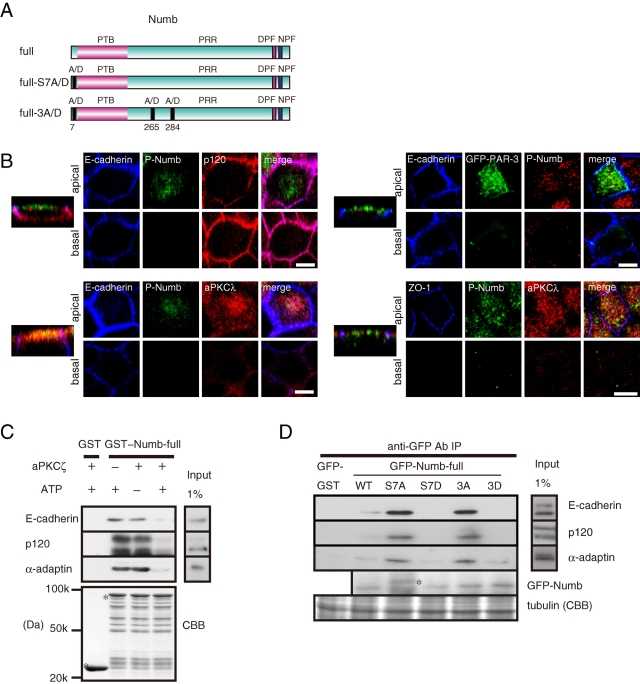
aPKC regulates the interaction of Numb with E-cadherin and p120 by phosphorylation
Our group and others previously found that aPKC phosphorylates Numb at three serine residues (Ser-7, Ser-265, and Ser-284) in a PAR-3-dependent manner (Figure 7A; Nishimura and Kaibuchi, 2007; Smith et al., 2007). To generate phosphomimetic mutants, Ser-7 was mutated to aspartic acid (GFP-Numb-full-length S7D), and all three residues were mutated to aspartic acid (GFP-Numb-full-length 3D; Nishimura and Kaibuchi, 2007). To generate phosphodeficient mutants, Ser-7 was mutated to alanine (GFP-Numb-full-length S7A), and all three residues were mutated to alanine (GFP-Numb-full-length 3A). We also used a previously characterized antibody that specifically recognizes Numb phosphorylated at Ser-7 (Nishimura and Kaibuchi, 2007). Numb and GFP-Numb localized to the apical membrane region and the lateral membrane in polarized MDCKII cells (Figure 5C and Supplemental Figure 5), whereas Numb phosphorylated at Ser-7 localized to the apical membrane region but not the lateral membrane (Figure 7B and Supplemental Figure 8A). GFP-Numb-full-length S7A and GFP-Numb-full-length 3A localized to the apical and lateral membrane, whereas GFP-Numb-full-length S7D and GFP-Numb-full-length 3D localized to the apical membrane but not the lateral membrane (Supplemental Figure 8B). GFP-PAR-3 and aPKC mainly localized to the apical membrane, including the apical junction area (Figure 7B), and partially overlapped with phosphorylated Numb at the apical membrane, suggesting that aPKC can efficiently phosphorylate Numb at the apical membrane.
FIGURE 7:
aPKC regulates Numb localization and the interaction of Numb with E-cadherin and p120 by phosphorylation. (A) Schematic representation of the phosphomimetic and phosphodeficient Numb mutants. (B) Localization of phosphorylated Numb in polarized MDCKII cells. Bar, 10 μm. (C) Purified GST proteins were incubated with recombinant aPKCζ in the presence or absence of ATP and then mixed with the MCF7 cell lysate. (D) Immunoprecipitation of Numb mutants from transfected cells. The asterisk indicates a nonspecific band.
The polarized localization of Numb led us to examine the effect of Numb phosphorylation on the interaction of Numb with E-cadherin, p120, and α-adaptin. Numb phosphorylation by aPKC impaired the association of Numb with E-cadherin, p120, and α-adaptin (Figure 7C). When the Numb mutants were precipitated from the MCF7 cells, E-cadherin, p120, and α-adaptin predominantly coprecipitated with the phosphodeficient Numb mutants, S7A and 3A, but not with the phosphomimetic Numb mutants, S7D and 3D (Figure 7D). Taken together, these results suggest that aPKC negatively controls Numb binding to E-cadherin and p120.
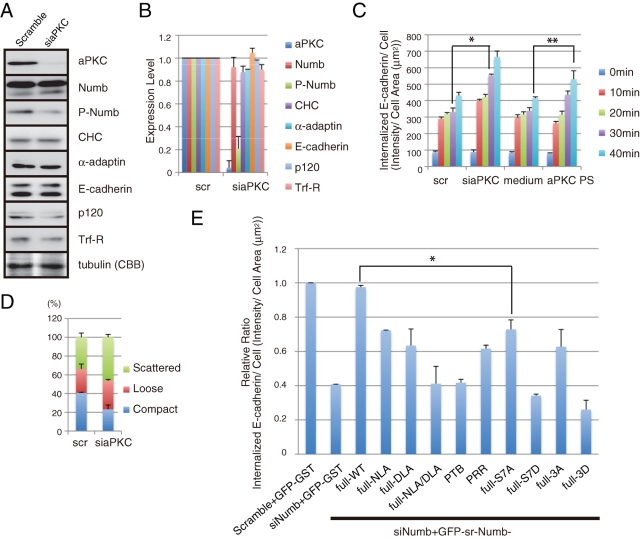
aPKC attenuates E-cadherin endocytosis
To test whether aPKC affects E-cadherin internalization, we performed an internalization assay with aPKC depletion and inhibition. The effectiveness of siRNA targeting aPKC was confirmed with immunoblot analysis (Figure 8, A and B). Transfection of mixed siRNAs targeting aPKCζ and aPKCλ specifically decreased their expression levels to ∼5% of that of the control scramble-transfected cells without noticeably affecting the level of Trf-R, which is an unrelated protein (Figure 8, A and B). Depletion of aPKC using siRNA or treatment with an aPKC inhibitor enhanced E-cadherin internalization (Figure 8C). These data raise the possibility that aPKC controls the internalization of E-cadherin through Numb phosphorylation. In support of this possibility, aPKC depletion increased the population of scattered colonies in MCF7 cells (Figure 8D).
FIGURE 8:
aPKC attenuates E-cadherin endocytosis. (A) Knockdown of aPKC in MCF7 cells by siRNA. Depletion of aPKC impaired Numb phosphorylation. (B) Quantification of (A). (C) Inhibition of aPKC accelerated E-cadherin endocytosis in MCF7 cells. The cells were either transfected with aPKC-targeting siRNA or treated with an aPKC inhibitor (aPKC pseudosubstrate) and subjected to an endocytosis assay for E-cadherin as in Figure 3. *, **, P < 0.001. (D) Categorization of colonies in the aPKC-depleted MCF7 cells. (E) Rescue experiments with Numb fragments and mutants in E-cadherin endocytosis. MCF7 cells were transfected with the indicated siRNA and siRNA-resistant (sr) Numb constructs. The ratio of total intensity of internalized to cell area (μm2) of scramble + GFP-GST was set to 1. *, P < 0.01. Expression level of each construct was biochemically measured and displayed in Supplemental Figure 7. All results are representative of more than three experiments.
To further confirm the physiological importance of Numb in E-cadherin endocytosis, we performed rescue experiments using various Numb mutants (Figures 1B and 7A). The expression of these mutants was monitored by immunoblot analysis (Supplemental Figure 7B). The expression of full-length sr-Numb rescued the inhibitory effect of Numb depletion on E-cadherin internalization, whereas sr-GFP-Numb-S7D and sr-GFP-Numb-3D failed to rescue it (Figure 8E). The phosphodeficient Numb mutants, S7A and 3A, could partially restore E-cadherin internalization, which suggests that a proper phosphorylation and dephosphorylation cycle is required for full restoration. Furthermore, Numb mutants defective in binding to endocytic proteins, such as NLA, DLA, and NLA/DLA, also failed to compensate for the Numb depletion. Thus, E-cadherin internalization appears to require Numb association with endocytic proteins.
DISCUSSION
AP-2 containing α-adaptin associates with E-cadherin through its dileucine motif in the absence of p120 and induces E-cadherin endocytosis in a clathrin-dependent manner (Figure 9, A and B; Miyashita and Ozawa, 2007; Yap et al., 2007; Ishiyama et al., 2010; Reynolds, 2010). Furthermore, p120 associates with the membrane-proximal region of E-cadherin and masks its dileucine motif, thereby preventing internalization (Figure 9B; Miyashita and Ozawa, 2007). We found that Numb partially overlapped with E-cadherin and p120 at cell–cell contact sites (Figure 1A). Numb interacted with p120 and E-cadherin both in vitro and in vivo (Figure 2). The C-terminal region of Numb interacted with the C-terminal region of p120 (Figures 2D and 6B). The PRR containing the C-terminal region of Numb was required for its localization to sites of cell–cell contact (Figure 1C). Numb depletion impaired E-cadherin internalization and strengthened cell–cell adhesive activity, whereas p120 depletion accelerated the internalization and weakened cell–cell adhesive activity (Figures 3 and 5, A and B). Expression of the Numb-binding, C-terminal fragment of p120 inhibited E-cadherin internalization in a dominant-negative manner (Figure 6, D and E). The internalized E-cadherin formed a complex with p120, Numb, α-adaptin, and clathrin (Figure 4C). Taken together, these observations indicate that Numb primarily associates with p120 and promotes the endocytosis of E-cadherin as a complex with p120 and other endocytic proteins, such as α-adaptin and clathrin. The weak interaction of Numb with E-cadherin raises the possibility that Numb is also involved in the endocytosis of the E-cadherin fraction not bound to p120 (Figure 9B).
FIGURE 9:
Numb controls E-cadherin endocytosis through p120 with aPKC, and Numb is involved in polarized E-cadherin trafficking. (A) Schematic representation of E-cadherin. E-cadherin consists of a signal peptide, extracellular cadherin domains 1–5 (EC1-5), transmembrane domain (TMD), and a cytoplasmic tail that contains a juxtamembrane domain (JMD) and cytoplasmic β-catenin-binding domain (CBD). In the E-cadherin cytoplasmic region, several motifs are prominent. The dileucine motif binds to cargo proteins, such as α-adaptin. NVYYY contains phosphorylated tyrosine residues that bind to Numb and Hakai. GGG is a core component that binds to p120 (Miyashita and Ozawa, 2007; Ishiyama et al., 2010). (B) The epithelial cell is highly polarized with an apical surface and basal membrane. E-cadherin composes adherens junctions and maintains epithelial polarity. E-cadherin endocytosis is essential for maintaining adherens junctions and cell polarity. By interacting with E-cadherin and p120, Numb plays a significant role in E-cadherin endocytosis, and its interactions are regulated through Numb phosphorylation by aPKC. Phosphorylated Numb cannot interact with E-cadherin and p120 and exists in the cytosol. There are two possible ways that Numb mediates E-cadherin endocytosis. First, Numb binds to p120 and mediates internalization of the entire E-cadherin complex containing p120. Alternatively, Numb binds directly to the NVYYY motif in E-cadherin, which is usually masked by p120, and internalizes p120-unbound E-cadherin. With these mechanisms, Numb controls E-cadherin endocytosis through p120 and aPKC. (C) Polarized E-cadherin trafficking by Numb establishes adherens junctions and epithelial cell polarity. The PAR complex with Cdc42, which localizes to tight and adherens junctions, has also recently emerged as an important regulator of endocytosis at adherens junctions (Balklava et al., 2007; Georgiou et al., 2008; Leibfried et al., 2008). Numb is phosphorylated by aPKC and thus loses its ability to mediate E-cadherin endocytosis. As a result, aPKC prevents E-cadherin endocytosis and may stabilize cell–cell adhesion at adherens junctions. This polarized endocytosis could account for the restricted distribution of E-cadherin at the lateral membrane.
PAR-3 associates with Numb and enhances the aPKC-mediated phosphorylation of Numb (Nishimura and Kaibuchi, 2007). This phosphorylation prevents the association of Numb with integrin-βs and α-adaptin, thereby inhibiting integrin endocytosis and controlling polarized cell migration. We found that the phosphorylation of Numb by aPKC blocked the association of Numb with α-adaptin, E-cadherin, and p120 (Figure 7, A, C, and D). Depletion or inhibition of aPKC enhanced the internalization of E-cadherin (Figure 8C). A phosphomimetic Numb mutant, which is defective in binding to E-cadherin and p120, could not rescue the Numb knockdown phenotype under the conditions in which full-length Numb rescued the phenotype (Figure 8E). These results indicate that aPKC phosphorylates Numb and negatively regulates the Numb-mediated endocytosis of E-cadherin, as described for integrin (Figure 9B). The endocytosis of E-cadherin at the lateral membrane and of integrin at the basal membrane appears to be coordinately regulated during collective cell migration (Nelson, 2009). Depletion of Numb and PAR-3 impairs the wound-induced collective migration of epithelial cells (Nishimura and Kaibuchi, 2007; Nakayama et al., 2008). Thus it is possible that Numb coordinately regulates the endocytosis of E-cadherin and integrin for collective migration under the control of PAR-3/aPKC.
In polarized MDCKII cells and 3D cysts, E-cadherin and p120 localized to the basolateral membrane where Numb accumulated (Figure 5C and E). The depletion of Numb induced the mislocalization of E-cadherin and p120 to the apical membrane (Figure 5, D and F), suggesting that Numb is required for the proper localization of E-cadherin and p120 to the basolateral membrane. This finding is consistent with the recent observation that clathrin is required for the proper localization of basolateral proteins, including E-cadherin, to the basolateral membrane (Deborde et al., 2008). Clathrin depletion depolarizes most basolateral proteins by interfering with their biosynthetic delivery and recycling, slowing the exit of basolateral proteins from the Golgi complex and promoting their abnormal sorting into apical carrier vesicles. In this regard, Numb has been implicated not only in the endocytosis of target proteins but also in the recycling of the target proteins from the recycling endosome to the plasma membrane (Smith et al., 2004). Thus Numb depletion may induce the abnormal sorting of E-cadherin and p120 to apical carrier vesicles.
Immunoelectron microscopic studies have shown substantial E-cadherin accumulation at the apical adherens junctions in the lateral membrane of the intestinal epithelium (Horiguchi et al., 1994). The PAR complex has been implicated in the establishment of the apicobasal polarity in epithelial cells (Suzuki and Ohno, 2006). PAR-3 localizes to apical junctions, such as adherens and tight junctions in the epithelium (Izumi et al., 1998; Suzuki and Ohno, 2006). PAR-3 and aPKC are necessary for the formation of adherens junctions and tight junctions in MDCK cells (Ooshio et al., 2007). We found that aPKC prevented E-cadherin endocytosis, presumably through Numb phosphorylation. Thus it is tempting to speculate that aPKC phosphorylates Numb at adherens and tight junctions and thereby impairs the Numb-mediated endocytosis of E-cadherin. This polarized endocytosis by Numb/aPKC could account for the polarized enrichment of E-cadherin at adherens junctions in the lateral membrane and ensure apicobasal polarity (Figure 9C).
Chemical materials and antibody
The cDNAs encoding Numb, E-cadherin, CLC, and α-adaptin were described previously (Kuroda et al., 1996; Nishimura et al., 2003; Nishimura and Kaibuchi, 2007). Mouse p120 cDNA was described elsewhere (Ohkubo and Ozawa, 1999). The rabbit polyclonal anti–Numb antibody and anti–pS7 Numb-specific antibody were generated and characterized as described previously (Nishimura and Kaibuchi, 2007). The anti–Numb peptide antibody (92425) was provided by A. Tokunaga and H. Okano (Keio University, Japan). The following commercial antibodies were obtained: anti-aPKCζ (Santa Cruz Biotechnology, Santa Cruz, CA); anti-Numb (Cell Signaling Technology, Danvers, MA); anti–E-cadherin (ECCD2) and anti-ZO-1 (Invitrogen, Carlsbad, CA); anti–E-cadherin (HECD1; Takara Bio, Osaka, Japan); anti–E-cadherin, anti-p120, anti–β-catenin, anti–α-adaptin, anti-CHC, anti-p190RhoGAP, anti-aPKCλ, and anti-EEA1 (BD Bioscience, San Jose, CA); anti–Trf-R and anti–Na+/K+-ATPase (Zymed, San Francisco, CA); mouse monoclonal anti-GFP (Roche, Barsel, Switzerland); rat polyclonal anti-GFP (Nacalai Tesque, Kyoto, Japan); rabbit polyclonal anti-GFP (MBL, Nagoya, Japan); and anti-His (Nacalai Tesque). Alexa488-, Alexa555-, and Alexa647-conjugated secondary antibodies against mouse, rabbit, or rat immunoglobulin G (IgG), and phalloidin were obtained from Molecular Probes (Invitrogen). Other materials and chemicals were obtained from commercial sources.
Plasmid construction
Fragments of cDNA were amplified using PCR and subcloned into pGEX (GE Healthcare, Buckinghamshire, UK), pRSET-c1, and pEGFP (Clontech Laboratories, Mountain View, CA) vectors. Point mutations of Numb were generated using PCR-based site-directed mutagenesis. All fragments were confirmed with DNA sequencing. GST-fusion and His-fusion proteins were produced in BL21 (DE3) cells and purified on Glutathione-Sepharose 4B beads (GE Healthcare) and Ni-NTA (QIAGEN, Venlo, Netherlands), respectively.
Cell culture and immunofluorescence analysis
MCF7 cells were cultured in MEM (Invitrogen) with 1× NEAA (Invitrogen) and 10% fetal bovine serum (Nichirei Biosciences, Tokyo, Japan). MDCKII cells were cultured in DMEM (Sigma, St. Louis, MO) with 10% calf serum (Hyclone, Logan, UT). The transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The cells were fixed at the indicated time point with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature followed by permeabilization for 10 min with 0.2% Triton X-100 containing 2 mg/ml BSA (Sigma). The cells were observed with a confocal laser microscope (LSM5 PASCAL; Carl Zeiss, Jena, Germany). All presented images represent a single confocal section (thickness of 0.8–1.0 μm), except as noted in the figure legends.
siRNA transfection
Oligonucleotide siRNA duplexes were synthesized by Greiner Japan. The siRNA sequences were as follows: scramble, 5′-CAGUCGCGUUUGCGACUGG-3′; siNumb#1, 5′-GGACCUCAUAGUUGACCAG-3′; siNumb#2, 5′-GAUGUCACCCUUUAAACGC-3′; sip120#1, 5′-CGAGGUUAUCGCUGAGAAC-3′; and sip120#2, 5′-GCCAGAGGUGGUUCGGAUA-3′. The siRNA sequences for μ2-adaptin, CHC, aPKCζ, and aPKCλ were described previously (Nishimura and Kaibuchi, 2007). The cells were analyzed 3 d after transfection. The rescue experiments and siRNA-resistant Numb were described previously (Nishimura and Kaibuchi, 2007). Of note, in our condition, transfection efficiency of siRNA to MCF7 and 2D-cultured MDCKII was 70–90% based on the fluorescence of FITC-conjugated siRNA (data not shown). To acquire the images, we identified knocked down cells that showed decreased target immunofluorescence and selected them surrounded with nontransfected cells for easy comparison, expect for our indication. In the case of MDCK 3D cysts (see below), one cyst is generally originated from a single transfected and nontransfected cell. In the similar way to the 2D culture, we identified the knocked down cells for the images. Consequently, all cells in the cyst showed the phenotype (Figure 5, E and F).
Coimmunoprecipitation assay
The MCF7 cells were extracted in a lysis buffer (20 mM Tris/HCl, pH 7.4, 1.0% NP-40, 1 mM EDTA, 150 mM NaCl, 50 μg/ml PMSF, 10 μg/ml leupeptin) and isolated with centrifugation at 100,000 × g for 1 h at 4°C. The soluble supernatants were incubated with the indicated antibodies for 1 h at 4°C. The immunocomplexes were then precipitated with protein A-Sepharose 4B (GE Healthcare), washed three times with lysis buffer, eluted by boiling in sample buffer for SDS–PAGE, and then subjected to immunoblot analysis with the indicated antibodies.
Pulldown assay
GST-fused Numb proteins were immobilized onto Glutathione-Sepharose 4B beads. The coated beads were incubated with the MCF7 cell lysate for 1 h at 4°C, washed three times with lysis buffer, eluted by boiling in sample buffer for SDS–PAGE, and then subjected to immunoblot analysis with the indicated antibodies.
In vitro binding assay
Purified GST-p120 and GST-E-cadherin cytoplasmic region were immobilized onto Glutathione-Sepharose 4B beads. The beads were incubated with purified His-Numb deletion fragments for 1 h at 4°C. Then the beads were washed three times, eluted by boiling in sample buffer for SDS–PAGE, and subjected to immunoblot analysis with a monoclonal anti–His antibody.
Phosphorylation assay of Numb with aPKC
The reaction was carried out in a 50-μl assay mixture containing 20 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 1 mM EGTA, 1 mM DTT, 50 ng recombinant aPKCζ (Upstate), 10 μM ATP, and 0.4 μM purified GST-Numb fragment for 30 min at 30°C. The assay mixture was incubated with Glutathione-Sepharose beads and then mixed with MCF7 cell lysate as described above for 1 h at 4°C. Bound proteins were eluted with SDS sample buffer.
Internalization assay
To analyze E-cadherin internalization, we adapted an immunohistochemical assay based on those described previously (Paterson et al., 2003; Nishimura and Kaibuchi, 2007). MCF7 cells were seeded onto PDL-coated coverslips. After 12 h, the culture medium was changed to Hanks' balanced salt solution supplemented with 5 mM Ca2+ and 50 μg/ml BSA, and the cells were equilibrated for 1 h. The cells were transferred to the chamber at 4°C. After incubation for an additional 1 h, the cells were incubated with the HECD1 antibody, which recognizes the extracellular domain of E-cadherin, for 1 h at 4°C. Afterward, the coverslips were washed with ice-cold PBS to remove the unbound antibody, and the medium was replaced with Hanks' balanced salt solution prewarmed to 37°C. After incubation at 37°C for various time periods, the cells were washed with ice-cold PBS three times and then fixed and incubated with an excess amount of unconjugated anti–mouse IgG (Jackson Laboratories, Bar Harbor, ME) to block the antibody remaining on the cell surface. Subsequently, the cells were permeabilized with 0.2% Triton X-100. The internalized E-cadherin was visualized with a fluorescence-conjugated secondary antibody. To quantify the internalization, images of cells were captured with the LSM5 PASCAL with apical to basal z-axis sections, and the projected images were used for quantification. The total fluorescent intensity within a cell was measured and divided by its area (sum of pixel intensity/μm2). The data represent the mean and the SE of the mean (SEM) of more than 30 cells in each condition. All images were acquired under identical parameters. The data are representative of three independent experiments. Of note, it is possible that the antibody treatment might affect the internalization rate.
Endocytosis assay with cell-surface biotinylation
Biotinylation of cell-surface E-cadherin followed methods described previously (Le et al., 1999). In brief, MCF7 cells grown on PDL-coated slips were incubated with 50 μg/ml cycloheximide for 30 min at 37°C and further incubated at 4°C for 1 h to stop cellular activity. The cells were then incubated with 1.5 mg/ml sulfo-NHS-SS-biotin (Pierce Chemical, Rockford, IL) for 30 min followed by washing with the blocking reagent (50 mM NH4Cl in PBS containing 1 mM MgCl2) and 0.1 mM CaCl2 to quench the free sulfo-NHS-SS-biotin, followed by several further washes in PBS. The cells were then incubated in the culture medium at 37°C for the indicated time period. To measure total E-cadherin, the cells were lysed in RIPA buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, 5 mM EDTA). To measure intracellular E-cadherin, the cells were incubated twice for 20 min at 4°C with glutathione solution (60 mM glutathione, 0.83 M NaCl, 0.83 M NaOH, 1% BSA) to remove all cell-surface biotin and then lysed with RIPA buffer. Biotinylated intracellular E-cadherin was recovered on streptavidin beads, eluted by boiling in sample buffer for SDS–PAGE, and then subjected to immunoblot analysis with anti–E-cadherin or Trf-R antibody.
Internalization assay with HECD1 antibody and immunoprecipitation
To immunoprecipitate intracellular internalized E-cadherin, the HECD1 antibody remaining at the surface was removed by acid washing (0.5 M acetic acid, 0.5 M NaCl; Paterson et al., 2003). Then the immunoprecipitation assay was performed.
Categorization of MCF7 colonies based on cell adhesion
One thousand cells were plated per 100-mm plate and allowed to form small colonies for 5 d. siRNA was added after 48 h of seeding. The number of cells maintaining contact with their neighbors was counted for each colony according to previous reports (Shtutman et al., 2006; Gross et al., 2009). The data consisted of three independent experiments.
Cyst formation and immunostaining
Cyst formation and immunostaining were performed as reported previously (Debnath et al., 2003). In brief, Growth Factor Reduced Matrigel (BD Biosciences) was spread onto coverslips and solidified at 37°C for 15 min. Then, 1 × 104 cells/ml cells mixed with medium containing 2% Matrigel were seeded onto the coverslips and cultured for the indicated number of days. The samples were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature, permeabilized with 0.5% Triton X-100 for 15 min, and blocked with 4% BSA in PBS for 30 min. After blocking, they were incubated with the indicated primary antibodies for 3 h, washed with 0.5% Triton X-100 twice, and then incubated with the secondary antibodies. The cysts were observed with a confocal laser microscope (LSM5 PASCAL). All presented images represent a single confocal section (thickness of 0.8–1.0 μm), except as noted in the figure legends.
Supplementary Material
Acknowledgments
We thank S. Ohno (Yokohama City University) for aPKC cDNA, T. Nishimura (RIKEN CDB, Japan) for Numb materials, W. J. Nelson and W. I. Weis (Stanford University, CA) for E-cadherin construct, R. Kobayashi and H. Tokumitsu (Kagawa University, Japan) for anti-phosphorylated Numb antibody, all members of the Kaibuchi lab for discussion and technical support, T. Sakata for initiating this work, and T. Ishii for secretarial assistance. Supported by JST CREST to K.K., KAKENHI (20227006 to K.K., 20790225 and 22790279 to T.W.), GCOE to K.K., and SCF to T.W.
Abbreviations used:
- aPKC
atypical protein kinase C
- AP-2
adaptor protein complex 2
- CHC
clathrin heavy chain
- CLC
clathrin light chain
- MDCK
Madin-Darby canine kidney
- PRR
proline-rich region
- PTB
phosphotyrosine-binding
- Trf-R
transferrin receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-03-0274) on July 20, 2011.
REFERENCES
- Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell. 2001;12:847–862. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- Berdnik D, Török T, González-Gaitán M, Knoblich JA. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell. 2002;3:221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol. 2004;14:R674–R685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Bryant D, Mostov K. Development: inflationary pressures. Nature. 2007;449:549–550. doi: 10.1038/449549a. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Fujioka Y, de Pereda JM, García-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, Pece S, Di Fiore PP. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E, Kowalczyk AP. Regulation of cadherin trafficking. Traffic. 2009;10:259–267. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JC, Schreiner A, Engels K, Starzinski-Powitz A. E-cadherin surface levels in epithelial growth factor-stimulated cells depend on adherens junction protein Shrew-1. Mol Biol Cell. 2009;20:3598–3607. doi: 10.1091/mbc.E08-12-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell EJ. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J Biol Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y, Furukawa F, Fujita M, Imamura S. Ultrastructural localization of E-cadherin cell adhesion molecule on the cytoplasmic membrane of keratinocytes in vivo and in vitro. J Histochem Cytochem. 1994;42:1333–1340. doi: 10.1177/42.10.7930515. [DOI] [PubMed] [Google Scholar]
- Ireton RC, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- Lau KM, McGlade CJ. Numb is a negative regulator of HGF dependent cell scattering and Rac1 activation. Exp Cell Res. 2011;317:539–551. doi: 10.1016/j.yexcr.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- Liu H, Komiya S, Shimizu M, Fukunaga Y, Nagafuchi A. Involvement of p120 carboxy-terminal domain in cadherin trafficking. Cell Struct Funct. 2007;32:127–137. doi: 10.1247/csf.07023. [DOI] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Ozawa M. Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J Biol Chem. 2007;282:11540–11548. doi: 10.1074/jbc.M608351200. [DOI] [PubMed] [Google Scholar]
- Mostov K, Martin-Belmonte F. Developmental biology: the hole picture. Nature. 2006;442:363–364. doi: 10.1038/442363a. [DOI] [PubMed] [Google Scholar]
- Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 2003;5:819–826. doi: 10.1038/ncb1039. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J Biol Chem. 1999;274:21409–21415. doi: 10.1074/jbc.274.30.21409. [DOI] [PubMed] [Google Scholar]
- Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci. 2007;120:2352–2365. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J Biol Chem. 2003;278:21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, Zurrida S, Maisonneuve P, Viale G, Di Fiore PP. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167:215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Yap AS. Traffic control: p120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells. J Cell Biol. 2003;163:437–440. doi: 10.1083/jcb.200310090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasin MR, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- Reynolds AB. Exposing p120 catenin's most intimate affair. Cell. 2010;141:20–22. doi: 10.1016/j.cell.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Roegiers F, Jan YN. Asymmetric cell division. Curr Opin Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, Di Fiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP. Numb is an endocytic protein. J Cell Biol. 2000;151:1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res. 2006;66:11370–11380. doi: 10.1158/0008-5472.CAN-06-2106. [DOI] [PubMed] [Google Scholar]
- Smith CA, Dho SE, Donaldson J, Tepass U, McGlade CJ. The cell fate determinant numb interacts with EHD/Rme-1 family proteins and has a role in endocytic recycling. Mol Biol Cell. 2004;15:3698–3708. doi: 10.1091/mbc.E04-01-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, et al. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J. 2007;26:468–480. doi: 10.1038/sj.emboj.7601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr Opin Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci USA. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Toyoshima M, Tanaka N, Aoki J, Tanaka Y, Murata K, Kyuuma M, Kobayashi H, Ishii N, Yaegashi N, Sugamura K. Inhibition of tumor growth and metastasis by depletion of vesicular sorting protein Hrs: its regulatory role on E-cadherin and beta-catenin. Cancer Res. 2007;67:5162–5171. doi: 10.1158/0008-5472.CAN-06-2756. [DOI] [PubMed] [Google Scholar]
- Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Heisenberg CP. Trafficking and cell migration. Traffic. 2009;10:811–818. doi: 10.1111/j.1600-0854.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sandiford S, Wu C, Li SS. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J. 2009;28:2360–2373. doi: 10.1038/emboj.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Omar FM, Das K, Ng WH, Lim C, Shiuan K, Yap CT, Salto-Tellez M. Characterization of Numb expression in astrocytomas. Neuropathology. 2008;28:479–484. doi: 10.1111/j.1440-1789.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.