The MARCH family of proteins are membrane-associated E3 ubiquitin ligases that down-regulate surface membrane proteins. Expression of MARCH8 in cells causes the ubiquitination and down-regulation of surface CD98 and CD44—cargo proteins that enter cells by clathrin-independent endocytosis and are usually routed to recycling, not degradation.
Abstract
Following endocytosis, internalized plasma membrane proteins can be recycled back to the cell surface or trafficked to late endosomes/lysosomes for degradation. Here we report on the trafficking of multiple proteins that enter cells by clathrin-independent endocytosis (CIE) and determine that a set of proteins (CD44, CD98, and CD147) found primarily in recycling tubules largely failed to reach late endosomes in HeLa cells, whereas other CIE cargo proteins, including major histocompatibility complex class I protein (MHCI), trafficked to both early endosome antigen 1 (EEA1) and late endosomal compartments in addition to recycling tubules. Expression of the membrane-associated RING-CH 8 (MARCH8) E3 ubiquitin ligase completely shifted the trafficking of CD44 and CD98 proteins away from recycling tubules to EEA1 compartments and late endosomes, resulting in reduced surface levels. Cargo affected by MARCH expression, including CD44, CD98, and MHCI, still entered cells by CIE, suggesting that the routing of ubiquitinated cargo occurs after endocytosis. MARCH8 expression led to direct ubiquitination of CD98 and routing of CD98 to late endosomes/lysosomes.
INTRODUCTION
The protein and lipid composition of the plasma membrane (PM) is balanced and maintained through the addition of new membrane from the secretory pathway and removal of membrane by endocytosis. There are two types of endocytosis, distinguished by the requirement for clathrin and the dynamin GTPase. Clathrin-dependent endocytosis (CDE) is the best-characterized form of endocytosis, in which specific amino acid sequences in the cytoplasmic tails of transmembrane proteins are recognized by adaptor proteins and packaged into clathrin-coated vesicles that are released into the cytoplasm following the action of dynamin (Conner and Schmid, 2003; Traub, 2009). Less studied is clathrin-independent endocytosis (CIE), in which no protein coat has been described, dynamin is not required, and the machinery necessary for endocytosis is unknown (Mayor and Pagano, 2007). In HeLa cells, major histocompatibility complex class I protein (MHCI), a PM cargo protein, enters cells by CIE, and studies following the trafficking of MHCI have helped to define the endosomal membrane system fed by CIE (Naslavsky et al., 2003, 2004; Robertson et al., 2006; Lau and Chou, 2008). After endocytosis, MHCI-containing vesicles fuse with sorting endosomes containing cargo that enters by CDE. From there, MHCI can traffic to late endosomes and lysosomes for degradation or recycle back to the PM in tubular recycling endosomes. The list of PM proteins entering cells by CIE is growing rapidly (Mayor and Pagano, 2007; Eyster et al., 2009), underscoring the importance of CIE and associated recycling pathways (Grant and Donaldson, 2009).
A major function of endocytosis is as a quality control mechanism for regulating PM proteins and lipids. Endocytosed proteins and lipids can be selected for degradation, allowing efficient turnover of the PM, or they can be targeted for recycling back to the cell surface, maintaining the proper protein and lipid composition of the PM (Maxfield and McGraw, 2004; Grant and Donaldson, 2009). In most cases, it is not clear how and where PM proteins are monitored and selected for degradation (Arvan et al., 2002). However, the addition of ubiquitin, a small, 8.5-kDa protein that can be covalently attached to lysine residues on target proteins (Hershko and Ciechanover, 1998), has been shown to target some PM proteins, in particular the epidermal growth factor (EGF) and growth hormone receptors, for degradation (d'Azzo et al., 2005; Acconcia et al., 2009). The addition of ubiquitin onto these proteins is mediated by E3 ubiquitin ligases, which specify the interaction between the ubiquitination machinery and their substrates (Pickart, 2001). PM proteins ubiquitinated on their cytoplasmic tails are recognized by the endosomal sorting complex required for transport (ESCRT) machinery and sorted into intralumenal vesicles forming multivesicular bodies or late endosomes, which fuse with lysosomes, resulting in degradation of the protein (Piper and Katzmann, 2007; Raiborg and Stenmark, 2009). This process is well characterized for the ligand-bound EGF receptor, which is ubiquitinated by the E3 complex c-CBL and targeted for degradation as a means to control signal output (Levkowitz et al., 1998, 1999; Sigismund et al., 2008). There is increasing evidence that other PM proteins, including G protein–coupled receptors and the cystic fibrosis conductance regulator CFTR, are also ubiquitinated and routed to degradation (Shenoy et al., 2001; Geetha et al., 2005; Okiyoneda et al., 2010) and that these ubiquitin enzyme systems might be critical for quality control mechanisms governing PM turnover. As there are hundreds of E3 ligases in the human genome, investigators are interested in identifying which E3 ligases target particular substrates for degradation and how these enzymes are regulated (d'Azzo et al., 2005).
Many viruses express proteins that down-regulate surface MHCI to evade host cell immune function (Lehner et al., 2005). Two of these viral proteins, known as K3 and K5, identified a new class of E3 ubiquitin ligases, the RING-CH family, which remove MHCI from the cell surface (Ohmura-Hoshino et al., 2006). Similar viral E3 ligases were discovered in disparate viruses, suggesting a common human host source (Fruh et al., 2002). Eleven mammalian membrane-associated RING-CH (MARCH) proteins were subsequently identified and shown to possess the ability to ubiquitinate PM proteins (Goto et al., 2003; Bartee et al., 2004; Morokuma et al., 2007; Nathan and Lehner, 2009). Indeed, two mammalian MARCH proteins, MARCH4 and MARCH9, can ubiquitinate MHCI, leading to its removal from the cell surface and degradation in lysosomes (Bartee et al., 2004). Although the normal physiological role of MARCH proteins in many cell types is unknown, in dendritic cells MHC class II surface stabilization is mediated by down-regulation of expression of MARCH1, an important step in dendritic cell maturation (Matsuki et al., 2007; De Gassart et al., 2008; Thibodeau et al., 2008).
Because MHCI is a CIE cargo protein and subject to down-regulation by certain MARCH proteins, we wanted to determine whether any of the MARCH E-3 ligases would affect the trafficking of other CIE cargo proteins. We recently identified new CIE cargo proteins and found in HeLa cells that the trafficking of a subset of these proteins, CD44, CD98, and CD147, diverged from the itinerary followed by MHCI (Eyster et al., 2009). CD44, CD98, and CD147 did not reach sorting endosomes associated with the early endosomal antigen (EEA1) but instead entered tubular recycling endosomes directly (Eyster et al., 2009). EEA1-positive endosomes have been suggested to be a selection point for entry into the multivesicular body pathway and lysosomal degradation (Leonard et al., 2008). In addition, a recent study using quantitative proteomics identified CD44 as being a possible substrate for MARCH8 (Bartee et al., 2010). In this study, we examine the fate of the divergent CIE cargo proteins and demonstrate that they do not traffic to late endosomes to the same extent as MHCI. We show that multiple CIE cargo proteins are affected by MARCH expression, leading to increased trafficking to late endosomes. Most strikingly, expression of MARCH8 for CD44 and MARCH1 or MARCH8 for CD98 causes a drastic change in their trafficking after endocytosis, switching them from robust recycling with little delivery to EEA1 compartments and late endosomes to rapid delivery to EEA1 compartments and degradation in late endosomes. Thus MARCH-mediated ubiquitination can alter the intracellular trafficking and fate of CIE cargo proteins.
RESULTS
CD44, CD98, and CD147 avoid trafficking to late endosomes
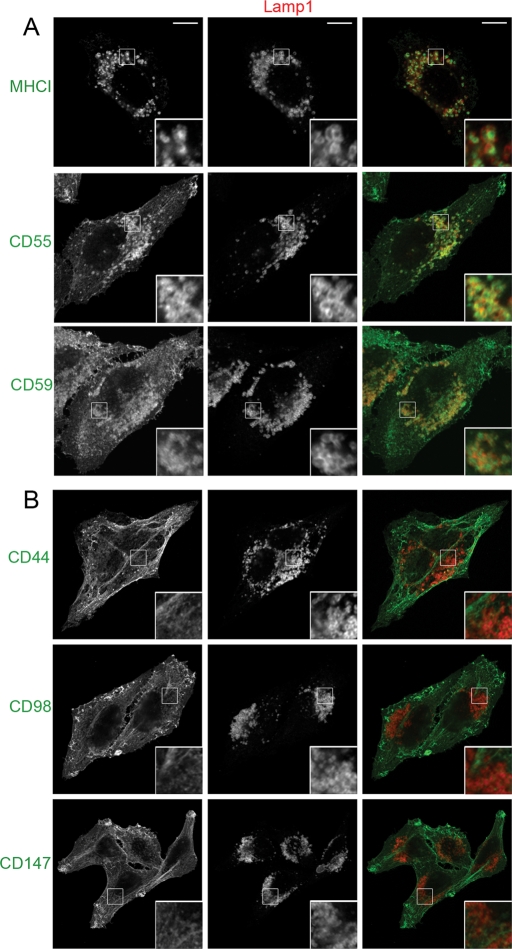
Previous work from our laboratory demonstrated that a new group of endogenous CIE cargo proteins, CD44, CD98, and CD147, follow a different intracellular itinerary than MHCI and other CIE cargo proteins, including CD55, CD59, and GLUT1 (Eyster et al., 2009). Although MHCI reaches endosomes associated with EEA1, the proteins CD44, CD98, and CD147 do not appreciably reach these endosomes. Given that EEA1-associated compartments have been associated with routing to lysosomes (Leonard et al., 2008), we hypothesized that the delivery of CD44, CD98, and CD147 to late endosomes and lysosomes might be reduced compared with that of other CIE cargo proteins. To track CIE cargo endocytosis, recycling, and delivery to a variety of intracellular compartments, HeLa cells were incubated with monoclonal antibodies directed to the extracellular portions of these proteins for 1 h. Then, to follow the trafficking of CIE cargo proteins to late endosomes, we treated cells overnight with the proton ionophore NH4Cl to neutralize the pH of the late endosome and block degradation, allowing visualization of delivered cargos. In HeLa cells treated with NH4Cl, MHCI accumulated inside enlarged late endocytic structures marked by Lamp1 after 24 h (Figure 1A). CD55 and CD59, two GPI-anchored CIE cargo proteins, also accumulated in late endosomes after 24 h. Of interest, CIE cargo proteins CD44, CD98, and CD147 did not accumulate in late endosomes over the same time period but remained on the cell surface and in CIE recycling tubules (Figure 1B). Similar results were obtained when 0.5 mM leupeptin, a protease inhibitor, was added to the culture media instead of NH4Cl to block lysosomal degradation (unpublished data). In addition to analyzing the delivery of CIE cargo proteins using antibody internalization, we also visualized the steady-state distributions of CIE cargo proteins after overnight NH4Cl treatment to be sure that the localization of these proteins in late endosomes was not due to surface antibody binding inducing altered trafficking of the cargo. Indeed, this was not the case, as MHCI colocalized with Lamp1 after cells were incubated for 24 h with NH4Cl (Supplemental Figure S1A), whereas CD44, CD98, and CD147 did not colocalize with Lamp1 after 24 h with NH4Cl (Supplemental Figure S1B). CD59 and GLUT1, a CIE cargo for which we lack an antibody suitable for internalization assays, also accumulated in late endosomes after 24 h (Supplemental Figure S1A).
FIGURE 1:
Clathrin-independent cargo proteins show differential trafficking to late endosomes. HeLa cells were incubated with antibodies to MHCI, CD55, or CD59 (A) and CD44, CD98, or CD147 (B) for 1 h to allow endocytosis to occur. Cells were washed with media and then transferred to media containing 25 mM NH4Cl for 24 h. Cells were then fixed as described in Materials and Methods, and the internalized antibodies were detected with Alexa 488 (green) goat anti–mouse secondary antibodies. Lamp1 was immunolabeled with a rabbit antibody and an Alexa 594 (red) goat anti–rabbit secondary antibody to detect late endosomal and lysosomal structures. Images were acquired as described in Materials and Methods. Bars, 10 μm.
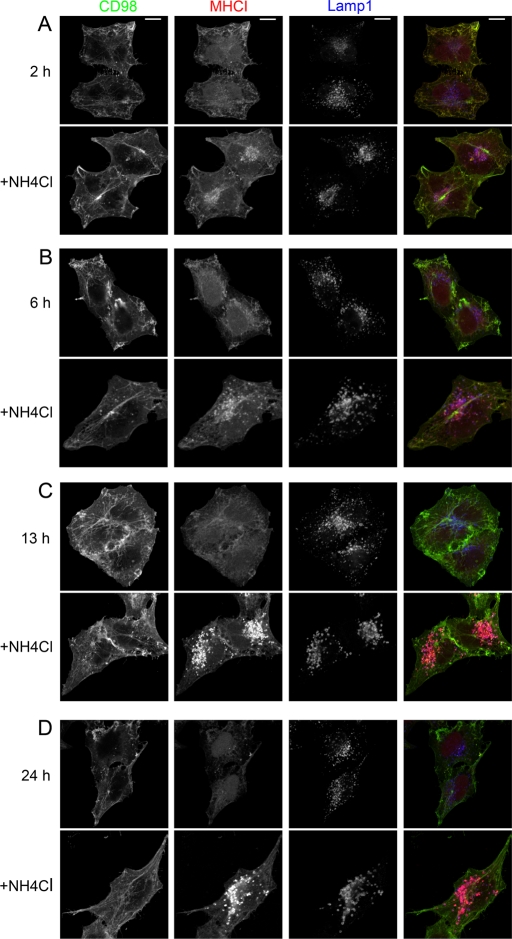
We also sought to visualize the separation of the two types of CIE cargo proteins by following the fate of internalized CD98 and MHCI together in the presence and absence of NH4Cl over time. In the absence of NH4Cl, we would predict that the antibody signal for degraded proteins would be reduced over time. Indeed, in cells where degradation was not inhibited, we observed a substantial reduction of MHCI antibody signal at 13 h (Figure 2C, top) and 24 h (Figure 2D, top), whereas CD98-bound antibody signal remained strong and associated with the cell surface and in tubular endosomal compartments. In contrast, in the presence of NH4Cl to inhibit degradation, as early as 2 and 6 h, MHCI antibody could be observed in enlarged juxtanuclear late endosomes, identified by labeling with antibody to Lamp1 (Figure 2, A and B, bottom). The distribution and the signal of CD98 antibody are not altered with this treatment at 2 and 6 h (Figure 2, A and B, bottom), although at 24 h (Figure 2D, bottom) and later (unpublished data) a small amount of CD98 can be observed colocalized with MHCI in enlarged juxtanuclear late endosomes. Thus, as shown by visualizing CIE cargo proteins directly or by following their trafficking, CD44, CD98, and CD147 did not appreciably accumulate in late endosomes after 24 h, in contrast to other CIE cargo proteins, MHCI, CD55, CD59, and GLUT1.
FIGURE 2:
CD98 does not accumulate in late endosomes over similar time periods as MHCI. Primary antibodies to CD98 or MHCI were added to HeLa cells to allow internalization for 1 h. Cells were washed and transferred to fresh media without (top) or with 25 mM NH4Cl (bottom). After 2 (A), 6 (B), 13 (C), and 24 (D) h cells were washed and fixed. A rabbit antibody to Lamp1 was used with an Alexa 633 (blue) secondary antibody to detect LE. Isotype-specific secondary antibodies to IgG1 488 (green) and to IgG2a 594 (red) were used to detect CD98 and MHCI, respectively. Bars, 10 μm.
MARCH protein expression causes specific removal of CIE cargo proteins from the cell surface
We hypothesized that the differential delivery of select CIE cargo proteins to late endosomes might be mediated by ubiquitination of the cytoplasmic domains of these proteins. Because expression of either the MARCH4 or MARCH9 E3 ubiquitin ligase causes ubiquitination of the MHCI cytoplasmic tail and down-regulation through a lysosomal mediated pathway (Bartee et al., 2004), we tested to see whether other CIE cargo proteins could be affected by MARCH protein overexpression.
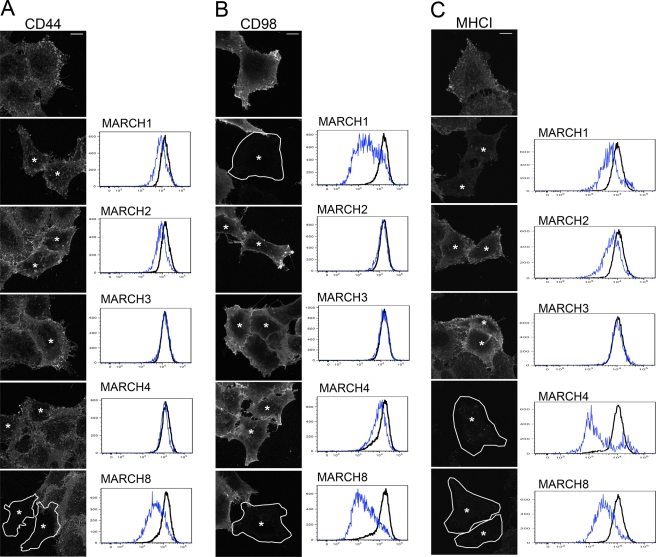
We examined the effects of expression of FLAG-tagged MARCH1, 2, 3, 4, and 8 on surface levels of CIE cargo proteins. These MARCH proteins were selected because they are predicted to be two-pass transmembrane proteins and have structures similar to those of K3 and K5 (Lehner et al., 2005). We monitored endogenous cell surface protein levels using immunofluorescence and flow cytometry analysis in cells overexpressing MARCH proteins. We found that CD44 was specifically removed from the cell surface by MARCH8 expression (Figure 3A) and that surface staining for CD98 was down-regulated by the expression of either MARCH1 or MARCH8 (Figure 3B). This differential sensitivity to MARCH proteins was somewhat unexpected because MARCH1 and MARCH8 are highly homologous proteins, and CD44 and CD98 reside in the same endosomal compartments, suggesting that slight differences between MARCH1 and MARCH8 can provide differences in substrate specificity. MARCH8 was also recently observed to down-regulate CD44 by using stable isotope labeling of amino acids in cell culture (Bartee et al., 2010), consistent with our findings here. As expected, we observed that expression of MARCH4 drastically diminished cell surface levels of MHCI (Figure 3C). We also observed that expression of untagged MARCH9 (but not tagged versions) led to diminished levels of surface MHCI (unpublished data); however, we were unable to assess its effect on CD44 or CD98, as we lacked suitable antibodies for double labeling. In addition, MARCH8 expression also diminished surface MHCI (Figure 3C). The same MARCH substrate specificity was observed in a human retinal pigment epithelial cell line, ARPE19; surface CD98 was reduced in cells expressing MARCH1 or MARCH8, and CD44 was reduced only in cells expressing MARCH8 (Supplemental Figure S2). Neither CD44 nor CD98 was affected by expression of MARCH4. Therefore we can conclude that MARCH protein expression results in reductions of cell surface staining of specific CIE cargo proteins.
FIGURE 3:
Effect of MARCH protein expression on surface levels of CD44, CD98, and MHCI. HeLa cells were transfected with the indicated MARCH-FLAG construct as described. After 18 h, the surface of the cells was labeled with mouse antibodies to CD44, CD98, and MHCI and processed for either immunofluorescence or flow cytometry. In fluorescence images, MARCH-transfected cells (as detected by rabbit anti-FLAG staining; not shown) are indicated by an asterisk and outlined in some cases. Graphs show the Alexa 488 staining intensity (x-axis) versus cell number (y-axis) for surface CD44 (A), CD98 (B), or MHCI (C) in control cells (black line) vs. indicated MARCH-expressing cells (blue line) from flow cytometry analysis. Bars, 10 μm.
Next we examined whether MARCH protein expression would affect other CIE cargo proteins. Given that the suggested mode of action of MARCH proteins is the ubiquitination of the cytoplasmic tails of transmembrane proteins, we tested whether the GPI-anchored, CIE cargo proteins CD55 and CD59, which lack cytoplasmic tails, would be affected by MARCH expression. Indeed, as would be predicted, the surface staining of neither CD55 (Supplemental Figure S3A) nor CD59 (unpublished data) was reduced by overexpression of any MARCH protein tested. Surface CD147, the other CIE cargo that lacks significant colocalization with EEA1, was relatively unaffected by MARCH protein expression as tested via immunofluorescence, although some reduction with MARCH4 and MARCH8 expression was observed by flow cytometry (Supplemental Figure S3B). The transferrin receptor (TfnR), a CDE cargo protein, was previously shown to be down-regulated by expression of MARCH1, MARCH2, or MARCH8 (Bartee et al., 2004; Nakamura et al., 2005). We also detected significant down-regulation of TfnR in cells expressing MARCH1, MARCH2, or MARCH8 in addition to those expressing MARCH4 (Supplemental Figure S3C). We did not detect any significant changes in any cell surface protein examined in cells expressing MARCH3.
MARCH expression changes trafficking of CIE cargo proteins
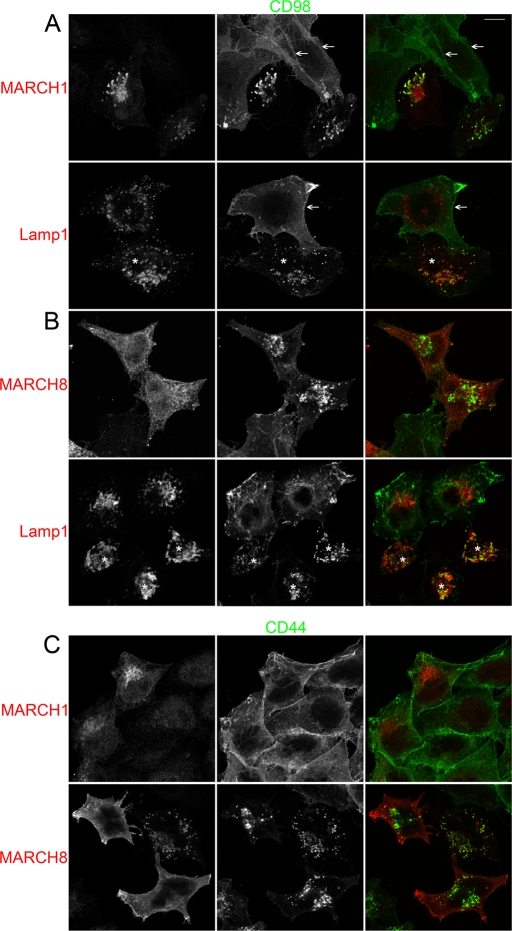
Previous work suggested that MARCH expression caused increased routing of MHCI to lysosomes and degradation (Bartee et al., 2004). Therefore we tested by antibody internalization for 1 h, followed by chase for 2 h in NH4Cl, whether accumulation of cargo in late endosomes was increased in MARCH-expressing cells. Indeed, we detected increased MHCI delivery to late endosomes in MARCH4-expressing cells at 2 h after the addition of NH4Cl (Supplemental Figure S4A), consistent with previous findings (Bartee et al., 2004). We then tested to see whether expression of specific MARCH proteins would also enhance delivery to late endosomes of CD98 and CD44. We found extensive colocalization of internalized CD98 antibody with Lamp1-positive compartments after only 2 h of chase with NH4Cl in MARCH1- and MARCH8-expressing cells (Figure 4, A and B). This is a dramatic change in trafficking of CD98 compared with nontransfected cells, where CD98 continues to be present at the PM and in tubular endosomal compartments (Figure 4A, arrows), and from the minimal CD98 delivery to late endosomes after 24 h in the absence of MARCH expression (Figure 1B and Supplemental Figure S1B). MARCH1 and MARCH8 are often observed in late endosomes (Supplemental Figure S4B), suggesting that the intracellular colocalization between MARCH1 and MARCH8 with CD98 is indeed in late endosomes. We detected increased CD44 delivery to late endosomes, similar to CD98, in cells expressing MARCH8 but not MARCH1 (Figure 4C). Therefore the specificity that we observed for MARCH-induced accelerated trafficking to late endosomes correlates with the specificity observed for MARCH-induced removal from the cell surface. The effect of specific MARCH protein expression on CD44 and CD98 is particularly striking as it completely alters their trafficking itinerary, causing accumulation in late endosomes within 2 h, whereas normally these proteins are barely detectable in late endosomes after 24 h.
FIGURE 4:
MARCH expression increases CIE cargo delivery to late endosomes. HeLa cells were transfected with either MARCH1-FLAG (A, C) or MARCH8-FLAG (B, C) constructs for 18 h. Primary antibodies to CD44 (C) or CD98 (A, B) were added to cells and allowed to internalize for 1 h. Cells were washed and transferred to media containing 25 mM NH4Cl for 2 h. Mouse antibodies to CD44 and CD98 were detected with anti–mouse Alexa 488 (green) and rabbit anti-FLAG or rabbit anti-Lamp1 with anti–rabbit Alexa 594 (red). Cells indicated with an asterisk in A and B, bottom, are assumed to be MARCH1-FLAG and MARCH8-FLAG transfected, respectively, due to the large change in CD98 localization only observed in MARCH1-FLAG– or MARCH8-FLAG–transfected cells as shown at the top. Cell surface and recycling tubule staining for CD98 are indicated with arrows in A. Bars, 10 μm.
The localizations observed by immunofluorescence for expressed MARCH proteins varied from cell to cell, with MARCH proteins localized throughout the secretory pathway (endoplasmic reticulum [ER], Golgi, endosomes, and lysosomes). We did observe in some cells localization of MARCH1 and MARCH8 on the tubular recycling endosomes that carry CIE cargo back to the PM. However, the phenotypes observed upon MARCH expression in individual cells did not seem to depend on the particular localization observed nor on level of expression of the MARCH protein.
CIE cargo proteins still enter by CIE in MARCH-expressing cells
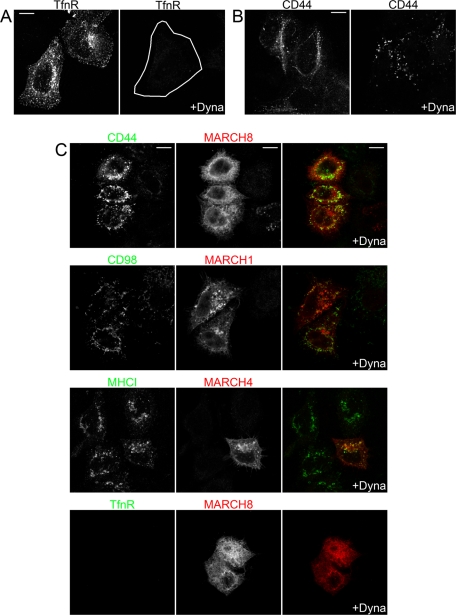
The rapid rerouting of CD44 and CD98 to late endosomes in cells expressing MARCH proteins raised the question as to whether these cargo proteins still entered cells by CIE or whether CDE would be used. Ubiquitination was suggested as a recognition motif for certain clathrin endocytic adaptors (Acconcia et al., 2009), and a previous report, using small interfering RNA (siRNA) knockdown of clathrin heavy chain or the ubiquitin adaptor protein epsin1, suggested that down-regulation of MHCI in cells expressing the viral K3 E3 ligase required clathrin and epsin1 for internalization (Duncan et al., 2006). However, at short times (5 min) of internalization in MARCH8-expressing cells, CD98 (Figure 5A) and CD44 (unpublished data) were found in endosomes distinct from those containing transferrin (Tfn), consistent with CD44 and CD98 still entering cells by CIE in MARCH-expressing cells. Furthermore, we tested directly whether CIE cargo proteins colocalized with clathrin heavy chain or epsin 1 on the surface of MARCH-expressing cells, which would suggest their entry by CDE. In HeLa cells expressing MARCH1, MARCH4, or MARCH8, we detected no significant colocalization between surface-stained CD98, MHCI, or CD44, respectively, with clathrin heavy chain (Figure 5, B–D). Indeed, the surface staining pattern for CD44, CD98, and MHCI in MARCH-expressing cells is similar to their normal staining, albeit at a much reduced level, and does not resemble the punctate appearance of clathrin heavy chain. In addition, we saw no colocalization between epsin1 and surface-stained CD44, CD98, or MHCI in MARCH-expressing HeLa cells (Supplemental Figure S5, A–C). By contrast, TfnR, a CDE cargo protein, colocalized with both clathrin heavy chain and epsin1 in cells expressing MARCH8 (Figure 5E and Supplemental Figure S5D), similar to its colocalization in untransfected cells. Finally, we tested whether acute inhibition of CDE using the dynamin inhibitor Dynasore (Macia et al., 2006) would block endocytosis in MARCH-expressing cells. Pretreatment for 1 h with 80 μM Dynasore blocked TfnR antibody uptake (Figure 6A), as shown by others (Macia et al., 2006), but endocytosis of CD44 (Figure 6B), CD98 (unpublished data), and MHCI (nontransfected cells in Figure 6C) was not affected by Dynasore treatment. In MARCH-expressing cells, CD44, CD98, and MHCI were still internalized in the presence of Dynasore (Figure 6C), whereas TfnR endocytosis was blocked (Figure 6C, bottom). The lack of significant colocalization between CIE cargo proteins and either clathrin or transferrin in MARCH-expressing cells and the lack of Dynasore sensitivity suggest that CD44, CD98, and MHCI continue to be internalized by CIE, and not CDE, in MARCH-expressing cells.
FIGURE 5:
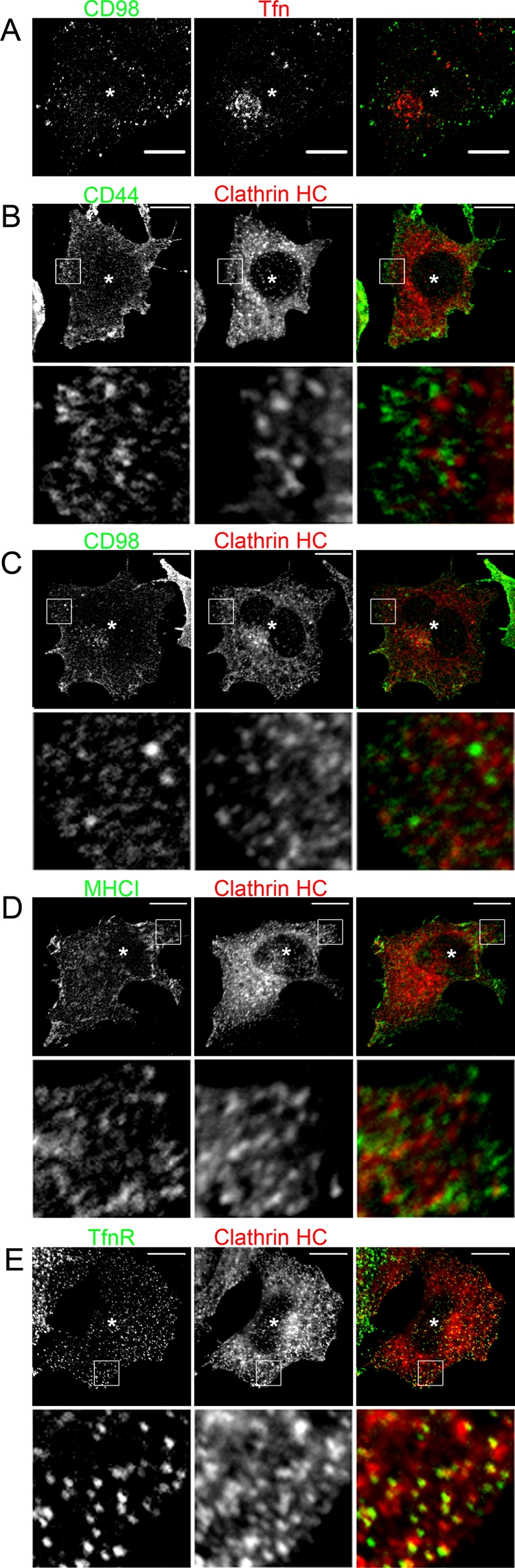
CIE cargo proteins do not internalize with Tfn or colocalize with surface clathrin in MARCH-expressing cells. (A) HeLa cells were transfected with MARCH8-FLAG. After 18 h, CD98 antibody and Tfn633 were added to cells and allowed to internalize for 5 min. Surface CD98 antibody and Tfn633 were removed with acid wash and fixed. MARCH8-FLAG was stained with a rabbit anti-FLAG antibody. CD98 and FLAG antibodies were detected with Alexa 488 anti–mouse and Alexa 594 anti–rabbit secondary antibodies, respectively. Tfn633 is pseudo-colored red, and asterisk denotes a MARCH8-FLAG–stained cell. In B–E, HeLa cells were transfected with plasmids encoding MARCH1-FLAG (C), MARCH8-FLAG (B, E), or MARCH4-FLAG (D). After 18 h, cells were fixed and incubated with primary antibodies to CD44 (B), CD98 (C), MHCI (D), and TfnR (E) in the absence of saponin to label surface antigen. Clathrin heavy chain (HC) was detected using a rabbit antibody in the presence of saponin. Surface antibodies were detected with Alexa 488 anti–mouse secondary and rabbit antibodies to clathrin HC with an Alexa 594 anti–rabbit secondary. Asterisk denotes MARCH-expressing cells identified as in Figure 4. Bars, 10 μm.
FIGURE 6:
Dynasore inhibits internalization of CDE but not CIE cargo proteins in MARCH-expressing cells. (A, B) Untransfected HeLa cells were pretreated with 80 μM Dynasore (+Dyna) or vehicle control for 1 h, and then monoclonal antibodies to TfnR (A) or CD44 (B) were added and allowed to internalize for 30 min in the continued presence of Dynasore or vehicle control. Surface antibody to TfnR and CD44 was removed by acid wash, cells were then fixed, and internalized CD44 and TfnR antibodies were detected with Alexa 594 anti–mouse secondary antibodies. (C) HeLa cells transfected with indicated MARCH-FLAG constructs were allowed to internalize monoclonal antibodies to indicated CIE cargos or TfnR in the presence of Dynasore as before. Surface antibodies were removed with acid wash, and cells were fixed and stained with a rabbit anti-FLAG antibody. Internalized antibodies to cargos and the rabbit anti-FLAG antibody were detected with Alexa 488 anti–mouse secondary and Alexa 594 anti–rabbit secondary antibodies. Bars, 10 μm.
MARCH expression leads to increased localization in EEA1-marked sorting endosomes
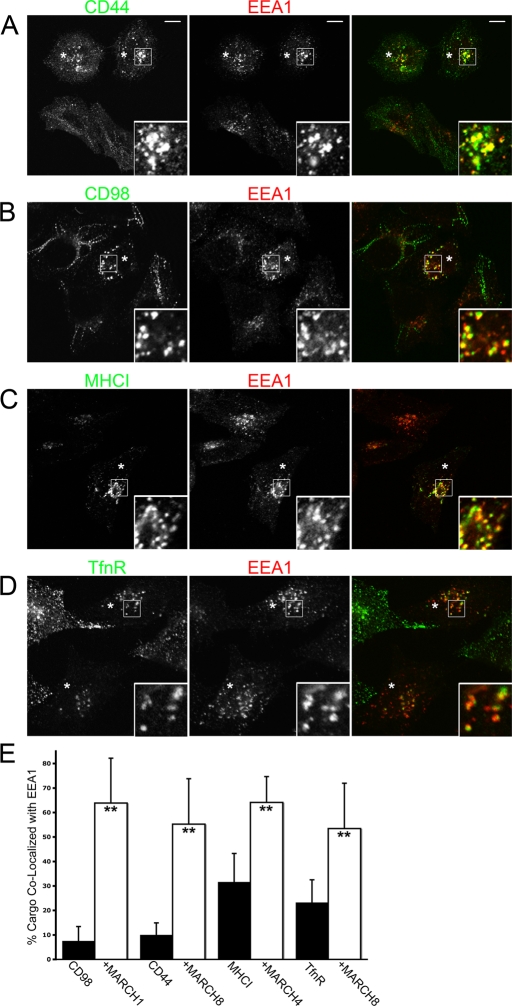
As we previously reported, internalized CD44, CD98, and CD147 show minimal localization with EEA1-associated sorting endosomes compared with MHCI in HeLa cells (Eyster et al., 2009). This reduced localization of CD44, CD98, and CD147 to EEA1 compartments correlates with their minimal delivery to late endosomes (Figure 1). Therefore we tested whether expression of MARCH proteins capable of downregulating CD44 and CD98 would now divert their trafficking to EEA1 compartments at short (30 min) times of internalization. Indeed, we found that CD44 colocalized with EEA1 only in MARCH8-expressing cells, and CD98 colocalized with EEA1 in MARCH1- or MARCH8-expressing cells (Figure 7, A and B, where MARCH-expressing cells, inferred by altered cargo distribution, are indicated with an asterisk). In the nontransfected, control cells, there was little to no colocalization of CD44 and CD98 with EEA1, as observed previously (Eyster et al., 2009), in contrast to MHCI, which colocalized with EEA1 in the absence (Eyster et al., 2009) and in the presence of MARCH4 expression (Figure 7C). We also tested whether TfnR would show increased localization with EEA1 in the presence of MARCH8, suggesting a diversion from recycling to degradation. In cells expressing MARCH8, we saw a greater degree of colocalization (Figure 7D) when compared with nontransfected controls. We quantified the change in degree of colocalization of each cargo with EEA1 in MARCH-expressing cells. As expected, in untransfected cells, there was minimal colocalization of CD44 and CD98 with EEA1 (<10%), but this increased to greater than 50% in MARCH1- and MARCH8-expressing cells, respectively (Figure 7E). Furthermore, for MHCI and TfnR, which both exhibit some colocalization with EEA1 in untransfected cells (20–30%), there was a twofold increase (50–60%) in colocalization with EEA1 in MARCH4 and MARCH8-expressing cells (Figure 7E). Thus the expression of MARCH proteins initiates a change in the endosomal trafficking itinerary of their substrates, with the trafficking of CD44 and CD98 dramatically altered such that these proteins now are routed to EEA1-associated endosomes and onto lysosomes for degradation.
FIGURE 7:
CD44, CD98, MHCI, and TfnR show increased localization with EEA1 in MARCH-expressing cells. HeLa cells transfected with MARCH1-FLAG (B), MARCH4-FLAG (C), or MARCH8-FLAG (A, D) were identified as in Figure 4 and are indicated with an asterisk. Primary antibodies to CD44 (A), CD98 (B), MHCI (C), or TfnR (D) were added to cells and allowed to internalize for 30 min. Mouse antibodies to CD44, CD98, MHCI, and TfnR were detected with anti–mouse Alexa 488 (green) and rabbit anti-EEA1 with anti–rabbit Alexa 594 (red). Bar, 10 μm. (E) The percentage of each cargo colocalizing with EEA1 in the absence (black bars) or presence (clear bars) of the indicated MARCH protein was quantified using Metamorph software. **Statistical significance at p < 0.001 between indicated cargo in control (black bars) and MARCH-expressing cells (clear bars). Bars, 10 μm.
TSG101 is required for MARCH8-induced trafficking of CD44 and CD98 to late endosomes
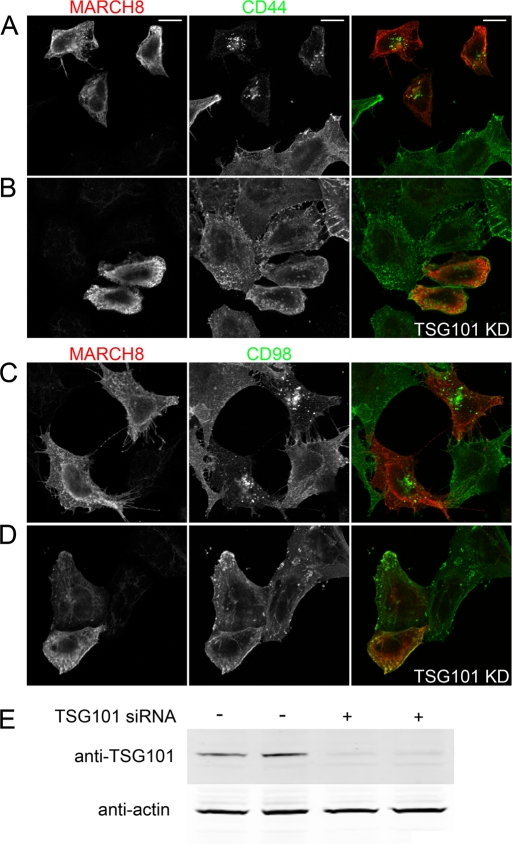
Having determined that MARCH overexpression leads to a dramatic change in the trafficking of CIE cargo proteins, routing them toward late endosomes and lysosomes for degradation, we examined whether the ESCRT complex machinery was involved. The ESCRT machinery consists of multiple protein complexes that sequentially recognize ubiquitinated transmembrane proteins on endosomes and package those cargos into multivesicular bodies (MVBs) that can fuse with lysosomes to complete the degradation of the designated cargo protein (Saksena et al., 2007). If the ESCRT complex is involved in MARCH-induced CIE cargo down-regulation, this would provide a mechanistic link between the increased EEA1 localization for CIE cargos in MARCH-expressing cells (Figure 7) and the increased delivery of CIE cargos to late endosomes in MARCH-expressing cells (Figure 4). TSG101, an essential component of the ESCRT-1 complex, is required for efficient recognition of ubiquitinated cargo and trafficking into the intralumenal vesicles of MVBs (Razi and Futter, 2006). We knocked down TSG101 expression to 25% of control cells (Figure 8E) and examined whether MARCH8-induced effects would be abrogated. Examining the steady-state distribution of CD44 and CD98 in MARCH8-expressing cells, we found that they localized to large late endosomal structures in mock-depleted cells (Figure 8, A and C). However, in cells depleted of TSG101, the MARCH8-induced phenotype was inhibited, and CD44 and CD98 localized primarily to the cell surface, similar to nontransfected cells (Figure 8, B and D). Thus TSG101 function is required for MARCH-induced trafficking of CIE cargo proteins to late endosomes. This is in agreement with findings of ESCRT involvement in K3-mediated down-regulation of MHCI (Hewitt et al., 2002), K5-mediated down-regulation of ALCAM (Bartee et al., 2006), and MARCH9-mediated down-regulation of SLAM (Hor et al., 2009).
FIGURE 8:
TSG101 knockdown reverses the effect of MARCH expression on CIE cargo proteins. HeLa cells were mock transfected (A, C) or transfected with an siRNA to TSG101 for 24 h (B, D). Cells were subsequently transfected with MARCH8-FLAG for 18 h (A–D). Total CD44 (A, B) or CD98 (C, D) distribution was detected with mouse antibodies and MARCH8-FLAG transfectants with a rabbit anti-FLAG antibody. Primary antibodies were detected with Alexa 488 (green) anti–mouse secondary and Alexa 594 anti–rabbit secondary, respectively, in mock (A, C) and TSG101 knockdown cells (B, D). (E) Western blot of mock and TSG101 knockdown lysates from replicate cultures. TSG101 protein levels (relative to actin) were reduced to 25% of control levels in siRNA-treated cells. Bars, 10 μm.
CD98 is ubiquitinated in MARCH8-expressing cells
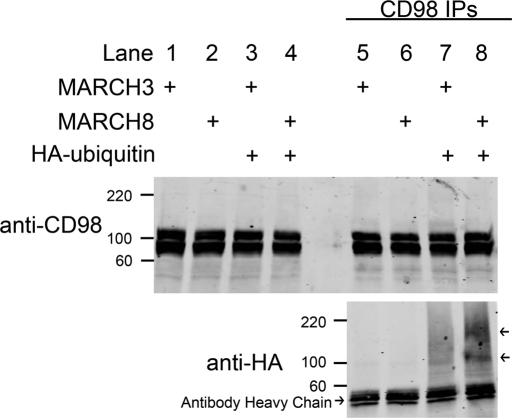
To test whether MARCH8 expression led to the ubiquitination of our endogenous CIE cargo proteins, we examined whether CD98 was ubiquitinated in MARCH8-expressing cells. CD98 was chosen since antibodies capable of immunoprecipitation were available. We transfected HeLa cells with MARCH8 and hemagglutinin (HA)-tagged ubiquitin for 18 h and then treated the cells with concanamycin A (Kataoka et al., 1994) overnight to accumulate ubiquitinated cargo proteins. As a transfection control, we transfected MARCH3 and HA-ubiquitin into other cells since MARCH3 expression was not observed to affect any aspect of CD98 trafficking (Figure 3). CD98 was then immunoprecipitated and probed for the presence of the HA-ubiquitin. HA-immunoreactive bands were readily detected in CD98 immunoprecipitates from MARCH8-expressing cells (Figure 9, arrows) but not from MARCH3-expressing cells. These bands migrated in the gel with molecular weights consistent with CD98 with multiple ubiquitin resides attached. This finding suggests that endogenous CD98, or a protein tightly associated with CD98, is ubiquitinated in MARCH8-expressing cells. To demonstrate that CD98 is directly ubiquitinated, proteins are typically solubilized from cells in denaturing buffer to release associations with other proteins and then immunoprecipitated with specific antibodies. Unfortunately, our antibody to CD98 would not immunoprecipitate under these conditions, so we turned to examining the direct ubiquitination of an expressed CD98 chimeric protein containing the SNAP tag.
FIGURE 9:
Ubiquitin is present in immunoprecipitated endogenous CD98 complexes in MARCH8-expressing cells. HeLa cells were transfected with MARCH3-FLAG or MARCH8-FLAG with or without HA-ubiquitin. After 18 h, CD98 was immunoprecipitated from whole-cell lysates as described in Materials and Methods. Ten percent of whole-cell lysates were immunoblotted with anti-CD98 antibodies (lanes 1–4). Molecular weights are identified in kDa. CD98 immunoreactive bands were detected at ∼90 and 110 kDA. Twenty percent of recovered CD98 immunoprecipitates were immunoblotted with anti-CD98 or anti-HA antibodies (lanes 5–8). Two HA-reactive bands were present in the MARCH8-expressing CD98 immunoprecipitates (lane 8 arrows).
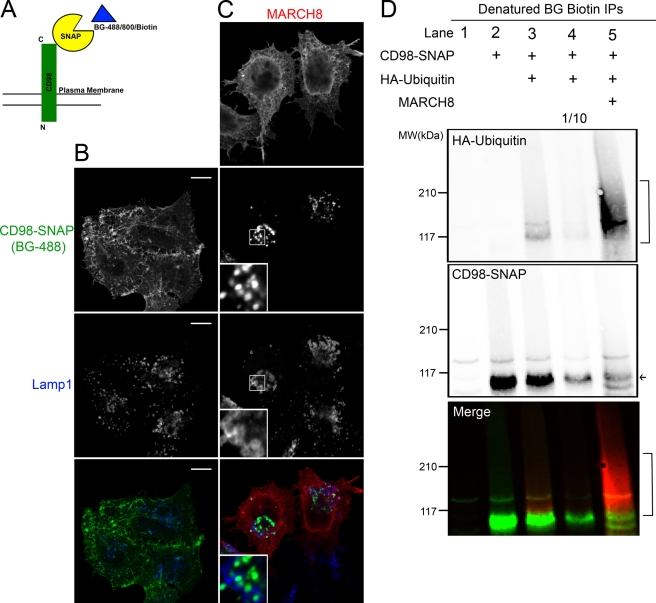
The SNAP tag is an engineered form of a 20-kDa DNA repair enzyme (O6-alkylguanine-DNA alkyltransferase) that binds covalently to O6-benzylguanine (BG) (Gautier et al., 2008), which can be attached to Alexa dyes and biotin (see Materials and Methods). We appended the SNAP tag to the carboxy terminus of CD98, a type II membrane protein, allowing labeling with multiple BG-conjugated ligands (Figure 10A). HeLa cells expressing CD98-SNAP were exposed to BG-488 for 1 h at 37°C to label the surface and load the endosomal compartment. Then the free BG was removed by multiple washes, and the cells were chased for 2 h in media containing 15 mM NH4Cl to follow the trafficking of CD98-SNAP similar to experiments with endogenous CD98. Similar to our observations for endogenous CD98 (Figure 4A), CD98-SNAP, when expressed alone, was primarily observed at the PM and in tubular endosomes with little colocalization with Lamp1 late endosomal compartments (Figure 10B). In cells expressing CD98-SNAP and MARCH8, however, CD98-SNAP was observed colocalized with Lamp1-associated compartments (Figure 10C), similar to the altered trafficking observed for endogenous CD98.
FIGURE 10:
CD98-SNAP traffics as CD98 does and is directly ubiquitinated in MARCH8-expressing cells. (A) Schematic diagram of CD98-SNAP interaction with BG ligands. Binding of BG to SNAP produces a covalent bond between the SNAP and BG. HeLa cells expressing CD98-SNAP with (C) or without (B) MARCH8-FLAG were labeled with BG-488 for 1 h and chased in NH4Cl for 2 h. A rabbit anti-Lamp1 and mouse anti-FLAG were used with Alexa 633 anti–rabbit and Alexa 594 anti–mouse secondary antibodies to visualize late endosomes and MARCH-transfected cells respectively. Bars, 10 μm. (D) BG-biotin–labeled CD98-SNAP was immunoprecipitated from denatured lysates of cells expressing the indicated proteins and separated on SDS–PAGE and immunoblotted as described in Materials and Methods. HA-ubiquitin was detected with mouse anti-HA and an Alexa 680 anti–mouse secondary antibody (red in merged blot). The biotin-labeled CD98-SNAP was detected with NeutrAvidin, DyLight-800 Conjugated (green in merged blot). Lane 4 was loaded with 1/10 of the total protein of lane 3, which is equivalent to the amount of CD98-SNAP in lane 5 (in MARCH8-expressing cells), to directly compare the ubiquitination levels for equivalent amounts of CD98-SNAP with and without MARCH8 expression. The arrow shows CD98-SNAP, and brackets indicate ubiquitinated forms of CD98-SNAP.
Next we set out to examine whether CD98 itself was directly ubiquitinated in MARCH8-expressing cells by transfecting cells with CD98-SNAP, MARCH8, and HA-ubiquitin. HeLa cells were labeled with BG-biotin for 4 h, and then the biotin-labeled CD98-SNAP was isolated on streptavidin beads after SDS denaturation and boiling of the lysate to ensure that CD98-SNAP alone was isolated. In the absence of HA-ubiquitin and MARCH expression, CD98-SNAP was visible on the blot (Figure 10D, lane 2) at the expected size of ∼115 kDa. When HA-ubiquitin was also expressed, a band of HA-ubiquitin that runs slightly above the size of the CD98-SNAP was visible (Figure 10D, lane 3). However, when MARCH8 was also expressed, a large amount of HA-ubiquitin was observed associated with the isolated CD98-SNAP (Figure 10D, lane 5), suggesting that CD98-SNAP is heavily ubiquitinated in MARCH8-expressing cells. The efficiency of MARCH8 down-regulation of CD98-SNAP was also revealed by the low level of labeling of the CD98-SNAP in MARCH8-expressing cells (Figure 10D, lane 5). When we loaded equivalent amounts of CD98-SNAP from cells not expressing MARCH8 (Figure 10D, lane 4), it was clear that MARCH8 caused elevated levels of ubiquitination (compare HA-ubiquitin in lanes 4 and 5). Taken together, these findings demonstrate that MARCH8 directly ubiquitinates CD98, and this likely leads to its routing to late endosomes for degradation.
DISCUSSION
Little is known about how membrane proteins and lipids at the cell surface are monitored and selected for degradation in late endosomes and lysosomes. Here we show that the CIE cargo proteins (MHCI, CD59, CD55) that reach EEA1 compartments (Eyster et al., 2009) are routed to late endosomes for degradation, and the CIE cargo proteins (CD44, CD98, and CD147) that avoid EEA1 compartments (Eyster et al., 2009) also avoid degradative compartments. In an attempt to understand this differential sorting and turnover of CIE cargo proteins, we expressed E3 ubiquitin ligases of the MARCH family to see whether they would affect trafficking and degradation of CIE proteins. We found that expression of MARCH8 dramatically altered the itinerary of CD44 and CD98, now causing these proteins to pass through EEA1 compartments on their way to late endosomes and degradation. These findings have given us new insight into how PM proteins turn over in cells and the role of ubiquitination in altering endosomal membrane traffic.
We used MARCH protein overexpression as a way to explore whether ubiquitination could alter CIE cargo trafficking. We found that surface CD44 is down-regulated by overexpression of MARCH8, surface CD98 by MARCH1 and MARCH8, and surface MHCI by MARCH 4 and 8 as previously documented (Bartee et al., 2004). We showed for endogenous CD98 and CD98-SNAP that MARCH8 expression leads to the appearance of ubiquitinated forms of CD98. In addition, we showed direct ubiquitination of CD98-SNAP in MARCH8-expressing cells. This suggests that MARCH proteins can alter the trafficking of their substrates by direct ubiquitination of cytosolic lysines. The altered trafficking for ubiquitinated CD44 and CD98 leads them to now visit EEA1 compartments en route to late endosomes.
Whether endogenous MARCH proteins are responsible for the turnover of CIE cargo proteins remains to be determined. MARCH proteins are in general widely expressed, with the exception of MARCH1, which is more restricted to immune cells (Bartee et al., 2004). There is evidence that MARCH1 levels are up-regulated in interleukin-10–treated monocytes (Thibodeau et al., 2008) and down-regulated during maturation of dendritic cells (De Gassart et al., 2008). MARCH1 appears to have a short half-life of <30 min (Jabbour et al., 2009), allowing changes in mRNA levels to be quickly evident at the protein level. Little is known about the regulation of expression of other MARCH proteins, but given that MARCH8 can specifically ubiquitinate and down-regulate CD44 and CD98, it would be interesting to examine whether MARCH8 expression levels are regulated in nonimmune cells.
The issue of where MARCH substrates are ubiquitinated and where and how the ubiquitinated cargo is recognized by the cell and sorted into the degradative pathway has not been thoroughly examined and is difficult to study. In addition to numerous E3 ubiquitin ligase activities in cells, there are active deubiquitinylation enzyme systems that continually remove ubiquitin from the substrates (Clague and Urbe, 2006; Acconcia et al., 2009). Some have suggested that cellular localization of MARCH proteins could be important for determining substrate specificity (Bartee et al., 2004). Unfortunately, there are as yet no antibodies capable of localizing endogenous MARCH proteins. We found that expressed MARCH1 and 8 localized predominantly at the PM and endosomes, MARCH2 and 3 at the ER, and MARCH4 and 9 primarily at the Golgi and trans-Golgi network, in agreement with previous observations (Bartee et al., 2004). However, each MARCH protein tested could be observed on all parts of the secretory pathway, and differential MARCH localization did not match with differential substrate specificity. Thus MARCH substrate specificity cannot be solely explained by differential subcellular localization.
The high sequence homology between MARCH1 and MARCH8, MARCH4 and MARCH9, and MARCH2 and MARCH3 was suggested to lead to overlapping functions for these pairs of similar proteins (Bartee et al., 2004). Indeed, in our hands this pairing holds true for CD98, as both MARCH1 and MARCH8 have similar effects on its trafficking and degradation. Because CD44 and CD98 have identical cellular localizations in HeLa cells, it seems likely that CD44 would encounter overexpressed MARCH1, yet it is not significantly down-regulated in MARCH1-expressing cells. Therefore sequence differences between MARCH1 and MARCH8 must provide the necessary specificity for CD44 to be efficiently down-regulated by MARCH8 but not by MARCH1. We are planning experiments to probe sequence differences between MARCH1 and MARCH8 in order to determine how MARCH8 specificity is generated for CD44.
Similar to what has been observed by others (Coscoy and Ganem, 2000; Ishido et al., 2000; Bartee et al., 2004; Duncan et al., 2006; De Gassart et al., 2008), we find that MARCH proteins down-regulate their targets from the PM via the endosomal/lysosomal system and not through the biosynthetic pathway. Using antibody internalization experiments, we determined that a labeled cohort of PM cargo proteins is being rapidly shifted toward degradation in lysosomes within 2 h. This also correlates with the increased colocalization with EEA1 at 30 min of internalization seen for multiple cargo proteins in the presence of MARCH (Figure 7). This increased localization in EEA1-positive endosomes correlates with late endosomal delivery and agrees with the observations made for CDE cargos low-density-lipoprotein receptor and activated EGF receptor, which are enriched in EEA1-positive early endosomes and selected for degradation, versus TfnR, which has lower EEA1 localization and is recycled (Leonard et al., 2008). Furthermore, we believe that for the ubiquitinated CIE cargo proteins, the alteration in their trafficking occurs after endocytosis by CIE. For CD44 and CD98, MARCH expression profoundly shifts the trafficking of CD44 and CD98 away from their normal recycling route toward late endosomes and lysosomes via EEA1-marked sorting endosomes. This shift in CD44 and CD98 trafficking is further supported by the need for a functional ESCRT complex as evidenced by TSG101 knockdown, where CD44 and CD98 revert toward a normal cellular distribution even in the presence of MARCH8 (Figure 8). Taken together, the data suggest that MARCH expression routes specific CIE cargo proteins to degradation via EEA1 compartments and the ESCRT-requiring MVB to late endosomes and lysosomes.
Our findings provide evidence that CIE cargo proteins subject to down-regulation by MARCH expression are still internalized via CIE. First, we observed that in cells expressing MARCH8, CD44 and CD98 still entered cells in endocytic vesicles distinct from those carrying clathrin cargo like TfnR. Second, we directly tested for colocalization of surface CD44 and CD98 with both clathrin heavy chain and the ubiquitin-recognizing protein adaptor epsin1 and detected no significant increase in MARCH-expressing cells (Figure 5 and Supplemental Figure S5). Third, we determined that acute inhibition of CDE endocytosis by the dynamin inhibitor Dynasore had no effect on CIE cargo internalization in MARCH-expressing cells (Figure 6). This finding is similar to that observed for the MARCH1 effect on MHC class II trafficking after endocytosis (Walseng et al., 2010). By directly assaying the colocalization of surface-stained CIE cargos with clathrin heavy chain or acutely inhibiting CDE endocytosis, we provide evidence that CIE cargo proteins and the CDE cargo TfnR still enter cells via their respective pathways in MARCH-expressing cells.
Our data support the model in which CIE cargo proteins are endocytosed and reach an endosome where some proteins, such as CD44 and CD98, are sorted and directed toward recycling, whereas others, such as MHCI, continue on toward an EEA1 marked endosome where selection for degradation can occur. Our demonstration that MARCH proteins can ubiquitinate CIE cargo proteins and alter their trafficking toward degradation suggests that MARCH proteins may regulate the turnover of PM proteins by altering their intracellular itinerary. How and where MARCH activity is regulated and the consequences for endogenous CIE cargo protein turnover and trafficking are questions remaining to be answered.
MATERIALS AND METHODS
Antibodies and plasmids
Mouse monoclonal antibodies to MHCI from hybridoma cell clones w6/32 (immunoglobulin G2a [IgG2a]) were used for immunofluorescence and internalization experiments. Mouse monoclonal antibodies to CD44 (clone BJ18; IgG1), CD55 (clone JS11; IgG1), CD59 (clone p282; IgG1), CD98 (clone MEM-108; IgG1), and CD147 (clone HIM6; IgG1) were purchased from Biolegend (San Diego, CA) and used for immunofluorescence and antibody internalization. Mouse monoclonal antibodies to TfnR (clone 236-15375; IgG1) were purchased from Invitrogen Molecular Probes (Eugene, OR). Goat polyclonal IgG for CD98 (used for Western blotting) and for epsin 1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CD98 (clone MEM-108; IgG1) was used for immunoprecipitations. Rabbit anti-HA was purchased from Covance Research Products (Princeton, NJ). Rabbit anti-FLAG, M2 mouse anti-FLAG, and rabbit anti-actin antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit anti-Lamp1 and rabbit anti–clathrin heavy-chain antibodies were purchased from Abcam (Cambridge, MA). Mouse monoclonal anti-GLUT1 antibody (clone 202915; IgG2B) was purchased from R&D Systems (Minneapolis, MN). Mouse anti-TSG101 antibody was purchased from GeneTex (Irvine, CA). All Alexa-conjugated secondary antibodies were purchased from Invitrogen Molecular Probes (Eugene, OR).
MARCH1-FLAG, MARCH2-FLAG, MARCH3-FLAG, MARCH4-FLAG, and MARCH8-FLAG were as described (Bartee et al., 2004). HA-ubiquitin was from James Hurley (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD).
Transfections
HeLa or ARPE-19 cells were grown on glass coverslips (for immunofluorescence), six-well dishes (for flow cytometry), or 35- and 10-cm plates (for whole-cell lysate generation) and transfected using FuGene (Roche, Indianapolis, IN) according to manufacturer's specifications. Transfections with multiple plasmids were performed with 1 μg of each plasmid per transfection. Experiments were carried out 18 h after transfection. TSG101 siRNA (Thermo Scientific, Dharmacon, Chicago, IL) was prepared according to manufacturer's instructions and transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) for 24 h.
Immunofluorescence and flow cytometry
HeLa or ARPE-19 cells were plated on class coverslips (immunofluorescence) or six-well dishes (flow cytometry) 48 h prior to use. Cells were trypsinized for 10 min and transferred to 2058 Falcon tubes (Becton Dickinson Labware, Franklin Lakes, NJ) (flow cytometry). For antibody internalization experiments, cells were preincubated with primary antibodies (diluted to 10 μg/ml in media) to CD44, CD98, MHCI, TfnR, or Tac for 1 h at 37°C and then transferred to fresh media containing 15–25 mM NH4Cl for 2, 6, 13, or 24 h. Dynasore (Sigma-Aldrich) was prepared in dimethyl sulfoxide and used according to manufacturer's instructions. For internalization experiments, antibodies to CD44, CD98, MHCI, or TfnR were added to cells to allow internalization for indicated times, and surface antibody was removed with 0.5% acetic acid in 0.5 M NaCl for 30 s (acid wash) prior to fixation. Tfn633 (Invitrogen Molecular Probes) internalizations were conducted in serum-free DMEM, and cells were serum starved for 1 h prior to internalizations. Cells were fixed for 10 min in 2% formaldehyde in phosphate-buffered saline (PBS), rinsed in PBS, and incubated with primary antibodies in PBS/10% fetal calf serum in the presence (total) or absence (surface) of 0.2% saponin as indicated. Alexa dye–conjugated secondary antibodies were used to detect primary antibody localizations. All images were obtained using a 510 LSM confocal microscope (Zeiss, Thornwood, NY) with a 63× 1.3–numerical aperture PlanApo objective. MetaMorph (Molecular Devices, Sunnyvale, CA) was used to quantify colocalization. Individual cells were outlined, and the overlapping fluorescence pixels were quantified using the colocalization function. Adobe Photoshop (San Jose, CA) was used for image processing. Flow cytometry samples were run on an LSR2 cytometer with the help of the National Heart, Lung, and Blood Institute Flow Cytometry Core Facility. Data analysis was performed using FloJo Software (Tree Star, Ashland, OR).
Whole-cell lysis and immunoprecipitation
Sixty-millimeter plates of transfected HeLa cells were washed twice in cold PBS and scraped into lysis buffer (1% IGEPAL [Sigma-Aldrich], 10% glycerol, 50 mM Tris, pH 7.5, 100 mM NaCl) with complete Mini Protease Inhibitor Cocktail (Roche, Indianapolis, IN) and 20 μM N-ethylmaleimide (Sigma-Aldrich). Samples were sonicated three times for 5-s pulses at 4°C with a Microson Ultrasonic Cell Disruptor (Misonix, Newtown, CT), power level 2. Samples were centrifuged at 1300 × g for 10 min at 4°C and supernatants removed. For immunoprecipitations, protein G–Sepharose beads (GE Healthcare, Uppsala, Sweden) were prepared per manufacturer's instructions, and lysates were added with 5 μg of anti-CD98 antibody (clone MEM-108). Samples were rocked end over end for 1 h at 4°C. Samples were washed four times with Lysis buffer and solubilized in SDS–PAGE sample buffer. Samples were run on 4–20% Tris-HCl polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose (Whatman, Sanford, ME). Western blots were probed with designated antibodies and analyzed using n Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE) and accompanying software.
CD98-SNAP immunofluorescence and immunoprecipitation
CD98-SNAP was constructed by fusing the SNAP open reading frame (pSEMS1-26m from Covalys/New England Biolabs, Ipswich, MA) onto the C-terminus of the type II protein CD98 (4F2 cell-surface antigen heavy-chain isoform b) by standard two-stage PCR protocols. A (GGGS)2 linker was placed between CD98 and the SNAP tag. The PCR fusion was cloned into the TA mammalian expression vector pTarget (Clontech, Mountain View, CA).
For immunofluorescence, SNAP-Surface-488 (New England Biolabs) (called here BG-488) was added to media to label the surface of HeLa cells expressing CD98-SNAP (with and without MARCH8 cotransfection), and the cells were then incubated at 37°C for 1 h. Cells were then washed and transferred to fresh media containing 15 mM NH4Cl and treated as described earlier.
For pull-down of CD98-SNAP, a non–cell-permeable BG substrate was prepared by reacting O6-[4-(aminomethyl)benzyl]guanine (BG-NH2) (1.3 Eq; Toronto Research Chemicals, North York, Canada) with EZ-Link NHS-PEG4-Biotin (Thermo Scientific, Waltham, MA). Reactions were performed at 30°C for 16 h in N,N-dimethylacetamide in the presence of 2 Eq triethylamine. BG-PEG4-Biotin was analyzed by high-resolution mass spectrometry and purity checked by high-performance liquid chromatography.
For expression, HeLa cells (10-cm dishes) were transfected with CD98-SNAP with or without HA-ubiquitin and MARCH8-FLAG. After 18 h, cells were labeled with BG-PEG4-Biotin substrate (3 mM) for up to 4 h at 37°C. Cells were rinsed three times with PBS, lifted, and pelleted at 300 × g. Cell pellets were incubated with 200 mM BG-NH2 for 15 min at 4°C, then solubilized in 0.2 ml of lysis buffer (50 mM Tris-Cl, pH 7.4, 0.25 M NaCl, 0.1% Triton X-100, 1 mM EDTA, 50 mM NaF, 1 mM dithiothreitol, and 100 mM Na3VO4) plus Complete Protease Inhibitor tablets (Roche, Indianapolis, IN). The cell extract was denatured by adding SDS to obtain a 1% final concentration and boiled for 15 min. The SDS was quenched by adding 0.1 ml of 20% Triton X-100 and 1.8 ml of lysis buffer and placed on ice for 30 min. The lysate was centrifuged at 13,000 × g for 5 min, and 50 μl of a 1:1 slurry of streptavidin–agarose (Sigma-Aldrich) was added to the supernatant. The lysate was rocked at room temperature for 1 h, and the beads were washed three times with lysis buffer and once with water. Thirty microliters of 2× SDS sample buffer was added, and the beads were boiled for 10 min before protein separation by SDS–PAGE (4–12% Tris-glycine; Novex, Invitrogen, Eugene, OR), transfer to nitrocellulose, and immunoblotting.
HA-ubiquitin was detected with monoclonal HA.11 (Covance). Alexa Fluor 680 secondary antibodies (Invitrogen) were used for HA detection. Biotinylated CD98-SNAP was detected with NeutrAvidin, DyLight-800 Conjugated (Thermo Scientific). The membrane was incubated with primary and secondary antibodies, each for 1 h at room temperature. The membrane was washed three times with 0.1% Tween 20 in phosphate-buffered saline and then quantitatively visualized by scanning with an Odyssey infrared scanner (Li-Cor Biosciences,).
Supplementary Material
Acknowledgments
We thank Lois Greene, Edward Korn, Lymarie Maldonado-Baez, Natalie Porat-Shliom, and Paul Roche for comments on the manuscript and critical discussions. We also acknowledge the assistance of Philip McCoy and Pradeep Dagur at the National Heart, Lung, and Blood Institute Flow Cytometry Core. This work was supported by the Intramural Research Program in the National Heart, Lung, and Blood Institute at the National Institutes of Health.
Abbreviations used:
- CDE
clathrin-dependent endocytosis
- CIE
clathrin-independent endocytosis
- EEA1
early endosome antigen 1
- MHCI
major histocompatibility complex class I protein
- Tfn
transferrin
- TfnR
transferrin receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-11-0874) on July 14, 2011.
REFERENCES
- Acconcia F, Sigismund S, Polo S. Ubiquitin in trafficking: the network at work. Exp Cell Res. 2009;315:1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Arvan P, Zhao X, Ramos-Castaneda J, Chang A. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic. 2002;3:771–780. doi: 10.1034/j.1600-0854.2002.31102.x. [DOI] [PubMed] [Google Scholar]
- Bartee E, Eyster CA, Viswanathan K, Mansouri M, Donaldson JG, Fruh K. Membrane-associated RING-CH proteins associate with Bap31 and target CD81 and CD44 to lysosomes. PLoS One. 2010;5:e151325. doi: 10.1371/journal.pone.0015132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K, Fruh K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J Virol. 2004;78:1109–1120. doi: 10.1128/JVI.78.3.1109-1120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartee E, McCormack A, Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2:e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol. 2006;16:551–559. doi: 10.1016/j.tcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Coscoy L, Ganem D. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci USA. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- De Gassart A, Camosseto V, Thibodeau J, Ceppi M, Catalan N, Pierre P, Gatti E. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci USA. 2008;105:3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruh K, Bartee E, Gouveia K, Mansouri M. Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses. Virus Res. 2002;88:55–69. doi: 10.1016/s0168-1702(02)00120-x. [DOI] [PubMed] [Google Scholar]
- Gautier A, Juillerat A, Heinis C, Correa IR, Jr, Kindermann M, Beaufils F, Johnsson K. An engineered protein tag for multiprotein labeling in living cells. Chem Biol. 2008;15:128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Goto E, Ishido S, Sato Y, Ohgimoto S, Ohgimoto K, Nagano-Fujii M, Hotta H. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J Biol Chem. 2003;278:14657–14668. doi: 10.1074/jbc.M211285200. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hewitt EW, Duncan L, Mufti D, Baker J, Stevenson PG, Lehner PJ. Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation. EMBO J. 2002;21:2418–2429. doi: 10.1093/emboj/21.10.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor S, Ziv T, Admon A, Lehner PJ. Stable isotope labeling by amino acids in cell culture and differential plasma membrane proteome quantitation identify new substrates for the MARCH9 transmembrane E3 ligase. Mol Cell Proteomics. 2009;8:1959–1971. doi: 10.1074/mcp.M900174-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74:5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour M, Campbell EM, Fares H, Lybarger L. Discrete domains of MARCH1 mediate its localization, functional interactions, and posttranscriptional control of expression. J Immunol. 2009;183:6500–6512. doi: 10.4049/jimmunol.0901521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Takaku K, Magae J, Shinohara N, Takayama H, Kondo S, Nagai K. Acidification is essential for maintaining the structure and function of lytic granules of CTL. Effect of concanamycin A, an inhibitor of vacuolar type H(+)-ATPase, on CTL-mediated cytotoxicity. J Immunol. 1994;153:3938–3947. [PubMed] [Google Scholar]
- Lau AW, Chou MM. The adaptor complex AP-2 regulates post-endocytic trafficking through the non-clathrin Arf6-dependent endocytic pathway. J Cell Sci. 2008;121:4008–4017. doi: 10.1242/jcs.033522. [DOI] [PubMed] [Google Scholar]
- Lehner PJ, Hoer S, Dodd R, Duncan LM. Downregulation of cell surface receptors by the K3 family of viral and cellular ubiquitin E3 ligases. Immunol Rev. 2005;207:112–125. doi: 10.1111/j.0105-2896.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S. Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J Cell Sci. 2008;121:3445–3458. doi: 10.1242/jcs.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Matsuki Y, et al. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- Morokuma Y, Nakamura N, Kato A, Notoya M, Yamamoto Y, Sakai Y, Fukuda H, Yamashina S, Hirata Y, Hirose S. MARCH-XI, a novel transmembrane ubiquitin ligase implicated in ubiquitin-dependent protein sorting in developing spermatids. J Biol Chem. 2007;282:24806–24815. doi: 10.1074/jbc.M700414200. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Fukuda H, Kato A, Hirose S. MARCH-II is a syntaxin-6-binding protein involved in endosomal trafficking. Mol Biol Cell. 2005;16:1696–1710. doi: 10.1091/mbc.E04-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell. 2003;14:417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan JA, Lehner PJ. The trafficking and regulation of membrane receptors by the RING-CH ubiquitin E3 ligases. Exp Cell Res. 2009;315:1593–1600. doi: 10.1016/j.yexcr.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Ohmura-Hoshino M, Goto E, Matsuki Y, Aoki M, Mito M, Uematsu M, Hotta H, Ishido S. A novel family of membrane-bound E3 ubiquitin ligases. J Biochem. 2006;140:147–154. doi: 10.1093/jb/mvj160. [DOI] [PubMed] [Google Scholar]
- Okiyoneda T, Barriere H, Bagdany M, Rabeh WM, Du K, Hohfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17:3469–3483. doi: 10.1091/mbc.E05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SE, Setty SR, Sitaram A, Marks MS, Lewis RE, Chou MM. Extracellular signal-regulated kinase regulates clathrin-independent endosomal trafficking. Mol Biol Cell. 2006;17:645–657. doi: 10.1091/mbc.E05-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Thibodeau J, et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. 2008;38:1225–1230. doi: 10.1002/eji.200737902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- Walseng E, Furuta K, Bosch B, Weih KA, Matsuki Y, Bakke O, Ishido S, Roche PA. Ubiquitination regulates MHC class II-peptide complex retention and degradation in dendritic cells. Proc Natl Acad Sci USA. 2010;107:20465–20470. doi: 10.1073/pnas.1010990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.