Rit knockout mice and D-Ric null Drosophila were used to identify the Rit/RIC subfamily of Ras-related GTPases as regulators of an evolutionarily conserved, p38-dependent signaling cascade that functions as a survival mechanism for cells in response to reactive oxygen species exposure.
Abstract
Ras-related small GTP-binding proteins control a wide range of cellular processes by regulating a variety of effector pathways, including prominent roles in the control of mitogen-activated protein kinase (MAPK) cascades. Although the regulatory role(s) for many Ras family GTPases are well established, the physiological function for the Rit/Rin subfamily has been lacking. Here, using both knockout mice and Drosophila models, we demonstrate an evolutionarily conserved role for Rit subfamily GTPases (mammalian Rit and Rin, and the Drosophila RIC homologue) in governing survival in response to oxidative stress. Primary embryonic fibroblasts derived from Rit knockout mice display increased apoptosis and selective disruption of MAPK signaling following reactive oxygen species (ROS) exposure but not in response to endoplasmic reticulum stress or DNA damage. These deficits include a reduction in ROS-mediated stimulation of a p38-MK2-HSP27 signaling cascade that controls Akt activation, directing Bad phosphorylation to promote cell survival. Furthermore, D-RIC null flies display increased susceptibility to environmental stresses and reduced stress-dependent p38 signaling, extending the Rit-p38 survival pathway to Drosophila. Together, our studies establish the Rit GTPases as critical regulators of an evolutionarily conserved, p38 MAPK–dependent signaling cascade that functions as an important survival mechanism for cells in response to oxidative stress.
INTRODUCTION
Cells are constantly exposed to a variety of environmental stresses, including oxidative stress, γ-irradiation, and endoplasmic reticulum (ER) stress. Aerobic organisms are constantly exposed to reactive oxygen species (ROS), and ROS is known to influence a range of physiological processes, from cell survival to aging (Beckman and Ames, 1998; Chong et al., 2005). However, generation of ROS beyond a cell's antioxidant capacity results in oxidative stress and leads to molecular and cellular damage. Indeed, excessive ROS contributes to the pathogenesis of numerous human diseases, and the ability to adapt to oxidative stress is critical for cell and tissue survival.
ROS-activated signaling mechanisms have evolved to promote cell survival and homeostasis in response to oxidative damage (Trachootham et al., 2008; Runchel et al., 2011). Hydrogen peroxide exposure activates all of the conventional mitogen-activated protein kinases (MAPKs), with ROS-mediated ERK and ERK5 activation associated with cell growth and survival, whereas inhibition of JNK and p38 signaling suppresses apoptosis induced by an array of cellular stresses (Runchel et al., 2011). However, in addition to modulating apoptotic signaling, both p38 and JNK kinase pathways function in a context-specific manner to regulate cell growth, differentiation, and migration (Kyriakis and Avruch, 2001; Pearson et al., 2001; Wagner and Nebreda, 2009). The contribution of p38 signaling to these processes is complex, considering the established roles for p38 in the modulation of both apoptosis and cell survival. p38 acts by directly phosphorylating cellular targets, but also by regulating the activity of a collection of subordinate kinases (Roux and Blenis, 2004), which in turn control additional cellular substrates, including transcription factors and small heat shock proteins (HSPs; Kostenko and Moens, 2009). Ultimately, the cellular balance between the duration and strength of MAPK signaling appears key to determining cell fate, although the molecular mechanisms that control whether oxidative stress-mediated signaling results in programmed cell death or recovery remain incompletely understood.
Ras-related small GTP-binding proteins function as molecular switches to control a wide range of physiological processes through the regulation of diverse effector pathways, including the MAPK and Akt signaling cascades. Although the regulatory role of many Ras family GTPases is well established, there are a large number of “orphan” GTPases whose physiological functions remain to be determined (Colicelli, 2004). This is particularly true within the Ras branch, in which structurally related GTPase proteins, despite often sharing common downstream effector targets and overlapping regulatory modulators, have been found to play nonredundant cellular functions (Reuther and Der, 2000; Raaijmakers and Bos, 2009). Studies in primary neurons established that Rit activates signaling pathways that control axonal and dendritic morphology (Lein et al., 2007; Andres et al., 2008), whereas analysis in pheochromocytoma cell lines demonstrated a role for Rit signaling in the regulation of neural differentiation and survival (Spencer et al., 2002; Shi and Andres, 2005; Shi et al., 2010, 2011); however, the principal physiological function of Rit remained uncharacterized. Here, using a genetic approach, we identify a fundamental role for the Rit GTPase in stress-activated MAPK regulation and prosurvival signaling. Rit deficiency renders primary fibroblasts susceptible to apoptosis and leads to a selective disruption of MAPK and Akt signaling cascades following oxidative-stress exposure. The prosurvival effect of Rit is likely due in part to its ability to activate a p38-dependent MK2-HSP27-Akt signaling cascade. We also find that Rit signaling is evolutionarily conserved, contributing to stress response signaling in Drosophila. Flies lacking the RIC GTPase, a Rit orthologue, are sensitive to a variety of environmental stresses, a phenotype that overlaps those caused by deletion of D-p38. Taken together, our studies define a fundamental and evolutionarily conserved signaling link between the Rit GTPase and p38-Akt kinase cascade, revealing a critical prosurvival role for this cascade in cells adapting to oxidative stress.
RESULTS
Drosophila RIC GTPase signaling is required for environmental stress responses
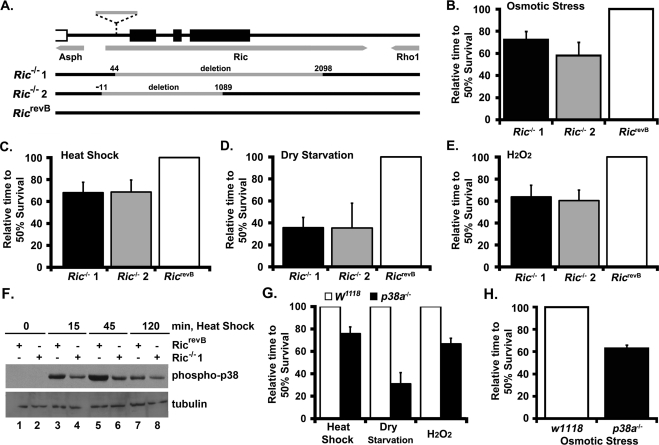
The restricted number of small GTP-binding proteins expressed in Drosophila has made it a valuable model for analyzing the physiological roles of Ras family GTPases. Whereas vertebrates express 35 members of the Ras subfamily, Drosophila express only 14 orthologues, including RIC, which shares a G2-effector domain and >65% amino acid identity with the vertebrate Rit and Rin GTPases (Wes et al., 1996; Shao et al., 1999; Colicelli, 2004). To address the physiological function of RIC signaling in vivo, we generated a series of D-Ric null strains by imprecise excision of a nearby transposon (P{RS5}5-HA-1205; Ryder et al., 2004), which is located 40 base pairs downstream of the presumed transcriptional start site and 128 base pairs upstream of the D-Ric CDS (Figure 1A). This work generated a series of alleles, including two strains (Ric1 and Ric2) with deletions of 2.1 and 1.1 kb removing all, or most, of the D-Ric locus, respectively, but leaving the flanking genes intact as determined by direct sequencing. Lines carrying precise excisions of the original P-element and restoring D-Ric structure (RicrevB) were recovered and used as control strains.
FIGURE 1:
Drosophila D-Ric mutants are sensitive to environmental stress. (A) Schematic of the mutagenesis strategy for the D-Ric locus. Gray arrows indicate gene structure; black boxes indicate RIC coding regions. Dashed line indicates the P-element insertion site. The gray bars indicate the size and position of deletions (numbers indicate the boundary of the deletions within D-Ric), as determined by genomic DNA sequencing. Neither neighboring gene was disrupted. (B) Adult Drosophila were placed in vials containing growth medium supplemented with 0.2 M NaCl and placed at 25°C, and flies were counted twice a day. The time to reach 50% survival for each genotype was compared. Similar relationships were seen at 0.1 and 0.3 M NaCl, with a decreasing time of survival as osmotic stress increased. (C) Adult Drosophila were placed at 38°C in vials containing normal growth medium and analyzed as in B. (D) Adult Drosophila were placed in empty culture vials at 25°C and analyzed as in B. (E) Adult Drosophila were placed in vials at 25°C containing a modified growth medium containing 2% sucrose and 1% H2O2 and analyzed as in B. (F) Adult Drosophila were placed at 38°C in vials containing normal growth medium for the indicated times. Total lysates were prepared from wild-type (RicrevB) and Ric KO (Ric−/−1) flies and analyzed by immunoblotting with the indicated antibodies. Note that levels of phosphorylated p38 were reduced in Ric−/− following heat shock. (G) Adult wild-type (W1118) or p38a KO (p38a−/−) Drosophila were exposed to heat shock, dry starvation, and oxidative stress and analyzed as in C– E. (H) Adult wild-type (W1118) or p38a KO (p38a−/−) Drosophila were exposed to osmotic stress and analyzed as in B.
D-Ric null flies are viable and fertile and display no apparent defects in patterning or apoptosis in developing embryonic or larval tissues. On the basis of a suggested role for Rit signaling in cell survival (Spencer et al., 2002), we tested the susceptibility of D-Ric mutants to a variety of environmental stresses. Adult D-Ric mutants showed a reduced resistance to osmotic stress (n = 120 per genotype; similar results were observed at three different sodium chloride concentrations), to 37°C heat shock (average of three trials, with n = 120 per genotype per trial), to dry starvation (average of three trials, with n = 200 per genotype per trial), and to oxidative stress (hydrogen peroxide [H2O2]) (average of two trials; with n = 90 per genotype per trial), suggesting a role for RIC in stress response signaling (Figures 1, B–E). These findings are reminiscent of those observed in D-p38a null flies (Craig et al., 2004), in which D-p38a mutants are susceptible to certain environmental stresses but show no observable developmental defects. Direct analysis of homozygous D-Ric and D-p38a knockout flies yielded approximately the same reduction in viability following stress exposure in both mutant strains (Figure 1, G–H). To examine whether Ric signaling contributes to p38 MAPK activation in Drosophila, D-Ric mutants were exposed to 37°C heat shock and subjected to anti–phospho-specific p38 immunoblotting (Figure 1F). Ric knockout altered both the amplitude and duration of p38 activation in response to heat when compared with the kinetics of p38 activation in RicrevB animals. Together these data provide genetic evidence that RIC signaling promotes survival in response to cellular stress in a manner that cannot be complemented by other Drosophila Ras family GTPases and suggest that p38 signaling contributes to this process.
Selective vulnerability to oxidative stress in Rit−/− fibroblasts
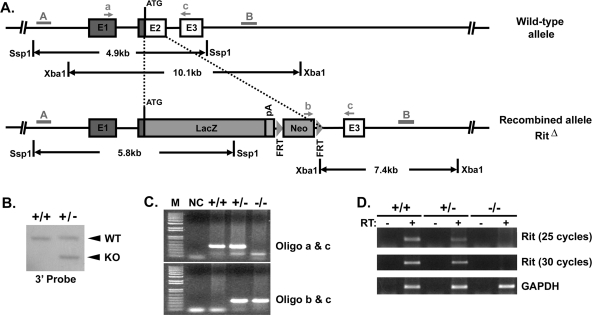
To examine the physiological role of Rit signaling, we generated a Rit1 knockout mouse (Figure 2). Rit null mutant mice were born at the expected Mendelian ratio and grew to adulthood without showing any discernible abnormalities. Examination of various tissues did not reveal any gross morphological or anatomical abnormalities, suggesting that Rit protein is not essential for proliferation or embryonic development.
FIGURE 2:
Rit knockout mouse. (A) Schematic representation of the strategy used to generate the Rit knockout mouse. Part of exon 2 was targeted and replaced by a LacZ-expressing cassette, followed by the neomycin gene flanked by FRT sites. The DNA probe (3′ probe) used for Southern analysis is indicated by the gray bar (B), and arrows indicate the location of genotyping primers (a, b, and c). (B) Genomic DNA isolated from heterozygous Rit (Rit+/−) or wild-type (WT) embryonic stem (ES) cells were subjected to XbaI digestion, and the resulting genomic DNA fragments were resolved by agarose gel electrophoresis and transferred to nylon membrane. The Rit genomic locus was detected by Southern blot using the radiolabeled 3′ probe. As illustrated in A, WT ES cells are expected to contain a 10.1-kb fragment, whereas a 7.4-kb fragment should also be detected from Rit+/− ES cells. (C) Genomic DNA was isolated from mouse tail biopsies and subjected to PCR analysis using the oligonucleotide primer pairs indicated in A. A 347–base pair band represents the mutant allele (bottom; amplification using primer pair b/c), whereas a 462–base pair band represents the wild-type allele (top; amplification using primer pair a/c). (D) Total RNA was isolated from cultured embryonic fibroblasts obtained from Rit mutants or WT littermates and subjected to RT-PCR analysis. GAPDH was included as a loading control. Note that Rit mRNA levels are completely eliminated in Rit−/− MEFs, whereas they are reduced in Rit+/− MEFs.
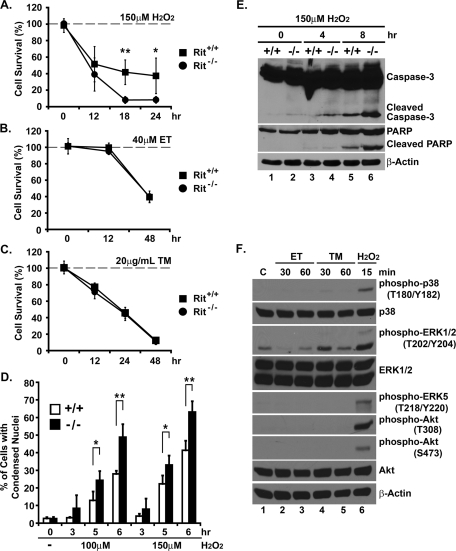
To evaluate cell survival, primary day 13.5 mouse embryonic fibroblasts (MEFs) were cultured from both Rit null and wild-type littermates. These cultures were left untreated or were exposed to a variety of cellular stresses, including a DNA-damaging agent (40 μM etoposide [ET]), ROS (150 μM H2O2), or ER stress (20 μg/ml tunicamycin [TM]). Cell numbers were analyzed over time using the MTS assay, and cell viability decreased in a time-dependent manner with each of the stresses (Figure 3, A–C). However, hydrogen peroxide exposure resulted in a dramatic reduction in the number of Rit null fibroblasts compared with similarly stressed wild-type cells (18-h exposure: 41.8% [±14.9] and 8.2% [±4.2] for wild type and Rit−/−, respectively, p < 0.001; Figure 3A). Apoptosis was increased in hydrogen peroxide–treated Rit null MEFs as monitored by the presence of condensed nuclei (Figure 3D) and cleaved caspase-3 and poly(ADP-ribose) polymerase (PARP; Figure 3E). This was not the case for cells exposed to either etoposide (48-h exposure: 39.4% [±4.1] and 40.2% [±3.3] for wild type and Rit−/−, respectively, p > 0.6) (Figure 3B) or tunicamycin (24-h exposure: 45.8% [±6.9] and 44.6% [±7.9] for wild type and Rit−/−, respectively, p > 0.7) (Figure 3C), in which both wild-type and Rit null fibroblasts displayed an equivalent reduction in cell viability.
FIGURE 3:
Rit loss sensitizes MEFs to oxidative stress but not DNA damage or ER stress. (A–C) Cell viability of wild-type (Rit+/+) and Rit−/− MEFs was determined by MTS metabolism at the indicated times after initial H2O2 (150 μM), ET (40 μM), and TM (20 μg/ml) exposure (t test; *p < 0.05, **p < 0.01, n = 3). (D) Hoechst staining was used to visualize nuclear morphology in wild-type and Rit−/− MEFs following exposure to H2O2 (0–150 μM) for the indicated durations. The results are presented as mean ± SD (t test: *p < 0.05, ** p< 0.01, n = 4). At least 300 cells were scored for each data point. (E) Lysates from wild-type and Rit−/− MEFs following exposure to H2O2 (150 μM) were analyzed by immunoblotting with the indicated antibodies. Note that basal levels of cleaved caspase-3 are slightly elevated in Rit−/− MEFs. (F) Lysates from wild-type MEFs were prepared following ET (40 μM), TM (20 μg/ml), and H2O2 (100 μM) exposure for the indicated times and analyzed by immunoblotting with the indicated antibodies.
Analysis of the MAPK signaling in wild-type MEFs following hydrogen peroxide (100 μM) treatment resulted in activation of p38, ERK, and ERK5. In addition, Akt was found to be activated in response to ROS stimulation (Figure 3F). In contrast, exposure to etoposide or tunicamycin failed to stimulate p38, ERK5, and Akt signaling in wild-type MEFs (Figure 3F), and ERK was only modestly activated by tunicamycin, suggesting that Rit-mediated regulation of one or more of these pathways might contribute to cell survival in response to oxidative stress.
Rit regulates ROS-mediated MAPK activation and p38-dependent survival signaling
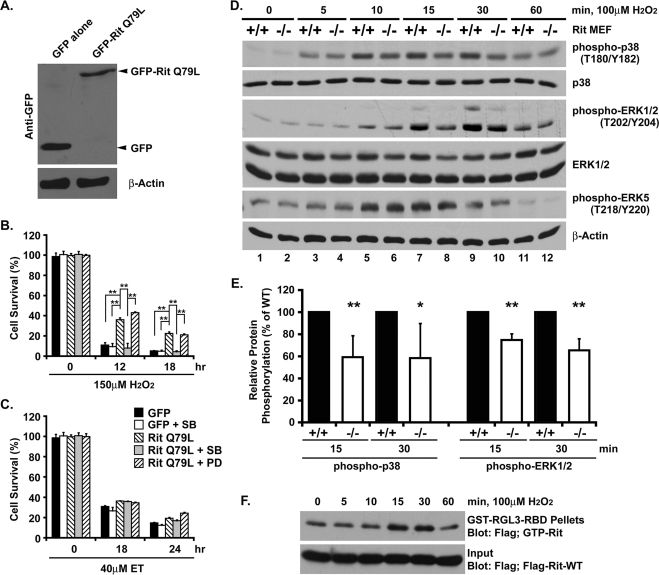
To confirm that the survival defect was the result of Rit loss and to examine the cellular signaling pathways underlying this effect, we established stable Rit−/−MEFs, immortalized with SV40 large T antigen, expressing either green fluorescent protein (GFP) or GFP-tagged RitQ79L, a constitutively active Rit mutant (Figure 4A). Exposure of GFP control cells to hydrogen peroxide (150 μM) induced robust cell death, whereas cells expressing active Rit (RitQ79L) were significantly protected (12-h exposure: 10.9% [±2.4] and 36% [±1.9] GFP and GFP-RitQ79L, respectively, p < 0.001; Figure 4B). In contrast, RitQ79L expression had only a modest effect on cell survival following etoposide (40 μM) exposure (Figure 4C). These data support the notion that Rit regulates an oxidative stress survival mechanism.
FIGURE 4:
p38 MAPK activity is required for Rit-mediated protection from oxidative stress. (A) Lysates from immortalized Rit−/− MEFs stably transfected with GFP alone or GFP-RitQ79L were immunoblotted with GFP and β-actin antibodies. (B, C) Cell viability of GFP- and GFP-RitQ79L–overexpressing Rit−/− MEFs was determined by MTS metabolism after initial H2O2 (150 μM) or ET (40 μM) exposure in the presence or absence of the p38 inhibitor SB203580 (10 μM) or MEK inhibitor PD98059 (10 μM). The results are presented as mean ± SD (t test; **p < 0.01, n = 3). (D) Lysates from wild-type and Rit−/− MEFs were prepared following H2O2 exposure (100 μM) for the indicated times and analyzed by immunoblotting with the indicated antibodies. (E) The relative phosphorylation of p38 and ERK1/2 after 15 and 30 min of H2O2 exposure was compared between wild-type (black bars) and Rit−/− (white bars) MEFs. The results are presented as mean ± SD (t test: *p < 0.05, **p < 0.01, n≥4). (F) Immortalized wild-type MEFs transfected with FLAG-Rit-WT were serum starved for 3 h prior to stimulation with H2O2 (100 μM) for the indicated times, and GTP-bound Rit was recovered by GST-RGL3-RBD pull down. Precipitated GTP-Rit was detected by anti-FLAG immunoblotting.
We next assessed the contribution of Rit to ROS-mediated intracellular signaling. Stimulation of serum-starved, wild-type MEFs with hydrogen peroxide led to the activation of p38, ERK, and ERK5 MAPK kinase cascades, whereas ROS-dependent p38 and ERK pathway activation was significantly reduced in Rit−/− mutant cells (Figure 4, D and E; 15-min exposure [n = 4]: 59.2% ± [19.6] and 74.8% ± [5.6] for p38 and ERK, respectively, p < 0.01). Of importance, Rit does not participate in all ROS-mediated MAPK signaling, as ERK5 activation was not altered in mutant fibroblasts. Because Ras-related GTPases respond to extracellular stimuli by exchanging GTP for bound GDP (Colicelli, 2004), we next tested whether hydrogen peroxide exposure triggered Rit activation. Rit-GTP loading studies are technically difficult in primary cells because of low levels of endogenous Rit and the lack of high-affinity anti-Rit antibodies. Thus, we performed Rit-GTP pull-down assays using MEFs transiently transfected with 3xFlag-Rit-WT as described previously (Shi and Andres, 2005; Lein et al., 2007). Stimulation with hydrogen peroxide resulted in a transient increase in GTP-bound Rit levels (Figure 4F), with activation kinetics consistent with a role for Rit in ROS-dependent p38 activation.
Rit is known to regulate both ERK and p38 MAP kinases following mitogen stimulation (Shi and Andres, 2005; Shi et al., 2008; Lein et al., 2007; Andres et al., 2008), prompting studies to examine the contribution of these pathways to survival. Although pharmacological blockade of MEK/ERK signaling (10 μM PD98059) had no effect on RitQ79L-dependent survival following hydrogen peroxide exposure, p38 inhibition (10 μM SB203580) almost completely blocked the survival advantage afforded by Rit signaling (12-h exposure: 43% [±0.9] and 8% [±4.7] for PD98059 and SB203580, respectively, p < 0.001; Figure 4B). As expected, these same inhibitors had no effect on cell survival following etoposide treatment in either wild-type or mutant MEFs (Figure 4C). Taken together, these data suggest that p38 signaling is a central feature of Rit-dependent survival signaling.
Rit-dependent survival involves p38-mediated Akt signaling
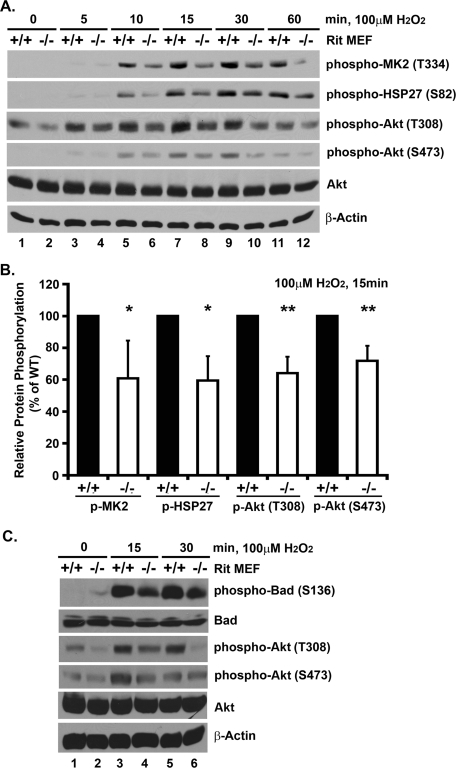
Although stress-mediated p38 activation is commonly associated with cell death induction, recent work has identified a survival cascade in which p38 promotes MK2 kinase activation within a novel HSP27 scaffolded complex. This allows MK2 to phosphorylate targets, including HSP27 and Akt, leading to cellular responses including the inhibition of apoptosis (Rane et al., 2003; Zheng et al., 2006; Wu et al., 2007). Using RNA interference (RNAi) approaches, we recently found that Rit contributes to stress-mediated regulation of this cascade in cultured cells (Shi et al., 2011). Consistent with a role for Rit in the regulation of this cascade in primary MEFs, MK2 (60.8% [±23.8], p < 0.05), HSP27 (59.5% [±15.5], p < 0.05), and Akt phosphorylation (64.2% [±10.3] and 71.9% [±9.4] for T308 and S473, respectively, p < 0.01) were decreased in Rit−/− MEFs following hydrogen peroxide exposure when compared with similarly treated wild-type cells (Figure 5, A and B). These results suggest that Rit mediates oxidative stress survival in a p38-Akt-dependent manner.
FIGURE 5:
Rit contributes to p38-MK2-HSP27-AKT prosurvival signaling. (A) Lysates from wild-type and Rit−/− MEFs were prepared following H2O2 exposure (100 μM) for the indicated times and analyzed by immunoblotting with the indicated antibodies. (B) The relative phosphorylation of MK2, HSP27, and AKT after 15 min of H2O2 exposure was compared between wild-type (black bars) and Rit−/− (white bars) MEFs. The results are presented as mean ± SD (t test: *p < 0.05, **p < 0.01, n ≥ 4). (C) Lysates from wild-type and Rit−/− MEFs were prepared following H2O2 exposure (100 μM) for the indicated times and analyzed by immunoblotting with the indicated antibodies.
Bad, a proapoptotic member of the Bcl-2 family, is regulated by Akt, which specifically phosphorylates Bad at serine 136 (Datta et al., 1997; del Peso et al., 1997), resulting in the sequestration of phospho-Bad in the cytosol. This prevents Bad from associating with mitochondria, where it displaces Bax from binding the antiapoptotic Bcl-2 and Bcl-XL proteins, leading to outer membrane permeability and apoptosis (Yang et al., 1995). Consistent with a role for Akt signaling in MEF survival following oxidative stress, stimulation of wild-type MEFs with hydrogen peroxide resulted in Akt (T308/S473) activation and a dramatic increase in phosphorylated Bad (S136) (Figure 5C). However, in Rit null cells, the phosphorylation of both Akt and Bad proteins was significantly reduced following hydrogen peroxide exposure. These data provide additional evidence that Rit loss selectively alters the coupling of oxidative stress to intracellular signaling pathways, particularly p38-dependent Akt activation.
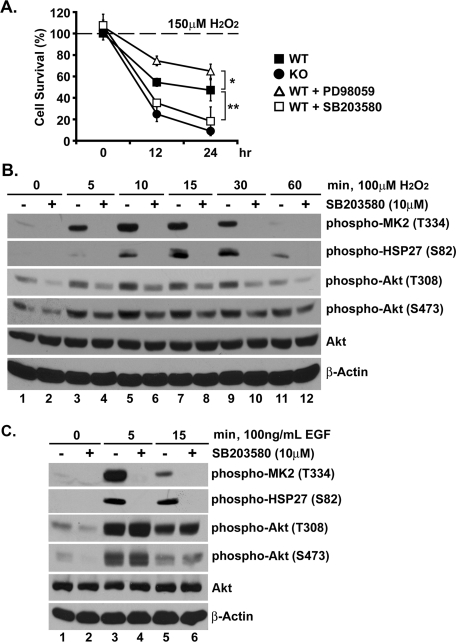
To test the foregoing hypothesis, wild-type MEFs were treated with SB203580 (10 μM) prior to hydrogen peroxide exposure, based on the reasoning that p38 blockade should disrupt Akt phosphorylation and increase oxidative stress–dependent apoptosis. As shown in Figure 6A, p38 inhibition, but not MEK/ERK blockade, resulted in a dramatic reduction in cell viability, rivaling that seen in Rit−/− MEFs, following hydrogen peroxide exposure. As expected, p38 inhibition was found to disrupt hydrogen peroxide–mediated MK2 and HSP27 phosphorylation in wild-type MEFs (Figure 6B). As a control, we also examined the effect of p38 blockade on epidermal growth factor (EGF)-mediated signaling. Whereas inhibition of p38 signaling blocked EGF-mediated MK2 and HSP27 phosphorylation, SB203580 treatment had no effect on EGF-dependent Akt signaling (Figure 6C), but it did suppress Akt activity following hydrogen peroxide exposure (Figure 6B). These results support a critical role for p38-dependent Akt activation in the survival of fibroblasts responding to oxidative stress.
FIGURE 6:
p38 signaling promotes AKT in response to ROS. (A) Viability of wild-type MEFs was determined by MTS metabolism at 12 and 24 h after initial H2O2 exposure (150 μM) in the presence or absence of SB203580 (10 μM) or PD98059 (10 μM). The results are presented as mean ± SD (t test; *p < 0.05, **p < 0.01, n = 3). (B) Lysates from wild-type MEFs pretreated with or without SB203580 (10 μM, 30 min) and exposed to H2O2 (100 μM) for the indicated duration were analyzed by immunoblotting with the indicated antibodies. (C) Lysates from wild-type MEFs pretreated with or without SB203580 (10 μM, 30 min) and stimulated with EGF (100 μg/μl) for the indicated duration were analyzed by immunoblotting. Note that whereas SB203580 treatment inhibits both MK2 and HSP27 phosphorylation, Akt activation is not affected.
DISCUSSION
Reactive oxygen species participate in a diverse array of biological processes, including cell growth, cellular senescence, and programmed cell death (Beckman and Ames, 1998; Chong et al., 2005; Runchel et al., 2011). In large part, the diversity of these cellular actions results from ROS-mediated activation of canonical intracellular signaling pathways, including multiple MAPK cascades (Chong et al., 2005; Runchel et al., 2011). In this study, we describe an evolutionarily conserved signaling link between the Rit GTPase subfamily and the p38 MAPK cascade that represents a novel survival mechanism. The identification of p38 as a major target of Rit signaling, particularly in response to oxidative stress, suggests that Rit–p38 signaling may be critical in determining whether ROS-dependent p38 signaling results in cell death or recovery.
The creation of mice lacking expression of the Rit GTPase and D-Ric null Drosophila provided the systems necessary to define the essential physiological function of the Rit/RIC GTPases. Although previous studies using ectopic expression of active and dominant-negative mutants or RNAi-based gene silencing identified a role for Rit signaling in the regulation of neural morphology (Lein et al., 2007; Andres et al., 2008) and for Rit and RIC in differentiation (Spencer et al., 2002; Harrison et al., 2005; Shi and Andres, 2005; Shi et al., 2008), these genetic models establish Rit family GTPases as crucial regulators of an evolutionarily conserved, prosurvival cascade. The marked, selective effect of Rit loss on ROS-mediated cell death in MEFs indicates that Rit provides a function that cannot be compensated by any other Ras family GTPase. As important, this function appears to be evolutionarily conserved, as D-Ric deletion increased susceptibility to environmental stress in Drosophila.
A major conclusion of this study is that the Rit-p38 survival pathway is conserved. D-Ric null strains are sensitive to environmental stresses, and Rit and RIC appear to have the conserved ability to regulate cell survival in a p38-dependent manner. Recent work has described a novel signaling module in which HSP27 serves as a scaffold to couple p38/MK2 signaling to Akt-dependent survival (Rane et al., 2001, 2003; Zheng et al., 2006; Wu et al., 2007). It is known that Drosophila express both HSP27 and MK2 orthologues, and thus the possibility exists that this p38 survival cascade will be preserved in flies. Indeed, Drosophila HSP27 has been shown to increase lifespan and resistance to oxidative stress (Hao et al., 2007). As a high–molecular mass homo-oligomer, mammalian HSP27 is known to direct the refolding of denatured proteins (Stetler et al., 2009). Our data indicate that Rit functions as a regulator of HSP27 phosphorylation in response to oxidative stress and in the control of p38/MK2/HSP27/Akt signaling (Shi et al., 2011). Numerous studies have demonstrated that phosphorylated HSP27 displays antiapoptotic activity (Kostenko and Moens, 2009; Stetler et al., 2009). Determining whether the contribution of HSP27 to Rit/RIC-dependent survival signaling is mediated primarily by coordinating ROS-dependent Akt activation or whether phosphorylated HSP27 supports additional distinct survival mechanisms awaits further experimentation.
Hydrogen peroxide has the capacity to function as a proliferative signal (Burdon, 1995), a second messenger (Bae et al., 1997), or as a signal to promote apoptosis or cell survival (Martindale and Holbrook, 2002; Runchel et al., 2011). Hydrogen peroxide exposure promotes activation of all of the classic MAPK cascades (Wang et al., 1998), and it appears that cell fate is dictated by the interplay of these individual kinase pathways (Pearson et al., 2001; Roux and Blenis, 2004; Wagner and Nebreda, 2009). Our data suggest that Rit contributes to this process by directing p38-dependent cell survival. How might Rit achieve this effect? Scaffolding proteins are known to confer specificity, promoting distinct spatial and temporal control over p38 activation and directing coupling to specific downstream effector pathways (Morrison and Davis, 2003). The coordinated loss of hydrogen peroxide–mediated, but not EGF-dependent, p38, MK2, HSP27, and Akt activation suggests that Rit plays a critical role in coordinating p38-mediated Akt activation and survival. A large number of regulatory kinases are known to contribute to p38 activation, including MAPK kinase (MAPKK)-dependent and MAPKK-independent pathways (Kyriakis and Avruch, 2001; Wagner and Nebreda, 2009). An important goal of future studies is to determine the molecular nature of the coupling between Rit and p38. Just as the recently identified OSM scaffold is known to specify Rac GTPase-mediated p38 activation following hyperosmotic stress by generating a Rac-OSM-MEKK3-MKK3 regulatory module (Uhlik et al., 2003), our data suggest a model in which Rit functions as an upstream regulator of a scaffolded p38-MK2-HSP27 complex involved in ROS-dependent Akt activation. Studies are ongoing to test this model.
Identifying a role for a Ras GTPase in Akt-mediated cell survival is perhaps not surprising. A number of Ras subfamily GTPases are known to direct PI3-kinase-dependent Akt activation (Rodriguez-Viciana et al., 2004; Yuan and Cantley, 2008), with Akt in turn controlling the balance between survival and apoptosis (Engelman et al., 2006). Indeed, we find that oxidative stress activates Akt, resulting in Bad phosphorylation (Figure 5C), and that both Rit and p38 contribute to this signaling cascade (Figures 3 and 4). Akt is activated by two sequential phosphorylation events, with PDK1 directing Thr308 phosphorylation, and a less well defined “PDK2” activity directing Ser473 phosphorylation (Vanhaesebroeck and Alessi, 2000). A complex interplay has been described for Akt, HSP27, p38, and MK2 kinases in which p38/MK2 signaling induces both Akt-Ser473 phosphorylation and the release of activated Akt from phosphorylated HSP27, although the molecular mechanisms regulating the signaling and dynamics of the HSP27 scaffolded complex remain to be established (Rane et al., 2001, 2003; Zheng et al., 2006; Wu et al., 2007). Therefore, although activation of Akt is known to underlie the ability of a number of Ras GTPases to promote cell survival, Rit appears to control a distinct p38-mediated survival signaling cascade, which relies in part on ROS-dependent p38-MK2-HSP27-Akt activation. Studies are underway to determine whether Rit uniquely contributes to p38/MK2–Akt signaling, which might explain why other Ras family GTPases cannot compensate for Rit or RIC loss. Stress-mediated p38 activation can lead to either cell death or cell recovery, depending on both the severity of the stimulus and the nature of the cell (Wagner and Nebreda, 2009). It will be important to examine the contribution of Rit to cell survival in additional cell systems, especially, neurons, cardiomyocytes, and granulocytes, in which p38 signaling has been shown to support survival (Thornton and Rincon, 2009).
In summary, the data presented here demonstrate that the Rit GTPase is a key participant in oxidative stress signal propagation through the regulation of a p38–Akt cascade. The evolutionary conservation of this stress signaling pathway between mammals and Drosophila indicates its importance to cell survival and potentially also for the regulation of additional p38-mediated biological processes (Wagner and Nebreda, 2009) such as the regulation of cell cycle progression (Thornton and Rincon, 2009) and the lifespan of hematopoietic stem cells in response to oxidative stress (Ito et al., 2006). p38 signaling has also been implicated in the regulation of additional prosurvival transcriptional cascades, including the activation of MEF2 (Mao et al., 1999), β-catenin via GSK3 inactivation (Thornton et al., 2008), and MSK1/2-CREB cascades (Arthur et al., 2004), expanding the potential role for Rit in regulating whether ROS-dependent p38 activity results in cell death or recovery.
MATERIALS AND METHODS
Plasmids and reagents
FLAG- and GFP-tagged human Rit expression vectors have been described previously (Shi and Andres, 2005; Shi et al., 2008). H2O2 and TM (Sigma-Aldrich, St. Louis, MO), ET (CalBiochem, La Jolla, CA), kinase-specific inhibitor SB203580 (Tocris Bioscience, Ellisville, MO), PD98059 (CalBiochem), and antibodies against caspase-3, PARP, phospho-p38, p38, phospho-ERK1/2, ERK1/2, phospho-ERK5, phospho-Akt (Ser-473 and Thr-308), Akt, phospho-MK2 (Thr-334), phospho-HSP27 (Ser-82), phospho-Bad (Ser-136), Bad (Cell Signaling Technology, Beverly, MO), HSP27 (CalBiochem), GFP (UC Davis/NIH NeuroMab Facility, Davis, CA), β-actin, and FLAG (Sigma-Aldrich) were purchased.
Generation of rit knockout mice
Mice were housed in a pathogen-free facility and handled in accordance with standard use protocols, animal welfare regulations, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the University of Kentucky Institutional Animal Care and Use Committee. A targeting vector (Figure 2) containing ∼5.7 kb of the murine Rit1 gene was constructed from 129/Sv strain mouse genomic DNA by the Gene Targeted Mouse Service Core at the University of Cincinnati (Cincinnati, OH), replacing the coding region of exon 2 with the lacZ gene together with a neomycin cassette (with flanking Flippase recognition target [FRT] sites). The targeting vector was transfected into embryonic stem (ES) cells derived from 129S6/SvEv Tac mice, and correctly recombined G418-resistant clones were identified by PCR and Southern blot analysis. Two independent ES clones were injected into blastocysts, and male mice with a high degree of chimerism were crossed to wild-type Black Swiss females to generate Rit+/− mice. Heterozygous offspring were mated to generate homozygous Rit−/−mice.
Mouse genomic DNA extraction and genotyping PCR
Genomic DNA was extracted from tail snips by incubation in tail lysis buffer (100 mM Tris-HCl, pH 8.8, 5 mM EDTA, 0.2% SDS, and 200 mM NaCl) containing 0.4 mg/ml proteinase K (Invitrogen, Carlsbad, CA) at 55°C overnight, followed by incubation with 60 μg/ml RNase (Invitrogen) at 37°C for 1 h. DNA was precipitated and resuspended in 10 mM Tris-HCl, pH 8.0, prior to genotyping analysis. Primers used for Rit genotyping were as follows: wild-type allele, forward primer 5′-GTGAAGG CCGAGGATGTAGG-3′ (oligo a), reverse primer 5′-GGTCATGGTCTTCTGGGAATCG-3′ (oligo c); knockout allele, forward primer 5′-ACCCGTGATATTGCTGAAGAGC-3′ (oligo b), reverse primer 5′-GGTCATGGTCTTCT GGGAATCG-3′ (oligo c; see Figure 2). The PCR parameters were Tm = 53°C for 35 cycles.
Reverse transcription-PCR analysis
To determine whether expression of Rit was eliminated in Rit-knockout mice, reverse transcription (RT)-PCR was performed. Briefly, total RNA was isolated with TRIzol reagent (Invitrogen) from Rit+/+, Rit+/− and Rit−/− MEF cultures. Total RNA (2 μg) was subjected to reverse transcription using the Enhanced Avian HS RT-PCR Kit (Sigma-Aldrich), and the levels of Rit expression were then examined by PCR using first-strand cDNA as template. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a control. The primer pairs were as follows: mouse Rit, forward, 5′-CAATGCAGTTCATC AGCCACC, and reverse, 5′-CGTGGAAAACG TCGTCGATG; and mouse GAPDH, forward, 5′-AAGCCCATCA CCATCT TCCAG, and reverse, 5′-AGGGGCCATCCACAGTCTTCT.
Cell culture
Primary MEFs were isolated from E13.5 embryos following standard protocols (Serrano et al., 1997) and maintained in DMEM supplemented with 10% (vol/vol) fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. All MEF experiments were performed at passage 3–5 unless otherwise noted.
Immortalization of primary MEFs
The day before infection with a retrovirus expressing SV40 large T-antigen (a gift of Andrei V. Budanov, University of California, San Diego, La Jolla, CA), 3×105 primary MEFs (passage 2) were seeded in 60-mm dishes and cultured overnight. On the day of infection, 1.5 ml of virus supernatant (SV40 large T-antigen) supplemented with polybrene (10 μg/ml) (Sigma) was applied to MEFs for 3.5 h, after which monolayers were washed and returned to culture medium. Twenty-four hours later, a second round of infection was performed, and immortalized MEFs were selected by continuous passage until a stable population doubling time was obtained. To generate MEFs stably expressing GFP or GFP-RitQ79L, immortalized MEFs were transfected and subjected to G418 (1 mg/ml) selection, and single colonies were picked and expanded. GFP and GFP-RitQ79L expression was confirmed by Western blot (Figure 4A).
Protein phosphorylation analysis
Whole-cell lysates were prepared using kinase lysis buffer [20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4), 150 mM NaCl, 50 mM KF, 50 mM β-glycerolphosphate, 2 mM ethylene glycol tetraacetic acid (pH 8.0), 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, and 1× protease inhibitor cocktail] and subsequently subjected to experimental analysis (Shi and Andres, 2005). Protein phosphorylation was determined by immunoblotting with appropriate phospho-specific antibodies, and band intensity was quantified by Image J (National Institutes of Health, Bethesda, MD).
Rit-GTP loading assays
GST fusion proteins containing the Rit-binding domain (RBD) of RGL3 (residues 610–709) were used to precipitate GTP-loaded Rit as described previously (Shi and Andres, 2005; Shi et al., 2006). Briefly, immortalized cells were transfected (Superfect; Qiagen, Valencia, CA) with 3xFLAG-Rit-WT (1 μg) and serum starved prior to stimulation. The cells were harvested and total cell lysates prepared. Total protein (400 μg) was subjected to GST-RGL3-RBD agarose pull-down with end-over-end rotation at 4°C for 2 h. The GST-RGL3-RBD agarose pellets were recovered after extensive washing, and GTP-Rit levels (active Rit) were determined by immunoblotting with anti-FLAG monoclonal antibody.
Apoptosis analysis
Cell apoptosis was examined by caspase-3 and PARP cleavage or nuclear condensation (Tewari et al., 1995). The levels of caspase-3 and PARP cleavage were determined by immunoblotting total cell lysates with anti–caspase-3 and anti-PARP antibodies. Nuclear condensation was analyzed by Hoechst staining following fixation, with >300 cells in 9–12 random fields counted for each individual experiment. A nonradioactive cell proliferation assay (MTS) (Promega, Madison, WI) was used according to manufacturer's directions to determine cell viability.
Stress assays in flies
Flies for stress resistance assays were grown to adulthood on semidefined medium, as described by the Bloomington Drosophila Stock Center (Backhaus et al., 1984), under uncrowded conditions to minimize stress during development. For heat shock stress, 2- to 3-d-old adults were placed in vials with basic Bloomington malt medium and put in a 38°C water bath. The number of dead flies was counted every 30 min until all were dead. For dry starvation assays, 2- to 3-d-old flies were placed in empty vials and kept in a 25°C humidified incubator. The number of live flies was counted until all were dead. Osmotic stress resistance was determined by placing 2- to 3-d-old adult flies in vials containing basic Bloomington malt medium supplemented with 0.1, 0.2, or 0.3 M NaCl and kept in a 25°C humidified incubator. Viability was monitored until all flies were dead. Oxidative stress was performed using either H2O2 or Paraquat to generate ROS. Two-day-old adult males were placed on medium consisting of agarose, 2% sucrose, and 1% H2O2 in vials or in vials containing cellulose acetate plugs (Flugs; Genesee Scientific, San Diego, CA) saturated with 2% sucrose plus 5 or 10 mM Paraquat and kept in a 25°C humidified incubator. Viability was monitored until all flies were dead.
Acknowledgments
We thank A. Budanov for the retroviral vectors and help in immortalization of MEFs, R. Cagan (Washington University, St. Louis, MO) for p38a null Drosophila, and G.-X. Shi, M. Gentry, and C. Moncman for helpful discussions. This work was supported by Public Health Service Grant NS045103 from the National Institute of Neurological Disorders and Stroke (D.A.) and 2P20 RR020171 from the National Center for Research Resources (D.A.). The article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Center for Research Resources.
Abbreviations used:
- E13.5
embryonic day 13.5
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- ES
embryonic stem cell
- ET
etoposide
- FRT
flippase recognition target
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFP
green fluorescent protein
- HSP
heat shock protein
- KO
knockout
- MAPK
mitogen-activated protein kinase
- MAPKK
mitogen-activated protein kinase kinase
- MEF
mouse embryonic fibroblast
- MK2 (MAPKAPK2)
MAP kinase-activated protein kinase 2
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PARP
poly (APD-ribose) polymerase
- RBD
Ras-binding domain
- ROS
reactive oxygen species
- RT-PCR
reverse transcriptase-PCR
- TM
tunicamycin
- WT
wild-type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-05-0400) on July 7, 2011.
REFERENCES
- Andres DA, Shi GX, Bruun D, Barnhart C, Lein PJ. Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation. J Neurochem. 2008;107:1436–1447. doi: 10.1111/j.1471-4159.2008.05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, Impey S. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus B, Sulkowski E, Schlote FW. A semi-synthetic, general-purpose medium for Drosophila melanogaster. Drosophila Inf Serv. 1984;60:210–212. [Google Scholar]
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxideRole in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CR, Fink JL, Yagi Y, Ip YT, Cagan RL. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 2004;5:1058–1063. doi: 10.1038/sj.embor.7400282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Hao X, Zhang S, Timakov B, Zhang P. The Hsp27 gene is not required for Drosophila development but its activity is associated with starvation resistance. Cell Stress Chaperones. 2007;12:364–372. doi: 10.1379/CSC-308.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Rudolph JL, Spencer ML, Wes PD, Montell C, Andres DA, Harrison DA. Activated RIC, a small GTPase, genetically interacts with the Ras pathway and calmodulin during Drosophila development. Dev Dyn. 2005;232:817–826. doi: 10.1002/dvdy.20346. [DOI] [PubMed] [Google Scholar]
- Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289–3307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun D, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. J Neurosci. 2007;27:4725–4736. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Raaijmakers JH, Bos JL. Specificity in Ras and Rap signaling. J Biol Chem. 2009;284:10995–10999. doi: 10.1074/jbc.R800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr Opin Cell Biol. 2000;12:157–165. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Sabatier C, McCormick F. Signaling specificity by Ras family GTPases is determined by the full spectrum of effectors they regulate. Mol Cell Biol. 2004;24:4943–4954. doi: 10.1128/MCB.24.11.4943-4954.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runchel CF, Matsuzawa A, Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal. 2011;15:205–218. doi: 10.1089/ars.2010.3733. [DOI] [PubMed] [Google Scholar]
- Ryder E, et al. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics. 2004;167:797–813. doi: 10.1534/genetics.104.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shao H, Kadono-Okuda K, Finlin BS, Andres DA. Biochemical characterization of the Ras-related GTPases Rit and Rin. Arch Biochem Biophys. 1999;371:207–219. doi: 10.1006/abbi.1999.1448. [DOI] [PubMed] [Google Scholar]
- Shi GX, Andres DA. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol Cell Biol. 2005;25:830–846. doi: 10.1128/MCB.25.2.830-846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GX, Jin L, Andres DA. Pituitary adenylate cyclase-activating polypeptide 38-mediated Rin activation requires Src and contributes to the regulation of HSP27 signaling during neuronal differentiation. Mol Cell Biol. 2008;28:4940–4951. doi: 10.1128/MCB.02193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GX, Jin L, Andres DA. Src-dependent TrkA transactivation is required for pituitary adenylate cyclase-activating polypeptide 38-mediated Rit activation and neuronal differentiation. Mol Biol Cell. 2010;21:1597–1608. doi: 10.1091/mbc.E09-12-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GX, Jin L, Andres DA. A rit GTPase-p38 MAPK survival pathway confers resistance to cellular stress. Mol Cell Biol. 2011;31:1938–1948. doi: 10.1128/MCB.01380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GX, Rehmann H, Andres DA. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol Cell Biol. 2006;26:9136–9147. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer ML, Shao H, Andres DA. Induction of neurite extension and survival in pheochromocytoma cells by the Rit GTPase. J Biol Chem. 2002;277:20160–20168. doi: 10.1074/jbc.M201092200. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27: mechanisms of cellular protection against neuronal injury. Curr Mol Med. 2009;9:863–872. doi: 10.2174/156652409789105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Thornton TM, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell'Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Yu M, Montell C. RIC, a calmodulin-binding Ras-like GTPase. EMBO J. 1996;15:5839–5848. [PMC free article] [PubMed] [Google Scholar]
- Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Lin Z, Zhao ZJ, Yang Y, Niu H, Shen X. MAPK-activated protein kinase-2 (MK2)-mediated formation and phosphorylation-regulated dissociation of the signal complex consisting of p38, MK2, Akt, and Hsp27. J Biol Chem. 2006;281:37215–37226. doi: 10.1074/jbc.M603622200. [DOI] [PubMed] [Google Scholar]