Abstract
Background. The efficacy of barrier precautions to prevent influenza transmission is unknown.
Methods. Twenty-eight participants were exposed to monodispersed live attenuated influenza vaccine (LAIV) particles (4.9 μm) in 6 groups: group 1, no precautions; group 2, ocular exposure only; group 3, surgical mask without eye protection; group 4, surgical mask with eye protection; group 5, fit-tested N95 respirator without eye protection; and group 6, fit-tested N95 respirator with eye protection. Influenza was detected by reverse-transcription polymerase chain reaction (RT-PCR) and culture in nasal washes. Exact 95% confidence intervals (CIs) were calculated.
Results. Influenza was detected in 4 of 4 participants in group 1 (95% CI, 0–.60), 3 of 4 in group 2 (95% CI, .006–.806]), 5 of 5 in group 3 (95% CI, 0–.522), 5 of 5 in group 4, (95% CI, 0–.522), 3 of 5 in group 5 (95% CI, .053–.853), and 1 of 5 in group 6 (95% CI, .05–.72). RT-PCR revealed significant differences between group 1 and all other groups except group 3.
Conclusions. Transocular transmission of LAIV occured in most participants suggesting the necessity of eye protection. An N95 respirator provided the best guard further enhanced by eye protection.
Airborne transmission represents a potentially efficient and poorly understood dissemination mechanism for pathogens, including viral infections such as the common cold and influenza. Influenza has caused 3 pandemics in the last century alone, with an overall death toll reaching tens of millions, and continues to cause annual epidemics of varying severity worldwide [1].
Strategies to prevent and control the often explosive outbreaks associated with these pathogens are limited to vaccination and treatment, if available, or isolation and barrier precautions [2, 3]. The latter include the use of face masks in the prevention of virus transmission. However, scientific evidence regarding the efficacy of personal protective equipment has been solely based on studies using mannequin head models and, more recently, hospital-population and several household-based studies [3–14]. The generalizability of mannequin studies to humans is unclear. On the other hand, both hospital and household studies are influenced by participant adherence to mask use and hand hygiene practices and by influenza exposures that are not work related and are uncontrolled.
We have developed a new testing methodology that allows the accurate, reproducible delivery of defined, monodispersed concentrations of live viruses to human participants in a fully controlled environment [15]. Here we report the first data regarding the efficacy of commonly used barrier precautions against influenza.
METHODS
Participants
In 2008, healthy employees and students at Wake Forest University were invited to participate in this study. Nineteen women and 9 men with an average age of 30.5 years agreed to participate (Table 1). None had received the seasonal influenza vaccine before enrollment. Informed consent was obtained from all participants, and participants were reimbursed for their time.
Table 1.
Summary of PCR and Cell Culture Results for LAIV Exposure Groups
| Intervention group | Participant number | Age | Gender | PCR and cell culture |
|
| Preexposure nasal wash | Postexposure nasal wash | ||||
| 1. LAIV transmission | 1 | 27 | M | Neg. | Pos. |
| 2 | 27 | F | Neg. | Pos. | |
| 3 | 45 | F | Neg. | Pos. | |
| 4 | 29 | F | Neg. | Pos. | |
| 2. Transocular transmission | 5 | 31 | M | Neg. | Neg. |
| 6 | 39 | F | Neg. | Pos. | |
| 7 | 25 | F | Neg. | Pos. | |
| 8 | 21 | F | Neg. | Pos. | |
| 3. SM w/o eye protection | 9 | 34 | F | Neg. | Pos. |
| 10 | 34 | M | Neg. | Pos. | |
| 11 | 24 | M | Neg. | Pos. | |
| 12 | 40 | F | Neg. | Pos. | |
| 13 | 22 | M | Neg. | Pos.a | |
| 4. SM with eye protection | 14 | 26 | M | Neg. | Pos. |
| 15 | 28 | F | Neg. | Pos. | |
| 16 | 32 | F | Neg. | Pols. | |
| 17 | 19 | M | Neg. | Pos. | |
| 18 | 21 | F | Neg. | Pos.a | |
| 5. N95 w/o eye protection | 19 | 48 | F | Neg. | Pos. |
| 20 | 24 | F | Neg. | Neg. | |
| 21 | 20 | F | Neg. | Neg. | |
| 22 | 36 | F | Neg. | Pos. | |
| 23 | 55 | M | Neg. | Pos. | |
| 6. N95 with eye protection | 24 | 23 | F | Neg. | Neg. |
| 25 | 24 | F | Neg. | Neg. | |
| 26 | 31 | F | Neg. | Pos. | |
| 27 | 45 | M | Neg. | Neg. | |
| 28 | 20 | F | Neg. | Neg. | |
NOTE. Exact confidence intervals (CIs): group 1: 95% exact CI (0–.60), group 2: 95% exact CI (.006–.806), groups 3 and 4: 95% exact CI (0–.522), group 5: 95% exact CI (.053–.853), group 6: 95% exact CI (.05–.72). LAIV, live attenuated influenza vaccine; PCR, polymerase chain reaction; SM, surgical mask.
Cell culture positive in hemadsorption assay test.
Live Attenuated Influenza Vaccine
The cold-adapted live attenuated influenza vaccine (LAIV) was purchased from MedImmune (2009/10 seasonal Flumist), which contains 2 influenza A strains and 1 influenza B strain (A/South Dakota/6/2007 [H1N1], an A/Brisbane/59/2007-like), A/Uruguay/716/2007 (H3N2) (an A/Brisbane/10/2007-like), and B/Brisbane/60/2008 [16]. Virus concentration was determined by standard plaque titration assay using MDCK cells [17, 18]. Plaque titration assays were performed at 32°C for these cold-adapted virus strains. All LAIV concentrations are given in plaque-forming units (PFUs)/mL of Hanks balanced salt solution (Cambrex BioScience).
Production of Carrier Particles
The particle size was produced by the vibrating-orifice aerosol generator (VOAG; Model 3450, TSI). In short, a liquid stream containing viable virus particles is chopped into uniform pieces (carrier particles) by high-frequency mechanical disruption, for example, ultrasound. The aerosol was adjusted at 4.9 μm (geometric mean diameter), with a geometric standard deviation of <1.13 confirming monodispersion. The carrier particle size was assessed in real time by an aerodynamic particle sizer based on the inertia of the carriers (Model 3321, TSI). A particle size of <5 μm was chosen to represent the greatest challenge to the barrier precautions tested, since smaller particles are more likely to pass through filter materials or face seal leaks.
The total exposure dosage of the individual participant equaled the LAIV dosage recommended by the manufacturer (106.5–7.5 fluorescent focus-forming units [FFUs] of each of the 3 strains per 0.2 mL). The fluid amount dispersed into the air was 2.6 mL over 20 minutes (liquid feed rate of fluid: 0.15 cm3/min, ultrasound frequency: 340 kHz, orifice diameter: 8 μm). To avoid agglomeration of smaller carrier particles, an electrostatic neutralizing step was introduced before release into room air. The total exposure amount was determined over 3 runs by a 6-stage Andersen sampler, revealing an average virus concentration of 1.13 × 106 ± 5.43 × 105 copies RNA/m3 (.01 pg RNA/m3).
Test Chamber
An airtight chamber, approximately 3.1 m3 in volume, was built around the front of a class II biologic safety hood (Purifier, Labconco Corp), with sufficient room for a volunteer to sit in front of the workbench. When activated, the air inside the chamber recirculates through a high-efficiency particulate air (HEPA) filter to remove any airborne pathogens. This chamber is equipped with a half-mask respirator with a backpack adaptor (North Safety Products). This allows a study subject wearing this gear to breathe clean air from outside the chamber, and to restrict virus exposure to the ocular route only.
Interventions
Participants were assigned to 1 of 6 groups: group 1, no barrier precautions (4 participants); group 2, ocular exposure only (4 participants); group 3, tie-on surgical mask with nose clip (Tie-On Surgical Mask 1818, 3M) without eye protection (5 participants); group 4, surgical mask with eye protection (nonvented Z87 Uvex Goggles; 5 participants); group 5, fit-tested N95 respirator with nose clip (3M 1860/1860S Health Care Particulate Respirator) without eye protection (5 participants); and group 6, fit-tested N95 respirator with eye protection (5 participants). The nonfiberglass, 3-layer surgical mask met the bacterial filtration efficiency (BFE >99%) and particle filtration efficiency (PFE >99% at 0.1 μm) standards according to the manufacturer. The nonwoven, fluid-resistant N95 respirator was approved by the National Institute for Occupational Safety and Health (NIOSH) and the Occupational Safety and Health Administration (OSHA), and meets the Centers for Disease Control and Prevention (CDC) guidelines for tuberculosis exposure control (filter efficiency level >95% against particulate aerosols free of oil, BFE >99%). All participants were exposed only once.
Aerosolization Runs
On the day of exposure, study subjects were checked for contraindications to LAIV vaccination. A nasal wash (2.5 mL of 0.9% saline solution in each nostril) was performed in each participant before exposure to control for active influenza infection. The participants changed into disinfected surgical scrubs, gowns, gloves, cap, and shoe covers. Barrier precautions were provided according to group assignment. N95 respirator fit-testing was performed using the TSI PortaCount Plus [19].
Participants were placed in front of the VOAG while remaining silent and avoiding any brisk body movements. The distance of the volunteer to the VOAG was held constant within 1 foot. After a 2-minute clearance run of the safety hood, the VOAG was turned on for a total of 20 minute. During the run there was no air exchange, and the temperature and relative humidity were held at 22°C and 60%, respectively (433 MHz Cable Free Pro Temperature and Humidity Monitor, Thermo Fisher Scientific).
After the exposure run, the VOAG was switched off and the safety hood was activated for a 5-minute clearance run. After stepping out, the participant was asked to remove the gloves followed by the mask without touching the face/nose area, and a nasal wash was performed. Subjects were then asked to undress and to wash hands and face with an antiviral washing solution. Participants were guided through the process to minimize the risk of cross-contamination.
After each exposure session, all surfaces potentially in contact with LAIV were thoroughly cleaned with antiviral decontamination solutions to avoid cross-contamination.
Endpoint Measures
Nasal Washes.
Nasal washes (2.5 mL of saline solution for each nostril) were performed on day 1 prior to and immediately after LAIV aerosol exposure to determine the presence of influenza vaccine strains. Samples were immediately used for reverse-transcription polymerase chain reaction (RT-PCR) and viral culture. Successful exposure was defined as 1 postexposure nasal wash positive for 1 of the challenge influenza A viruses. The amount of RT-RNA detected by PCR was used for quantitative comparisons of the intervention groups.
RT-PCR.
Extraction was carried out using the Qiagen viral RNA extraction kit (catalog number 52906). For quantitative reverse transcription-PCR (qRT-PCR) detection of the influenza A strains in the aerosolized samples of LAIV, the M gene of the master donor virus strains ca A/Ann Arbor/6/60 (accession number M23978) was the amplification target for A strains, using the following primer set:
• Primer AF: 5′ -AAA GCC GAG ATC GCA CAG AGA CTT -3′
• Primer AR: 5′ -GGC ACG GTG AGC GTG AAT ACA AAT -3′
DNA vectors containing the M gene regions for influenza A were synthesized by GeneArt in a pMA(ampR) vector.
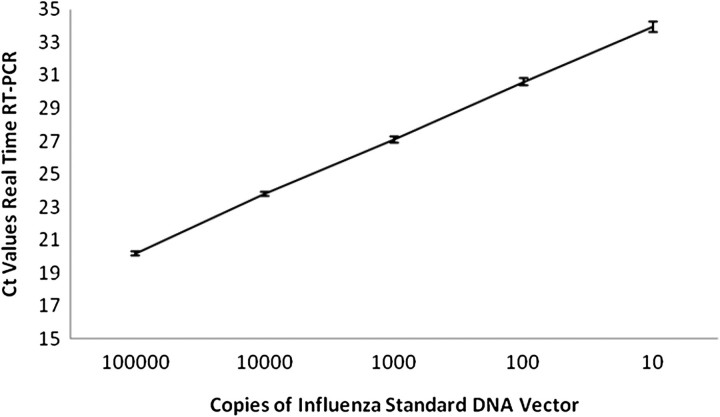
Standard curves (DNA vectors) produced for influenza A were used to quantify the amount of viral RNA present in the samples produced from the aerosolized runs of LAIV (Figure 1) [20]. All dilutions of standards, master mix assembly, and qRT-PCR 96-well plate setup were performed with an Eppendorf robot (epMotion 5070; QuantiTect SYBR Green RT-PCR Kit [catalog number 204243, Qiagen]). Plates were assayed in an Eppendorf MasterCycler ep Realplex 2. The thermocycler conditions were as follows: RT-step at 50°C for 30 minute, Taq activation at 95°C for 15 minute, followed by 40 cycles at 94°C for 15 seconds (denaturation), 55°C for 30 seconds (primer annealing), 72°C for 30 seconds (extension), and primer melting curve step, which allowed for monitoring of the amplification of product for influenza.
Figure 1.
Standard curves for influenza A real time reverse-transcription polymerase chain reaction. Data represent results from 4 independent runs.
We calculated the average number of RNA copies and the weight of RNA in picograms per PCR reaction in nasal washes for the intervention groups and compared them to the total LAIV exposure dosage dispersed into the air.
Viral Culture.
To confirm the viability of LAIV in the nasopharynx of exposed subjects, a selected sample from subjects in groups 3–6 was cultured by inoculation of Rhesus Monkey Kidney cells and incubated at 32°C for 5 days. Viral growth was detected by qualitative hemadsorption of guinea pig red blood cells. Hemadsorption assay was performed as follows: Cell media was replaced with 1 mL of 0.1% guinea pig red blood cells (BioLink) and incubated for 30 minute at 4°C, and aggregation of red blood cells was determined by direct light microscopy [21].
Statistical Analysis.
Percentage rates for the transmission of LAIV to the nasopharyngeal mucosa of aerosol-exposed study participants were calculated for each group. To calculate the 95% confidence interval (CI) for these transmission rate percentages, we used the exact binomial distribution computational algorithm (SAS software, version 9.2). To compare the average RNA copies observed per intervention group, pairwise comparisons were done using the nonparametric Wilcoxon rank sum test.
RESULTS
Interventions
All study participants in group 1 experienced transmission of aerosolized LAIV to the nasopharynx (Table). An exact 95% CI for this binomial outcome, based on our observed rate of 100% positive responses, would include the interval of (0%–60%); the interpretation of this interval is that if this experiment were conducted 100 times, approximately 95% of the times, the observed percentage of patients carrying LAIV in their mucous membranes would fall within this range. Three of 4 study subjects in group 2 showed transmission of aerosolized LAIV to the nasopharynx despite exposure being limited to the transocular route. The efficacy of barrier precautions against LAIV aerosol transmission was tested in groups 3 to 6, each group with 5 study subjects. All subjects wearing surgical masks without eye protection (group 3) showed transmission of LAIV to the nasopharynx. The addition of eye protection did not decrease transmission.
Two of 5 study subjects wearing fit-tested N95 respirators showed transmission of LAIV to the nasopharynx. One of 5 study subjects wearing eye protection plus fit-tested N95 respirators showed transmission of LAIV to the nasopharynx.
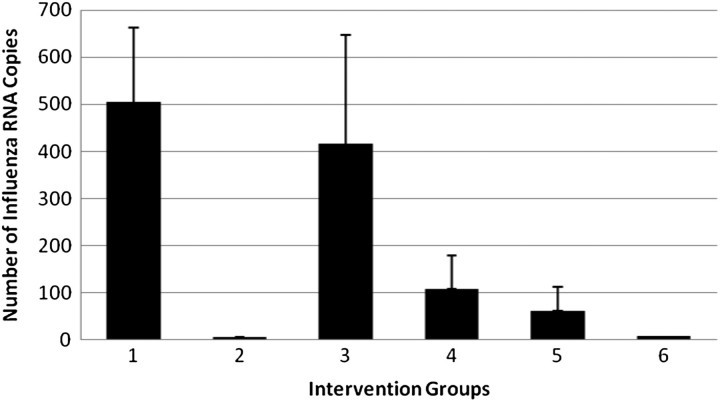
In the nasal washes, RT-PCR revealed 504 copies, or 5.49 × 10−5 pg RNA in group 1, followed by group 3 (416 copies, 7.95 × 10−5 pg RNA), group 4 (108 copies, 2.47 × 10−5 pg RNA), group 5 (62 copies, 1.74 × 10−5 pg RNA), group 6 (8 copies, 1.38 × 10−6 pg RNA), and group 2 (5 copies, 9.20 × 10−7 pg RNA) (Figure 2). Significant differences were detected between group 1 (no mask) and all other groups (P < .05) except group 3 (surgical mask without eye protection, P = .62) (Figure 2). Comparison of the effects of eye protection within the surgical and the N95 respirator groups revealed no statistically significant differences.
Figure 2.
Quantitative reverse-transcription polymerase chain reaction results of influenza A in nasal washes by intervention group.
Safety
None of the 28 participants reported any influenza symptoms following LAIV exposure.
DISCUSSION
In the past, assessment of mask/respirator efficacy by certification entities such as NIOSH has been limited to filter material testing and mannequin head models [22]. However, the emergence of new pathogens such as severe acute respiratory syndrome (SARS) and most recently the H1N1 virus have called into question the applicability of these findings to real-world practice [23]. Two recent field studies have attempted to address this question. Loeb et al enrolled 446 nurses in 8 tertiary care centers who were randomly assigned to wear a medical mask or a fit-tested N95 respirator [7]. No differences between the 2 groups could be detected. Similar results were observed by Ang et al [8] during the 2009 H1N1 pandemic in Singapore. Groups of persons wearing N95 respirators and surgical masks experienced similar rates of acute respiratory illness. Several problems are inherent to these field studies. Individual exposure risks of health-care workers are not limited to patient contacts in the hospital but are affected by exposures outside the work setting. These risks may be highly variable and difficult to account for in field studies. Furthermore, compliance to other infection control activities such as hand hygiene, isolation/cohorting of patients, or triage may greatly influence individual exposure risks. This was demonstrated during the 2002–2004 SARS outbreaks [24].
We have developed a novel approach to test the efficacy of face masks and respirators. In it, human subjects were exposed to live viruses in defined concentrations and particle sizes challenging the barrier precautions by using small, filter- and face-leak–penetrating particles. Safety of the participants is assured by using attenuated viral pathogens in the form of Food and Drug Administration (FDA)–approved vaccine strains, such as LAIV. This novel setup allows a cost-efficient, timely, and accurate way to study infection routes and the efficacy of barrier precautions in controlling airborne and droplet viral transmission in humans.
The most surprising finding of this study is very high rates of transocular transmission in subjects exposed to aerosols containing LAIV. Nasal washes from 3 of 4 participants were positive for LAIV immediately following exposure. This strongly suggests that LAIV is reaching the nasopharynx by way of the nasolacrimal duct. It should also be noted that the lag time between exposure to aerosolized LAIV and detection in nasal washes was less than 30 min, indicating a fast transfer rate of virus to the nasopharynx. Indirect evidence of transocular transmission has previously been reported for the common cold and respiratory syncytial virus (RSV). Winther et al showed that virus can be detected in the nasopharynx after eye inoculation with a rhinovirus suspension (human rhinovirus [HRV] 39; 300 median tissue culture infective dose; first sample taken 24 hour after inoculation) [25]. Gala et al found a decrease in RSV infections due to disposable eye-nose goggles on an infant ward, indicating an effect of eye protection [26]. However, this is the first study presenting direct evidence of transocular delivery of influenza viruses in airborne form.
In contrast to our findings using LAIV, we could not detect a common cold virus (HRV 39) in nasal washes in 10 subjects after eye exposure [27]. We used the same testing environment as in the present study, and the only difference was a much lower exposure dosage for the common cold virus (human infective dose 100 for HRV without protection: 560 PFU total exposure) compared with LAIV (106.5–7.5 FFU total exposure). This raises the question if factors such as specific virus characteristics (enveloped vs nonenveloped) or exposure amounts may influence transocular delivery.
Efficacy testing of the 2 respiratory barrier types against the mechanically generated aerosol revealed the superiority of a fit-tested N95 respirator over a surgical mask; the surgical mask did not provide protection from LAIV transmission whether or not eye protection was also employed. This is in contrast to the recent findings from field studies [7, 8]. The addition of eye protection to the fit-tested N95 respirator increased protection from LAIV transmission, suggesting a combination of transocular and direct respiratory transmission. However, due to the small sample size, this trend remains to be confirmed.
Human exposure studies using viral pathogens raise concerns regarding the safety of participants. To minimize the risk of adverse events, we decided to use seasonal LAIV approved by the FDA for intranasal spray application. None of the study subjects reported any influenza symptoms or adverse events.
Another risk associated with live virus studies is cross-contamination of samples. Close monitoring, guidance of the participants, and thorough cleaning of the testing environment after the exposure sessions assured compliance with the removal of contaminated clothing and disinfection steps. In addition, our previous study with a common cold virus using the same stringent protocol did not show any evidence of cross-contamination [27].
In a recent study, Lindsley et al described the overall distribution of airborne influenza virus in an urgent care medical center [28]. They found that about 42% of influenza A RNA in particles were ≤4.1 μm, indicating potential transmission by aerosols, as opposed to larger droplets. This finding affirms the importance of studying influenza transmission in aerosols, as we have done in this study. Interestingly, influenza A was detected in concentrations ranging from 0.1 pg RNA/m3 to 75.4 pg RNA/m3, confirming the presence of viral RNA in a patient care setting. In our study, subjects were exposed to 0.01 pg RNA/m3, at least 10-fold lower than concentrations of viral RNA found by Lindsley et al. Our decision to expose study subjects to a complete vaccine dosage did not result in influenza virus exposures higher than those likely encountered in clinical settings.
Our study has several limitations. Potential differences between the vaccine strains, wild-type seasonal influenza, and the pandemic H1N1 strain should be considered. To date, only 1 case of person-to-person LAIV transmission following vaccination has been reported, suggesting that this influenza strain may have different transmission characteristics than wild-type influenza strains [29]. There are similar concerns regarding the contents of bioaerosols. Proteins and other substances expelled with virus particles may change their survival and infectivity properties. However, our study detected influenza present in the nasopharynx immediately following exposure, and we did not measure viral infection or growth in the nasopharynx of study subjects. Thus, the applicability of our findings to influenza virus transmission are not likely influenced by differences in transmission dynamics or growth kinetics between wild-type strains and the cold-adapted, attenuated vaccine strains of this study.
Because we primarily used RT-PCR detection methods in our analysis, we wished to confirm the viability of LAIV vaccine strains used. Therefore, we performed viral cultures in a selection of nasal wash specimens, with the caveat that identification of virus in the samples does not imply infection. We detected LAIV by culture in 2 of 4 samples. Our findings are consistent with those of other groups that showed greatly enhanced influenza detection sensitivity of RT-PCR techniques [30].
Our limited sample size did not allow calculation of P values for the group count outcomes. However, exact 95% confidence intervals were calculated to establish an estimate of certainty for the observed results. We also compared the quantitative RT-PCR results revealing significant differences between no protection, surgical masks plus eye protection, and N95 respirator groups. Wearing the surgical mask with or without eye protection did not reduce virus transmission. We were also limited to 2 brands of masks and respirators. In particular, surgical masks have shown filter efficiencies ranging from 10% to 90% in sodium chloride tests [31]. We tested only 1 N95 respirator, fit-tested to the individual participant as required by the CDC. The effect of fit-testing on respirator efficacy may be an important variable to measure in future studies incorporating larger sample sizes. During the H1N1 pandemic the efficacy of surgical masks compared with N95 respirators for prevention of aerosol transmission of influenza was widely debated, resulting in differing recommendations [32, 33]. Recently, the CDC has updated its recommendations for the 2010/2011 influenza season, requiring surgical masks for all patient care activities except aerosol-producing procedures. The latter require fit-tested N95 respirators [34]. However, evidence regarding the efficacy of masks or respirators against influenza is only beginning to emerge. Our model provides a novel approach to evaluate the preventive qualities of these barrier precautions in a controlled testing environment, allowing the manipulation of key variables such as viral load, particle size, temperature, and humidity during human exposure.
According to our results, the eyes could be an entry route for influenza, allowing viral particles easy and fast access to the upper respiratory tract. The type of surgical mask tested was inferior to a fit-tested N95 respirator in preventing aerosol delivery; however, none of the tested barrier precautions provided complete protection, including a CDC-recommended fit-tested N95 respirator and the addition of eye protection.
The conclusions drawn from this study are influenced by the current lack of understanding regarding the viral particle load necessary to infect an individual with influenza, and the dispersal pattern of influenza produced by affected patients. Mechanically produced aerosol represents a high-risk exposure situation using small aerosol particles, and will need to be compared with concentrations and size distributions that represent human-generated aerosols to allow an understanding of actual risk. However, our study introduces a novel testing approach and points to the potential need for combining effective respirator types with eye protection to successfully interrupt transmission of influenza in aerosol form. These outcomes may provide at least first insights in the transocular transmission dynamics of influenza and the efficacy of the barrier precautions tested.
Funding
The influenza exposure study was sponsored by an intramural grant from the Center for Worker Health at Wake Forest University School of Medicine, Winston-Salem, North Carolina. Development of the aerosolization and detection technology was funded by a CDC/Department of Health and Human Services (grant 5U01CI000474-02).
Acknowledgments
The authors thank Dr Michael Bell, CDC Division of Healthcare Quality Promotion, for supporting the development of the aerosolization technology.
References
- 1.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous Respiratory protection, 29 CFR §1910.134. 1998;63:1151–200. [Google Scholar]
- 3.Musher DM. How contagious are common respiratory tract infections? N Engl J Med. 2003;348:1256–66. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan A, Perl TM. Respiratory protection against influenza. JAMA. 2009;302:1903–4. doi: 10.1001/jama.2009.1494. [DOI] [PubMed] [Google Scholar]
- 5.Mermel LA. Preventing the spread of influenza A H1N1 2009 to health-care workers. Lancet Infect Dis. 2009;9:723–4. doi: 10.1016/S1473-3099(09)70299-3. [DOI] [PubMed] [Google Scholar]
- 6.Jefferson T, Del Mar C, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2009;339:b3675. doi: 10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–71. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 8.Ang B, Poh BF, Win MK, Chow A. Surgical masks for protection of health care personnel against pandemic novel swine-origin influenza A (H1N1)-2009: results from an observational study. Clin Infect Dis. 2010;50(7):1011–14. doi: 10.1086/651159. [DOI] [PubMed] [Google Scholar]
- 9.Gralton J, McLaws ML. Protecting healthcare workers from pandemic influenza: N95 or surgical masks? Crit Care Med. 2010;38:657–67. doi: 10.1097/ccm.0b013e3181b9e8b3. [DOI] [PubMed] [Google Scholar]
- 10.Larson EL, Ferng YH, Wong-McLoughlin J, Wang S, Haber M, Morse SS. Impact of non-pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep. 2010;125:178–91. doi: 10.1177/003335491012500206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15:233–41. doi: 10.3201/eid1502.081167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiello AE, Murray GF, Perez V, et al. Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. J Infect Dis. 2010;201:491–8. doi: 10.1086/650396. [DOI] [PubMed] [Google Scholar]
- 13.Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151:437–46. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 14.Cowling BJ, Zhou Y, Ip DK, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138:449–56. doi: 10.1017/S0950268809991658. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff WE. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, K-1615a, 12–15 September. 2009. Novel technique to study live influenza and common cold virus in mono-dispersed aerosols. [Google Scholar]
- 16.MedImmune FluMist 2009/2010 Package Insert. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm123743.pdf. Accessed 16 June 2010. [Google Scholar]
- 17.Matrosovich M, Matrosovich T, Garten W, Klenk HD. New low-viscosity overlay medium for viral plaque assays. Virol J. 2006;3:63. doi: 10.1186/1743-422X-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youil R, Su Q, Toner TJ, et al. 2004.Comparative study of influenza virus replication in Vero and MDCK cell lines. J Virol Methods. 2004;120:23–31. doi: 10.1016/j.jviromet.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 19. Standard 29 CFR: Part 1910.134 respiratory protection. http://www.osha.gov/pls/oshaweb/owastand.display_standard_group?p_toc_level=1&p_part_number=1910. Accessed 16 June 2010. [Google Scholar]
- 20.Ngaosuwankul N, Noisumdaeng P, Komolsiri P, et al. Influenza A viral loads in respiratory samples collected from patients infected with pandemic H1N1, seasonal H1N1 and H3N2 viruses. Virol J. 2010;7:75. doi: 10.1186/1743-422X-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talbot TR, Crocker DD, Peters J, et al. Duration of virus shedding after trivalent intranasal live attenuated influenza vaccination in adults. Infect Control Hosp Epidemiol. 2005;26:494–500. doi: 10.1086/502574. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous Respiratory protective devices; final rules and notice, 42 CFR part 84. 1995;60:30335–98. [Google Scholar]
- 23.Jefferson T, Del Mar C, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: a Cochrane review. Health Technol Assess. 2010;14:347–476. doi: 10.1002/14651858.CD006207.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Gamage B, Moore D, Copes R, Yassi A, Bryce E BC Interdisciplinary Respiratory Protection Study Group. Protecting health care workers from SARS and other respiratory pathogens: a review of the infection control literature. Am J Infect Control. 2005;33:114–21. doi: 10.1016/j.ajic.2004.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winther B, Gwaltney JM, Jr., Mygind N, Turner RB, Hendley JO. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–7. [PubMed] [Google Scholar]
- 26.Gala CL, Hall CB, Schnabel KC, et al. The use of eye-nose goggles to control nosocomial respiratory syncytial virus infection. JAMA. 1986;256:2706–8. [PubMed] [Google Scholar]
- 27.Bischoff WE. Transmission route of rhinovirus type 39 in a mono-dispersed airborne aerosol. Infect Control Hosp Epidemiol. 2010;31:857–9. doi: 10.1086/655022. [DOI] [PubMed] [Google Scholar]
- 28.Lindsley WG, Blachere FM, Davis KA, et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50:693–8. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- 29.Vesikari T, Karvonen A, Korhonen T, et al. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J. 2006;25:590–5. doi: 10.1097/01.inf.0000220229.51531.47. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg GA, Erdman DD, Edwards KM, et al. New Vaccine Surveillance Network Study Group. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–10. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 31.Oberg T, Brosseau LM. Surgical mask filter and fit performance. Am J Infect Control. 2008;36:276–82. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC. Interim guidance on infection control measures for 2009 H1N1 influenza in healthcare settings, including protection of healthcare personnel. 2010. http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed 16 June 2010. [PubMed] [Google Scholar]
- 33.WHO. Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses interim guidance, 16 December 2009. http://www.who.int/csr/resources/publications/swineflu/swineinfinfcont/en/index.html. Accessed 16 June 2010. [Google Scholar]
- 34.CDC. Guidelines and recommendations prevention strategies for seasonal influenza in healthcare settings. 2010. http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed 2 November 2010. [Google Scholar]