Abstract
The lymphatic system plays a crucial role in the immune system’s recognition and response to disease, and most solid cancers initially spread from the primary site via the tumor’s surrounding lymphatics before hematological dissemination. Hence, the lymphatic system is an important target for developing new vaccines, cancer treatments, and diagnostic agents. Targeting the lymphatic system by subcutaneous, intestinal, and pulmonary routes has been evaluated and subsequently utilized to improve lymphatic penetration and retention of drug molecules, reduce drug-related systemic toxicities, and enhance bioavailability of poorly soluble and unstable drugs. Lymphatic imaging is an essential tool for the detection and staging of cancer. New nano-based technologies offer improved detection and characterization of the nodal diseases, while new delivery devices can better target and confine treatments to tumors within the nodal space while sparing healthy tissues. This manuscript reviews recent advances in the field of lymphatic drug delivery and imaging and focuses specifically on the development ofliposomes and solid lipid nanoparticles for lymphatic introduction via the subcutaneous, intestinal, and pulmonary routes.
1. Liposomal drug delivery and imaging
A wide spectrum of cancers, including breast, lung, and head and neck squamous cell cancers, metastasizes through lymphatics to enter systemic circulation and develop secondary tumors. The lymphatics and sentinel lymph node, the first tumor draining lymph node, play a significant role in early stage diagnostics and subsequent therapies. Lymphatic delivery of drug encapsulated liposomal formulations has been investigated extensively in the past decade. A liposome is a vesicle containing lipid bilayer, composed of unimers which usually have a hydrophilic head and a hydrophobic tail and oriented so that the hydrophobic headgroups are inside the bilayer. Hydrophilic, lipid-insoluble drugs, or DNA can be encapsulated in the aqueous core within the liposome to improve their stability or bioavailability, compared to the free drugs or DNA. Current research on the lymphatic retention and imaging of subcutaneously, intestinally, and pulmonary administration of engineered liposomes is discussed in this section. The primary improvements over conventional therapy are summarized in Table 1.
Table 1.
Summary of primary improvements of engineered liposomes over conventional therapy in vivo.
1.1 Liposomal formulations for lymphatic drug delivery via the subcutaneous route
Traditional liposomal formulations utilize the encapsulation of anti-cancer drugs into liposomes for intravenous infusion for the treatment of various types of cancers. Doxil, a pegylated liposomal doxorubicin chemotherapy formulation, is widely used as first-line therapy of AIDS-related Kaposi’s sarcoma, breast cancer, ovarian cancer, and other solid tumors [9–13]. The primary advantages of systemic liposomal chemotherapy encompass reduced side effects compared to the standard therapy, and prevention of the anti-cancer drug from enzymatic digestion in the systemic circulation. Another characteristic of pegylated liposomes is that their size can be adjusted through the modification of the PEG chains or lipid bilayer core of the carrier, promoting its potential use as a drug delivery vehicle to the lymphatics after subcutaneous injection [1].
In 2007, Moghimi et al. proposed that subcutaneously injected pentameric IgM may facilitate the lymphatic drainage and enhance lymphatic retention of IgG1-PEG-liposome conjugates; presumably attributed to the binding between IgM and macrophage Fc receptors in the draining lymph node sinuses, promoting the effective trapping of the therapeutic peptide or protein bound immune-PEG-liposomes [2]. In their previous study, they reported that pegylated immune-liposomes had more rapid lymphatic drainage from the injection site to the surrounding lymphatics due to the hydrophilic nature of the PEG moieties [3]. In their later study, they proved that concomitant administration of monoclonal IgM against rat IgG1 adjacent to the injection site of IgG1-PEG-liposomes could further enhance the lymphatic retention of the liposomal conjugates. It may be ascribed to the formation of IgM antibody-mediated vesicular aggregates in the lymphatic vessels. Harrington et al. reported improved delivery of anticancer drugs to tumors using pegylated liposomes as vehicles for intratumoral and subcutaneous injection [1]. The authors compared the pharmacokinetics and tissue distribution, of subcutaneously or intratumorally injected pegylated liposome-encapsulated 111In-labeled diethylenetriaminepentaacetic acid (IDLPL) with the unencapsulated IDLPL. The encapsulated form increased the carrier residence time and concentration in the head and neck tumors and their nodal metastases (both inguinal and axillary lymph nodes) in a nude mouse xerograph model, yielding a 11.6-fold increase in the area-under-the-curve (AUC) (96 hours).
A major issue of conventional liposomal formulations is that liposomes are poorly retained in the draining lymph nodes following injection (<2% injected dose). In order to enhance the retention of liposomes to lymph nodes, Phillips et al. developed an avidin activated biotin-coated liposome, resulting in prolonged lymphatic retention and extended lymph node localization, in an experimental rabbit model [4]. Biotin-coated liposomes were injected subcutaneously into both hind feet of rabbits, followed by an adjacent subcutaneous injection of avidin, causing the formation of liposomal aggregates in the lymphatic vessels. Twenty-four hours post injection, the retention of the liposomes was increased by 7.1 and 6.7-fold in the popliteal nodes and iliac nodes, respectively.
Intravenous liposomal doxorubicin (i.e. Doxil) has substantially improved pharmacokinetics compared to doxorubicin via the intravenous route, including improved AUC and half life. Ling et al. compared subcutaneous administration of liposomal doxorubicin with the standard intravenous doxorubicin treatment in a rabbit breast cancer model [14]. Female New Zealand rabbits were inoculated with VX2 carcinomas and treated three times with 1-mg/kg (doxorubicin equivalent) of subcutaneous liposomal doxorubicin, intravenous adriamycin, or saline, once the axillary lymph nodes reached 5 mm in diameter. Two days after the completion of the treatment, the nodal volume was measured and compared between different treatment groups. Data revealed that the liposomal doxorubicin treatment and intravenous doxorubicin decreased the nodal volume by 57% and 27%, respectively, and increased the number of apoptotic cells by 3.2- and 2.0-fold, respectively, compared to the saline control. The subcutaneous administration of doxorubicin demonstrated a more robust inhibitory effect in relative to systemic doxorubicin, with a p value equals to 0.002 between the two treatment groups. Site inflammation including allergy and skin ulceration was not observed, likely due to the low dose of the drug administered, as well as the relatively short treatment period. In summary, the liposomal doxorubicin treatment exhibited effective inhibition of tumor progression and induction of apoptosis of metastatic cells, which may be a promising method for the treatment of node-positive advanced breast cancer.
The lymphatic administration route can also improve access to the systemic space and improve plasma pharmacokinetics compared to intravenous formuations. Trubetskoy et al. showed that subcutaneously injected liposome-encapsulated drugs can be absorbed into systemic circulation in response to manual massage of the injection site [15]. A five minute manual massage could induce release of up to 40% of the subcutaneously injected 200-nm 111In-angiotensin II incorporated PEG-liposomes (made with egg phosphatidyl choline). In addition, when compared to the unmodified liposomes, the PEG-shielded liposomes had increased blood localization due to the reduced uptake by the lymph node macrophages. During the observation period of 45 minutes, the blood concentrations of the radioisotope-labeled, and drug-incorporated PEGylated liposomes remained constant, whereas the concentration of the plain liposomes decreased shortly post massage. The authors also suggested that the drug release pattern and the pharmacokinetic profile of the formulation could be modulated though the modification of the liposomal surface structure..
1.2 Liposomal formulations for lymphatic drug delivery via the intestinal route
Oral delivery of therapeutics is the most convenient and widely employed route of drug delivery. However, a number of drugs exhibit poor oral bioavailability due to the degradation of the active drugs in the gastrointestinal tract. The pH of stomach fluid may go as low as 1.0 in the fasted state and increase to 3.0 to 4.0 in the fed state. The extremely acidic environment facilitates the degradation of orally administered drugs. In the 1970s, liposomal formulations were developed as oral drug delivery carriers, shielding the labile drugs from the acidic environment via reduced rate of degradation and increased extent of uptake. Orally administered drug-incorporated liposomes enter the systemic circulation via the portal vein and intestinal lymphatics. Drugs entering the portal vein pass through the liver and undergo first-pass metabolism. However, drugs entering the intestinal lymphatic through the intestinal lumen first migrate to the lymphatic vessels and draining lymph nodes before entering systemic circulation; therefore, avoiding the liver and first-pass metabolism. Lymphatic uptake of therapeutics via the intestinal route plays a role in the increased bioavailability of a number of drugs [16].
Intestinal absorbability and stability are the primary formulation concerns when delivering drug-encapsulated liposomal formulations orally. In the 1980s, Hashida et al. studied the in vivo absorption characteristics of liposome-encapsulated carboxyfluorescein as a model liposomal drug and compared the plasma and lymph concentration profiles of the released carboxyfluorescein to that of the free dye [5]. There were no statistically significant differences in both the plasma and lymph between the two formulations, suggesting drug-encapsulated liposomes exhibited poor absorption across the intestinal mucosa. However, co-administration of lipid-surfactant mixed micelles greatly enhanced the permeability of the drug-trapped liposomes. This inductive effect was likely due to the interaction between the lipid-surfactant micelle and the lipid bilayer of the intestinal cell membrane.
A number of hydrophilic drugs exert poor lymphatic transport due to their low intestinal bioavailability, limiting their use in the clinic. In 2006, Ling et al. evaluated a liposome system for oral delivery of a poorly bioavailable hydrophilic drug, cefotaxime, in a rat model by comparing three forms of the drug: liposomal formulation, aqueous free drug, and a physical mixture of the drug and liposomes [16]. The data revealed that the liposomal formulation of the drug exhibited a 2.7-fold increase in its oral bioavailability compared to the aqueous dosage, and a 2.3-fold increase for the physical mixture. In addition, they also reported that the liposomal formulation lead to a significant enhancement of the lymphatic localization of the drug relative to the other two formulations. Therefore, liposome systems may be useful carriers for poorly bioavailable hydrophilic drugs, promoting their lymphatic transport in the intestinal lymph as well as their systemic bioavailability.
Oral delivery of DNA vaccination remains a challenge due to the unsatisfactory stability of DNA vaccines and the need to induce an immunological response after immunization. Perrie et al. reported a novel liposome-mediated DNA vaccination using a dehydration-rehydration method, encapsulating plasmid DNA encoded for an enhanced green fluorescent protein into the liposomes [6]. Stability studies in a simulated intestinal media suggested the dehydration-rehydration vesicles incorporating the DNA (DRV-DNA) had a greater intestinal stability compared to the small unilamellar vesicles (SUV-DNA). In addition, the DRV-DNA, when administered orally in a mouse model, invoked a greater and longer IgA response compared to the naked DNA, suggesting a prolonged DNA retention and improved DNA protection of the DRV-DNA formulation.
1.3 Liposomal formulations for lymphatic drug delivery via the pulmonary route
The respiratory system consists of an upper respiratory tract, including the nose, nasal cavity, and pharynx; and a lower respiratory tract, including the lungs and trachea. The lungs are rich in lymphatic vessels, and a series of lymph nodes encompassing the hilar, mediastinal, carinal, and aortic nodes. Therefore, the lymphatics and lymph nodes can be readily accessed by pulmonary delivered aerosolized formulations [17].
Chemotherapy delivered by the pulmonary route could concentrate anticancer drugs in the lungs and draining lymph, while avoiding significant issues associated with systemic chemotherapy including serious non-target toxicities, poor drug penetration into the lymphatic vessels and surrounding lymph nodes, and first pass clearance in the case of orals. Latimer et al. investigated the feasibility of delivering liposomal formulation of paclitaxel and a vitamin E analog (α-TEA) in an aerosol for treatment of murine mammary tumors and metastases [7]. The pulmonary delivery of sequential combination treatments significantly decreased the mammary tumor burden compared to controls as well as the individually administered liposomal drugs. In addition, the aerosolized combination regimen reduced the total number of cells staining positive for cell markers CD31 and Ki67, suggesting an improved inhibition of lymphatic and pulmonary metastases. In a similar study, Lawson et al. compared the anti-proliferative efficacy of a dilauroylphosphatidylcholine liposome delivered 9-nitro-camptothecin (9-NC), α-TEA, and a combination therapy of 9-NC and α-TEA in a metastatic murine mammary tumor model. Each individual treatment and the combination treatment was encapsulated in liposomes and delivered via an aerosol to treat lung and the surrounding lymph node metastases. The Ki-67 immunostaining results revealed that there were less proliferative cells present in the animals treated with the combination therapy compared to the animals treated with 9-NC alone. However, there were no statistically significant differences between the 9-NC + α-TEA group and α-TEA group alone. All three treatments (9-NC alone, α-TEA alone, and 9-NC + α-TEA) showed elevated number of apoptotic cells by TUNNEL assay, when compared to the empty liposome control group. In addition, the in vivo anti-cancer efficacy studies demonstrated the combination treatment greatly hindered the tumor progression compared to each treatment alone, leading to the prolonged survival rate. [18].
Another application of pulmonary delivered liposomal formulations is to visualize deep lung lymphatic drainage after incorporating radioactive markers such as 99mTc into liposomes. Botelho et al. administered an aerosolized formulation of nanoradioliposomes to wild boars, and successfully visualized their deep lymphatic lung network and surrounding lymph nodes (Fig. 1) [8]. The technique provided new information into the complex structure of the lymphatic network, which was not available through the use of conventional CT. In addition, it has become a new and non-invasive molecular imaging technique for the diagnosis of early dissemination of lung cancers.
Figure 1.
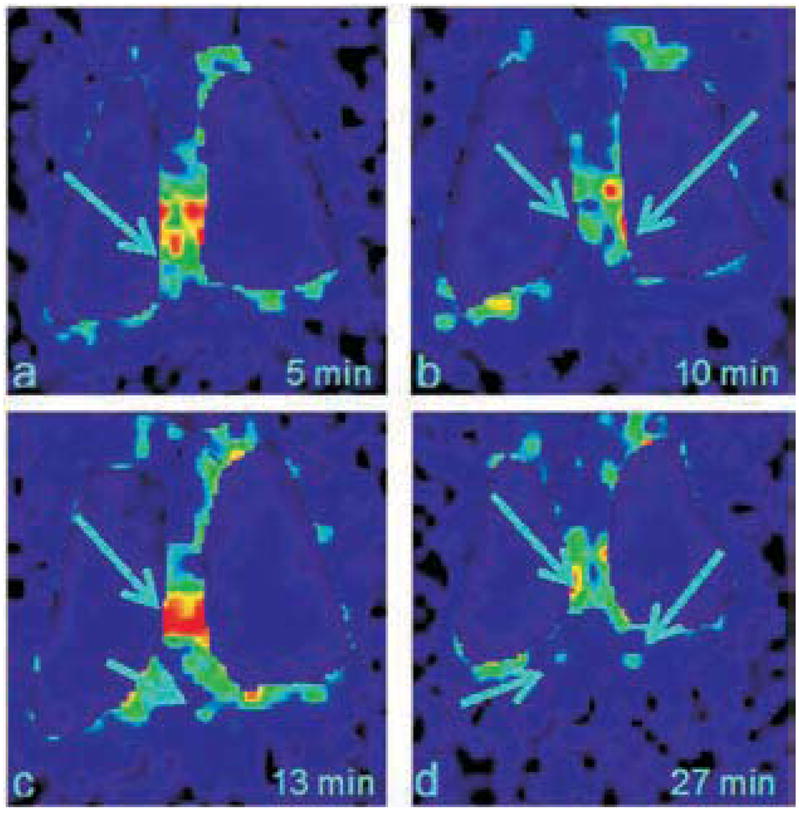
Thoracic images in posterior view obtained after 99mTc-Liposome aerosol inhalation, in the pig n° 18. a) 5 min after the inhalation – visualization of hilar draining chains; b) 10 min after the inhalation – individualization of hilar draining chains; c) 13 min after the inhalation – transdiaphragmatic drainage; d) 30 min after the inhalation – initial visualization of the aortic chain ganglia (Adapted from [8]).
1.4 Targeted lymphatic delivery of antibody conjugated liposomal drug carriers
Twenty-one therapeutic monoclonal antibodies have been approved by the FDA for immunological and oncological targeting since 1994, most of which belong to the IgG1 family. Lymphatic delivery of liposomal antibody formulations offers advantages over other routes due to the enhanced cellular uptake of the antibodies and the prolonged localization of the liposomal conjugates, resulting in improved efficacy. In 2004, Cui et al. developed a mannan-coated liposome protamine-DNA (LPD) nanoparticle formulation based on their previous studies of LPD nanoparticles [19]. The in vitro uptake of the mannan-coated LPD by antigen-presenting cells, DC2.4, was evaluated and compared to non-coated LDP. The data revealed that the mannan coating significantly enhanced cellular uptake of the nanoparticles by targeting the antigen-presenting cells; therefore, it may be a potential carrier for the delivery of biopharmaceuticals. In a separate experiment, they investigated the in vivo immunity of mannan-coated LPD against HPV-positive TC-1 lung cancer in C57BL/6 mice, by incorporating the major histocompatibility complex class-I (MHC class-I) restricted peptide antigen from HPV 16 E7 protein. It was reported that both preventive and therapeutic efficacy of the LPD/E7 formulation was greatly enhanced due to the mannan coating.
HIV is life-threatening disease worldwide. Approximately forty-two million people live with HIV worldwide, half of which are in African countries. HIV infection in humans causes immune system failure, resulting in millions of deaths due to pathogen infections. Therefore, anti-HIV treatment regimens are in great need worldwide. Desormeaux et al. described a method for the synthesis of PEG stabilized immunoliposomes conjugated to anti-HLA-DR antibodies, that effectively delivered indinavir to the lymphoid tissues of female C3H mice for the treatment of HIV-1 [20]. The subcutaneous administration of anti-HLA-DR immunoliposomes demonstrated a 2.9-fold greater accumulation in the cervical lymph nodes of C3H mice compared to the standard liposome formulation. In addition, the PEG stabilized immunoliposomes delivered high concentration of indinavir to the lymphoid tissues for at least 15 days post-injection. A single subcutaneous dose (2 μmol lipids/g tissue) of the sterically stabilized immunoliposomes demonstrated a 0.9 to 1.9-fold greater AUC in the cervical, brachial, mesenteric, inguinal, and popliteal lymph nodes of C3H mice compared to the conventional immunoliposomal formulation. The encapsulation of anti-viral drugs into sterically stabilized immunoliposomes introduced a novel drug delivery approach for the treatment of life-threatening HIV-1 disease by greatly increasing the drug concentration in the diseased lymph nodes. In 2000, Bestman-Smith et al. optimized the anti-HIV liposomal formulations for accumulation in lymphoid organs by targeting the primary HLA-DR expressing cellular reservoirs of HIV-1. The liposomal formulations of interest were made from a combination of dipalmitoylphosphatidylcholine, dipalmitoylphosphatidylglycerol, distearoylphosphatidylethanolamine-N-[poly(ethyleneglycol)-2000], and dipalmitoylphosphatidylethanolamine-N-(4-(p-maleimidophenyl)butyryl). They compared four types of liposomal formulations, including: conventional liposomes, sterically stabilized liposomes, conventional immunoliposomes bearing anti-HLA-DR, and sterically stabilized immunoliposomes bearing anti-HLA-DR Fab’ fragments at the end termini of PEG chains [21]. Greater accumulation of liposomes were observed for the PEGylated immunoliposomes compared to the other three treatments in most of the evaluated lymph nodes and the spleen in female C3H mouse model; introducing a novel approach to concentrate drugs in HIV-1 reservoirs and improve their efficacy.
Another potential clinical application of liposomal formulations is cancer vaccination. Although cancer diagnostic and therapeutic technologies have been greatly improved in the past two decades, there is still a critical need for cancer prevention medications, such as effective and safe cancer vaccinations. In 2001, Mui et al. reported their study of a liposomal nanoparticle-encapsulated oligodeoxynucleotides (ODN)-cytosine-guanine (CpG) formulation (LN-ODN-CpG); which demonstrated enhanced stimulatory activity of LN-ODN-CpG against the immune response, by triggering the Toll-like receptor 9 in an ICR mouse model. The lipid particle carriers were synthesized using distearoylphosphatidylcholine/cholesterol/dioleoyl-3-N,N-dimethylammoniumpropane/1-O-(2′-(ω-methoxypolyethyleneglycol)succinoyl-2-N-myristoylsphingosine with a molar ratio of 20:45:25:10 [22]. In a following study from their group in 2007, they explored the feasibility of using subcutaneously injected LN-ODN-CpG formulation as a cancer vaccine adjuvant along with co-administration of tumor-associated antigens to induce antigen specific adaptive immune response in murine cancer models of thymoma and melanoma. They also compared the lymphatic uptake of fluorescently labeled free and liposome-encapsulated ODN-CpG conjugates. Post subcutaneous injection of either free ODN-CpG or LN-ODN-CpG (5 mg/kg on an ODN-CpG basis), mice were sacrificed and their draining lymph nodes were excised at pre-determined time intervals. The results revealed that the LN-ODN-CpG treatment demonstrated a two to nine-fold increase in lymph node uptake of the ODN-CpG compared to the free ODN-CpG, suggesting the possibility of using the liposomal nanoparticles as a cancer vaccination for lymphatically metastatic cancers [23].
A similar study was conducted by Kojima et al in 2008 using an ovalbumin (OVA) -encapsulated oligomannose-coated liposomal (OML) vaccination against OVA-expressing E.G7-OVA tumor in a C57BL/6 mouse model. All of the mice previously immunized with the OVA-OMLs developed effective anti-cancer immunity and completely rejected the tumor. In addition, a single subcutaneous injection of the OVA-OML treatment led to significantly reduced tumor progression in tumor-bearing mice [24].
There has been a series of clinical trials to evaluate the efficacy and safety of liposomal cancer vaccinations [25–26]. A phase IIB trial of the L-BLP25 liposome vaccine was conducted in patients with stage IIIB and IV non-small cell lung cancer. The L-BLP25 liposome vaccine is a liposome-based peptide vaccine derived from the mucin 1 antigen. In the trial, patients were randomly divided into two groups: the L-BLP25 group, in which patients were treated with weekly immunizations of the liposomal vaccine following a single intravenous dose of cyclophosphamide; or the control group, in which patients were treated with cyclophosphamide alone. Although the results demonstrated a 4.4-month increase in survival for the patients that were treated with the vaccination, the difference was not statistically significant [25]. Nevertheless, no vaccine-related toxicities were observed in the patients in the L-BLP25 treatment group.
1.5 Lymphatic imaging of liposomal formulations
Lymphatic system is a complex network, consisting of lymph vessels and lymph nodes, which are connected by the lymph vessels. Imaging of the lymphatics plays a role in disease discovery and diagnosis. In addition to the conventional computed tomography (CT) technique, newer tools such as scintigraphy and lymphography have also been widely utilized in the past two decades. Scintigraphy, a valuable tool to capture two-dimensional images of a body radiation source after intravenous injection of a readily cleared radioactive chemical into the patient, has been used extensively in the past decade for the development and evaluation of gamma-emitting radionuclide labeled liposomal formulations. Pillips et al. reported a series of applications of scintigraphic imaging using technetium-99m (99mTc) labeled liposomes [27], including examination of bone fracture healing, detection of viral infection, and intraarticular and intralymphatic delivery of liposomal formulations. In addition, Phillips et al. also compared a series of 99mTc labeled liposomes, including: 99mTc sulfur colloid, filtered 99mTc sulfur colloid, filtered 99mTc sulfur colloid with reduced heating time, and 99mTc human serum albumin, in rabbits for optimal detection of the sentinel lymph nodes (Fig. 2) [28]. Similar radioactive imaging techniques may be used for the evaluation and optimization of new radioactive liposomes for improved sentinel lymph node diagnostics.
Fig 2.
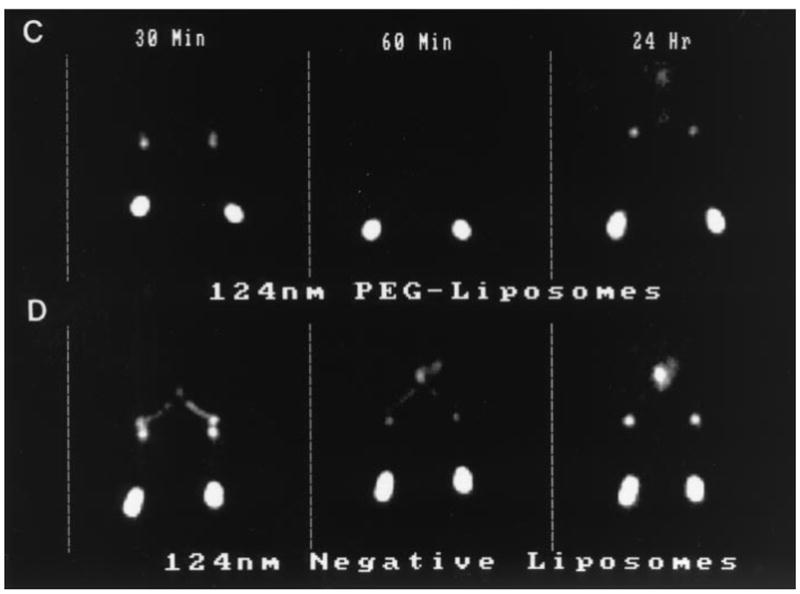
Gamma camera images of the lower portion of rabbits following subcutaneous injection (0.3 ml) of various [99mTc] liposomes in each hind foot at either 30 minutes, 60 minutes or 24 hours post injection. The images show the injection site in the hind feet, the popliteal node region and iliac node region. (C) 124 nm PEG-liposomes (both feet); (D) 124 nm Negative-liposomes (both feet).
Lymphoscintigraphy is a relatively new technology, which is only been widely used clinically since the 1990s. It is a type of nuclear medicine imaging, generating two-dimensional scintigrams of the lymphatic system including lymph nodes and vessels. A radioactive substance is administered as an intravenous injection, oral, or pulmonary dosage. The radioactive material is taken up by the draining lymph nodes after passing through lymph ducts; in the meantime, it generates gamma rays that can be detected by a gamma camera or positron emission tomography scanner. It is commonly used as a tool to identify sentinel lymph nodes [29] and diagnose disease conditions such as lymphoma [30] and lymphedema [31–32]. In 2002, Plut et al. developed a formulation kit by chemically combining a pyrogen-free low molecular weight isosulfan blue dye solution and 99mTc labeled liposomes, producing a radioactive blue liposomal formulation for pre-surgical injections clinically [33]. This formulation avoids the non-synchronized distribution and clearance kinetics of the small molecular weight dye and the radioactive liposomes when they are administered separately. In the clinic, patients usually receive an injection of a colored dye solution before operation, providing visualization of the lymphatic network, followed by another injection of a radioactive formulation. The radiotracer can be detected using lymphoscintigraphy, leading to the identification of draining lymph nodes, including the sentinel lymph node. However, the two injections do not occur simultaneously, thus causing the differing distribution and clearance kinetics of the two substances. In order to overcome this issue and simplify the pre-surgical injection procedure, the kit was developed by combining the isosulfan blue dye with the 99mTc labeled liposomes.
Magnetic resonance imaging (MRI) could become another useful tool for imaging the lymphatic system. MRI has the potential to overcome the poor resolution of 99mTc, but lymphcentric contrast agents are required to provide clinically useful imaging of the lymphatic system. Fujimoto et al. developed gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) labeled liposomes using long-circulating palmityl-D-glucuronide liposomes. After a subcutaneous injection of the liposomal formulation into the hind feet of rabbits, the popliteal lymph nodes as well as the deep retroperitoneal lymph node were detected. The enhanced lymphatic liposomal accumulation was likely due to the trapping of liposomes by the macrophages [34–36].
Rapid clearance post injection is the major drawback of liposomal formulations; therefore, a liposomal drug delivery system with prolonged retention is of high interest to physicians. Avidin/biotin-liposome systems overcome this limitation by forming avidin biotin-liposome aggregates after subsequent injections into the primary intraperitoneal of biotin coated liposomes followed by avidin [37–38]; thus, retaining the liposomes in the diseased tissues and the draining lymph nodes. Zavaleta et al. compared three avidin/biotin-liposome systems with different dosing sequences of avidin and biotin-liposomes in a rodent ovarian cancer xenograft model [37]. Lymphatic uptake of the liposomes was significantly enhanced in the rats treated with the subcutaneous injection of biotin-liposomes 2 hours post avidin injection. A similar study was conducted using a slightly modified avdin/99mTc-blue-biotin-liposome system by Medina et al. to target the mediastinal node, and to determine the most effective injection site and tissue biodistribution of 99mTc-labeled liposomal formulations in rats [39]. The pharmacokinetics and movement pattern of the avdin/99mTc-blue-biotin-liposome formulation post intraperitoneal injected was evaluated using scintigraphic imaging. Their results suggested that lymph node targeting effect was independent of the cavity in which the formulation was inected, however, was closely related to the dosage administered.
2. Solid lipid nanoparticle formulation for lymphatic drug delivery
Solid lipid nanoparticles (SLN) are a class of particulate drug carriers [40] made from lipids that remain in the solid state at room and body temperatures. Lipids utilized for SLN are typically physiological lipids, including: fatty acids, steroids, waxes, mono-, di-, or triglyceride mixtures. A wide variety of biocompatible surfactants are used to stabilize SLN; therefore, SLN have the advantage of physical stability with low toxicity. SLN are becoming increasingly used for the protection of labile drugs from degradation in the body and for controlled sustained release [41].
The application of SLN formulations for anticancer drug delivery has overcome many obstacles commonly seen in conventional cancer chemotherapy, such as limited specificity, high toxicity and tendency of drug resistance [42]. Various anticancer drugs, including: etoposide [43], methotrexate [44], and idarubicin [45] have been incorporated into SLN by different research groups and evaluated. Conventional administration routes (e.g. intravenous route) have shown relatively low tumor uptake [43], due to the hindrance of drug-loaded SLN to access the solid tumor [46], and reduced circulation time, due to the fast clearance by mononuclear phagocyte system, which in turn decrease the specific targeting effect. For this reason, various alternative application routes, such as duodenal [45, 47–49], subcutaneous [43] and pulmonary [50] routes have been studied.
2.1 SLN formulations for lymphatic drug delivery via the duodenal route
Drugs with poor oral bioavailability due to low solubility in GI tract or pre-systemic hepatic metabolism (first-pass effect) can be incorporated into SLN; allowing transported into systemic circulation via the intestinal lymphatics and bypassing first-pass metabolism, enhancing the drug bioavailability. A series of studies related to the absorption and distribution of SLN after duodenal administration has been carried out by Bargoni, Cavalli, and their colleagues [45, 47–49]. In one of the above studies [47], 131I-17-iodoheptadecanoic acid labeled drug-free SLN were administered into the duodenal lumen of fed rats. Transmission electron microscopy and photon correlation spectroscopy analysis of the lymph and blood samples indicated that SLN were taken up and transported predominantly by the lymphatics rather than by systemic circulation, confirming the transmucosal transport of SLN. However, the author suggested that the significant difference in the SLN uptake between the lymphatic system and blood was probably the result of the totally blocked passage of the SLN from the lymph to the blood, because of the designed experimental conditions for sample collection and the physiologically impaired transportation and absorption of SLN caused by surgery-related paralysis of the intestine of the animals. A later comparison study [48] of the drug release, absorption, and distribution, of tobramycin-loaded SLN (tobra-SLN), prepared from stearic acid, phosphatidylcholine, and taurocholate, in the plasma and lymph after duodenal administration, revealed that the AUC for tobra-SLN was 24-fold higher than intravenous administration of the same SLN formulation and 120-fold higher than free tobramycin solution after intravenous injection. In addition, this study suggested the potential of SLN as sustained release system, as the half-life of tobramycin increased from 57 min (tobramycin solution) to 283 min (tobra-SLN intravenously administered) to 1371 min (tobra-SLN duodenally administered). The enhancement of drug absorption and bioavailability for tobra-SLN after duodenal administration was ascribed mostly to the preferred transmucosal transport of tobramycin SLN to the lymph compared to the blood. In a similar study applying idarubicin loaded SLN (IDA-SLN) conducted by the same group, the enhancement in drug bioavailability was observed also when the IDA-SLN was administered through the duodenal route when compared to the intravenous route [45].
2.2 SLN formulations for lymphatic drug delivery via the duodenal route
The influence of the subcutaneous route on the tumor uptake and biodistribution of drug-loaded SLN was evaluated by Reddy et al [43]. They prepared etoposide -loaded tripalmitin (ETPL) SLN radiolabeled with 99mTc, and the ETPL nanoparticles were administered subcutaneously, intraperitoneally, and intravenously, to mice bearing Dalton’s lymphoma tumors. Gamma scintigraphy and the radioactivity measurements were used to determine biodistribution and tumor uptake of the ETPL SLN in the blood and organs. The data 24 hours after subcutaneous administration indicated that the ETPL SLN exhibited a noticeably higher degree of tumor uptake (8 and 59 fold higher than that of the intraperitoneal and intravenous routes, respectively) and reduced build-up in RES organs (i.e. liver, lung and spleen) compared to the other two routes. The tumor concentration of the ETPL SLN one hour after subcutaneous injection was 23.3% of that at 24 hours post injection, showing a lower extent of initial uptake compared to intraperitoneal and intravenous routes, whose initial uptake ratios were 29.7% and 48.1%, respectively. The gradual increase in tumor uptake of the drug from the subcutaneous injection site after the low initial uptake indicated that the drug-loaded SLN could be used for sustained drug release therapy. Therefore, a proximal subcutaneous injection could be proposed as the optimal route for lymphatic chemotherapy over intraperitoneal and intravenous routes.
2.3 SLN formulations for lymphatic drug delivery via the pulmonary route
Since lymphatic drainage takes great part in alveolar clearance of foreign materials with submicron size, targeted delivery of anticancer drug to the lymphatic from the pulmonary route could be developed. The potential of targeted therapies and imaging agents to the lungs are great, considering intrathoracic lymph node metastasis are common in small cell lung cancer, occurring in about 70% of the limited stage patients and to nearly 80% of the extensive stage patients [51]. In the case of the other more common type of lung cancer- non small cell lung cancer, greater than 80% of stage IV patients showing extensive metastasis to the lymphatics [52].
Videira et al. [50] evaluated the bio-distribution of inhaled 99mTc-HMPAO-radiolabelled SLN (rl-SLN) with an average size of 200 nm, and compared the results with the distribution of the free tracer (99mTc-HMPAO) administered via the same route. The gamma scintigraphic images showed significant accumulation of the rl-SLN in the lungs followed by rapid distribution into the inguinal lymph nodes via alveolar clearance, which indicated that the rl-SLN were mainly cleared from lungs via the lymphatics. In addition, about 24% of the 99mTc-HMPAO activity remained in the lungs four hours after administration, demonstrating a significant degree of retention of the intact SLN. However, the free tracer was cleared from lungs primarily by the systemic circulation. The long retention and specific clearance mechanism of the 99mTc-HMPAO in rl-SLN was consistent with the proposition by Müller et al. [40] that drug molecules are widely dispersed into the solid lipid matrix during production, and due to the reduced mobility of the drug moiety in the lipid crystal lattice, prolonged drug release from SLN can be achieved.
2.4 Influence of physicochemical characteristics of SLN on lymphatic uptake
Although the uptake of SLN into the lymphatic system and the distribution within the lymphatics varies with the different administration routes, they are largely determined by the physicochemical characteristics of the SLN particles [53–54], including: particle size, surface charge, and hydrophobicity. When the SLN are injected subcutaneously, the particle size becomes the major determinant of lymphatic uptake from interstitial fluid [53, 55]. The interstitium is structured as narrow aqueous channels of about 100 nm in diameter, so SLN sized between 10 to 100 nm can easily travel through these channels from the injection site into the lymph. Particles larger than 100 nm will be retained at the injection site, and those smaller than 10 nm tend to undergo re-absorption back into the blood capillaries. A similar size dependence was observed by Oussoren et al [55] using liposomes to study lymphatic flow. In addition to particle size, a negative surface charge and hydrophobicity have been shown to facilitate the lymphatic drainage of SLN from the injection site and localization in the lymph nodes [53].
Solid lipid nanoparticles are complex colloidal particle systems in which the physicochemical characteristics of SLN are impacted by the components and the composition, including the intermolecular interactions between the loaded drug and SLN matrix. In a study of methotrexate (MTX) SLN formulation for oral lymphatic delivery, Paliwal et al. [44] assessed the influence of the lipid on the characteristics of the SLN. The SLN were prepared using four different lipids (stearic acid, monostearin, tristearin and Compritol 888 ATO) by a solvent diffusion method. Particle size, zeta potential, shape, drug entrapment efficiency, in vitro release profile, and pharmacokinetics characteristics, of the four MTX SLNs were compared. The MTX-loaded SLN prepared from Compritol 888 ATO, a highly lipophilic atomized mixture of mono-, di-, tri-behenates of glycerol, generated the smallest, most uniform SLN with the highest entrapment efficiency. The Compritol 888 ATO-based SLN (MTX-CA) yielded a 10% higher MTX loading; the longer chain length of glyceryl behenate in the Compritol 888 ATO could enhance the intermolecular entrapment of the MTX by interchain intercalation. In addition, the crystal lattice of SLN prepared from Compritol 888 is less perfect in comparison with those made from relatively pure lipids, thus leaving more space for the drug to be loaded. The bioavailability and intestinal lymphatic uptake of MTX SLNs were evaluated after intraduodenal administration to albino rats. All four MTX SLN formulations exhibited a significant increase in the AUC and lymphatic drug concentration compared to the free drug solution, with MTX-CA having the highest bioavailability and lymphatic absorption. The author ascribed these results to the MTX-CA having the smallest size and the solubilization characteristics of the mono-glyceryl behenate in Compritol 888 ATO. The Compritol 888 ATO formulation was proposed as the optimal oral carrier; however, the Compritol 888 ATO formulation also had a relatively low negative zeta potential (−12mV). A similar finding was observed in the study of 99mTc-radiolabelled SLN [50] using the same lipid. Low zeta potential is an indicator of reduced storage stability, due to the possible conversion of the SLN dispersion into a viscous gel. This transformation could be related to a change in the lipid crystals [41]. However, this phenomenon could be hindered by addition of the proper surfactant and optimizing the lipid concentration.
The emulsifier composition and concentration influence the partitioning of the drug between the oil and aqueous phases during the SLN formation, which has direct effects on the SLN quality. For this reason Sanjula et al. [56] compared the physiochemical characteristics, in vitro release, and in vivo absorption, of Carvedilol-loaded SLN with varied ratios of the nonionic emulsifier poloxamer 188. A high poloxamer 188 concentration resulted in reduced drug entrapment efficiency and aggregation of the SLN, which in turn decreased the amount of drug that could be released from the SLN and lowered the bioavailability. This observation was consistent with a study by the Paliwal et al., who used soya lecithin as an emulsifier in methotrexate SLN [44]. In the latter study, as the amount of soya lecithin increased from 1.0% to 1.5%, the drug entrapment efficiency and the percentage release of drug decreased. A possible explanation is that when the concentration of the emulsifier is above a level at which it can sufficiently cover the lipid core, the SLN dispersion is stabilized, and the extra surfactant tends to form micelles that entrap the hydrophobic drug within their cores. Therefore, the portion of drug that partitioned into the lipid matrix decreased, resulting in the reduced entrapment efficiency of the SLN formulation. Furthermore, the increased size and aggregation introduced by the additional surfactant reduced the hydrophobicity of the SLN, thus decreasing the lymphatic uptake [53–54].
Adjusting the amount of drug loaded in the SLN can change the physicochemical characteristics of SLN, including: particle size, total particle number, and total surface area of the SLN dispersion. These variations could impact the in vitro release behavior and in vivo pharmacokinetic parameters. In a comparative study of SLN formulations with different percentages of the drug tobramycin (1.25%, 2.50% and 5.00%), Cavalli et al. [49] found that after duodenal administration, lower percentage tobramycin formulations resulted in slower drug release, higher bioavailability, and longer residence time. The SLN with 1.25% drug loading yielded the smallest particle sizes (less than 100 nm); hence, this SLN dispersion had the largest total number of particles in the given volume of SLN dispersion. Analysis of the lymph and lymph nodes revealed that the tobramycin concentration was significantly higher in lymph nodes than in lymph after duodenal administration; indicating that the drug retention in the lymph nodes was followed by slow drug release to the lymphatic system.
3. Conclusions and future challenges
Lymphatic chemotherapy holds great promise as a new therapy for patients with metastatic cancers. Recurrence is frequent in many cancers, especially those diagnosed at advanced stages. The failure to address these cases is due in part to occult disease residing in the deep tissues and distant lymph nodes and dose-limiting toxicities of existing protocols that prevent patients from receiving full recommended regimens. Lymphatic therapy using drug-encapsulated liposomes and solid lipid nanoparticles emerges as a new technology to provide better penetration into the lymphatics where residual disease exists. It can be used in the clinic either as a neoadjuvant to achieve early disease control, or be administered post treatment to serve as a cleanup therapy to eradicate micro- and nano-metastases, preventing cancer recurrence. In addition, these nanoparticle formulations provide a number of advantages for delivery of poorly water-soluble, unstable, and cytotoxic drugs, to the lymphatic system. By optimizing the preparation procedure and choosing the proper administration route, significant enhancements in bioavailability and lymphatic uptake can be achieved.
Acknowledgments
This work was supported by awards from the National Institutes of Health (R21 CA132033), the American Cancer Society (RSG-08-133-01-CDD), the Susan G. Komen Foundation (KG090481), the University of Kansas General Research Fund, and an Eli Lilly Predoctoral Fellowship to SC.
References
- 1.Harrington KJ, Rowlinson-Busza G, Syrigos KN, Uster PS, Vile RG, Stewart JS. Pegylated liposomes have potential as vehicles for intratumoral and subcutaneous drug delivery. Clin Cancer Res. 2000;6:2528–2537. [PubMed] [Google Scholar]
- 2.Moghimi SM, Moghimi M. Enhanced lymph node retention of subcutaneously injected IgG1–PEG2000–liposomes through pentameric IgM antibody–mediated vesicular aggregation. Biochim Biophys Acta. 2008;1778:51–55. doi: 10.1016/j.bbamem.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Moghimi SM. The effect of methoxy–PEG chain length and molecular architecture on lymph node targeting of immuno–PEG liposomes. Biomaterials. 2006;27:136–144. doi: 10.1016/j.biomaterials.2005.05.082. [DOI] [PubMed] [Google Scholar]
- 4.Phillips WT, Klipper R, Goins B. Novel method of greatly enhanced delivery of liposomes to lymph nodes. J Pharmacol Exp Ther. 2000;295:309–313. [PubMed] [Google Scholar]
- 5.Hashida N, Murakami M, Yoshikawa H, Takada K, Muranishi S. Intestinal absorption of carboxyfluorescein entrapped in liposomes in comparison with its administration with lipid–surfactant mixed micelles. J Pharmacobiodyn. 1984;7:195–203. doi: 10.1248/bpb1978.7.195. [DOI] [PubMed] [Google Scholar]
- 6.Perrie Y, Obrenovic M, McCarthy D, Gregoriadis G. Liposome (Lipodine)–mediated DNA vaccination by the oral route. J Liposome Res. 2002;12:185–197. doi: 10.1081/lpr-120004792. [DOI] [PubMed] [Google Scholar]
- 7.Latimer P, Menchaca M, Snyder RM, Yu W, Gilbert BE, Sanders BG, Kline K. Aerosol delivery of liposomal formulated paclitaxel and vitamin E analog reduces murine mammary tumor burden and metastases. Exp Biol Med (Maywood) 2009;234:1244–1252. doi: 10.3181/0901-RM-8. [DOI] [PubMed] [Google Scholar]
- 8.Rabaca Roque Botelho MF, Tavares Marques MA, Freitas Gomes CM, Marques Ferreira da Silva A, Andrade Figueiredo Bairos VA, de Matos Santos Rosa MA, Pena Abrunhosa A, Pedroso de Lima JJ. Nanoradioliposomes molecularly modulated to study the lung deep lymphatic drainage. Rev Port Pneumol. 2009;15:261–293. doi: 10.1016/s2173-5115(09)70109-5. [DOI] [PubMed] [Google Scholar]
- 9.Frenkel V, Etherington A, Greene M, Quijano J, Xie J, Hunter F, Dromi S, Li KC. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed–high intensity focused ultrasound exposure. Acad Radiol. 2006;13:469–479. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first–line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 11.Tejada-Berges T, Granai CO, Gordinier M, Gajewski W. Caelyx/Doxil for the treatment of metastatic ovarian and breast cancer. Expert Rev Anticancer Ther. 2002;2:143–150. doi: 10.1586/14737140.2.2.143. [DOI] [PubMed] [Google Scholar]
- 12.Prescott LM. Doxil offers hope to KS sufferers. J Int Assoc Physicians AIDS Care. 1995;1:43–44. [PubMed] [Google Scholar]
- 13.Soundararajan A, Bao A, Phillips WT, Perez Rr, Goins BA. [(186)Re]Liposomal doxorubicin (Doxil): in vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft model. Nucl Med Biol. 2009;36:515–524. doi: 10.1016/j.nucmedbio.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling R, Li Y, Yao Q, Chen T, Zhu D, Jun Y, Chen J. Lymphatic chemotherapy induces apoptosis in lymph node metastases in a rabbit breast carcinoma model. J Drug Target. 2005;13:137–142. doi: 10.1080/10611860400027725. [DOI] [PubMed] [Google Scholar]
- 15.Trubetskoy VS, Whiteman KR, Torchilin VP, Wolf GL. Massage–induced release of subcutaneously injected liposome–encapsulated drugs to the blood. J Control Release. 1998;50:13–19. doi: 10.1016/s0168-3659(97)00104-1. [DOI] [PubMed] [Google Scholar]
- 16.Ling SS, Magosso E, Khan NA, Yuen KH, Barker SA. Enhanced oral bioavailability and intestinal lymphatic transport of a hydrophilic drug using liposomes. Drug Dev Ind Pharm. 2006;32:335. doi: 10.1080/03639040500519102. [DOI] [PubMed] [Google Scholar]
- 17.Kraft SL, Dailey D, Kovach M, Stasiak KL, Bennett J, McFarland CT, McMurray DN, Izzo AA, Orme IM, Basaraba RJ. Magnetic resonance imaging of pulmonary lesions in guinea pigs infected with Mycobacterium tuberculosis. Infect Immun. 2004;72:5963–5971. doi: 10.1128/IAI.72.10.5963-5971.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson KA, Anderson K, Snyder RM, Simmons-Menchaca M, Atkinson J, Sun LZ, Bandyopadhyay A, Knight V, Gilbert BE, Sanders BG, Kline K. Novel vitamin E analogue and 9–nitro–camptothecin administered as liposome aerosols decrease syngeneic mouse mammary tumor burden and inhibit metastasis. Cancer Chemother Pharmacol. 2004;54:421–431. doi: 10.1007/s00280-004-0817-y. [DOI] [PubMed] [Google Scholar]
- 19.Cui Z, Han SJ, Huang L. Coating of mannan on LPD particles containing HPV E7 peptide significantly enhances immunity against HPV–positive tumor. Pharm Res. 2004;21:1018–1025. doi: 10.1023/b:pham.0000029292.66792.4f. [DOI] [PubMed] [Google Scholar]
- 20.Desormeaux A, Bergeron MG. Lymphoid tissue targeting of anti–HIV drugs using liposomes. Methods Enzymol. 2005;391:330–351. doi: 10.1016/S0076-6879(05)91019-7. [DOI] [PubMed] [Google Scholar]
- 21.Bestman-Smith J, Gourde P, Desormeaux A, Tremblay MJ, Bergeron MG. Sterically stabilized liposomes bearing anti–HLA–DR antibodies for targeting the primary cellular reservoirs of HIV–1. Biochim Biophys Acta. 2000;1468:161–174. doi: 10.1016/s0005-2736(00)00254-6. [DOI] [PubMed] [Google Scholar]
- 22.Mui B, Raney SG, Semple SC, Hope MJ. Immune stimulation by a CpG–containing oligodeoxynucleotide is enhanced when encapsulated and delivered in lipid particles. J Pharmacol Exp Ther. 2001;298:1185–1192. [PubMed] [Google Scholar]
- 23.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, Yuan N, Hope M, Cullis P, Tam Y. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti–tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007;56:1251–1264. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima N, Biao L, Nakayama T, Ishii M, Ikehara Y, Tsujimura K. Oligomannose–coated liposomes as a therapeutic antigen–delivery and an adjuvant vehicle for induction of in vivo tumor immunity. J Control Release. 2008;129:26–32. doi: 10.1016/j.jconrel.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulières D, Cormier Y, Ellis P, Price A, Sawhney R, Davis M, Mansi J, Smith C, Vergidis D, Ellis P, MacNeil M, Palmer M. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non–small–cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 26.Neidhart J, Allen KO, Barlow DL, Carpenter M, Shaw D, Triozzi PL, Conry RM. Immunization of colorectal cancer patients with recombinant baculovirus–derived KSA (Ep–CAM) formulated with monophosphoryl lipid A in liposomal emulsion, with and without granulocyte–macrophage colony–stimulating factor. Vaccine. 2004;22:773–780. doi: 10.1016/j.vaccine.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Phillips WT, Goins B. Assessment of liposome delivery using scintigraphic imaging. J Liposome Res. 2002;12:71–80. doi: 10.1081/lpr-120004779. [DOI] [PubMed] [Google Scholar]
- 28.Phillips WT, Andrews T, Liu H, Klipper R, Landry AJ, Blumhardt R, Goins B. Evaluation of [(99m)Tc] liposomes as lymphoscintigraphic agents: comparison with [(99m)Tc] sulfur colloid and [(99m)Tc] human serum albumin. Nucl Med Biol. 2001;28:435–444. doi: 10.1016/s0969-8051(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 29.Bagaria SP, Faries MB, Morton DL. Sentinel node biopsy in melanoma: technical considerations of the procedure as performed at the John Wayne Cancer Institute. J Surg Oncol. 2010;101:669–676. doi: 10.1002/jso.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Tamura M, Hamauzu T, Nakayama A, Kawasugi K, Kamakura M, Kinoshita T, Kuyama Y, Yamanaka M, Wang LM, Sanaka M, Mineshita S. Intestinal Behçet’s disease associated with non–Hodgkin’s lymphoma. J Gastroenterol. 1997;32:241–245. doi: 10.1007/BF02936375. [DOI] [PubMed] [Google Scholar]
- 31.Luongo JA, Scalcione LR, Katz DS, Yung EY. Progression of clinically stable lymphedema on lymphoscintigraphy. Clin Nucl Med. 2009;34:585–588. doi: 10.1097/RLU.0b013e3181b06cc5. [DOI] [PubMed] [Google Scholar]
- 32.Bourgeois P, Dargent JL, Larsimont D, Munck D, Sales F, Boels M, De Valck C. Lymphoscintigraphy in angiomyomatous hamartomas and primary lower limb lymphedema. Clin Nucl Med. 2009;34:405–409. doi: 10.1097/RLU.0b013e3181a7d013. [DOI] [PubMed] [Google Scholar]
- 33.Plut EM, Hinkle GH, Guo W, Lee RJ. Kit formulation for the preparation of radioactive blue liposomes for sentinel node lymphoscintigraphy. J Pharm Sci. 2002;91:1717–1732. doi: 10.1002/jps.10170. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto Y, Okuhata Y, Tyngi S, Namba Y, Oku N. Magnetic resonance lymphography of profundus lymph nodes with liposomal gadolinium–diethylenetriamine pentaacetic acid. Biol Pharm Bull. 2000;23:97–100. doi: 10.1248/bpb.23.97. [DOI] [PubMed] [Google Scholar]
- 35.Misselwitz B, Sachse A. Interstitial MR lymphography using Gd–carrying liposomes. Acta Radiol Suppl. 1997;412:51–55. [PubMed] [Google Scholar]
- 36.Trubetskoy VS, Cannillo JA, Milshtein A, Wolf GL, Torchilin VP. Controlled delivery of Gd–containing liposomes to lymph nodes: surface modification may enhance MRI contrast properties. Magn Reson Imaging. 1995;13:31–37. doi: 10.1016/0730-725x(94)00083-f. [DOI] [PubMed] [Google Scholar]
- 37.Zavaleta CL, Phillips WT, Soundararajan A, Goins BA. Use of avidin/biotin–liposome system for enhanced peritoneal drug delivery in an ovarian cancer model. Int J Pharm. 2007;337:316–328. doi: 10.1016/j.ijpharm.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Medina LA, Calixto SM, Klipper R, Phillips WT, Goins B. Avidin/biotin–liposome system injected in the pleural space for drug delivery to mediastinal lymph nodes. Journal of Pharmaceutical Sciences. 2004;93:2595–2608. doi: 10.1002/jps.20163. [DOI] [PubMed] [Google Scholar]
- 39.Medina LA, Calixto SM, Klipper R, Li Y, Phillips WT, Goins B. Mediastinal node and diaphragmatic targeting after intracavitary injection of avidin/99mTc–blue–biotin–liposome system. J Pharm Sci. 2006;95:207–224. doi: 10.1002/jps.20516. [DOI] [PubMed] [Google Scholar]
- 40.Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 41.Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 42.Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RS. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release. 2005;105:185–198. doi: 10.1016/j.jconrel.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 44.Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, Mehta A, Vyas SP. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine. 2009;5:184–191. doi: 10.1016/j.nano.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Zara GP, Bargoni A, Cavalli R, Fundarò A, Vighetto D, Gasco MR. Pharmacokinetics and tissue distribution of idarubicin–loaded solid lipid nanoparticles after duodenal administration to rats. J Pharm Sci. 2002;91:1324–1333. doi: 10.1002/jps.10129. [DOI] [PubMed] [Google Scholar]
- 46.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 47.Bargoni A, Cavalli R, Caputo O, Fundarò A, Gasco MR, Zara GP. Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm Res. 1998;15:745–750. doi: 10.1023/a:1011975120776. [DOI] [PubMed] [Google Scholar]
- 48.Cavalli R, Zara GP, Caputo O, Bargoni A, Fundarò A, GMR Transmucosal transport of tobramycin incorporated in SLN after duodenal administration to rats. Part I––a pharmacokinetic study. Pharmacol Res. 2000;42:541–545. doi: 10.1006/phrs.2000.0737. [DOI] [PubMed] [Google Scholar]
- 49.Cavalli R, Bargoni A, Podio V, Muntoni E, Zara GP, Gasco MR. Duodenal administration of solid lipid nanoparticles loaded with different percentages of tobramycin. J Pharm Sci. 2003;92:1085–1094. doi: 10.1002/jps.10368. [DOI] [PubMed] [Google Scholar]
- 50.Videira MA, Botelho MF, Santos AC, Gouveia LF, de Lima JJ, Almeida AJ. Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J Drug Target. 2002;10:607–613. doi: 10.1080/1061186021000054933. [DOI] [PubMed] [Google Scholar]
- 51.Elliott JA, Osterlind K, Hirsch FR, Hansen HH. Metastatic patterns in small–cell lung cancer: correlation of autopsy findings with clinical parameters in 537 patients. J Clin Oncol. 1987;5:246–254. doi: 10.1200/JCO.1987.5.2.246. [DOI] [PubMed] [Google Scholar]
- 52.Stenbygaard LE, Sørensen JB, Larsen H, Dombernowsky P. Metastatic pattern in non–resectable non–small cell lung cancer. Acta Oncol. 1999;38:993–998. doi: 10.1080/028418699432248. [DOI] [PubMed] [Google Scholar]
- 53.Hawley AE, Davis SS, Illum L. Targeting of colloids to lymph nodes: influence of lymphatic physiology and colloidal characteristics. Adv Drug Deliver Rev. 1995;17:129–148. [Google Scholar]
- 54.Charman WN, Stella VJ. Lymphatic Transport of Drugs. CRC Press; 1992. [Google Scholar]
- 55.Oussoren C, Zuidema J, Crommelin DJ, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection.: II. Influence of liposomal size, lipid composition and lipid dose. Biochim Biophys Acta. 1997;1328:261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 56.Sanjula B, Shah FM, Javed A, Alka A. Effect of poloxamer 188 on lymphatic uptake of carvedilol–loaded solid lipid nanoparticles for bioavailability enhancement. J Drug Target. 2009;17:249–256. doi: 10.1080/10611860902718672. [DOI] [PubMed] [Google Scholar]


