Abstract
OBJECTIVE
To investigate whether the odds of pelvic organ prolapse vary significantly with the number of vaginal births and whether cesarean birth is associated with prolapse.
STUDY DESIGN
In this cross-sectional study of women over the age of 40, pelvic organ prolapse was defined as descent to or beyond the hymen. Logistic regression was used to estimate the relative odds of pelvic organ prolapse for each vaginal birth or cesarean birth, controlling for confounders.
RESULTS
Two hundred ninety women underwent a pelvic organ prolapse quantification POPQ examination, and 72 were found to have pelvic organ prolapse. A single vaginal birth significantly increased the odds of prolapse (OR 9.73, 95% CI 2.68-35.35). Additional vaginal births were not associated with a significant increase in the odds of prolapse. Cesarean births were not associated with prolapse (OR 1.31, 95% CI 0.49-3.54).
CONCLUSION
The odds of pelvic organ prolapse were almost 10 times higher after a single vaginal birth. The mnrginal impact of additiotull births on this association was small.
Keywords: cesarean section, childbirth, uterine prolapse
Pelvic organ prolapse is a common problem, resulting in > 200,000 surgical require additional operations later in life.2 Therefore, prevention strategies are fundamental in attempting to reduce the public health burden associated with prolapse.
Prolapse is more common among parous women than nulliparous women.3 Obstetric management might provide an opportunity for prevention. Research in this area is limited by the long latency between childbirth and prolapse. In addition, although an examination is required for most accurate classification of support, much of the published research relies on surrogate measures of prolapse, such as prolapse symptoms4-6 or surgical intervention for prolapse.3 There have been few studies with physical classification of prolapse in large cohorts of women.7-9
In this research, we used data from a cross-sectional study of women in an ambulatory population of women seeking gynecologic care, some for pelvic floor disorders.10 We compared structured physical examinations with childbirth history under the hypothesis that vaginal birth is associated with poorer pelvic organ support. We also investigated whether the odds of prolapse increase with increasing numbers of vaginal births and whether cesareans are associated with prolapse.
Materials and Methods
This was an analysis of a cross-sectional study of women with and without pelvic floor complaints.10 This study was approved by the Johns Hopkins Institutional Review Board.
We recruited women over 40 years of age who were scheduled for gynecologic or urogynecologic care. We included patients from 5 outpatient sites affiliated with Johns Hopkins Medical Institutions in metropolitan Baltimore, Maryland. Women under 40 years of age were excluded because the prevalence of pelvic floor disorders would be too low among younger women to draw meaningful conclusions. We also excluded pregnant women, those who could not complete questionnaires in English and women who had never been sexually active because the primary study evaluated sexual function and pelvic floor dysfunction. Between January 1, 2006, and April 1, 2007, we approached 420 women for participation in this research, and 344 (82%) enrolled. Of those, 298 (87%) completed a research questionnaire and constituted our study population.
After obtaining informed consent, subjects underwent a gynecologic examination. Each woman reported her total number of cesarean and vaginal births. Women were asked to report the weight of the largest infant and whether any of their children had been delivered by vacuum or forceps. We also included participant's age, race, hysterectomy status and history of prior pelvic surgery.
On examination, each participant's height was recorded in inches, and weight was measured in pounds. Body mass index as calculated as kilograms per meter squared. Pelvic organ support was measured and described according to the pelvic organ prolapse quantification (POPQ) system.11 All POPQ exams were performed with the patient performing maximal Valsalva effort in the lithotomy position. Uterovaginal descent was recorded to the nearest half-centimeter.
To ensure consistency with the POPQ examinations at all clinic sites, all physicians performing this examination were trained with an instructional video (produced by the American Urogynecologic Society). Competency in performing the examination was demonstrated prior to the study and re-confirmed throughout.
To provide the most comprehensive analysis of the relationship between childbirth history and prolapse, we classified prolapse in 3 different ways. First, we defined prolapse as vaginal or cervical de-census to or beyond the hymen,12,13 the threshold where prolapse symptoms have been reported to occur.12-14 Therefore, this was thought to be the most clinically meaningful threshold for the definition of prolapse. Second, we analyzed prolapse by stage (i.e., as an ordinal variable, with 5 categories from stage 0 to IV). Third, we quantified prolapse with the position of the most dependent vaginal segment, measured in centimeters, relative to the hymen.
For each of these 3 classifications, we investigated the association between prolapse and childbirth history. Because our initial analyses indicated that the relationship behveen prolapse and parity is not linear, we considered separately the impact of the first birth and subsequent births. We therefore considered pelvic organ support as a function of the first vaginal delivery and each additional vaginal delivery. We also considered a history of any cesarean as a separate independent variable.
Because we were interested in considering different measures of prolapse (i.e., as binary, ordinal and continuous), we used 3 different types of regression analysis. For the binary definition of prolapse (de-census to or beyond the hymen), we used logistic regression. To quantify the relative odds of increasing pelvic organ prolapse stage (e.g., the relative odds of a 1-stage increase), we used multivariable ordinal regression. For each analysis, we calculated the OR associated with the first vaginal delivery, as well as each additional vaginal birth or cesarean, with a 95% CI. Third, to estimate the increase in the extent of prolapse at the most dependent point, we used linear regression. The linear regression coefficients provided the mean change in prolapse severity associated with each unit increase in the independent variable of interest (e.g., vaginal births). Because age fulfilled the criteria of being a confounder, all analyses controlled for age.
Descriptive statistics were computed using standard methods for means, medians and proportions. Forcategorical data we used χ2 or Fisher's exact test where appropriate. To compare continuous variables across groups, we used a 1-way ANOVA. Statistical analysis was performed with Stata 9.2 (Stata-Corp, College Station, Texas). A p value of <0.05 was considered significant.
Results
Of the 298 participants, 8 were excluded who did not undergo POPQ examinations. The remaining participants are described in Table I. Increasing prolapse stage was significantly associated with age and parity. There were no statistically or clinically significant associations between prolapse stage and the other variables considered, including history of hysterectomy.
Table I.
Characteristics of the Population, by Stage of Pelvic Organ Support
| Characteristic | Stage 0 (n = 39) | Stage I (n = 132) | Stage II (n = 89) | Stage III (n = 30) | p Value |
|---|---|---|---|---|---|
| Age (yr) | 51.1 ± 9.8 | 56.8 ± 11.4 | 55.4 ± 9.6 | 62.0 ± 13.4 | < 0.001 |
| Parity | 1.4 ± 1.2 | 2.0 ± 1.44 | 2.3 ± 1.3 | 3.1 ± 1.9 | < 0.001 |
| Body mass index (kg/m2) | 28.6 ± 7.3 | 28.2 ± 6.6 | 29.6 ± 6.5 | 28.4 ± 5.1 | 0.446 |
| Race (n, %) | 0.638 | ||||
| Black | 6 (15.4%) | 25 (19.1%) | 9 (10.2%) | 4 (14.3%) | |
| White | 32 (82.1%) | 101 (77.1%) | 75 (85.2%) | 22 (78.6%) | |
| Other | 0 | 5 (3.8%) | 0 | 0 | |
| Weight of largest child delivered vaginally (g) | 8 ± 1.2 (n = 22) | 7.8 ± 1.3 (n = 86) | 8.1 ± 1.2 (n = 76) | 8.1 ±12 (n = 26) | 0.60 |
| History of hysterectomy | 6 (15.4%) | 40 (30.3%) | 16 (18.0%) | 10 (30.3%) | 0.082 |
Data are presented as either mean ± SD or total numbers and percentages.
Of the 290 participants, 41 were nulliparous. There were 202 participants who had a history of vaginal birth only, 31 had a history of cesarean birth only, and 16 reported both vaginal and cesarean birth. The number of vaginal births ranged from 0 to 8, with 50% of women reporting between 1 and 3 births. The number of cesareans ranged from 0 to 3 (27 women reporting 1 cesarean, 17 reporting 2 cesareans and 3 reporting 3 cesareans).
Of the 218 women with at least 1 vaginal birth, 75 (34.4%) reported a history of at least one operative vaginal delivery (forceps or vacuum birth). However, data regarding operative delivery were missing for 71 (32.6%) women, presumably because the participants either did not understand the question or could not recall this aspect of their history. Because one-third of women did not report whether they had undergone operative vaginal birth, we did not consider the impact of operative delivery in our analyses.
Prolapse to or beyond the hymen was observed in 72 (24.8%). Stage of pelvic organ support was 0 in 39 women, I in 132, II in 89 and 111 in 30. No woman in our study population had stage IV support. Twenty-four women reported a history of prior surgery for prolapse and/or urinary incontinence.
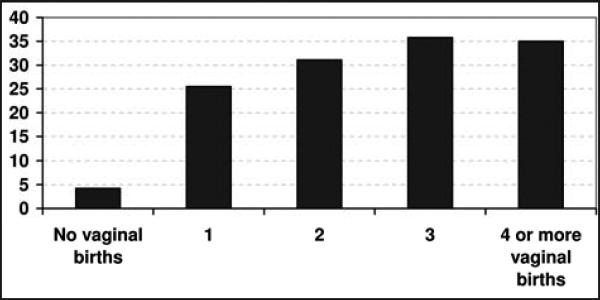
The relationship between the number of vaginal births and prolapse to or beyond the hymen is illustrated in Figure 1. As shown, the proportion of women with prolapse was much greater for those with at least 1 vaginal birth. In a multi variable analysis controlling for age (Table II), the first vaginal birth had a significant association with the odds of prolapse. Specifically, the first vaginal birth was associated with a 10-fold increase in the odds of prolapse to or beyond the hymen (OR 9.73, 95% CI 2.68-35.4). Additional births were not associated with a further increase the odds of prolapse (OR 1.09 for each additional birth, 95% CI 0.87-1.38). In addition, cesarean birth was not significantly associated with prolapse. More specifically, in a multivariable analysis controlling for number of vaginal births, women with a history of at least 1 cesarean birth did not have greater odds of prolapse than nulliparous women (OR 1.31, 95% CI 0.49-3.54).
Figure 1.
Percentage of women with prolapse to or beyond the hymen as a function of the number of vaginal births.
Table II.
Multivariable Logistic Regression for Pelvic Organ Prolapse, as a Function of Age and Obstetric History
| History and age | Relative odds (95% CI) |
|---|---|
| First vaginal birth | 9.73 (2.68–35.35) |
| Each additional vaginal birth | 1.09 (0.87–1.38) |
| At least 1 cesarean birth | 1.31 (0.49–3.54) |
| Age (yr) | 1.01 (0.99–1.04) |
Prolapse was defined as decensus to or beyond the hymen.
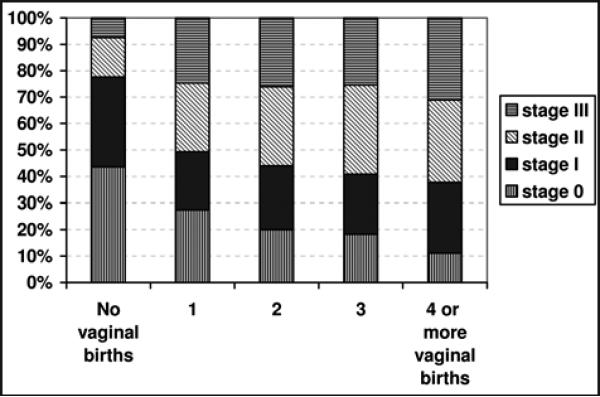
We found a similar relationship between prolapse stage and the number of vaginal births (Figure 2). Specifically, the largest increase in prolapse stage was seen with the first birth. In a multivariable ordinal regression, the odds of a one-stage increase in prolapse was almost 3 times higher after the first birth (OR 2.73, 95% CI 1.46-5.11). However, prolapse stage was not significantly increased with additional vaginal births (OR 1.21, 95% CI 0.98-1.49). Controlling for the number of vaginal births, there was no associated increased odds of prolapse with cesarean births (OR 1.60, 95% CI 0.84-3.04).
Figure 2.
Prolapse stage as a function of the number of vaginal births.
Comparing the measurement of the most dependent point of the vagina relative to the hymen, there was a trend toward poorer support among women with a greater number of vaginal births. Specifically, in a multivariable linear regression analysis, we found that the most dependent point was 0.88 cm lower after a single vaginal birth (95% CI 0.36-1.41 cm). Additional vaginal births were associated with 0.19 cm of additional descent (95% CI 0.02-0.36 cm). Compared to nulliparous women, a history of cesarean birth was associated with 0.49 cm of additional descent, although this difference was not statistically significant (95% CI-O.07-1.05 cm).
Discussion
In this research, we found that the odds of prolapse increased 10-fold with a single vaginal birth. We found no significant increase in the odds of prolapse for additional vaginal births. However, when we considered the impact of delivery on the quantitative severity of prolapse (e.g., the position of the most dependent point of the vagina), we were able to observe a statistically significant but very small impact of additional vaginal births. Specifically, the most dependent vaginal segment was 0.12 cm lower after each successive vaginal birth, after a 0.88-cm change with the first birth. We conclude that the first vaginal birth is associated with a significantly increased odds of prolapse, while additional vaginal births have a marginal impact on this association.
Our results extend the work of Larsson et al,6 who used data from the Swedish Hospital Discharge Registry to investigate the association between obstetric history and pelvic organ prolapse. They found that women who had only vaginal deliveries had a strong association between parity and the risk of surgery for prolapse, in an almost linear fashion. Our findings are slightly different in that we observed the greatest odds of prolapse from the first vaginal delivery. This difference is possibly explained by a difference in outcome measures. The Larsson study used surgeries for prolapse as an outcome measure, while in our study, prolapse was defined from actual POPQ examination data. The decision to perform surgery for prolapse is subjective and may be influenced by factors other than the degree of anatomic prolapse.
A strength of this study is that we used a structured, quantitative physical examination to assess prolapse and compare anatomic pelvic organ support to obstetric history. We have already reported our findings with respect to the correlation behveen symptoms and prolapse severity,14 which is generally poor for most prolapse symptoms. Therefore, in this article we focused on objective measures of prolapse rather than symptoms.
The relatively small size of this study population limited our ability to investigate some possible associations of interest. For example, the population included only 47 women with cesarean birth. With this relatively small number of women with cesarean birth, we could not investigate the impact of the number of cesarean deliveries on pelvic organ support. Also, we had limited power to investigate the association of prolapse with cesarean birth. Given the observed findings in this population, we had 66% power to investigate a significant association between cesarean and prolapse to or beyond the hymen.
Our data set included no details regarding the occurrence of labor prior to cesarean deliveries. There is some evidence that labor, rather than cesarean birth, may be the most relevant exposure with respect to prolapse risk. If some of the cesarean deliveries in this study were performed after active labor, we might underestimate the protective effect of cesarean birth.
A possible limitation of this research is that we relied on maternal recall of childbirth events that occurred many years prior to this study. Maternal recall is likely to be excellent for number of vaginal and cesarean births15 but may be unreliable for other obstetric exposures. We suspect that maternal recall may also be suboptimal for the weight of the largest child delivered vaginally,15,16 and this might have prevented us from observing an association between that variable and prolapse status. In addition, approximately one-third of women who delivered vaginally did not report whether they had undergone an operative delivery, suggesting that this exposure was not accurately captured in our research. We therefore were unable to investigate the possible effect of operative delivery on pelvic organ support. To our knowledge, there are no current data to suggest that operative deliveries significantly impact the risk of prolapse, and we suggest this factor be considered in future longitudinal studies.
Because of our research design, we cannot conclude that the relationship between vaginal birth and prolapse is causal. It is possible that factors that influence the exposure (in this case, delivery mode) might also influence the outcome (in this case, pelvic organ prolapse). In other words, cesarean birth might be a marker for lower risk of prolapse later in life. Other biologic factors may play a role in the association between childbirth and pelvic floor disorders. It is plausible that the same anatomic factors that contribute to a woman's ability to efficiently and safely deliver an infant vaginally may predispose her to evenhtal pelvic organ prolapse. Similarly, anatomic features predisposing women to a difficult labor may also impact the risk of future pelvic floor disorders. For example, in a study by Handa et al, a wide transverse inlet and narrow obstetric conjugate were associated with pelvic floor disorders.17 Features of bony pelvic architecture might confound the association between childbirth history and prolapse if the same features that protect against the development of prolapse also predispose to cesarean.
Our findings suggest an association between vaginal birth and pelvic prolapse; however, we do not presume this relation to be causal. Specifically, if we assume that vaginal birth is a direct cause of prolapse, these findings could have important implications for preventive strategies aimed at prolapse prevention. The practice of performing a cesarean to prevent future prolapse would be unacceptable to many mothers and would probably not be feasible with current health care resources. In addition, universal cesarean would potentially have other implications for the health of mothers, especially those planning multiple births. We conclude that further research should be aimed at identifying modifiable obstetric risk factors. We also advocate research to identify women at highest risk, for whom a planned cesarean birth might be an acceptable strategy.
The odds of prolapse increased 10-fold with a single vaginal birth.
We were able to observe a statistically significant but very small impact of additional vaginal births.
There are no current data to suggest that operative deliveries significantly impact the risk of prolapse.
Acknowledgments
Supported by the National Institutes of Health.
Footnotes
Presented as an oral poster at the 2008 Society of Gynecologic Surgeons Annual Clinical Meeting, Savannah, Georgia, April 14- 16,2008.
Financial Disclosure: The authors have no connection to any companies or products mentioned in this article.
References
- 1.Brown JS, Waetjen LE, Subak LL, et al. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol. 2002;4:712–716. doi: 10.1067/mob.2002.121897. [DOI] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstct Gynecol. 1997;4:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: Observations from the Oxford Family Planning Association Study. Br J Obstct Gynaecol. 1997;5:579–585. doi: 10.1111/j.1471-0528.1997.tb11536.x. [DOI] [PubMed] [Google Scholar]
- 4.Rortveit G, Brown JS, Thom DH, et al. Symptomatic pelvic organ prolapse: Prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;6:1396–1403. doi: 10.1097/01.AOG.0000263469.68106.90. [DOI] [PubMed] [Google Scholar]
- 5.Lukacz ES, Lawrence JM, Contreras R, et al. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;6:1253–1260. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 6.Larsson C, Kallen K, Andolf E. Cesarean section and risk of pelvic organ prolapse: A nested case-control study. Am J Obstet Gynecol. 2009;3:243.el–243.e4. doi: 10.1016/j.ajog.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Hendrix SL, Clark A, Nygaard I, et al. Pelvic organ prolapse in the Women's Heillth Initiative: Gravity and gravidity. Am J Obstet Gynecol. 2002;6:1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 8.Swift S, Woodman P, O'Boyle A, et al. Pelvic Organ Support Study (POSST): The distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;3:795–806. doi: 10.1016/j.ajog.2004.10.602. [DOI] [PubMed] [Google Scholar]
- 9.Kim CM, Jeon MJ, Chung DJ, et al. Risk factors for pelvic organ prolapse. Int J Gynaecol Obstet. 2007;3:248–251. doi: 10.1016/j.ijgo.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Handa VL, Cundiff G, Chang HH, et al. Female sexual function and pelvic floor disorders. Obstet Gynecol. 2008;5:1045–1052. doi: 10.1097/AOG.0b013e31816bbe85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gyoecol. 1996;1:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard I, Bradley C, Brandt D, et al. Pelvic organ prolapse in older women: Prevalence and risk factors. Obstet Gynecol. 2004;3:48–497. doi: 10.1097/01.AOG.0000136100.10818.d8. [DOI] [PubMed] [Google Scholar]
- 13.Tan JS, Lukacz ES, Menefee SA, et al. Predictive value of prolapse symptoms: A large database study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;3:203–209. doi: 10.1007/s00192-004-1243-8. discussion 209. [DOI] [PubMed] [Google Scholar]
- 14.Gutman RE, Ford DE, Quiroz LH, et al. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am J Obstet Gynecol. 2008;6:683.el–683.e7. doi: 10.1016/j.ajog.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yawn BP, Suman VJ, Jacobsen SJ. Maternal recall of distant pregnancy events. J Clin Epidemiol. 1998;5:399–405. doi: 10.1016/s0895-4356(97)00304-1. [DOI] [PubMed] [Google Scholar]
- 16.Catov JM, Newman AB, Kelsey SF, et al. Accuracy and reliability of maternal recall of infant birth weight among older women. Ann Epidemiol. 2006;6:429–431. doi: 10.1016/j.annepidem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Handa VL, Pannu HK, Siddique S, et al. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstet Gynecol. 2003;6:1283–1290. doi: 10.1016/j.obstetgynecol.2003.08.022. [DOI] [PubMed] [Google Scholar]