Abstract
New data support use of levodopa pharmacotherapy with behavioral contingency management (CM) as one efficacious combination in cocaine dependence disorder treatment. A potential mechanism of the combined treatment effects may be related to dopamine-induced enhancement of the saliency of contingently delivered reinforcers. Evidence to support this mechanism was sought by evaluating levodopa-enhancing effects across distinct CM conditions that varied in behavioral targets. A total of 136 treatment-seeking, cocaine dependent subjects participated in this 12-week, randomized, placebo-controlled trial of levodopa (vs. placebo) administered in combination with one of three behavioral CM conditions. In the CM-URINE condition, subjects received cash-valued vouchers contingent on cocaine-negative urine toxicology results. In the CM-ATTEND condition, the same voucher schedule was contingent on attending thrice weekly clinic visits. In the CM-MEDICATION condition, the same voucher schedule was contingent on Medication Event Monitoring Systems- and riboflavin-based evidence of pill-taking behavior. Primary outcomes associated with each CM target behavior were analyzed using generalized linear mixed models for repeated outcomes. CM responding in the CM-ATTENDANCE and CM-MEDICATION conditions showed orderly effects, with each condition producing corresponding changes in targeted behaviors, regardless of medication condition. In contrast, CM responding in the CM-URINE condition was moderated by medication, with levodopa-treated subjects more likely to submit cocaine-negative urines. These findings specify the optimal target behavior for CM when used in combination with levodopa pharmacotherapy.
Keywords: contingency management, levodopa, medication compliance, pharmacotherapy, behavior therapy
Contingency management (CM) procedures have been recommended as a behavioral therapy platform for evaluating cocaine pharmacotherapies (Carroll, Kosten, & Rounsaville, 2004; Carroll & Rounsaville, 2007). In addition to potency, CM procedures provide flexibility and specificity for targeting symptoms or problems that may not be addressed by medication alone. A growing number of studies have demonstrated benefit when combining a CM platform with pharmacotherapy for cocaine dependence (e.g., Kosten, 2003; Moeller, 2007; Schmitz et al., 2008, 1998). In these studies, CM has been used to reinforce abstinence from cocaine, based on negative urine specimens. Using an abstinence-based CM platform, we recently reported marked reduction in cocaine use for patients receiving levodopa pharmacotherapy compared to those receiving levodopa without CM (Schmitz et al., 2008).
In addition to drug use, CM has been utilized effectively to enhance retention (Pollastri et al., 2005) and medication compliance (Lussier et al., 2006; Prendergast et al., 2006); two highly salient target behaviors in cocaine clinical trials (Carroll & Rounsaville, 2007). To address the common problem of clinic nonattendance, Pollastri and colleagues (2005) tested a CM procedure that offered tangible incentives (low-cost retail items) to increase research appointment attendance in substance dependent outpatients. Adding the CM intervention resulted in a lower rate of unanticipated no-shows, as hypothesized, and in keeping with previous findings (e.g., Chutuape et al., 2001; Higgins et al., 2002). To the extent that medication compliance is related to treatment success, as some studies have shown (Namkoong et al., 1999; Rohsenow et al., 2000; Volpicelli et al., 1997), CM can be suitably applied to reinforce evidence of pill taking behavior. Sorenson and colleagues (2007) used CM to improve medication adherence among HIV-positive methadone maintenance patients. Compared to medication coaching alone, patients who were reinforced with CM for correct opening of electronic medication caps showed improved adherence to their antiretroviral medication regimen. Thus, CM is ideally suited for targeting behaviors that can negatively impact medication effectiveness, including ongoing drug use, treatment nonattendance, and medication noncompliance.
CM may be a particularly appropriate behavioral therapy platform for trials where the treatment medication is expected to enhance sensitivity to natural (nondrug) reinforcers. Agents that increase or enhance dopamine function, for example, might facilitate CM-responding by acting on neurobehavioral processes associated with reward salience, for example, learning, motivation (e.g., Vocci, 2007; Volkow, Fowler, & Wang, 2003; Volkow, Fowler, Wang, & Swanson, 2004). This synergism might explain the efficacy found when combining the dopamine precursor, levodopa, with a CM behavioral therapy platform (Schmitz et al., 2008). To support this explanation, the present study set out to demonstrate that the benefits of levodopa (vs. placebo) would be seen across CM platforms that target different behaviors (abstinence, medication compliance, attendance). It was reasoned that if the CM-levodopa treatment effect was because of nonspecific enhancement of reward saliency, increases in each target behavior would be observed in the presence of levodopa but not placebo. On the other hand, if the levodopa reward-enhancing effects were specific, increases in CM responding in the presence of levodopa would be observed only for a particular target behavior (i.e., abstinence).
Method
Study Design
This study was a planned component of a multiarm parallel-protocol design, testing a set of pharmacological and behavioral treatment interactions. The first protocol demonstrated levodopa treatment effects (vs. placebo) when combined with a behavioral therapy platform that included abstinence-based CM (Schmitz et al., 2008). The second protocol (present study) examined levodopa treatment effects across different CM platforms. The two protocols were run concurrently, with random and simultaneous assignment into one of the multiple treatment arms. The abstinence-based CM represented the “shared” active comparator for both protocols.
The six treatment arms consisted of levodopa/carbidopa (800/200 mg/d) or placebo administered in combination with CM targeting one of three different behaviors: clinic attendance (CM-ATTEND); medication compliance (CM-MEDICATION); cocaine negative urine toxicology (CM-URINE). In the CM-ATTEND condition, subjects received cash-valued vouchers contingent on attending thrice weekly clinic visits. In the CM-MEDICATION compliance condition, the same voucher schedule was contingent on electronic cap openings (Medication Event Monitoring Systems [MEMS]), and riboflavin-based evidence of pill-taking behavior. In the CM-URINE condition, vouchers were contingent on cocaine-negative urine toxicology results.
Following a 2-week intake evaluation phase, baseline information was used to urn randomize subjects to ensure even distribution of treatment groups with respect to sex, employment status, severity of cocaine addiction, motivation to change, and concurrent alcohol use (Stout, Wirtz, Carbonari, & Del Boca, 1994). Treatment began with a 1-week dose escalation schedule (Days 1–2, one 50/12.5 levodopa/carbidopa sustained-release tablet, Sinemet CR, b.i.d.; Days 3–4, one 100/25 tablet b.i.d.; Days 5–6, one 200/50 tablet b.i.d.; Day 7, one 400/100 tablet b.i.d.), followed by maintenance for 11 weeks, and a 7-day dose reduction at week 12. Medication and placebo were packed in identical capsules with riboflavin (100 mg) and dispensed in MEMS at each clinic visit (M, W, F). MEMS caps electronically record bottle openings (i.e., presumptive doses).
In addition to receiving medication, all subjects participated in weekly, brief (10–15 min), nurse-conducted, clinical management sessions and weekly 1-hr manual-driven individual cognitive–behavioral therapy sessions (see Schmitz et al., 2008, for details). CM, also referred to as voucher-based reinforcement therapy, was conducted by a research assistant who tracked targeted behaviors and delivered earned vouchers on a weekly basis.
Thrice weekly clinic visits (M, W, F) were required during treatment. Nonattendance on a scheduled clinic visit day because of illness, holidays, and so forth, could be rescheduled for alternate days of the week that the visit was missed (T, Th) without penalty. Absences that were not rescheduled were considered missed in terms of CM tracking. At intake, all subjects were informed of the general clinic policy that excessive missed visits (3 consecutive, 9 total) could be grounds for discharge from the study. At the end of 12 weeks, all participants were contacted to complete posttreatment assessments.
Subjects
All participants were enrolled at the outpatient Treatment Research Clinic (TRC) located at the Substance Abuse Research Center (Houston, TX). Inclusion required meeting Diagnostic and Statistical Manual of Mental Disorders-IV (DSM–IV) (American Psychiatric Association, 1994) criteria for current cocaine dependence and self-reported recent use of cocaine (confirmed by qualitative urine benzoylecgonine testing during the intake evaluation period). Exclusion criteria included: (a) dependence on alcohol or drugs other than cannabis or nicotine; (b) current nonsubstance induced Axis I psychotic, depressive, or anxiety disorder; (c) presence of significant suicidal or homicidal ideation; (d) having a major medical illness or condition (e.g., severe pulmonary or cardiovascular disease, renal function impairment); (e) concomitant medications interacting with levodopa/carbidopa (e.g., MAO inhibitors, anticonvulsants); (f) pregnancy or nursing; and (g) inability to read, write, or speak English.
Of the 543 patients who underwent initial consent and screening for eligibility, 132 did not return to complete the intake process, 193 were deemed ineligible based on medical or psychiatric exclusion criteria, and 218 were allocated to treatment arms not used in this protocol. A total of 136 participants were randomized into the six treatment arms of the present study. Following randomization, 35 participants failed to start treatment for reasons including work conflicts and not attending visits. A total of 101 participants received an initial dose of treatment.
The research protocol, consent form, and all assessment/advertising materials were reviewed and approved by the Committee for the Protection of Human Subjects (CPHS) of the University of Texas Medical School—Houston (Clinicaltrials.gov Identifier: NCT00218075).
CM Conditions
The same reinforcement schedule was applied across CM conditions and followed standard recommendations (Budney & Higgins, 1998) that we (Moeller, 2007) and others (Epstein, 2003; Petry et al., 2007) have used. Voucher values started at $2.50 and increasing by $1.25 for each consecutive occurrence of the target behavior. A $10 bonus voucher was awarded for evidence of three consecutive occurrences of the target behavior. Missing or refused observations were considered nonoccurrences of the target behavior and reset the voucher value to $2.50. Subjects received a weekly written and verbal statement indicating the frequency of occurrences of the target behavior at each scheduled clinic visit (M, W, F) of the previous week, with associated earnings. Vouchers earned could be exchanged at any time for gift certificates (e.g., local restaurants, movie theater) or redeemed as direct cash payments. Participants who provided evidence of the target behavior consecutively throughout the 12 weeks of treatment could earn a total of $997.50.
As described previously (Schmitz et al., 2008), participants in the CM-URINE group earned vouchers for each urine sample that tested negative for benzoylecgonine (cutoff 300 ng/ml). Specimens were sent for onsite analysis using Syva EMIT and Varian Thin Layer chromatography Toxi-Lab systems. Participants in the CM-ATTEND group earned vouchers for each scheduled clinic visit (M, W, F) that they attended. Participants in the CM-MEDICATION group earned vouchers for each occurrence of correct pill-taking behavior, operationally defined as agreement between MEMS openings and urinary riboflavin testing (Del Boca, 1996). Thus, an occurrence was coded as compliant if the MEMS-data indicated exactly two bottle openings (for each day since previous scheduled clinic visit) and urine riboflavin level exceeded the cutoff level of 20 fluorescence units (Mooney, 2004).
Assessments
Psychiatric diagnostic and addiction severity information were collected at intake using the Structured Clinical Interview for DSM–IV (SCID; First, 1995) and the Addiction Severity Index (ASI; McLellan, 1992). Before starting medication, all subjects underwent a medical history and physical examination, laboratory tests (liver and thyroid function), and cardiac evaluation (i.e., 12-lead electrocardiogram). Vital signs (including heart rate, blood pressure, and weight) were obtained weekly during treatment. Adverse events were evaluated by the study nurse during clinical management sessions.
Data Analysis
All analyses were performed on the intent-to-treat sample using the Statistical Analysis System, Version 9.13 (SAS, 2006) with statistical significance designated as p < .05. Treatment groups were compared on baseline characteristics using analysis of variance for continuous data and Fisher’s exact test when data were categorical. Proportional hazards Cox regression analyses were used to test for differences in time to dropout from treatment with medication and CM group as factors.
Primary outcome measures corresponding to each CM target included attendance, medication compliance, and cocaine use. Each was analyzed using generalized linear mixed models for repeated outcomes. Analyses of dichotomous data (e.g., attendance/nonattendance) utilized binomial distribution with logit link. Post hoc inspection of differences examined simple effects or least squares means where appropriate, correcting for multiplicity using Holm-Bonferroni stepdown procedure. Effect sizes were expressed as odds ratios. Analysis of continuous outcomes (e.g., total voucher earnings) utilized a general linear model approach with similar post hoc procedures and effect sizes expressed as group means. The interaction of medication × CM platform, if significant for each of the three targeted outcome variables (attendance, medication compliance, cocaine use), was considered evidence supporting a general reward enhancement hypothesis.
Results
Sample Characteristics
The demographic and substance use characteristics of participants at randomization are presented in Table 1. The t1 mostly male (83%), African American (71%) sample had a mean age of 41 years (SD = 7.5) and mean education of 12.8 years (SD = 1.9). Fifty-three percent were employed. Recent cocaine use was reported to be 13.4 (SD = 8.6) days in the past 30, with lifetime cocaine use reported to be 12.0 (SD = 6.4) years. The majority of subjects (64%) reported previous drug abuse treatment. Cocaine was detected in 82% of the samples submitted on the first intake visit.
Table 1.
Demographic and Drug Use Characteristics of Participants at Baseline by Randomization Status
| Placebo |
Levodopa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CM- ATTENDa n = 21 |
CM- URINEb n = 27 |
CM- MEDICATIONc n = 20 |
CM- ATTENDa n = 23 |
CM- URINEb n = 23 |
CM- MEDICATIONc n = 22 |
|||||||
| Variable | N | % | N | % | N | % | N | % | N | % | N | % |
| Female | 5 | 23.8 | 3 | 11.1 | 5 | 25.0 | 4 | 17.4 | 3 | 13.0 | 3 | 13.6 |
| Race | ||||||||||||
| White | 5 | 23.8 | 8 | 29.6 | 1 | 5.0 | 3 | 13.0 | 5 | 21.7 | 6 | 27.3 |
| Black | 15 | 71.4 | 16 | 59.3 | 14 | 70.0 | 19 | 82.6 | 18 | 78.3 | 14 | 63.6 |
| Hispanic | 1 | 4.8 | 3 | 11.1 | 5 | 25.0 | 1 | 4.3 | 0 | 0 | 2 | 9.1 |
| Employed | 11 | 52.4 | 11 | 40.7 | 11 | 55.0 | 13 | 56.5 | 12 | 52.2 | 14 | 63.6 |
| Smoking crack cocaine | 17 | 81.0 | 25 | 92.6 | 17 | 85.0 | 17 | 73.9 | 20 | 87.0 | 18 | 81.8 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 40.2 | 6.5 | 41.2 | 6.9 | 38.2 | 7.5 | 40.2 | 8.5 | 43.4 | 7.0 | 43.5 | 8.1 |
| Education (years) | 12.0 | 1.3 | 12.8 | 2.1 | 12.8 | 1.9 | 12.7 | 2.0 | 13.1 | 1.5 | 13.4 | 2.1 |
| Cocaine use (past 30 days) | 13.3 | 8.4 | 12.6 | 10.0 | 12.6 | 10.4 | 13.3 | 10.3 | 12.4 | 7.6 | 15.9 | 9.3 |
| Lifetime cocaine (years) | 12.3 | 6.7 | 11.3 | 5.9 | 11.1 | 6.6 | 13.5 | 7.9 | 10.8 | 4.6 | 12.1 | 6.9 |
| Alcohol use (past 30 days) | 11.7 | 9.6 | 6.6 | 7.9 | 6.9 | 8.3 | 10.7 | 10.3 | 10.1 | 10.6 | 12.6 | 10.9 |
| Lifetime alcohol (years) | 17.5 | 10.7 | 19.8 | 8.8 | 16.0 | 11.5 | 15.9 | 9.2 | 16.5 | 11.3 | 20.9 | 10.5 |
Contingency management targeting attendance.
Contingency management targeting cocaine-negative urine samples.
Contingency management targeting medication compliance.
Retention and Attendance
There were 136 subjects randomized and 101 initiated treatment (i.e., began study medication). The overall proportion of subjects remaining in treatment at week 6 was (51%) and at week 12 was (35%), with nonsignificant but potentially meaningful higher retention in the CM-ATTEND condition, log rank χ2(1) = 3.01, p = .0790.
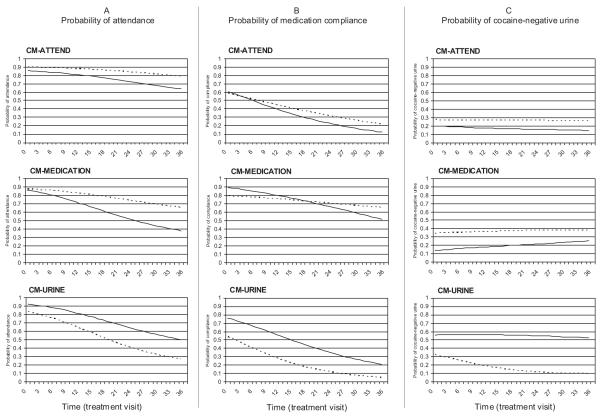
Clinic visit attendance during treatment declined over time in all groups, F(1, 95) = 14.83, p < .0002; however, the decline was steeper in the CM-MEDICATION and CM-URINE groups compared to the CM-ATTEND group, as indicated by the time by condition effect, F(1, 2,322) = 3.83, p < .02, see Figure 1. For participants in the CM-URINE (odds f1 ratio [OR] 0.92, 95% confidence interval [CI] 0.89–0.95) and CM-MEDICATION (OR 0.89, 95% CI 0.82–0.98) conditions, the odds of attendance decreased significantly for every additional day in treatment. For participants in the CM-ATTEND condition, the decrease in the odds of attendance was not significant (OR 0.98, 95% CI 0.94–1.03). Attendance rates did not differ by medication, F(1, 2,322) = 0.05, p < .82.
Figure 1.
Model-estimated probabilities of three targeted outcomes. Panel A (left) presents clinic attendance; Panel B (center) presents medication compliance; Panel C (right) presents cocaine-negative urines. Each graph represents a CM condition, with lines representing levodopa (solid) and placebo (dashed). CM-ATTEND, contingency management targeting attendance; CM-MEDICATION, contingency management targeting medication compliance; CM-URINE, contingency management targeting cocaine-negative urine samples.
Medication Compliance
A CM effect, F(2, 2,181) = 6.81, p < .0011, indicated that averaging across time and medication conditions, subjects in the CM-MEDICATION conditions had higher rates of compliance than subjects in the CM-ATTEND (OR 7.47, 95% CI 3.52–15.86) and CM-URINE (OR 8.09, 95% CI 3.83–17.06) conditions (see Figure 1). The medication effect, F(1, 2,181) = 5.45, p < .01, indicated that average compliance rates were higher in the levodopa condition than in placebo; however, when collapsing across all other variables in the equation (i.e., least squares means) this comparison no longer held (OR 1.46, 95% CI 0.77–2.75). Finally, the time effect, F(1, 89) = 55.89, p < .0001, indicated that overall rates of medication compliance declined over time. For each additional day in treatment the odds of compliance decreased by 0.93 (95% CI 0.91–0.95). The interaction of CM condition by medication was nonsignificant, F(2, 2,181) = 1.26, p < .28.
Cocaine Use
Analysis of the proportion of cocaine-negative urines during treatment resulted in a CM condition by medication interaction, F(2, 2,189) = 3.57, p < .03. For participants in the placebo condition, CM-URINE failed to demonstrate a difference relative to CM-ATTEND (OR 0.51, 95% CI 0.06–4.70) or CM-MEDICATION (OR 0.34, 95% CI 0.02–7.73). For participants in the levodopa condition, CM-URINE demonstrated a difference relative to CM-ATTEND (OR 26.38, 95% CI 1.74–398.95) and CM-MEDICATION (OR 13.50, 95% CI 1.24–149.02). Although the size of the 95% CIs indicate that these differences lack precision (likely a function of small sample size), the point estimates have clinical meaningfulness.
Voucher Earnings
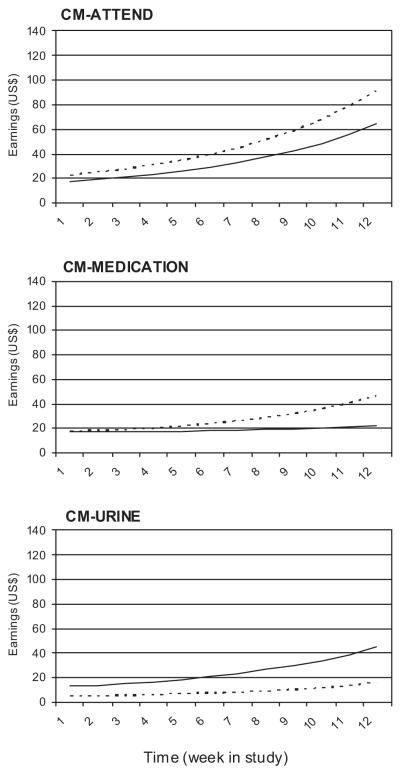
Total voucher earnings increased over time, as expected when using a cumulative reinforcement schedule, F(1, 75) = 7.14, p < .009 (see Figure 2). Voucher earnings differed as a function of CM, F(2, 502) = 12.14, p < .0001, with highest average earnings in the CM-ATTEND group (M = 28.97, 95% CI 21.11–39.77) compared to the CM-MEDICATION (M = 17.06, 95% CI 11.25–25.88) and CM-URINE (M = 9.54, 95% CI 6.52–13.97) groups. However, this effect was moderated by medication condition, F(2, 502) = 4.92, p < .008. For placebo, the CM-ATTEND condition resulted in higher earnings (M = 34.71, 95% CI 22.38–53.82) than the CM-URINE condition (M = 5.86, 95% CI 3.20–10.72), t(502) = 4.68, p ≤ .0001. For levodopa, no CM group differences were found.
Figure 2.
Voucher earnings over time. Each graph represents a CM condition, with lines representing levodopa (solid) and placebo (dashed). CM-ATTEND, contingency management targeting attendance; CM-MEDICATION, contingency management targeting medication compliance; CM-URINE, contingency management targeting cocaine-negative urine samples.
Discussion
This study tested the benefits of levodopa (vs. placebo) across CM platforms that targeted different behaviors (attendance, medication compliance, cocaine abstinence). Results produced orderly changes in treatment-related behaviors when performance of each was contingently reinforced. The hypothesis that levodopa would enhance CM responding regardless of the targeted behavior was not supported. Evidence of levodopa-enhancing effects were found only in the CM condition that reinforced cocaine-negative urines, providing further support for the appropriateness of this specific treatment combination.
Previously, we demonstrated levodopa treatment effects when combined with a behavioral therapy platform that included abstinence-based CM (Schmitz et al., 2008). The present study examined the effect of levodopa across different CM platforms in an attempt to determine whether this effect is specific to abstinence as a target behavior or related more generally to levodopa’s ability to enhance the saliency of CM rewards. The observed lack of levodopa versus placebo differences on CM effects for attendance and medication compliance outcomes fails to support a general reward enhancement explanation. That levodopa enhanced responding only under the urine-based CM intervention suggests a more nuanced synergy between levodopa and CM. It is possible that the saliency of CM rewards are facilitated by the dopamine-enhancing properties of levodopa, but only when there is a reduction in the rewarding value, or saliency, of cocaine, which is made possible by targeting abstinence with CM. This conceptualization follows the recommended multipronged treatment approach of using behavioral and pharmacological strategies to shift reward preference away from cocaine and toward nondrug rewards (Volkow et al., 2003; Volkow et al., 2004).
While most CM interventions target abstinence outcomes, this study provides evidence of improved outcomes when targeting therapeutic goals of clinic attendance and medication compliance, consistent with previous reviews of CM effectiveness (Griffith et al., 2000; Lussier et al., 2006). By comparing three behavioral targets within the same study, we demonstrated higher overall voucher earnings in the CM condition that reinforced attendance, suggesting that targeting this behavior resulted in more successful contact with available contingencies. In recent studies, shaping methods have been recommended as a way to increase the number of responders to CM treatments of substance abuse (Lamb et al., 2004a, 2004b; Preston et al., 2001). One example of shaping, suggested by our data, would be to successively increase task difficulty by starting with CM reinforcement for visit attendance, then building on this performance by adding medication compliance as an additional target behavior, toward the eventual desired target of abstinence.
There are some limitations of this study that should be noted. First, the small sample size, coupled with high attrition, cautions against overinterpretation of the findings. Second, although significant CM effects were found, actual rates of responding were less than robust, perhaps because of variations in the administration of CM. In this study vouchers were delivered once a week, rather than immediately (same clinic visit), a procedural variation known to influence CM effect size (Lussier et al., 2006). Finally, while the study design focused on manipulating different target behaviors under identical reinforcement schedules, doing so inherently manipulated the response requirement such that the three CM conditions were not equal in terms of effort or output needed to obtain reward. For example, to earn CM reinforcement in the medication compliance condition, subjects had to attend the clinic, provide a urine sample (riboflavin analysis), and submit their MEMS pill bottle; clearly requiring more effort than simply attending a clinic visit in the CM-ATTEND condition. These differences in response requirements may have resulted in differential reward saliency across the CM interventions.
Despite limitations, the study was conducted according to a strong experimental design that allowed testing of the independent and interactive effects of the treatment factors. Each CM arrangement was well-defined using objective measures of the target behavior. As the field searches for effective medication-by-behavioral therapy treatments, the present study provides a useful model for exploring combined mechanisms of action. The distinct interaction of levodopa and abstinence-based CM, shown here, may offer a new approach for coordinating reward-based interventions that effectively compete with the reinforcing effects of cocaine.
Acknowledgments
This study was funded by the National Institute on Drug Abuse (NIDA) Medications Development Center Grant (P50-DA-9262). Preliminary results were presented at the annual meeting of the College of Problems on Drug Dependence in 2007 by Joy M. Schmitz.
Contributor Information
Joy M. Schmitz, Department of Psychiatry and Behavioral Sciences, University of Texas—Houston
Jan A. Lindsay, Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine
Angela L. Stotts, Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine Department of Family and Community Medicine, University of Texas—Houston.
Charles E. Green, Center for Clinical Research & Evidence-Based Medicine, University of Texas—Houston.
F. Gerard Moeller, Department of Psychiatry and Behavioral Sciences, University of Texas—Houston.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed Author; Washington, DC: 1994. [Google Scholar]
- Budney AJ, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. Therapy Manuals for Drug Addiction. USDHHS NIH; 1998. [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug and Alcohol Dependence. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. A perfect platform: Combining contingency management with medications for drug abuse. American Journal of Drug and Alcohol Abuse. 2007;33:343–365. doi: 10.1080/00952990701301319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MA, Katz EC, Stitzer ML. Methods for enhancing transition of substance dependent patients from inpatient to outpatient treatment. Drug and Alcohol Dependence. 2001;61:137–143. doi: 10.1016/s0376-8716(00)00133-2. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcoholism, Clinical and Experimental Research. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM–IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) Biometric Research Department; New York: 1995. [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug and Alcohol Dependence. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives. A substance abuse treatment innovation. Addictive Behaviors. 2002;27:887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug and Alcohol Dependence. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Improving contingency management programs for addiction. Addictive Behaviors. 2004a;29:507–523. doi: 10.1016/j.addbeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004b;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: A double-blind, placebo-controlled trial. American Journal of Drug and Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Sayre SL, Green C, Rhoades H, Schmitz JM. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addictive Disorders & Their Treatment. 2004;3:165–173. [Google Scholar]
- Namkoong K, Farren CK, O’Connor PG, O’Malley SS. Measurement of compliance with naltrexone in the treatment of alcohol dependence: Research and clinical implications. Journal of Clinical Psychiatry. 1999;60:449–453. doi: 10.4088/jcp.v60n0706. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology. 2007;75:983–991. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Pollastri AR, Pokrywa ML, Walsh SJ, Kranzler HR, Gelernter J. Incentive program decreases no-shows in nontreatment substance abuse research. Experimental and Clinical Psychopharmacology. 2005;13:376–380. doi: 10.1037/1064-1297.13.4.376. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. Journal of Consulting and Clinical Psychology. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Colby SM, Monti PM, Swift RM, Martin RA, Mueller TI, Eaton CA. Predictors of compliance with naltrexone among alcoholics. Alcoholism, Clinical and Experimental Research. 2000;24:1542–1549. [PubMed] [Google Scholar]
- SAS . The SAS System for Windows. Version 9.13 SAS Institute Inc.; Cary, NC: 2006. [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: Choosing the optimal behavioral therapy platform. Drug and Alcohol Dependence. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Rhoades HM, Elk R, Creson D, Hussein I, Grabowski J. Medication take-home doses and contingency management. Experimental and Clinical Psychopharmacology. 1998;6:162–168. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, Hall S. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: A randomized trial. Drug and Alcohol Dependence. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol Supplemental. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Vocci FJ. Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction. 2007;102:1888–1889. doi: 10.1111/j.1360-0443.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. Journal of Clinical Investigation. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Molecular Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Archives of General Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]