Abstract
Schizophrenia subjects’ smooth pursuit abnormalities may reflect a problem with perception of motion, although evidence comes primarily from reports using stimuli that differ from standard smooth pursuit stimuli (i.e moving gratings or coherent dots). This study presented schizophrenia and healthy subjects with a forced choice speed discrimination paradigm using smooth pursuit-like stimuli. The schizophrenia subject’s motion processing was impaired, as evidenced by their significantly higher speed discrimination thresholds (a difference that was 1.7 larger for the patient group). Abnormalities of motion perception in schizophrenia, even for simple stimuli, suggest a problem with motion processing in area MT.
Keywords: schizophrenia, motion perception, motion discrimination, smooth pursuit, MT
1. Introduction
Schizophrenia subjects have abnormalities of eye movement control, with problems during smooth pursuit tasks being a consistent finding (Hutton & Kennard, 1998). In the simplest terms (see, e.g., Lisberger et al., 1987; Stanton et al., 2005), generating an accurate smooth pursuit response requires adequate motion perception (primarily an extrastriate cortex function) and generation of the correct motor response based on that perceptual information (primarily a frontal cortex function). Investigating whether schizophrenia patients’ motion processing abilities are intact, therefore, is important for understanding the cause of their smooth pursuit-related deficits.
When presented with dynamic random dot patterns, schizophrenia patients require an unusually high proportion of coherently moving dots to discern the pattern’s global direction (Stuve et al., 1997; Chen et al., 2003; Chen et al., 2005; Kim et al., 2006; O'Donnell et al., 2006). Schizophrenia patients also are worse at discerning speed differences between two paired moving gratings (Chen et al., 2004; O'Donnell et al., 2006; Slaghuis et al., 2005). These results at least suggest that schizophrenia patients have impairments in motion perception.
Motion perception deficits could underlie schizophrenia patients’ performance difficulties during smooth pursuit tasks. Stimuli employed in the perceptual studies (dynamic random dot patterns and gratings) differ markedly from those employed in smooth pursuit studies (small, ~1°, targets moving across a dark background), however, making links between these two literatures uncertain. In the current study, we measured motion perception abilities using stimuli and conditions highly similar to those used previously to show smooth pursuit deficits in schizophrenia (e.g. Clementz & McDowell, 1994). Specifically, we employed a speed discrimination task, a straightforward means for assessing motion analysis (see Nakayama, 1985) that is closely related to actual smooth pursuit abilities (Kowler & McKee, 1987).
2. Method
Eighteen right-handed chronic outpatients with DSM-IV (American Psychiatric Association, 1994) schizophrenia (Median Age=36 yrs, 25th–75th %tile=28–44; 6 females) and 17 right-handed healthy subjects (Median Age=37 yrs, 25th–75th %tile=28–46; 10 females) participated in this study after providing written informed consent. All patients were clinically stable on antipsychotic medications (12 on atypical and 6 on typical) for >8 weeks prior to participation. Antipsychotic medications are not believed to drastically affect motion processing (see, e.g., Avila et al., 2006; Kim et al., 2006), and the patients on typicals and atypicals did not differ on performance. Subjects were interviewed with the SCID (First et al., 1995) by two psychologists (BAC and JEM) to either verify their clinical diagnosis (schizophrenia) or rule out Axis I disorders (healthy subjects). Participants were absent of neurological hard signs, clinically confounding treatments, history of head trauma and current psychoactive substance use disorders.
The stimulus was a 1° diameter dot (~20 cd/m2) that was rear-projected on a black screen (~0.2 cd/m2) using a red helium neon lazar driven by a closed-loop mirror galvanometer. Subjects viewed the stimulus from a chin rest situated 1 m away, and were required to maintain fixation on a cross at screen center that was 2 deg below the stimulus path. On each trial, two stimulus intervals were presented that were 200–250 ms (rectangular distribution) in duration and had a 250 ms ISI. Both intervals contained stimulus motion in the same direction (leftward or rightward; randomized across trials). One interval contained a “standard” speed (12 or 24 deg/s), and the other contained a “test” speed that differed from the standard by −25%, −16.67%, −8.33%, 0%, +8.33%, +16.67%, or +25% [differential speed = (test speed - standard speed)/ standard speed)]. At the end of each trial, subjects reported (verbally) which of the two intervals (first or second) contained the faster stimulus motion (a standard 2-alternative forced-choice design), and the experimenter provided feedback on response accuracy. Randomization of stimulus duration reduced the ability of subjects to use this variable when making their judgments. The 2-alternative forced-choice design, and use of test speeds both faster and slower than the standard, minimizes biases in discrimination judgments.
The two standard speeds were tested in separate blocks, and 210 trials (30 for each differential speed; half in each direction) were obtained for each block. For each test speed, the percentage of trials for which the subject perceived the test speed faster than standard speed was calculated (from 0% to 100%). These data were then fit with a cumulative normal function using probit analysis (Finney, 1971; McKee at al., 1985) to obtain a speed discrimination threshold (threshold = half the difference in differential speeds corresponding to the 25% and 75% levels of the psychometric function). Three subjects (2 schizophrenia, 1 healthy) did not meet a minimal performance criterion of 75% correct, so their data were not used in hypothesis testing.
3. Results
A group (schizophrenia, normal) by standard speed (12, 24) repeated measures ANOVA was conducted on the speed discrimination threshold data. The interaction was not statistically significant (F<1), so data were combined across the two standard speeds to get a single speed discrimination threshold for each subject (doubling the number of trials improved the probit fit and provided a better estimate of speed discrimination threshold for each subject).
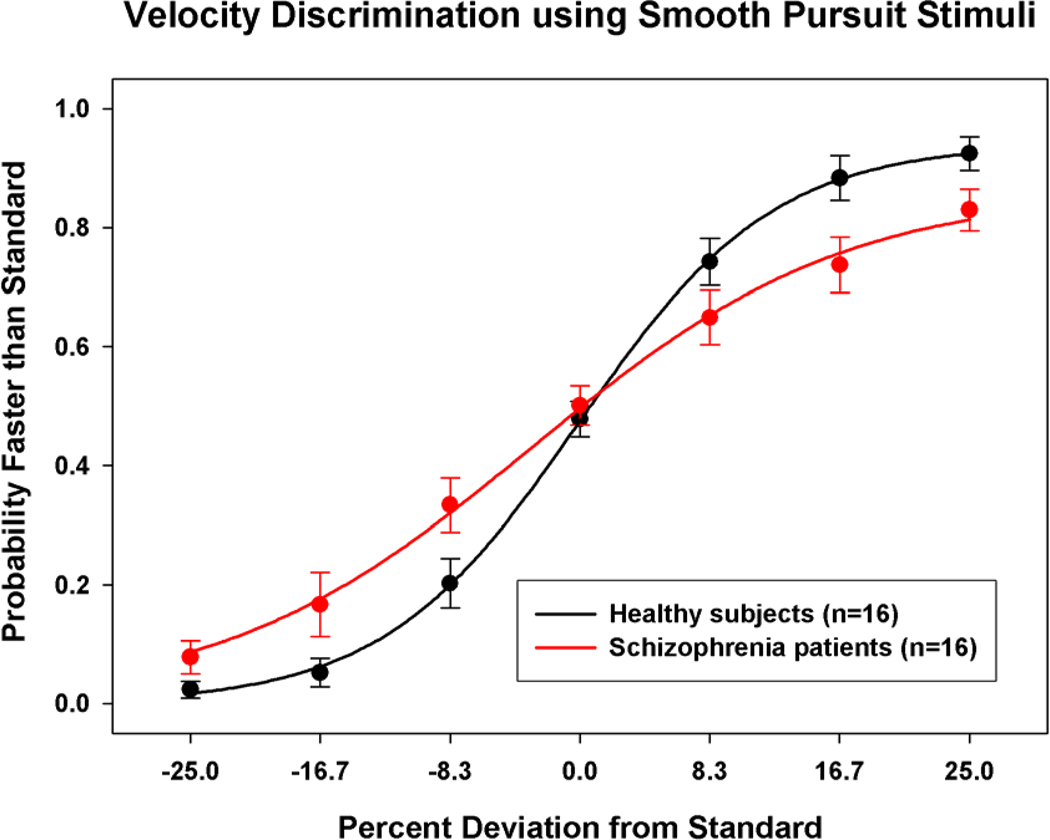
The schizophrenia subjects (M=14.7%, SD=4.2) had significantly higher speed discrimination thresholds than the healthy subjects (M=8.9%, SD=3.1), t(30)=4.4, p<.001, with this difference being 1.7 larger for the patient group. There was not a group difference on “bias”, the percent speed difference yielding 50% reports of test speed being faster than the standard, t(30)=1.1, p=.281. To more directly visualize group differences, Figure 1 shows the groups mean psychometric functions, which were obtained by averaging data for the different test speeds across subjects. The function for the schizophrenia patients is shallower than that of the healthy subjects, reflecting poorer speed discrimination performance.
Figure 1.
Plot of the psychometric function for schizophrenia (red) and healthy (black) subjects for speed discrimination averaged across standard speeds (12 and 24 deg/sec). On the abscissa is percent deviation from the standard speed and on the ordinate is the proportion (mean, ±1 SE) of trials on which the target was perceived as faster than the standard velocity. The best fitting sigmoidal function is also shown for both groups.
4. Discussion
The purpose of this study was to evaluate motion perception among schizophrenia patients with the types of stimuli used to evaluate smooth pursuit ability. Consistent with other motion perception studies, schizophrenia subjects had a significantly elevated speed discrimination threshold when compared to healthy subjects. This study, therefore, demonstrates a motion perception deficit with stimuli similar to those employed to show deficits during smooth pursuit among schizophrenia patients. These data may provide evidence for a more direct link between the perceptual and eye movement data, and are consistent with the notion that problems during smooth pursuit in schizophrenia are secondary to or concomitant with a perceptual deficit.
An issue to consider in schizophrenia studies is whether an observed deficit is the result of more basic perceptual and/or motivation limitations. It seems unlikely that elevated motion perception in schizophrenia can be accounted for by these factors. First, in other studies of motion detection and analysis, schizophrenia patients have had normal abilities to detect either the presence/absence or direction of motion using grating stimuli (e.g., Chen et al., 2003; Chen et al., 2005). Second, in smooth pursuit studies, schizophrenia patients have normal pursuit maintenance if prediction is not required even though pursuit onset is abnormal (Clementz & McDowell, 1994). Third, in other visual target detection studies requiring saccadic eye movements, schizophrenia patients can actually perform better than healthy subjects under specific task conditions (e.g., Clementz, 1996a).
It also is important to consider whether other, higher level, cognitive factors could account for group differences on motion processing. Avila et al. (2006) suggested that initial velocity perception may be unaffected in schizophrenia, and that schizophrenia patients’ problems with pursuit may “reflect an inability to accurately generate, store, and/or access these ‘remembered’ velocity signals” (p. 599). In some motion perception studies, subjects must keep information about a perceptual event “in mind” for comparison with a subsequent event. Schizophrenia patients have working memory impairments that are modality independent and may be manifest at brief delay intervals, although typically not as short as the 250 ms interval we used here (Lee & Park, 2005). It may be useful in future studies, therefore, to assess whether higher level cognitive control rather than basic perceptual abilities determines group differences in motion perception when matching/comparison operations are required (see, e.g., Chen et al 1999; Kim et al 2006).
The present finding is consistent with the conclusion that schizophrenia patients have a motion perception deficit attributable to dysfunction in extrastriate cortex area MT (see, e.g., Chen et al., 2005; Lencer et al., 2005). These data also are consistent with the theory of specific magnocellular pathway dysfunction in schizophrenia (Kim et al., 2006), since this pathway provides majority input to motion area MT (Maunsell et al., 1990). A possible complication for this interpretation is that schizophrenia patients have accurate saccades to slowly moving smooth pursuit targets (Clementz, 1996b; Kim et al., 1997), indicating that at least some MT-supported functions are normal in schizophrenia (Dursteler & Wurtz, 1988; Newsome et al., 1985). Future work on motion perception in schizophrenia, including functional brain imaging studies (e.g., Hong et al., 2005; Lencer et al., 2005), and studies comparing motion perception, smooth pursuit, and predictive pursuit abilities using the same stimuli (e.g., Hong et al., 2006), are needed to clarify this interesting, complex, literature that has possibly critical implications for understanding the neuropathology of this illness (Kim et al., 2006).
Acknowledgement
This work was support by a grant from the Unites States Public Health Service (MH51129).
Role of the Funding Source
The grant agency (NIH) provided funds to support collection of data and manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No Conflicts.
Contributors
Only contributors are the authors.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Avila MT, Hong LE, Moates A, Turano KA, Thaker GK. Role of anticipation in schizophrenia-related pursuit initiation deficits. J. Neurophysiol. 2006;95(2):593–601. doi: 10.1152/jn.00369.2005. [DOI] [PubMed] [Google Scholar]
- Chen EY, Hui CL, Chan RC, Dunn EL, Miao MY, Yeung WS, Wong CK, Chan WF, Tang WN. A 3-year prospective study of neurological soft signs in first-episode schizophrenia. Schizophr. Res. 2005;75(1):45–54. doi: 10.1016/j.schres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Nakayama K, Matthysse S, Palafox G, Holzman PS. Dependence of impaired eye tracking on deficient velocity distrimination in schizophrenia. Arch. Gen. Psychiatry. 1999;56(2):155–161. doi: 10.1001/archpsyc.56.2.155. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage motion processing in schizophrenia. Biol. Psychiatry. 2004;55(8):834–841. doi: 10.1016/j.biopsych.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy D, Matthysse S, Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr. Res. 2003;61(2–3):215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Clementz BA. The ability to produce express saccades as a function of gap interval among schizophrenia patients. Exp. Brain Res. 1996a;111(1):121–130. doi: 10.1007/BF00229561. [DOI] [PubMed] [Google Scholar]
- Clementz BA. Saccades to moving targets in schizophrenia: evidence for normal posterior cortex functioning. Psychophysiology. 1996b;33(6):650–654. doi: 10.1111/j.1469-8986.1996.tb02360.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE. Smooth pursuit in schizophrenia: abnormalities of open- and closed-loop responses. Psychophysiology. 1994;31(1):79–86. doi: 10.1111/j.1469-8986.1994.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Dursteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J. Neurophysiol. 1988;60(3):940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit Analysis. third ed. Cambridge, UK.: Cambridge University Press; 1971. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Lorna B. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Hong LE, Mitchell BD, Avila MT, Adami H, McMahon RP, Thaker GT. Familial aggregation of eye-tracking endophenotypes in families of schizophrenia patients. Arch. Gen. Psychiatry. 2006;63(3):259–264. doi: 10.1001/archpsyc.63.3.259. [DOI] [PubMed] [Google Scholar]
- Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57(7):726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Hutton S, Kennard C. Oculomotor abnormalities in schizophrenia: a critical review. Neurology. 1998;50(3):604–609. doi: 10.1212/wnl.50.3.604. [DOI] [PubMed] [Google Scholar]
- Kim CE, Thaker GK, Ross DE, Medoff D. Accuracies of saccades to moving targets during pursuit initiation and maintenance. Exp. Brain Res. 1997;113(2):371–377. doi: 10.1007/BF02450336. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr. Res. 2006;82(1):1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E, McKee SP. Sensitivity of smooth eye movement to small differences in target velocity. Vision Res. 1987;27(6):993–1015. doi: 10.1016/0042-6989(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm. Psychol. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Heide W, Binkofski F. Reduced neuronal activity in the V5 complex underlies smooth-pursuit deficit in schizophrenia: evidence from an fMRI study. Neuroimage. 2005;24(4):1256–1259. doi: 10.1016/j.neuroimage.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu. Rev. Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Nealey TA, DePriest DD. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J. Neurosci. 1990;10(10):3323–3334. doi: 10.1523/JNEUROSCI.10-10-03323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SP, Klein SA, Teller DY. Statistical properties of forced-choice psychometric functions: Implications of probit analysis. Perception & Psychophysics. 1985;37(4):286–298. doi: 10.3758/bf03211350. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Biological image motion processing: a review. Vision Res. 1985;25(5):625–660. doi: 10.1016/0042-6989(85)90171-3. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J. Neurosci. 1985;5(3):825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell BF, Bismark A, Hetrick WP, Bodkins M, Vohs JL, Shekhar A. Early stage vision in schizophrenia and schizotypal personality disorder. Schizophr. Res. 2006;86(1–3):89–98. doi: 10.1016/j.schres.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Bowling AC, French RV. Smooth-pursuit eye movement and directional motion-contrast sensitivity in schizophrenia. Exp. Brain Res. 2005;166(1):89–101. doi: 10.1007/s00221-005-2347-1. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Friedman HR, Dias EC, Bruce CJ. Cortical afferents to the smooth-pursuit region of the macaque monkey's frontal eye field. Exp. Brain Res. 2005;165(2):179–192. doi: 10.1007/s00221-005-2292-z. [DOI] [PubMed] [Google Scholar]
- Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol. Med. 1997;27(1):143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]