Abstract
Cartilage failure in diarthrodial joints results in pain and a reduction in quality of life. The goal of cartilage tissue engineering is to replace or regenerate these mechanically loaded tissues to restore function to the joint. Recent advances in our laboratory have resulted in the production of cartilage and fibrocartilage with clinically relevant properties. A review of salient results will constitute the bulk of this manuscript, which serves as a companion to the Academy of Osseointegration’s 2010 presentation that shares the same title. After providing a brief background of the clinical problem, this review will highlight several specific tissue engineering tools. The approaches used in producing mechanically functional cartilage through tissue engineering have several parallels to the problems faced in osseointegration, e.g., the need for mechanically appropriate tissues at the implantation site. The discussion that follows will focus on how approaches developed in identifying alternative cell sources and various exogenous stimuli for producing neocartilages may be applicable to osseointegration.
Keywords: tissue engineering, articular cartilage, self-assembly, stem cells, regenerative medicine
Background to Articular Cartilage Tissue Engineering
Tissue engineering is defined as an interdisciplinary research field devoted to the repair or replacement of tissues or organs through the application of engineering methods, material science, chemistry, cells and other biological materials, and externally applied stimuli. To properly place into context the need for engineering articular cartilage, the clinical need for replacement cartilages and their desired functional properties will be presented first.
The Clinical Issue
The Centers for Disease Control and Prevention estimate osteoarthritis affects 27 million Americans, and this number is expected to grow as the population ages. While osteoarthritis can affect younger populations, many more elderly are stricken, with estimates of almost one-fifth of the population over 45 developing this debilitating disease (1). Damage to the joints and articular cartilage from blunt or accumulated trauma represents a common event during aging and results in severe pain and reduction in the quality of life.
Articular cartilage is recalcitrant to repair due to its low cellularity, and aneural and avascular nature. While traumatic disruption of the joint is understood to result in cartilage degeneration, the initial etiology of osteoarthritis in many cases still remains unknown. However, degradation of the matrix by multiple methods including wear and tear, overloading, and inflammatory/catabolic destruction results in loss of joint function. During the progression of osteoarthritis, cartilage typically decreases in collagen and proteoglycan content and increases in water content (2) with subsequent changes in biomechanical characteristics. Fibrillation and fissures may develop and extend to the subchondral bone (3). Regardless of cause, defects within articular cartilage can be divided into two groups based on depth: partial and full-thickness defects. Although of less apparent severity, partial-thickness defects penetrate only so far as the articular cartilage matrix, resulting in a defect isolated from the underlying blood supply and bone marrow progenitor cells within the subchondral bone. Therefore, repair activities of the defect only originate from the adjacent, metabolically quiescent chondrocytes incapable of more than a few days of increased matrix synthesis, resulting in a lasting defect which can affect the surrounding tissue mechanics and initiate further tissue degradation (4). Full-thickness defects (also known as osteochondral defects) penetrate into the subchondral bone, allowing for access to blood and mesenchymal progenitor cells to initiate a more robust healing response (5). However, the repair tissue that fills this defect does not last due to its more fibrous and mechanically inferior nature. Current treatment options that successfully replace cartilage are inconsistent (6), and many treatment options deal with management of pain symptoms (7). The gold standard in care remains the total joint replacement, which, while successful, has a usable life of approximately 10 to 15 years before revision. This is inadequate especially for young patients. The widespread occurrence and harmful nature of cartilage degeneration establish a strong need for development of replacement tissue engineered cartilage.
Native Cartilage Properties
Replication of the structure and function of the native cartilage is desired; to this end, an understanding of the properties of the native tissue is needed. Articular cartilage exists as a thin smooth tissue covering the ends of the bones, with liquid and solid fractions. The interactions of these two phases are described by the biphasic model, which describes the viscoelastic nature of the tissue (8). The biochemical composition of the tissue allows for its unique mechanical properties. In adult humans, 70–80% of the matrix wet weight is water, while the remaining solid fraction is primarily collagen type II (50–75%) and proteoglycans (15–30%) (9).
The cells in cartilage, chondrocytes, occupy less than 5% of the tissue volume in humans but are essential for maintaining the extracellular matrix (ECM). Isolated within the dense ECM, the chondrocyte source of nutrients is primarily through diffusion from the synovial fluid, assisted by joint movement. The articular chondrocyte phenotype varies by zonal depth, but all differentiate from mesenchymal progenitor cells and, in healthy adult tissues, have slow to no proliferation. Immediately surrounding the chondrocytes is the pericellular matrix which differs in composition from the bulk ECM. Within the pericellular matrix higher levels of decorin, aggrecan, and a network of type VI collagen are found (10). The chondrocytes are anchored to this matrix through the CD44 receptor to hyaluronan-proteoglycan aggregates and by integrins. These integrins can serve as receptors for ECM proteins such as collagens type II and IV, laminin, fibronectin, and vitronectin (11).
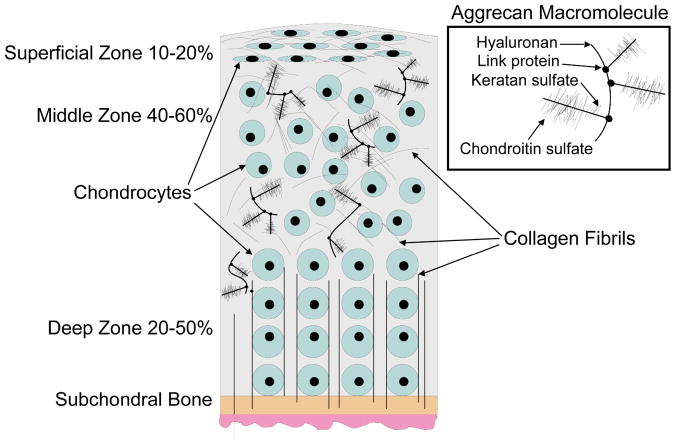
Three identifiable zones exist within articular cartilage, based on ECM content and cell phenotype, termed superficial, middle, and deep (Figure 1). The superficial zone of articular cartilage contains flattened discoid cells that secrete superficial zone protein, and collagen fibers oriented parallel to the surface in the direction of shear providing tensile strength and controlling fluid permeability (12). The middle zone consists of spherical cells arranged in perpendicular columns, the highest aggrecan (proteoglycan) content (13–14), and cartilage intermediate layer protein (15). The deep zone includes the calcified area of cartilage; the tidemark distinguishes between the non-calcified and calcified areas. This characteristic zonal architecture, which is intimately linked to the biology, mechanical function, and changing composition, results in zone specific mechanical properties of articular cartilage. The organization of the cartilage varies by depth as a result of differences in the forces experienced throughout the tissue. Tensile forces within cartilage result primarily from load redistribution to the surrounding tissue during compressive loading, and from the sliding motion of articulating surfaces upon each other. The mechanical properties of articular cartilage are dependent on the molecules composing the ECM and their organization; these properties are primarily determined by the interactions of collagen and aggrecan.
Figure 1.
Zones and macromolecules present in articular cartilage.
Collagen and Tensile Properties
Although collagen type II makes up the bulk of the collagens within cartilage, collagens VI, IX, X, and XI are also present. Collagens IX and XI can crosslink with collagen II to produce larger fibrils which can form an interconnected mesh network surrounding the aggrecan (16). The collagen network encapsulating the aggrecan provides tensile strength to resist the expansion of the proteoglycans (17). On a microscopic scale the water and collagen content in the tissue decreases with depth from the articulating surface, while the collagen fibril size increases. Collagen fibers are oriented tangential to the surface to resist shear and tension, while the organization of collagen fibrils in the middle of the tissue is more random. Fibers near the tidemark are arranged perpendicular to the surface interfacing with the underlying bone.
Proteoglycans and Compressive Properties
The bulk of the proteoglycans within cartilage are found in aggregates composed of aggrecan linked to hyaluronic acid via link protein. Aggrecan exists as a large highly glycosylated proteoglycan with long linear glycosaminoglycan (GAG) chains of chondroitin sulfate and keratan sulfate molecules radiating from a central protein core, resulting in a bottle brush structure. The carboxyl (COO−) and sulfate (SO3−) groups present on these GAGs produce a strong negative charge allowing it to absorb water and swell creating an osmotic pressure that resists compressive mechanical forces (12). Smaller proteoglycans (i.e., biglycan, fibromodulin, and decorin) occur in lower concentrations and contribute to the organization of the matrix and ligand sequestering (18–19). Compressive loading is one of the main forces encountered by cartilage. The movement of the interstitial fluid trapped by the aggrecan through the matrix dissipates the compressive load due to frictional drag, which is dependent on the hydraulic permeability of the tissue. As the permeability of healthy cartilage is low, this results in high interstitial fluid pressures during load. Over time, the interstitial fluid pressure decreases as a function of the permeability, resulting in the load being transferred to the solid portion of the extracellular matrix, resulting in the viscoelastic nature of cartilage. As this fluid is exuded from the joint during loading it also serves as a hydrodynamic lubricant to reduce friction (20).
Techniques for Articular Cartilage Tissue Engineering
Approaches to engineering articular cartilage can be broadly divided into in vivo and in vitro methods. The former stems from the belief that the in vivo environment contains all the necessary stimuli to direct tissue formation if a suitable cell source or scaffold were provided. A variety of polymer or biological scaffold materials for the filling of articular cartilage defects have been studied, often in combination with various growth factors, and cell sources (21). Clinically this has resulted in the autologous chondrocyte implantation technique, wherein chondrocytes are isolated from non-load bearing regions, expanded in vitro, and reimplanted under a periosteal flap (22) or in a collagen bilayer scaffold (23). Although this results in defect filling and short-term pain relief (24), the implanted material may not regain native tissue mechanical properties. This review focuses on in vitro tissue engineering, which seeks to complete the bulk of matrix production and organization before implantation to deliver a neotissue of sufficient properties to function, integrate, and remodel (Figure 2). The in vitro environment is defined by the bioengineer. This allows for the controlled examination and fine-tuning of relevant tissue engineering parameters to stimulate the formation of functional tissue; the classic paradigm of tissue engineering has involved the triad of cells, signals and scaffolds. As scaffolds can have many disadvantages including stress shielding, cell-cell contact inhibition, and biodegradability issues, a scaffold-less approach utilizing a self-assembling process has been developed (25). The self-assembling process is based on the differential adhesion hypothesis (26–27), and many encouraging results have been obtained using this method with different combinations of cells and signals, as described below.
Figure 2.
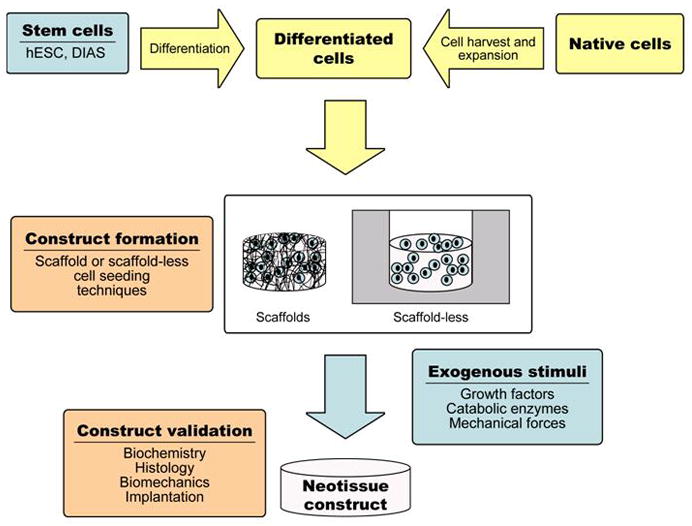
The paradigm of in vitro tissue engineering.
Scaffold-Less Self-Assembling Process
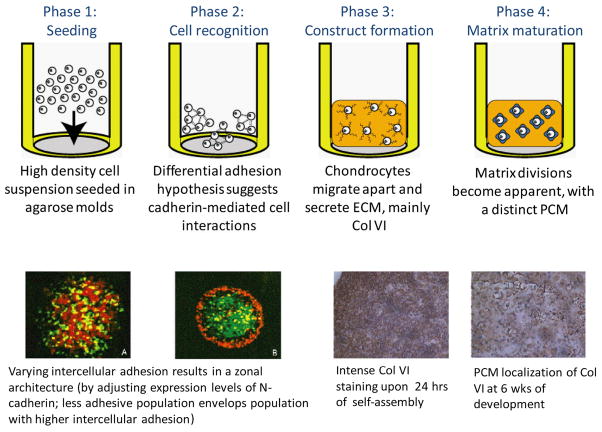
The self-assembling process employs high-density seeding of native chondrocytes in agarose molds, allowing for control of construct size and shape. Cartilage constructs with clinically relevant dimensions (~15 mm dia. by 1 mm thick) and properties approaching those of native cartilage have been created using this scaffold-less approach (25). The self-assembly of chondrocytes has been shown to closely recapitulate normal development and maturation (Figure 3). Abundant cadherin activity, when the cells are first seeded, levels off after the cells have coalesced to initiate matrix production, which consists of glycosaminoglycans and collagen VI. At four weeks, tensile properties reach a maximum and compressive properties level off, while biochemically the tissue matures with collagen VI localized pericellularly, increased amounts of collagen II in the interterritorial space, and an increased chondroitin 4-sulfate to 6-sulfate ratio (26).
Figure 3.
Phases in the self-assembly of differentiated articular chondrocytes mimics those of development. (Used under the Creative Commons Attribution License, from Ofek et al., PLoS ONE, 2008 (26).)
Cell Sources
Autologous chondrocytes are source limited, and harvesting healthy cartilage tissue results in site morbidity and mortality, as is common with most autologous sourced cells. Chondrocytes have low natural proliferation and expansion techniques result in phenotypic changes (28). Therefore, for the numbers of chondrocytes needed for tissue engineering, using autologous chondrocytes is impractical for clinical use (29). This has led to the investigation of other cell sources, such as human embryonic stem cells (hESC) and dermis-isolated aggrecan sensitive cells (DIAS). To employ these cells in tissue engineering, methods need to be determined to 1) isolate, 2) differentiate, and 3) purify them. These steps are investigated in parallel using a modular approach to accelerate the pace of discovery. For example, as protocols for stem cell differentiation are refined to reach higher efficiencies, purification protocols are also under development to enrich the percentage of relevant cells for self-assembly.
Embryonic stem cells (ESCs) are pluripotent as defined by their ability to indefinitely proliferate and differentiate into any of the three germ layers. Employing the modular approach we have induced chondrogenic differentiation of both BG01Vs and H9s hESC lines followed by tissue engineering of these cells. This was performed by differentiating hESC in chondrogenic media in embryoid bodies for up to six weeks, followed by enzymatic disassociation. These differentiated disassociated cells where self-assembled and cultured an additional four weeks. With both hESC lines, fibrocartilage constructs were produced (30). To improve chondrogenic differentiation, combinations of Transforming Growth Factor β 3 (TGF-β 3) and Bone Morphogenetic Protein-4 (BMP-4) were used in embryoid bodies. This growth factor combination increased cell surface marker CD44 and also increased GAG and total collagen by 6.7-fold and 4.8-fold, respectively. An alternative method of culturing embryoid bodies in the vicinity of fibrochondrocytes increased collagen II production 9.8-fold (31). Using these various differentiation techniques, hESC lines can be differentiated toward the chondrocyte phenotype.
Considering its relative abundance and ease of access, dermis is considered one of the best autologous source organs to isolate stem/progenitor cells for future therapeutic applications. A dermis derived sub-population, termed DIAS cells, has been identified that can be chondro-induced using aggrecan. Protocols have been developed to isolate, purify, expand, and chondro-induce DIAS cells in vitro. Specifically, exposing DIAS cells to aggrecan for 24 hours produced dense cell aggregates that contained higher levels of collagen type II then fibroblast controls for up to 14 days. Using self-assembly, these chondro-induced DIAS cells have shown promise in producing 3D constructs with cartilaginous properties (32).
Signals to Generate Functional Properties In Vitro
In vivo, cartilage is exposed to a milieu of mechanical and biochemical signals. The duration, magnitude and combination of these stimuli are not well defined, but nonetheless regulate the ECM components chondrocytes secrete. To better delineate how these stimuli contribute to improving the mechanical properties of neotissues, several stimuli have been examined in vitro, including growth factors, hydrostatic pressure and catabolic enzymes.
Using self-assembly, growth factors such as TGF-β, BMPs, and Insulin like Growth Factors (IGFs) have been shown to improve neotissue mechanical properties. The combined treatment of BMP-2 and IGF-I resulted in increases in GAG production and a greater than 1-fold increase in aggregate modulus. In contrast, TGF-β1 treatment increased both GAG and collagen content, and yielded 1-fold increases in both aggregate and tensile modulus (33). For cartilage tissue engineering, TGF-β1 demonstrated the most potency as it increased collagen content and tensile modulus, and outperformed a combination of growth factors.
Mechanical stimulation, such as hydrostatic pressure or direct compression, has repeatedly been shown to have positive effects on native and neotissue mechanical properties. In general, these stimuli have been chosen to have magnitudes at or below the physiological range. For instance, the application of 10 MPa static hydrostatic pressure, applied for 1 hour a day for 5 days, significantly increased the collagen content over 2-fold, the aggregate modulus by 1.4-fold, and the tensile modulus 1.9-fold (34). These increases in construct properties are similar to those obtained from using growth factors.
Combinations of these two different classes of stimuli, TGF-β1 and hydrostatic pressure, resulted in additive effects on mechanical properties, increasing the aggregate modulus by more than 1.6 fold and the tensile modulus by more than 2.3 fold. Furthermore, the combination treatment had a greater than additive effect; a synergistic increase in collagen content was observed (35). This combination of biochemical and mechanical stimuli resulted in constructs with mechanical properties overlapping those of native tissue.
Classically, most tissue engineering studies (cartilage and other tissues) have been driven by the addition of anabolic factors to increase production of extracellular matrix and tissue strength. Counterintuitively, catabolic factors, such as enzymes that digest the cartilage matrix, may improve mechanical properties by assisting matrix turnover. For example, application of chondroitinase ABC (C-ABC), which digests GAGs, has resulted in increased tensile mechanical properties in self-assembled cartilage constructs. Although a single four-hour C-ABC treatment depleted GAGs and reduced construct compressive properties, both of these recovered after two weeks. Furthermore, the treatment had resulted in an 80% increase in tensile modulus (36). Likely, the application of C-ABC mirrors that of native tissue matrix remodeling and suggests further uses of catabolic factors in tissue engineering.
Assaying Tissue Mechanical Properties
To determine if engineered tissues can replicate native tissue function, assays capable of determining mechanical properties are required. As the compressive and tensile properties of articular cartilage are necessary for its function, we have defined testing parameters that measure both compressive aggregate modulus and tensile modulus. To measure values for compressive properties, a form of creep indentation testing is used. A platen of known dimension and size is used to indent the sample under constant stress, and deformation is measured over time. A porous platen is used to allow for fluid to exude from the sample at the platen contact site. Data are collected until the sample reaches deformational equilibrium. Following testing, a numerical algorithm fits the data to the biphasic theory to compute three independent variables that describe construct material properties, the aggregate modulus, permeability, and Poisson’s ratio (37). To measure tensile modulus, a set of grips are fixed upon the tissue and a constant strain rate of 1% is used to pull the sample apart until failure. The tensile modulus can then be calculated from the linear region of the stress-strain curve. These assays allow for identification of not only native tissue properties that need to be replicated, but also can be used as quality control for the tissue engineered materials.
Summary
While the self-assembly process was initially developed based on the use of articular chondrocytes, the encouraging results has led it to be applied to a spectrum of cartilage tissues (38). By varying the cell source, the biochemical stimuli applied, and the mold geometry, the engineering of a range of fibrocartilage tissues have been produced (e.g. meniscus and TMJ). For example, circumferential collagen fibril alignment could be observed in meniscus constructs grown in ring shaped molds, resulting in a 3-fold increase in circumferential tensile properties compared to radial tensile properties (39). While this technique shows promise, current studies focus on continuing to improve the mechanical properties of the neotissues produced, by optimization of biochemical and mechanical stimuli. Due to its versatility and capability to increase functional (biochemical and biomechanical) characteristics, the self-assembly process has recently been combined with DIAS cells and chondro-differentiated hESCs to produce constructs with cartilage-specific ECM. This further demonstrates the ability to use this technique over multiple cell sources. As much as no one signal controls the development and maintenance of articular cartilage, optimization of stimuli combinations and a greater understanding of the mechanisms of gene regulation at work remain areas of current research. The objective of our research remains the engineering of cartilage with clinically relevant biochemical and biomechanical properties paralleling that of native tissue.
Insights Related to Dental Implant Osseointegration
As previously discussed, articular cartilage tissue engineering can be approached using in vivo or in vitro methods, each employing a combination of cells, signals, and scaffolds. Insights gained from these approaches can similarly be applied to osseointegration. For instance, failure to osseointegrate can be attributed to lack of necessary tissue at the implantation site; placing implants in patients with inadequate bone support (either of sufficient quality or quantity) remains a major obstacle. Increasing the available bone for implantation using autologous sourced grafts requires multiple surgeries which are undesirable. Autografts are also scarce, and their harvest can lead to donor site morbidity and pain. Engineered tissues may fill this need for autologous tissue. In articular cartilage tissue engineering, the quality and quantity of the neotissue has been increased through the use of growth factors, mechanical stimuli, and catabolic enzymes. Examples of how these stimuli may be or are used in in vivo and in vitro tissue engineering, as related to osseointegration, are discussed below.
The In Vivo Approach to Improving Bone Quality and Quantity
Similar to cartilage tissue engineering in vivo to fill defects, scaffolds, cells, and signals can be considered for osseointegration. With regard to scaffolds, various formulations of demineralized freeze-dried bone allograft putty or matrix have been used to fill intraosseous defects (40) to improve bone quality. As described above, one of the clinical strategies for in vivo articular cartilage engineering has been the implantation of autologous cells in concert with scaffolds for defect filling. Were this strategy to be applied to osseointegration, one may want to consider the previously described issues surrounding this approach (e.g., cost, cell sourcing, multiple surgeries, and no immediate load bearing). Finally, the signals currently employed in vivo for bone formation have consisted of growth factors. For example, Platelet Derived Growth Factor in combination with an osteoconductive material (GEM 21S®, Osteohealth, Shirley, NY, USA) has shown clinical use in dental practice (41). Also, BMP-2 in conjunction with a collagen sponge (INFUSE, Medtronic, Minneapolis, MN, USA), as well as BMP-7 in conjunction with a type I bone collagen carrier (OP-1, Stryker, Kalamazoo, MI) are other related products clinically available for bone formation.
However, the use of anabolic growth factors alone may not be the complete answer. As described above, one of the insights gained from engineering articular cartilage has been the use of catabolic enzymes to increase the quality (mechanical properties) of the tissue produced (42) and to enhance cartilage-to-cartilage integration (43). The in vivo use of appropriately selected catabolic agents used in concert with growth factors may similarly allow for increased functionality and/or implant integration.
The In Vitro Approach to Improving Bone Quality and Quantity
While growth factor enhanced biomaterials are effective, the use of these products requires time for both healing and growth of the new bone in vivo before an implant can be placed, which can be several months. To augment the site of implantation, in vitro growth of a neotissue using a combination of scaffold-less self-assembly, various cell sources, and signals may result in a construct that provides a starting point for further bone incorporation, thus reducing healing time. The in vitro approach offers other advantages in this regard. As previously described, the self-assembly process produces engineered tissues of controllable sizes and shapes. For cells, the in vitro setting also allows for the careful control of appropriate signals to differentiate or purify multiple cell sources. Although the cell sources identified in cartilage tissue engineering have been differentiated toward a chondrogenic potential, related technologies can be developed for identifying cells applicable to bony defect filling or for regenerating the periodontal ligament and gingiva. With regard to signals, stimuli that are impractical in the in vivo setting can be used and applied in a well-controlled manner, such as certain types of mechanical loading (e.g., hydrostatic pressure). Additionally, stimuli (growth factors) can be applied repeatedly without the need for complex sustained release materials. This allows for the use of dosing regimens involving combinations of growth factors and/or mechanical loading. Finally, an in vitro tissue engineering approach prevents the patient from being directly exposed to these growth factors, and the bulk of tissue growth can occur in an aseptic environment.
In addition to supplying implantable engineered tissues, one can also envision the enhancement of osseointegration in vitro. For instance, an implant may be pre-integrated with engineered bone and associated mucosal tissue, in vitro prior to implantation. This would potentially have several benefits, including optimization of implant-tissue integration, higher mechanical stability, and the ability to fill areas of highly resorbed bone. The quality of osseointegration and tissue formation could then be verified using non-invasive optical monitoring, (44) an approach we are currently applying to tissue engineered cartilage. Furthermore, bone-to-bone integration is a more favorable condition than implant osseointegration, thus resulting in increased resistance to load bearing during the early critical period of implantation when the implant can be overloaded.
The Role of Tissue Engineering Beyond Osseointegration
In the long-term, current implants have a useful life expectancy that is less than that of the natural teeth, which can last several decades. Therefore, a long term solution may be the regeneration of native tissues, possibly via the implantation of adult or stem cells differentiated to form a tooth bud that grows to the correct size, geometry and mechanical strength. The principal issue in tooth regeneration is similar to that faced in cartilage tissue engineering; i.e., a need to recapitulate a specific spatial and temporal series of events to achieve mechanically functional tissues.
Regeneration of the tooth is complicated by the need to form four distinct tissues with functional interfaces (pulp, dentin, cementum and enamel) including a root supported by the periodontal ligament and anchored into the alveolar bone. Current work on autologous cell sources (e.g., stem cells from adjacent tissues such as the root apical papilla and the periodontal ligament) has demonstrated promise in regenerating integrated living roots capable of supporting a crown in a pig model (45). However, control of tissue shape (necessary for correct function), and isolation of sufficient autologous cells remains elusive. The techniques outlined above for cartilage tissue engineering may help to elucidate the necessary steps to tissue engineer a functional tooth. The self-assembly technique can potentially be employed to create the complex shapes needed for dental function. The ability to differentiate skin (DIAS) cells toward a chondrogenic phenotype serves as an example of how non-dental tissue sources might yield cell sources useful in producing the various dental lineages required to engineer the tooth. While the exogenous stimuli identified for cartilage tissue engineering are unlikely to directly transfer to engineering other tissues, they nonetheless can serve as starting points for improving tissue mechanical properties.
Conclusion
Tissue engineering in implant dentistry necessarily focuses on the guided regeneration of bone. Already, some tissue engineered products have reached the market combining biological signals with osteoconductive scaffolds. The engineering of more complex dental structures can employ insights learned in regenerating cartilage. Further advancements in osseointegration will require continued dialogue between clinicians and tissue engineers to reap the benefits of cooperative feedback between these two groups.
Acknowledgments
The authors would like to acknowledge the support of NIH R01AR053286 for this work.
References
- 1.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis. 1994;53:565–568. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 3.Aluisio FV, Christensen CP, Urbaniak JR. Orthopaedics. Williams & Wilkins; 1998. [Google Scholar]
- 4.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Revell CM, Athanasiou KA. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defectst. Tissue Eng Part B Rev. 2008 doi: 10.1089/ten.teb.2008.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuels J, Krasnokutsky S, Abramson SB. Osteoarthritis: a tale of three tissues. Bull NYU Hosp Jt Dis. 2008;66:244–250. [PubMed] [Google Scholar]
- 8.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 9.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001:S26–33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 10.Johnston SA. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. 1997;27:699–723. doi: 10.1016/s0195-5616(97)50076-3. [DOI] [PubMed] [Google Scholar]
- 11.Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002;59:36–44. doi: 10.1007/s00018-002-8403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little K, Pimm LH, Trueta J. Osteoarthritis of the hip: an electron microscope study. J Bone Joint Surg Br. 1958;40-B:123–131. doi: 10.1302/0301-620X.40B1.123. [DOI] [PubMed] [Google Scholar]
- 13.Maroudas NG. On the low adhesiveness of fluid phospholipid substrata. J Theor Biol. 1979;79:101–116. doi: 10.1016/0022-5193(79)90259-5. [DOI] [PubMed] [Google Scholar]
- 14.Gu WY, Lai WM, Mow VC. Transport of fluid and ions through a porous-permeable charged-hydrated tissue, and streaming potential data on normal bovine articular cartilage. J Biomech. 1993;26:709–723. doi: 10.1016/0021-9290(93)90034-c. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo P, Bayliss MT, Heinegard D. A novel cartilage protein (CILP) present in the mid-zone of human articular cartilage increases with age. J Biol Chem. 1998;273:23463–23468. doi: 10.1074/jbc.273.36.23463. [DOI] [PubMed] [Google Scholar]
- 16.Responte DJ, Natoli RM, Athanasiou KA. Collagens of articular cartilage: structure, function, and importance in tissue engineering. Crit Rev Biomed Eng. 2007;35:363–411. doi: 10.1615/critrevbiomedeng.v35.i5.20. [DOI] [PubMed] [Google Scholar]
- 17.Stockwell RA. Cartilage failure in osteoarthritis: Relevance of normal structure and function. A review. Clinical Anatomy. 1990;4:161–191. [Google Scholar]
- 18.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302 ( Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 20.Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech. 2009;42:1163–1176. doi: 10.1016/j.jbiomech.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frenkel SR, Di Cesare PE. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32:26–34. doi: 10.1023/b:abme.0000007788.41804.0d. [DOI] [PubMed] [Google Scholar]
- 22.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 24.Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 25.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 26.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS One. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg MS. Mechanism of tissue reconstruction by dissociated cells. II. Time-course of events. Science. 1962;137:762–763. doi: 10.1126/science.137.3532.762. [DOI] [PubMed] [Google Scholar]
- 28.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Heng BC, Cao T, Lee EH. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells. 2004;22:1152–1167. doi: 10.1634/stemcells.2004-0062. [DOI] [PubMed] [Google Scholar]
- 30.Koay EJ, Hoben G, Athanasiou KA. Tissue engineering with chondrogenically-differentiated human embryonic stem cells. Stem Cells. 2007;25:2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 31.Hoben GM, Willard VP, Athanasiou KA. Fibrochondrogenesis of hESCs: Growth factor combinations and co-cultures. Stem Cells Dev. 2008;18:283–292. doi: 10.1089/scd.2008.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng Y, Hu JC, Athanasiou KA. Isolation and chondroinduction of a dermis-isolated, aggrecan-sensitive subpopulation with high chondrogenic potential. Arthritis Rheum. 2007;56:168–176. doi: 10.1002/art.22300. [DOI] [PubMed] [Google Scholar]
- 33.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2008;18:18. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue engineering. 2009;15:1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A. 2009;15:3119–3128. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage--II. A numerical algorithm and an experimental study. J Biomech. 1989;22:853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 38.Hoben GM, Athanasiou KA. Creating a spectrum of fibrocartilages through different cell sources and biochemical stimuli. Biotechnol Bioeng. 2008;100:587–598. doi: 10.1002/bit.21768. [DOI] [PubMed] [Google Scholar]
- 39.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 40.Bender SA, Rogalski JB, Mills MP, Arnold RM, Cochran DL, Mellonig JT. Evaluation of demineralized bone matrix paste and putty in periodontal intraosseous defects. J Periodontol. 2005;76:768–777. doi: 10.1902/jop.2005.76.5.768. [DOI] [PubMed] [Google Scholar]
- 41.Pellegrini G, Seol YJ, Gruber R, Giannobile WV. Pre-clinical models for oral and periodontal reconstructive therapies. J Dent Res. 2009;88:1065–1076. doi: 10.1177/0022034509349748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J Orthop Res. 2009;27:949–956. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Breevaart Bravenboer J, In der Maur CD, Bos PK, Feenstra L, Verhaar JA, Weinans H, et al. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6:R469–476. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Phipps J, Elson DS, Stoy H, Tinling S, Meier J, et al. Fluorescence lifetime imaging microscopy: in vivo application to diagnosis of oral carcinoma. Opt Lett. 2009;34:2081–2083. doi: 10.1364/ol.34.002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]