Abstract
Background
d-Amphetamine (AMPH) is a widely prescribed ADHD medication, but little is known about its effects on impulsive choice with escalated use.
Objective
The current study examined the effects of short and long access to AMPH self-administration on impulsive choice in a delay discounting task in which rats chose between a small immediate reward (1 sucrose pellet immediately) and a larger delayed reward (3 sucrose pellets after an adjusting delay).
Methods
Following choice stability in delay discounting, all rats received 15 1-hour sessions of AMPH self-administration (0.1 or 0.03 mg/kg/infusion); self-administration sessions began 45 min after each delay discounting session. Rats were then either maintained on the short access (ShA) self-administration session or were switched to a long access (LgA) 6-hour session for 21 days, followed by a 7-day withdrawal phase in which only the delay discounting task continued.
Results
LgA rats in the 0.03 mg/kg/infusion dose group escalated in total number of infusions across sessions, although rats in the 0.1 mg/kg/infusion dose group did not. LgA groups at both unit doses showed decreased mean adjusted delays across sessions compared to the ShA groups, indicating that long access to AMPH increases impulsive choice. During the AMPH withdrawal phase, LgA groups returned back to baseline mean adjusted delays, indicating that the effect on impulsive choice was reversible.
Conclusion
These results show that extended AMPH self-administration produces a transient loss of inhibitory control, which may play a role in the escalating pattern of drug intake that characterizes the addiction process.
Keywords: Delay Discounting, d-Amphetamine, Escalation, Impulsivity
Introduction
Impulsivity is a multi-dimensional trait that emerges in every major system of human personality (Whiteside and Lynam 2001). Impulsive choice, or preference for immediate over delayed reward, has been associated with stimulant abuse, which is characterized by a switch from regulated intake to compulsive intake (Koob and Kreek 2007). It is unclear, however, to what extent impulsivity is a determinant or a consequence of drug abuse (deWit 2009). As a determinant, increases in impulsive behaviors may increase drug use. As a consequence, maladaptive behaviors resulting from drug use may increase impulsive choice.
Delay discounting is a task used to measure impulsivity in human and nonhuman animals in which subjects choose between a small immediate reward and a large delayed reward. Impulsive choice is characterized as the extent to which consequences, or outcomes, decrease in their ability to control behavior due to a delay to their occurrence (Reynolds, 2006). There are, however, cross-species differences in delay discounting. For example, Mazur and Logue (1978) found that laboratory animals generally tend to be impulsive (unless a fading procedure is employed), whereas humans use a maximization strategy, and are more self-controlled (Logue et al. 1986). In a study comparing pigeons and rats, Green et al. (2004) also found that rates of discounting were higher in pigeons, but unlike humans, discounting rates of pigeons or rats did not vary systematically as a function of amount of delayed reward.
Previous studies have examined the effects of stimulant drugs on delay discounting in different species. In rats, Stanis et al. (2008) found that repeated exposure to experimenter-administered d-amphetamine (AMPH; via i.p. injection) does not increase impulsive choice in delay discounting. In contrast, in humans, de Wit et al. (2002) found that administration of acute oral AMPH decreases impulsivity on a delay discounting task. Human cocaine abusers also show higher discounting rates of monetary and crack/cocaine rewards than matched controls (Coffey et al. 2003). Similarly, Bickel et al. (1999) found that cigarette smokers discount the value of delayed money and cigarettes more than never-and ex-smokers. Ex-smokers, however, are indistinguishable from never-smokers, indicating that the increase in discounting due to cigarette smoking is reversible. Consistent with these results in humans, repeated nicotine treatment increases impulsive choice on a delay discounting task in rats and this effect is also reversible (Dallery and Locey 2005). This stimulant-induced loss of inhibitory control may be an important determinant of the escalating pattern of drug intake that characterizes the addiction process.
Models of the transition from moderate to excessive drug intake have been developed recently in laboratory animals. With extended access to cocaine or methamphetamine (6 hr/day), rats will increase the amount of drug they self-administer (Ahmed et al. 1998; 1999; 2004; Kitamura et al. 2006; Mandyam et al. 2008). This escalation effect occurs within 7–14 days, and can be seen in both the total and first hour of drug intake (Ahmed et al. 1998). Animal models of escalation are important for understanding the neurobiological mechanisms underlying the transition to excessive drug intake.
While AMPH is a medication prescribed widely for the treatment of attention deficit hyperactivity disorder (ADHD; Biederman et al. 1999; Robbins 2002; Wilens et al. 2001), little is known about its effect on impulsive choice with escalated use. Previous findings on the effects of experimenter-administered AMPH on impulsive choice are inconsistent. While some studies reported that AMPH increases the value of delayed rewards, (i.e., decreased impulsivity; Wade et al. 2000), other studies reported that AMPH decreases the value of delayed rewards (Evenden and Ryan 1996). These discrepant findings may reflect baseline (no drug) differences in performance, as well as differences in environmental history (Perry et al. 2008).
The main purpose of the current study was to determine if the effects of extended access to AMPH self-administration alters impulsive choice in a delay discounting task in rats. A secondary aim of this study was to determine if previous work showing escalation of cocaine and methamphetamine self-administration generalizes to AMPH. Rats were trained initially on an adjusting delay task in which they were required to choose between a small, immediate reward (1 sucrose pellet), and a larger, delayed reward (3 sucrose pellets). After stable choice responding was obtained, rats were also trained to self-administer AMPH (either 0.03 or 0.1 mg/kg/infusion) during 1-hr sessions that occurred following each delay discounting session. Rats from each dose group were then exposed to daily delay discounting sessions, followed each day by continued access the 1-hr AMPH self-administration session or to an extended 6-hr AMPH self-administration session. Thus, the effects of dose (0.03 vs. 0.1 mg/kg/infusion) and access duration (1 vs. 6 hr) on impulsive choice and AMPH intake were determined.
Materials and Methods
Subjects
Twenty four male Sprague-Dawley rats (250–275 g) were obtained from Harlan (Indianapolis, IN). They were acclimated to a colony room and handled 3–5 days prior to the experiment. Rats were single housed in a colony room held at a constant temperature. Light and dark phases were on a 12:12 hr cycle, and all experimentation occurred in the light phase. Rats were on food restriction (85% of free feed body weight), and had unlimited access to water in their home cage. Rats were cared for in accordance with the 1996 edition of the Guide for the Care and Use of Laboratory Animals (NIH), and procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
Eighteen experimental chambers (ENV-001; MED Associates, St. Albans, VT) located inside sound-attenuating chambers were used. The front and back walls of the experimental chambers were made of aluminum, while the side walls were made of Plexiglas. There was a recessed food tray (5×4.2 cm) located in the bottom-center of the front wall. A response lever was located on each side of the recessed food tray on the front wall. A 28-V white cue light was located 6 cm above each response lever. A white houselight was mounted in the center of the back wall of the chamber. All responses and scheduled consequences were recorded and controlled by a computer interface. A computer controlled the experimental session using Med-IV software.
Procedure
Delay Discounting Training Phase
Rats were trained initially for 15 days on an adjusting delay task used previously by Perry et al. (2008). Daily sessions began at 08:00 and ended following the completion of 60 trials or 2 hr, whichever occurred first. Each session included 15 blocks of 4 trials in which 2 trials were forced trials, and 2 trials were free choice trials. Each session began with the illumination of a houselight. Trial blocks began with 1 forced-left and 1 forced-right trial; the order of these two trials alternated randomly within- and between-sessions. Forced trials began with the extension of the active lever and the illumination of a white stimulus light above the lever. Following a lever press response, the lever was retracted immediately, followed by either 1 or 3 sucrose-based 45 mg pellets (F0021 dustless precision pellet, Bio-Serve, Frenchtown, NJ) delivered immediately or after a delay, respectively. The third and fourth trials in each block were free choice trials, which were signaled by the illumination of both stimulus lights above each lever. Following each 4-trial block, both levers were retracted. One response to one lever resulted in delivery of 1 sucrose pellet immediately, and one response to the other lever resulted in delivery of 3 pellets following an adjusting delay. The side of the lever associated with the immediate or delayed reinforcer alternated daily.
Following each trial, an adjusting intertrial interval (ITI) occurred such that each trial lasted 60 s. After 60 s elapsed, the next trial began. All lights were turned off and responses on the levers had no programmed consequences. The initial delay of the larger reinforcer was 0 s to allow rats to acquire choice to the delayed reinforcer as a function of their own behavior. Subsequent responses to the larger reinforcer resulted in an increased delay, depending on response patterns to the two choice alternatives on free choice trials. A response to the small immediate reinforcer resulted in a 1-s decrease in the delay to the larger delayed reinforcer (although a minimum delay of 0 s and a maximum delay of 45 s to the larger reinforcer was imposed). The delay to the larger reinforcer was adjusted according to responses on only the third and fourth trials in each block (i.e., the free choice trials). During the delay, the stimulus lights were turned off, although the houselight remained illuminated until the delivery of 3 pellets. The delay on the final free choice trial on each session was used as the initial delay on the next session. The main outcome measure, a mean adjusted delay (MAD), was calculated at the end of each session by averaging all adjusting delays on free choice trials. Delay discounting sessions lasted for either 60 trials or 2 hr, whichever occurred first. Rats remained on this procedure for 15 days prior to surgery to insure that all rats had stable MAD scores (varying by less than 5 s across 5 days). MAD scores were used as a quantitative measure of impulsive choice, with lower MADs indicating higher levels of impulsive choice.
Surgery
Following the initial delay discounting training phase, rats underwent surgery in which indwelling jugular catheters were implanted. One day prior to surgery and 3 days afterwards, rats were treated with the non-opioid analgesic carprofen (5 mg/kg, s.c.). While under anesthesia (100 mg/kg ketamine, 5 mg/kg diazepam, i.p.), a silastic indwelling catheter was inserted into the jugular vein and exited via a metal cannula stabilized in a headmount made of dental acrylic. A silastic leash attached an infusion pump to the headmount during the self-administration sessions. Rats were given one week to recover, and a mixture of gentamicin (0.2 ml), heparin (0.6 ml), and saline was used to flush catheters daily.
AMPH Self-Administration Training Phase
Following recovery from surgery, rats were given 3 days of delay discounting alone, followed by 15 days in which each delay discounting session was followed 45 min later by a 1-hr AMPH self-administration session. Rats were divided into two AMPH unit dose groups, 0.03 or 0.1 mg/kg/infusion, based on a median split of their MAD scores from the last 3 days of delay discounting prior to self-administration training. MAD scores were ordered according to delay length, and rats with the top half and the bottom half of the scores were considered low and high impulsive, respectively. Half of the low impulsive and half of the high impulsive rats were assigned to the ShA group; the other half of low and high impulsive rats were assigned to the LgA group within each unit dose. Daily delay discounting sessions began at 08:00 and rats were returned to the colony room for 45 min upon completion of this session. At 10:00, rats were again transported to the experimental room and the 1-hr self-administration session began.
Self-administration sessions consisted of 1-hr access to AMPH, either 0.03 or 0.1 mg/kg/infusion, on a fixed ratio-1 (FR-1) schedule. Sessions began with the presentation of both the active and inactive levers (counterbalanced for position among subjects). Responses to the active lever resulted in illumination of both cue lights and a 0.1 ml infusion of AMPH delivered across 5.9 s, followed by a 20-s time-out period in which both cue lights were illuminated. Responses to the inactive lever had no programmed consequences and responses to either lever during the time-out period also had no programmed consequences. Following the end of the time-out period, cue lights turned off and both active and inactive levers were again presented.
Extended Access Phase
Following initial 1-hr self-administration training sessions, rats were divided into ShA and LgA groups based on a median split of their MAD scores from the last 3 days of delay discounting during the AMPH training phase. Rats in the ShA group were given 21 daily sessions identical to those in training. Rats in the LgA group were treated similarly, except that the AMPH self-administration session length was increased to 6 hr.
Withdrawal Phase
Following the 21-day extended access period, rats were given daily delay discounting sessions alone for 7 days.
Statistical Analyses
Separate mixed-factor ANOVAs were performed on the delay discounting data from the extended access and withdrawal phases, with group as a between-subject factor and session as a within-subject factor. Paired-samples t-tests were conducted to determine significance between groups within each session. Separate mixed-factor ANOVAs and repeated-measures linear trend analyses were performed on the self-administration data from the extended access phase for each unit dose; data from each unit dose were analyzed separately because the two doses were assessed in separate experiments. A 3-way mixed-factor ANOVA was performed on the time course data, with group as a between-subject factor and time and session as within-subject factors. Subsequent paired-samples t-tests were performed on the time course data, comparing number of infusions in the ShA and LgA groups within 5-min bins.
Results
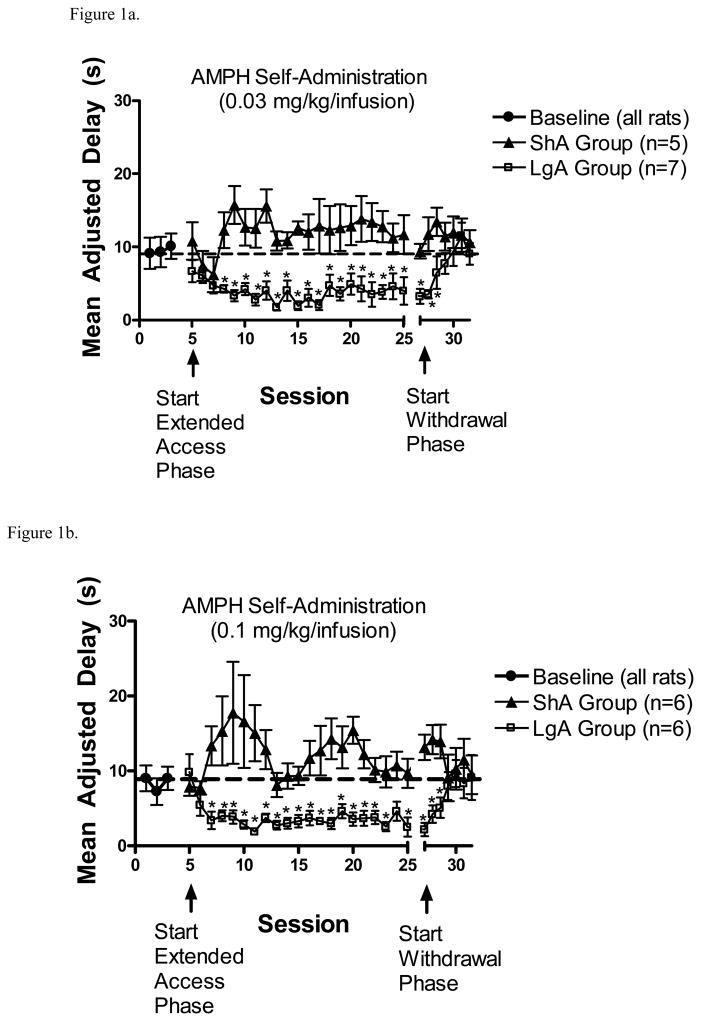
There was no effect of AMPH self-administration on MAD scores during the self-administration training phase (results not shown). However, the change in MAD scores for the ShA and LgA groups self-administering either 0.03 or 0.1 mg/kg/infusion AMPH across phases are plotted in Figures 1a and 1b. During the extended access phase, with the 0.03 mg/kg/infusion dose, a 2 X 21 ANOVA revealed a significant group x session interaction (F(20,240) = 1.9, p < .05). With the 0.1 mg/kg/infusion dose, there was also a significant group x session interaction (F(20,200) = 1.6, p <.05). Subsequent analyses revealed no significant change across sessions in ShA groups with either dose. However, paired samples t-tests comparing ShA and LgA groups within each session revealed that MAD scores decreased across sessions in the LgA groups compared to the ShA groups with both AMPH doses.
Figure 1.
(A). MAD scores (mean ± S.E.M.) from the ShA and LgA groups from baseline, extended access, and withdrawal phases following self-administration of 0.03 mg/kg/infusion AMPH. *p<0.05, compared to ShA group. (B). MAD scores (mean ± S.E.M.) from the ShA and LgA groups from baseline, extended access, and withdrawal phases following self-administration of 0.1 mg/kg/infusion AMPH. *p<0.05, compared to ShA group.
During the withdrawal phase (Figs 1a and 1b), with the 0.03 mg/kg/infusion dose, a 2 X 7 ANOVA revealed a significant group x session interaction (F(6,72) = 6.51, p < .001). With the 0.1 mg/kg/infusion dose, there was a significant main effect of session (F(6,60) = 3.51, p < .01), as well as a significant group x session interaction (F(6,60) = 2.66, p < .05). Subsequent evaluation of the interactions revealed that no significant change occurred in ShA groups with either dose. However, paired samples t-tests comparing ShA and LgA groups within each session revealed that MAD scores were lower in LgA groups compared to ShA groups only during the first three withdrawal sessions with both AMPH doses.
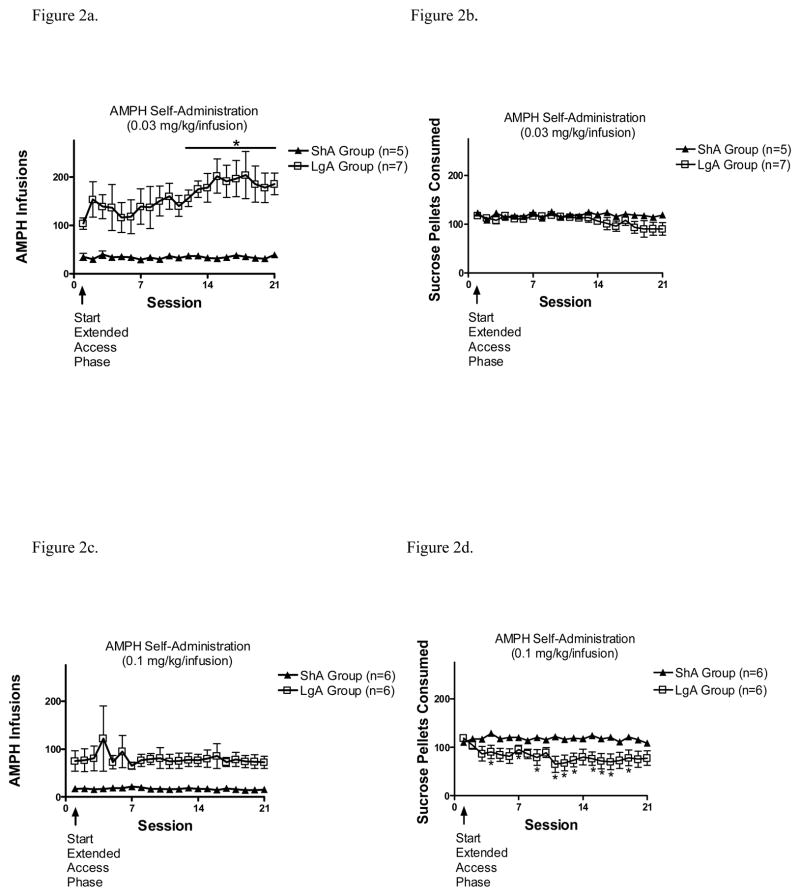
The total number of infusions from both the ShA and LgA groups at the 0.03 and 0.1 mg/kg/infusion doses are shown in Figures 2a and 2c, respectively. With the 0.03 and 0.1 mg/kg/infusion doses, 2 X 21 ANOVAs revealed no significant main effects or interactions. However, to assess more fully if escalation was obtained in any group, planned linear trend analyses were conducted on the number of infusions across the 21-day extended access phase. With the 0.03 mg/kg/infusion dose, there was a significant linear trend in the LgA group (t(126) = 3.46, p = .001), but not the ShA group. With the 0.1 mg/kg/infusion dose, there was no significant trend in either ShA or LgA groups. Further, within-subject t-tests comparing total number of infusions on each session relative to session 1 revealed that the LgA group self-administering 0.03 mg/kg/infusion AMPH showed a significant increase in number of infusions on sessions 12–21 compared to session 1.
Figure 2.
(A). Total number of AMPH infusions (mean ± S.E.M.) from the ShA and LgA groups during the 21-day extended access phase with 0.03 mg/kg/infusion dose. *p<0.05, compared to session 1. (B). Total number of sucrose pellets consumed (mean ± S.E.M.) during the delay discounting sessions from the ShA and LgA groups during the 21-day extended access phase with 0.03 mg/kg/infusion dose. (C). Total number of AMPH infusions (mean ± S.E.M.) from the ShA and LgA groups during the 21-day extended access phase with 0.1 mg/kg/infusion dose. (D). Total number of sucrose pellets consumed (mean ± S.E.M.) during the delay discounting sessions from the ShA and LgA groups during the 21-day extended access phase with 0.1 mg/kg/infusion dose. *p<0.05, compared to session 1.
Sucrose pellet consumption during the extended access phase for both the 0.03 and 0.1 mg/kg/infusion doses is also plotted in Figures 2b and 2d, respectively. With the 0.03 mg/kg/infusion dose, a 2 × 21 ANOVA revealed no significant main effects or interaction. With the 0.1 mg/kg/infusion dose, there was a significant main effect of session (F(20,200) = 1.9, p <.05), as well as a significant session x group interaction (F(20,200) = 2.45, p = .001). Subsequent evaluation of the interaction obtained with the 0.1 mg/kg/infusion dose revealed a decrease in number of pellets consumed in the LgA group (F(20,100) = 1.83, p < .05), but not in the ShA group. Within-subject t-tests comparing ShA and LgA groups within each session also revealed that the LgA group tended to consume fewer pellets than ShA after the first 2 sessions, although this difference was not significant across all sessions.
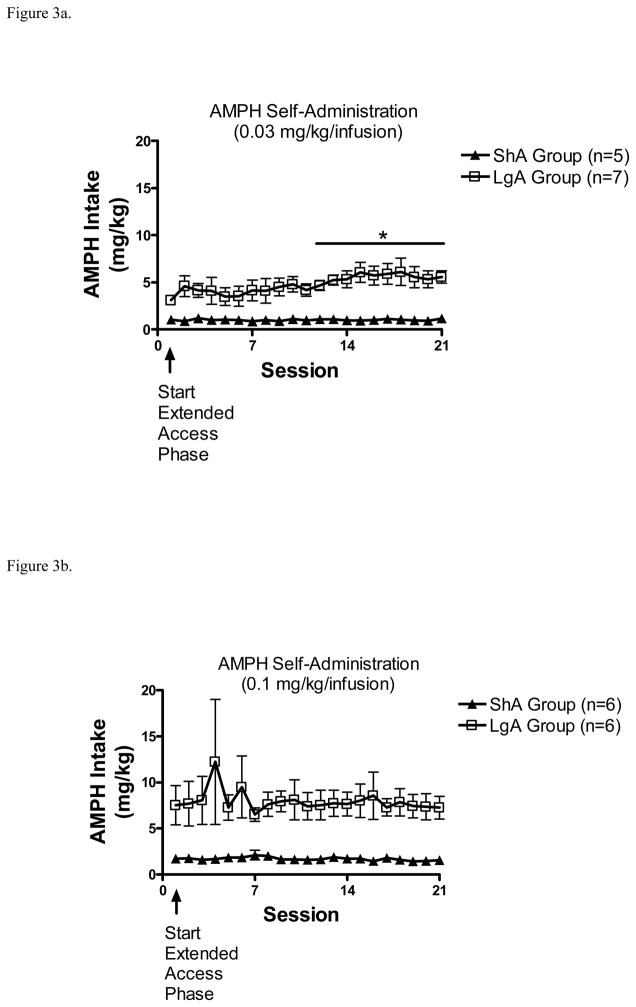
Total AMPH intake was calculated and plotted separately for the 0.03 and 0.1 mg/kg/infusion doses (Figures 3a and 3b). Separate 2 × 21 ANOVAs revealed no significant main effects or interactions with either AMPH dose. Planned linear trend analyses were conducted with each dose to determine if escalation was obtained with AMPH intake. With the 0.03 mg/kg/infusion dose, there was a significant linear trend in the LgA group (t(126) = 3.46, p = .001), but not in the ShA group. Subsequent paired samples t-tests were conducted comparing each session relative to session 1. Amount of AMPH intake on each session relative to session 1 revealed that the LgA group self-administering 0.03 mg/kg/infusion AMPH showed a significant increase in intake on sessions 12–21 compared to session 1. With the 0.1 mg/kg/infusion dose, there was no significant trend in either ShA or LgA groups.
Figure 3.
(A). Total AMPH intake (mean ± S.E.M.) from the ShA and LgA groups during the 21-day extended access phase with 0.03 mg/kg/infusion dose. *p<0.05, compared to session 1. (B). Total AMPH intake (mean ± S.E.M.) from the ShA and LgA groups during the 21-day extended access phase with 0.1 mg/kg/infusion dose.
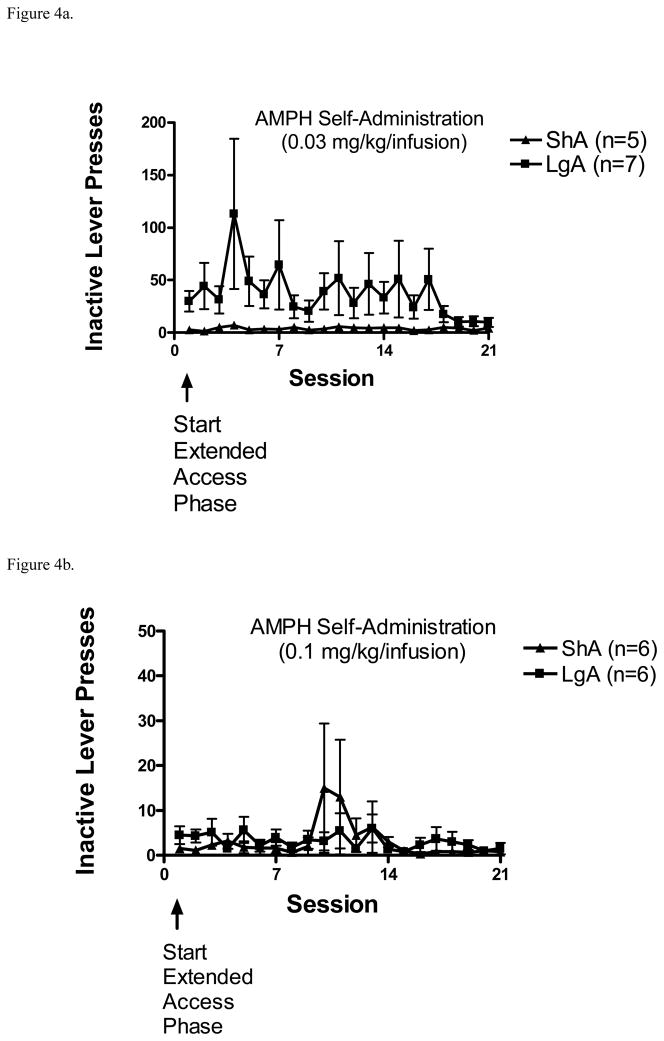
Inactive lever presses during the AMPH self-administration sessions for both the 0.03 and 0.1 mg/kg/infusion doses are shown in Figures 4a and 4b. Separate 2 × 21 ANOVAs revealed no significant main effects or interactions with either AMPH dose tested.
Figure 4.
(A). Total number of inactive lever presses (mean ± S.E.M.) from the ShA and LgA groups during the 21-day extended access phase with 0.03 mg/kg/infusion dose of AMPH. (B). Total number of inactive lever presses (mean ± S.E.M.) from the ShA and LgA groups during the 21-day extended access phase with 0.1 mg/kg/infusion dose of AMPH.
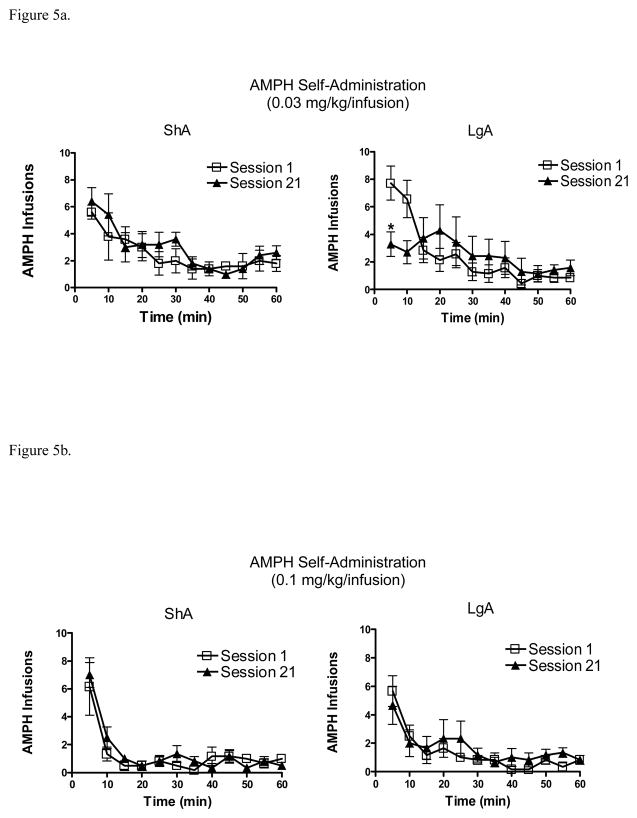
The time-dependent changes in AMPH infusions across the 60 min of ShA and the first 60-min of sessions 1 and 21 are shown for the 0.03 and 0.1 mg/kg/infusion doses in Figure 5. With the 0.03 mg/kg/infusion dose (Fig 5a), a 2 × 2 × 12 ANOVA revealed a significant main effect of time (F(11,132) = 16.41, p< .0001), a significant session x time interaction (F(11,132) = 1.94, p< .05), and a significant group x session x time interaction (F(11,132) = 3.73, p< .0001). Paired samples t-tests revealed that LgA rats self-administered significantly less AMPH on session 21 than session 1 only during the first 5-min bin (t(6) = 3.84, p < .01). With the 0.1 mg/kg/infusion dose (Fig 5b), ANOVA revealed only a significant main effect of time (F(11,110) = 25.4, p < .001).
Figure 5.
(A). Number of AMPH infusions (mean ± S.E.M.) on sessions 1 and 21 of extended access to 0.03 mg/kg/infusion dose across 5-min bins (left panel: ShA group; right panel: LgA group), * p<.01, compared to session 1. (B). Number of AMPH infusions (mean ± S.E.M.) on sessions 1 and 21 of extended access to 0.1 mg/kg/infusion dose across 5-min bins (left panel: ShA group; right panel: LgA group).
Data on number of trials completed, latency to respond on forced trials, and total nonreinforced responses on baseline, session 1, and session 21 of delay discounting in the extended access phase are shown in Table 1. With the 0.03 mg/kg/infusion dose, a 2 × 3 ANOVA revealed a significant main effect of session (F(2,24) = 4.25, p < .05) and a significant group x session interaction (F(2,24) = 4.25, p < .05) for number of trials completed. Subsequent evaluation of the interaction revealed a significant decrease in number of trials completed for the LgA group (F(2,12) = 4.25, p < .05) but no significant decrease in number of trials completed for the ShA group. There was no significant main effect of session or group x session interaction for latency to respond, likely due to the high variability among rats. There was, however, a significant main effect of session for total nonreinforced responses (F(2,24) = 10.74, p < .001) and a significant group x session interaction (F(2,24) = 6.24, p < .01). Further evaluation of this interaction indicated a significant decrease across sessions in total nonreinforced responses in the LgA group (F(2,12) = 10.53, p < .01) but not in the ShA group. With the 0.1 mg/kg/infusion dose, there was a only a significant main effect of session for latency to respond (F(2,20) = 5.4, p < .05), with LgA groups showing a longer latency than ShA groups with both AMPH doses; there was no significant main effects or interaction on number of trials completed or nonreinforced responses.
Table 1.
Number of Trials Completed, Latency to Respond on Forced Trials, and Total Nonreinforced Responses in Delay Discounting.
| Group | Trials Completed | Latency to Respond on Forced Trials (sec) | Total Non-Reinforced Responses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Session 1 | Session 21 | Baseline | Session 1 | Session 21 | Baseline | Session 1 | Session 21 | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| 0.03 ShA | 60±0 | 60±0 | 60±0 | 2.1±0.6 | 2.7±0.5 | 4.4±1.6 | 84.9±26.4 | 76.2±16.4 | 69.6±31.2 |
| 0.03 LgA | 60±0 | 60±0 | 49.3±5.1 | 1.8±0.2 | 7.2±3.4 | 146±92.4 | 96.0±26.7 | 96.2±26.7 | 15.8±4.7 |
| 0.1 ShA | 60±0 | 60±0 | 60±0 | 8.9±6.4 | 2.8±0.8 | 80.1±74.3 | 51.7±11.4 | 41.3±13.5 | 52.1±9.5 |
| 0.1 LgA | 60±0 | 60±0 | 45.1±8.7 | 9.4±4.4 | 4.1±2.0 | 206±95.4 | 27.1±8.8 | 37.1±8.9 | 14.8±7.5 |
Discussion
Extended access to AMPH self-administration decreased MAD scores compared to short access, indicating that impulsive choice increased at both the 0.03 and 0.1 mg/kg/infusion doses. This finding contrasts with previous research showing that repeated exposure to experimenter-administered AMPH (via i.p. injection) does not increase impulsive choice on a delay discounting task (Stanis et al. 2008). One possible explanation for the inconsistent findings may be that rats in the previous study did not have control over AMPH administration. Using a yoked control paradigm, in which a self-administering rat is yoked to a second rat that receives the drug infusion non-contingently, it has been shown that control over drug exposure may influence the neurobehavioral effects obtained (Jacobs et al. 2003). Previous studies have shown that animals that passively receive drug (yoked animals) have a lack of expectancy of the drug effects, as well as unaltered brain activity detected by electroencephalogram (Gresing and Szeto 1993). Smith and Dworkin (1986) also found that contingent administration of morphine produced more extensive and larger neurobiological alterations than non-contingent administration. According to Dworkin, Porrino, and Smith (1992), contingent relationships are important in defining aspects of reinforcement mechanisms. Thus, the disparity between the current findings and the findings of Stanis et al. (2008) may be due to differences between contingent and non-contingent AMPH administration.
The present results indicate that repeated extended access to AMPH increases impulsive choice, regardless of whether intake becomes excessive. There a number of factors that may have contributed to the increased impulsivity in LgA groups relative to ShA groups, including greater overall amphetamine intake per session, greater amount of time self-administering amphetamine, and/or a shorter interval between the self-administration and delay discounting session. Further work will be needed to determine which of these factors, if any, contributed the differences in MAD scores between ShA and LgA groups. In any case, the fact that LgA rats self-administering 0.1 mg/kg/infusion AMPH became more impulsive, but did not escalate intake, suggests that excessive intake does not depend directly on a loss of inhibitory control. However, since delayed discounting represents only one facet of behavioral inhibition (deWit 2009), we cannot rule out the possibility that other inhibitory tasks may be altered differentially in escalating and non-escalating groups.
Since previous studies have found that experimenter-administered AMPH increases sugar consumption (Evans and Vaccarino 1987; Sills and Vaccarino 1996), it is possible that the decrease in MAD scores with both AMPH unit doses in the current study may have reflected a devaluation of the sucrose pellet reward used in the delay discounting task. Even though extended access to 0.1 mg/kg/infusion AMPH decreased sucrose pellet consumption in the current study, reward devaluation is not a likely explanation for the decrease in MAD scores because no reliable change in number of pellets consumed or latency to respond for pellets was observed with 0.03 mg/kg/infusion AMPH. Nonetheless, since sucrose and AMPH were available in the same context each day, we cannot rule out the possibility that transfer effects between the food-reinforced delay discounting and AMPH self-administration tasks may have occurred, thus causing reward competition or interference in performance.
Consistent with previous human and non-human animal studies with nicotine (Bickel et al. 1999; Dallery et al. 2005), the effect of LgA AMPH self-administration on impulsivity was reversible following drug abstinence. Since the increase in impulsive choice was reversible, this finding appears to rule out long-term neural dysfunction or damage as an explanation of the behavior change in the delay discounting task. This finding is also consistent with clinical evidence showing reversal of neural changes seen in abstinent stimulant abusers (Volkow et al. 2001; Wang et al. 2004).
In the clinical literature, there are inconsistent reports suggesting that the treatment of ADHD with stimulant medications may or may not increase the risk for subsequent substance abuse. Some studies have reported increased risk (Lambert and Hartsough 1998; Vitiello 2001) and others have reported decreased risk (Wilens et al. 2003). In either case, the current results suggest that the clinical use of AMPH, which can lead to excessive use (Berman et al. 2008), may not have any long-term detrimental effects on impulsivity.
There was a dose-dependent escalation effect in LgA groups in the current report, with intake increasing across sessions using 0.03 mg/kg/infusion AMPH, but not using 0.1 mg/kg/infusion AMPH. This dose-dependent AMPH effect is generally consistent with a previous report showing that escalation of methamphetamine intake is more robust with lower unit doses than with higher unit doses (Kitamura et al. 2006). The difference in drug intake from the first to last session of extended access to 0.03 mg/kg/infusion is thought to be due to an alteration in reward threshold (Ahmed et al. 2005). At the higher dose (0.1 mg/kg/infusion) in the present study, it is likely that fewer self-infusions were required to reach the reward threshold, thus mitigating against an escalating pattern of intake. However, it is important to note that a ceiling effect may have prevented the LgA group from escalating intake at the higher dose. Examination of AMPH intake in LgA rats showed that intake was higher with 0.1 mg/kg/infusion AMPH than with 0.03 mg/kg/infusion AMPH on session 1 of extended access. Thus, it is possible that the LgA group with 0.1 mg/kg/infusion AMPH did not escalate because they had already approached maximal intake on session 1. It is also possible that aversive and/or stereotypic effects of 0.1 mg/kg/infusion AMPH may have prevented the LgA group from escalating intake.
Some caution is needed regarding the failure to observe escalation using 0.1 mg/kg/infusion AMPH, as several procedural details were unique to the current study. For example, rats in the current study were tested in the light cycle, and while at least one study found escalation during the light cycle (Allen et al. 2007), most escalation studies have been conducted during the dark cycle (Ahmed et al. 2003; 2004; Kitamura et al. 2006; Mandyam et al. 2007; 2008). In addition, rats in the current study were on food restriction during the extended access phase, which may have affected rates of AMPH intake. Since food restriction typically increases stimulant self-administration (Cabeza de Vaca and Carr 1998; Carroll and Meisch 1984), it is possible that food restriction elevated self-administration rates from the initial extended access session, thus blunting the escalation effect across sessions.
A surprising finding of the current study was that the initial increase in AMPH intake during the first portion of the session (i.e., “loading effect”) dissipated by session 21 in the LgA group self-administering 0.03 mg/kg/infusion AMPH. Since this was the only group that displayed an escalation of intake, it is possible that the loss of drug loading early in the session may have decreased any response suppressant effect of AMPH, thus allowing intake to increase across the total 6-hr session. However, this finding is inconsistent with a previous study with methamphetamine, in which LgA rats showed a gradual increase in the amount of drug they self-administered during the initial portion of the session, as well as an overall escalation of intake, as a function of experience with extended access (Kitamura et al. 2006). It is not clear why the current results contrast with Kitamura et al. (2006), but several procedural differences exist between these studies. Specifically, there may be differences in the temporal pattern of intake between AMPH and methamphetamine with extended access. These studies also were conducted in different portions of the diurnal cycle (light versus dark phase), and the current study involved an additional delay discounting task.
In conclusion, these results demonstrate that extended access to AMPH produces a reversible increase in impulsive choice. In addition, while escalation has been found with several illicit drugs, including methamphetamine (Kitamura et al. 2006; Mandyam et al. 2008), cocaine (Ahmed et al. 1998; 1999; 2004), and heroin (Ahmed et al. 2000), the current findings extend the generality of this finding to the widely used clinical medication, AMPH. A predictive preclinical model of escalation may be important in understanding the neurobiological causes of excessive drug use and impulsivity, as well as the preclinical screening of compounds for drug dependence therapy. Although the preclinical escalation model appears to have generality, the neural mechanisms that underlie the transient increase in impulsive choice following extended access to AMPH needs to be examined.
Acknowledgments
Supported by NIH grants P50 DA05312 and T32 DA007304.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology. 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Allen RM, Dykstra LA, Carelli RM. Continuous exposure to the competitive N-methyl-D aspartate receptor antagonist, LY235959, facilitates escalation of cocaine consumption in Sprague–Dawley rats. Psychopharmacology. 2007;191:341–351. doi: 10.1007/s00213-006-0661-3. [DOI] [PubMed] [Google Scholar]
- Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2000;14:123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. In: Thompson T, Dews PB, Barrett JE, editors. Advances in behavioral pharmacology. Academic; New York: 1984. pp. 47–88. [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-Amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Porrino LJ, Smith JE. Importance of behavioral controls in the analysis of ongoing events. Neurological Approaches to Brain-Behavior Interactions, National Institute on Drug Abuse Research Monogr. 1992;124:173–188. [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Effects of d- and 1-amphetamine on food intake: evidence for a dopaminergic substrate. Pharmal Biochem Behav. 1987;27:649–652. doi: 10.1016/0091-3057(87)90189-4. [DOI] [PubMed] [Google Scholar]
- Grasing K, Szeto H. EEG changes with different levels of morphine self-administration. Neuropharmacology. 1993;32:543–553. doi: 10.1016/0028-3908(93)90050-d. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect? J Exp Anal Behav. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer ANM. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learning Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Logue AW, Peña-Correal TE, Rodriguez ML, Kabela E. Self control in adult humans: variation in positive reinforcer amount and delay. J Exp Anal Behav. 1986;46:159–173. doi: 10.1901/jeab.1986.46-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam C, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self administration and voluntary exercise have opposing effects on medial prefrontal cortex gliosis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam C, Wee S, Crawford E, Eisch A, Richardson H, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Logue AW. Choice in a “self-control” paradigm: Effects of a fading procedure. J Exp Anal Behav. 1978;30:11–17. doi: 10.1901/jeab.1978.30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Robbins TW. ADHD and addiction. Nature Medicine. 2002;8:24–25. doi: 10.1038/nm0102-24. [DOI] [PubMed] [Google Scholar]
- Sills TL, Vaccarino FJ. Individual differences in sugar consumption following systemic or intraaccumbens administration of low doses of amphetamine in nondeprived rats. Pharmacol Biochem Behav. 1996;54:665–670. doi: 10.1016/0091-3057(96)00024-x. [DOI] [PubMed] [Google Scholar]
- Smith JE, Dworkin SI. Neurobiological substrates of drug self-administration. In: Brown RM, Clouet D, editors. Opiate Receptor Subtypes and Brain Function. Vol. 71. National Institute on Drug Abuse Research Monogr; 1986. pp. 127–145. [PubMed] [Google Scholar]
- Stanis JJ, Avila HM, White MD, Gulley JM. Dissociation between long-lasting behavioral sensitization to d-amphetamine and impulsive choice in rats performing a delay-discounting task. Psychopharmacology. 2008;199:539–548. doi: 10.1007/s00213-008-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B. Long-term effects of stimulant medications on the brain: possible relevance to the treatment of attention deficit hyperactivity disorder. J Child Adolescent Psychopharmacology. 2001;11:25–34. doi: 10.1089/104454601750143384. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, deWit H, Richards JB. Effects of dopaminergic drugs on delayed rewards as a measure of impulsive behavior in rats. Psychopharmacology. 2000;15:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality Individ Differences. 2001;30:669–689. [Google Scholar]
- Wilens TE, Spencer TJ, Biederman J. A review of the pharmacotherapy of adults with Attention-Deficit/Hyperactivity Disorder. J Attention Disorders. 2001;5:189–202. doi: 10.1177/108705470100500401. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]