Abstract
Purpose of the review
The Notch pathway is an evolutionary conserved cell–cell communication mechanism that plays a key role in kidney development. Here we will discuss a number of recently published papers describing the role of Notch signaling in kidney development, homeostasis, injury and repair.
Recent findings
Recent gene expression studies identified regulation of the Notch pathway in patients with chronic kidney disease (CKD). Mechanistic experiments performed using transgenic and knock-out mouse models indicate that Notch plays an important functional role in the development of proteinuria and renal fibrosis. Inhibition of the Notch pathway ameliorated diabetic kidney disease, nephrotic syndrome and fibrosis in different rodent models.
Summary
An increasing amount of evidence suggests that Notch plays a role in CKD development. Understanding the role of Notch signaling in the kidney can aid in the development of new therapeutics for CKD.
Keywords: chronic kidney disease, diabetic nephropathy, end-stage kidney disease, epithelial cells, fibrosis, glomerulus, Notch signaling, podocytes
Introduction
In the US, 20 million people suffer from chronic kidney disease (CKD) [1], which often manifests itself as fibrosis of the kidney. Renal fibrosis is characterized by the dysfunction of epithelial cells, loss of capillaries, increased collagen deposition, massive expansion of the activated myofibroblast population and an influx of inflammatory cells [2]. On the molecular level, several pathways have been proposed to contribute to CKD development. In order to better understand the pathogenesis of CKD, we and others performed genome-wide transcriptome analysis studies on human kidney tissue materials obtained from patients with CKD [3–6]. Several of these studies detected increased activity of the Notch pathway in CKD samples.
Notch signaling
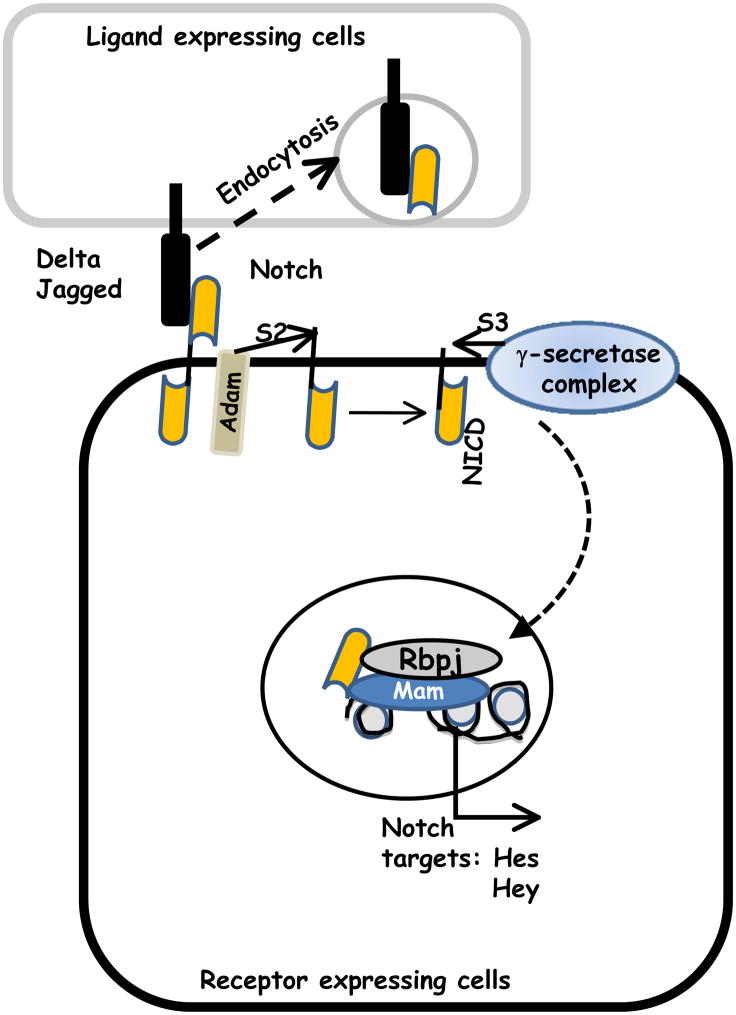
The Notch pathway was first described by John S. Dexter Thomas in Drosophila in the early 20th century, when a mutation of the Notch gene resulted in notches at the margins of the wing blades of fruit flies. The molecular analysis and sequencing of the pathway was undertaken in 1980 [7]. Notch is present in all metazoans, and mammals possess four different Notch receptors, referred to as Notch1–4 [8]. The Notch receptor is a single-pass transmembrane receptor protein [8,9,10]. Notch is initially produced in the endoplasmic reticulum as pre-Notch. Maturation of the Notch receptor requires the glycosyltransferase Fringe. Pre-Notch is then cleaved by a furin-like convertase into an intracellular and an extracellular domain and then transported to the plasma membrane. The extracellular domain is involved in ligand binding and the intracellular domain works in signal transduction. Notch ligands are also single-pass transmembrane proteins and are members of the DSL (Delta/Serrate/LAG-2) family of proteins. In mammals, the corresponding names for the ligands are Delta-like and Jagged. Interaction of ligands with the Notch receptors triggers a series of proteolytic cleavages, by a metalloprotease of the ADAM family (TACE, tumor necrosis factor-α-converting enzyme) and finally by the gamma secretase complex (Fig. 1). This final cleavage results in the release of Notch intracellular domain (NICD). NICD then translocates to the nucleus and binds to other transcriptional regulators (mainly of the CBF1/RBP-JK, SU(H), Lag1 family) to trigger the transcription of the target genes, mainly Hes and Hey family genes. This core signal transduction pathway is used in most Notchdependent processes and is known as the ‘canonical’ pathway. However, depending on the cellular context, the amplitude and duration of Notch activity can be further regulated at various points.
Figure 1. Notch receptor signaling.
A schematic view of two cells is shown, one expressing the Notch receptor, and an adjacent cell expressing the Notch ligand Jagged. Upon ligand binding, Notch is cleaved to release the extracellular domain, which is then endocytosed along with the ligand. Further cleavage of the receptor by the γ-secretase complex releases the Notch intracellular domain (NICD). NICD then translocates to the nucleus and regulates gene transcription (see text for details).
The general problem that Notch solves is the resolution of a binary decision in cell fate, one in which a few dispersed cells in a field of undifferentiated cells must acquire a new fate, and further, must communicate to neighboring cells that they should follow a different fate [9,11]. The function of the Notch-signaling pathway has been subdivided into three categories. The first is called lateral inhibition, by which a population of equivalent cells with very small differences are resolved into different fates. The second category is lineage decisions whereby Notch signaling between two daughter cells is dependent on asymmetrical inheritance of Notch regulators; for example B-cell versus T-cell fate decision in the immune system of mammals. Lastly, Notch signaling that occurs between two populations of cells can establish boundaries or segregate the two groups.
Notch plays a key role in kidney development
The mature human nephron consists of several different segments. During nephrogenesis, the formation of the S-shaped body is the first step in the nephron segmentation process [12_]. The lower part of the S-shaped body gives rise to future podocytes, the middle part gives rise to proximal tubular epithelial cells, the upper part becomes the thick ascending limb of the loop of Henle and the distal convoluted tubule.
Prior studies have established the expression pattern and key role of Notch signaling in nephron segmentation [13,14]. Notch1 was found to be expressed in the prospective mesangium and by the prospective distal tubule and collecting ducts. Notch2 was present in the primitive proximal tubule with very faint co-localization with podocyte progenitors. Notch ligand, Jagged1 showed faint expression in primary vesicles, comma-shaped bodies, and S-shaped bodies and later in developing distal tubules and the prospective loop of Henle. Jagged1 was also detected in proximal tubules; however, no expression was observed in podocytes. The Dll1 signal was strong in both the prospective proximal tubules and loop of Henle. Hes1 mRNA was expressed in both mesenchymal and epithelial structures early during nephron development but appeared to decrease with glomerular maturation, whereas Hes5 was specifically found in the ascending limb of Loop of Henle. Hey1 was expressed in pretubular aggregates and renal vesicles, suggesting its possible role in early nephron development. HeyL was expressed in the glomerular cleft, renal vesicles and endothelial cells around the developing glomerulus and also in segments of the prospective proximal tubules. These studies indicate a very carefully choreographed expression pattern for the different Notch ligands, receptors and downstream targets.
Functional studies performed by Cheng et al. [15] demonstrated the key role of Notch signaling in nephron development. In the presence of a gamma-secretase inhibitor (which blocks Notch signaling) fewer renal epithelial structures developed from cultured mouse metanephroi [15]. Similar results were obtained from mice with genetic deletion of genes that are part of the Notch processing–gamma secretase complex (Presenilin1, 2). Presenilin deficiency in the kidney led to severe nephrogenesis defects and virtually no comma or S-shaped bodies, or mature glomeruli [16]. Later studies performed by Kopan’s group indicated that whereas both Notch1 and Notch2 are expressed in developing kidneys Notch2 plays a non-redundant role in the process [17,18]. Deletion of Notch1 did not alter kidney development, whereas genetic ablation of Notch2 caused the loss of proximal kidney epithelium (podocyte, proximal tubule cells).
These results indicate the critical role of the Notch pathway in proximal tubule and podocyte development.
Role of notch in the glomerulus
Once kidney development is complete, very little Notch activity can be observed in mature rodent and human kidneys [6,19,20]. Immunostaining studies using cleavage site-specific antibodies or mice with genetic indicators for Notch activity showed the complete absence of Notch signaling in the glomerulus (Fig. 2). Interestingly, if Notch1 expression is kept elevated (using transgenic mouse models) in developing podocytes (in the capillary loop stage) animals rapidly develop albuminuria and glomerulosclerosis and die by 3 weeks of age [21__]. Conversely, genetic deletion of Notch transcriptional co-factor (Rbpj) in podocytes at and after the capillary loop stage did not induce any phenotypic changes in rodent kidneys [21__]. These studies highlighted the importance of Notch silencing in mature podocytes in maintaining the glomerular filtration barrier.
Figure 2. Expression of cleaved Notch1 in control and fibrotic kidneys.
Representative images from immunostaining experiments performed with cleaved Notch1 antibody of control ‘healthy’ and from fibrotic kidneys (obtained from a diabetic kidney disease sample). Note the absence of cleaved Notch1 expression in control kidneys and the increased expression of cleaved Notch1 in glomerular and tubular epithelial cells in CKD kidneys.
Recently performed antibody-based expression analysis using samples from 10 different renal disease groups found increased NOTCH1 and NOTCH2 expression in different renal disorders [22_]. Statistical analysis performed across all disease groups indicated that podocyte expression of NOTCH1, 2 and JAGGED1 correlates with the degree of albuminuria and glomerulosclerosis (Fig. 2). Transgenic and knock-out technology was used to interrogate the functional role of Notch in kidney disease. Niranjan et al. [6] generated mice with podocyte-specific inducible expression of the intracellular domain of Notch1 (ICN1) in adult animals (4 weeks old). These animals rapidly developed proteinuria and glomerulosclerosis and died a couple of weeks after Notch1 expression was turned on. On the mechanistic level, ICN1 induced p53 expression and subsequent podocyte apoptosis was thought to be responsible for glomerulosclerosis. Conversely, podocyte-specific genetic deletion of the Notch transcriptional partner (Rbpj) protected mice from diabetes-induced podocyte loss and albuminuria. Blocking the Notch pathway with a gamma-secretase inhibitor also ameliorated the puromycin aminonucleotide-induced nephrotic syndrome in rats [6]. In addition, the authors identified an interaction between Notch and Tgf-β 1 signaling. Tgf-β 1 regulates the Notch ligand Jagged1 expression leading to increased active Notch1 levels [23]. Notch expression in vivo or in vitro increased Tgf-β 1 levels indicating a positive feedback loop. Recently, Lin et al. [24__] using a pharmacological inhibitor of Notch signaling (DAPT, a gamma secretase inhibitor) reported the role of the Notch pathway in a rat model of diabetic nephropathy. They found increased Notch activity upon culturing podocytes in high glucose-containing medium. In cultured podocytes, Vegf treatment increased cleaved Notch1 levels, decreased nephrin expression and induced podocyte apoptosis. These studies highlighted the close connection between Notch and Vegf pathways. In summary, these recent studies describe increased podocyte Notch activity in proteinuric kidney diseases and suggest that podocyte Notch1 contributes to the pathogenesis of proteinuria and diabetic kidney disease. Notch, Vegf and Tgf-β pathways appear to interact to mediate podocyte injury.
Whereas it appears that expression of Notch1 in mature/differentiated podocytes plays a role in CKD development [6,21__], a recent study suggests that expression of Notch in renal progenitors might play a role in renal regeneration. Lasagni et al. [25__] suggest that the severity of glomerular damage depends upon a Notch1–3 regulated balance between podocyte death and regeneration provided by renal progenitors. The authors describe that Notch1 expression in mature podocytes causes podocyte apoptosis; however, they found that in-vitro differentiation of human renal progenitor cells was enhanced by Notch1–3 expression. Moreover, Notch inhibition (with the use of the gamma secretase inhibitor DAPT) in a mouse model of transient proteinuria (induced by doxorubicin) resulted in an early decrease of albuminuria and protection of Notch-induced podocyte apoptosis; however, it did reduce recovery at the later stages. Whereas these experiments indicate that Notch in renal progenitor cells can contribute to repair, it remains to be seen whether progenitor cell-mediated repair actually occurs in patients with glomerulosclerosis, as it is relatively rare to see a full remission in patients with glomerulosclerosis.
Role of Notch in tubular epithelial cells, acute kidney injury and fibrosis
It appears that few renal tubular epithelial cells remain positive for active Notch1 even after kidney development is complete. The role of these Notch-positive cells in renal homeostasis has not been studied. It seems, however, that short-term gamma secretase-based inhibition of Notch signaling does not induce obvious phenotypic changes in the kidney.
As we discussed earlier, Notch2 plays a key role in proximal tubule development. On the contrary, genetic deletion of Notch signaling from the ureteric bud (i.e. the prospective collecting duct) causes nephrogenic diabetes insipidus in mice [26]. As often seen, developmental pathways are re-activated in organs during acute injury and play a role in regeneration. While studying acute kidney injury using the ischemia reperfusion model, Kobayashi et al. [27] noted the increased renal expression of Notch2, its ligand Delta1 and Hes1. Co-culturing of Delta1-expressing stromal cells with renal tubular cells induced the proliferation of tubular epithelial cells, therefore, the authors hypothesized that Notch2 expression might play a role in tubular epithelial cell regeneration during acute kidney injury.
Gene expression studies performed on control and diseased human kidneys identified the increase in Notch and related genes in the tubulointerstitial portions of diabetic kidneys (Fig. 2). Walsh and his co-workers [4] were one of the first to describe elevated levels of Jagged1 and Hes1 in microdissected tubulointerstitial samples of renal biopsies from diabetic nephropathy patients, when compared to control living donors. Using in-situ hybridization hybridization, they demonstrated the specific up-regulation and co-expression of Jagged1 and Hes1 in renal tubuli. Notch-pathway-based gene clustering was able to distinguish diabetic nephropathy samples from control living donor samples or minimal change disease samples. Recent studies by Murea et al. [22_] suggest that the activation of the Notch pathway is not specific for DKD, but can be observed in wide range of renal diseases. Tubulointerstitial expression of cleaved Notch1 correlated with the degree of tubulointerstitial fibrosis and glomerular filtration rate across a spectrum of renal diseases. Increased Jagged1 expression was also observed in kidneys of mice with tubulointerstitial fibrosis using ureteral obstruction or folic acid-induced renal fibrosis models [28]. Recently, Bielesz et al. [29__] performed pharmacological, genetic in-vivo and in-vitro experiments to study the role of Notch in renal tubules during acute kidney injury, regeneration and chronic tubulointerstitial fibrosis. Folic acid given to mice in high doses precipitates in the renal tubules and causes acute kidney injury evident as a rise in serum creatinine after the acute injury renal fibrosis develops [29__]. Gamma secretase inhibitors did not interfere with creatinine decline in folic acid-induced acute kidney injury model, indicating that Notch might be dispensable during renal regeneration in acute kidney injury. More interestingly, using the folic acid-induced fibrosis model, they demonstrate that Notch blockade significantly reduced tubulointerstitial fibrosis. Similarly, genetic deletion of Notch partner Rbpj (using the PEPCKcre/Rbpjflox conditional knock-out mice) was able to ameliorate folic acid-induced fibrosis, whereas conditional inducible expression of cleaved Notch1 in tubular epithelial cells rapidly induced the development of tubulointerstitial fibrosis in mice. Interestingly, on the molecular level, whereas Notch induced apoptosis of mature glomerular podocytes, it promoted proliferation of tubular epithelial cells and fibroblasts. These findings indicate that the Notch pathway plays a key role in tubulointerstitial fibrosis.
Potential clinical relevance
In addition to Notch’s important role in organ development it also plays a key role in the pathogenesis of many different human disease conditions; including T-cell acute lymphoma leukemia, breast cancer, melanoma and other solid tumors, and pharmaceutical companies have already developed an entire arsenal of inhibitors [10__,30]. Most of them bind and block the gamma secretase enzyme complex which plays a key role in cleaving the Notch receptor following ligand binding [31]. Interestingly, the same complex mediates the release of β-amyloid in the brain and gamma-secretase inhibitors (GSIs) are in clinical trials for the treatment of Alzheimer’s disease [32,33].
The main side effect of these inhibitors is their gastrointestinal toxicity [34]. The use of GSIs are associated with secretory metaplasia manifested as a marked increase in intestinal goblet cells and the arrest of intestinal crypt cell proliferation, thus limiting their potential therapeutic use. However, a recent study suggests that this toxicity can be circumvented by concomitant administration of glucocorticoids. Real et al. [35] reported that for the treatment of T-cell acute lymphoblastic leukemia this combined therapy not only improved antileukemic effects, but also reduced gut toxicity in vivo. Such an approach could potentially be translated for the use of renal disorders, as glucocorticoid treatment is currently the mainstream therapy for most glomerular disorders. Another important alternative could be the use of soluble selective blocking antibodies [36_]. Recent studies suggest that selective blocking of Notch1 inhibits tumor growth in preclinical models through two mechanisms: inhibition of cancer cell growth and deregulation of angiogenesis. Whereas inhibition of Notch1 and Notch2 causes severe intestinal toxicity, inhibition of either receptor alone reduces or avoids this effect, demonstrating a clear advantage over pan-Notch inhibitors. This could be another potential strategy to reduce Notch signaling. Further studies are needed to determine the role of Notch1, 2 in CKD development.
Conclusion
Notch plays a key role in kidney development. The Notch pathway is mostly silenced once kidney development is complete with only a few cells remaining positive for Notch in the adult kidney. Several recent studies showed the reactivation of this pathway in different renal disorders. Transgenic and knock-out studies indicate that activation of Notch in glomerular or tubular epithelial cells causes renal fibrosis development. Pharmacological studies also suggest that inhibition of this pathway could also be an important therapeutic strategy for CKD.
Acknowledgments
Dr Susztak is supported by National Institutes of Health (5R01DK076077-04) and funds from the Juvenile Diabetes Research Foundation.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
_ of special interest
__ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Collins AJ, Gilbertson DT, Snyder JJ, et al. Chronic kidney disease awareness, screening and prevention: rationale for the design of a public education program. Nephrology (Carlton) 15(Suppl 2):37–42. doi: 10.1111/j.1440-1797.2010.01312.x. [DOI] [PubMed] [Google Scholar]
- 2.Schnaper HW, Kopp JB. Renal fibrosis. Front Biosci. 2003;8:e68–e86. doi: 10.2741/925. [DOI] [PubMed] [Google Scholar]
- 3.Kretzler M, Cohen CD, Doran P, et al. Repuncturing the renal biopsy: strategies for molecular diagnosis in nephrology. J Am Soc Nephrol. 2002;13:1961–1972. doi: 10.1097/01.asn.0000020390.29418.70. [DOI] [PubMed] [Google Scholar]
- 4.Walsh DW, Roxburgh SA, McGettigan P, et al. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim Biophys Acta. 2008;1782:10–21. doi: 10.1016/j.bbadis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Si H, Banga RS, Kapitsinou P, et al. Human and murine kidneys show genderand species-specific gene expression differences in response to injury. PLoS ONE. 2009;4:e4802. doi: 10.1371/journal.pone.0004802. Epub 2009 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe PE, Greenspan RJ. Local function of the Notch gene for embryonic ectodermal pathway choice in Drosophila. Cell. 1986;46:773–783. doi: 10.1016/0092-8674(86)90353-3. [DOI] [PubMed] [Google Scholar]
- 8.Ilagan MX, Kopan R. SnapShot: notch signaling pathway. Cell. 2007;128:1246. doi: 10.1016/j.cell.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- 10__.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. This is an excellent and comprehensive review on the regulation of the Notch pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopan R, Turner DL. The Notch pathway: democracy and aristocracy in the selection of cell fate. Curr Opin Neurobiol. 1996;6:594–601. doi: 10.1016/s0959-4388(96)80090-0. [DOI] [PubMed] [Google Scholar]
- 12_.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 18:698–712. doi: 10.1016/j.devcel.2010.04.008. Outstanding recent review on renal development also describes the role of Notch in kidney development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–F952. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- 14.Piscione TD, Wu MY, Quaggin SE. Expression of Hairy/Enhancer of Split genes, Hes1 and Hes5, during murine nephron morphogenesis. Gene Expr Patterns. 2004;4:707–711. doi: 10.1016/j.modgep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Cheng HT, Miner JH, Lin M, et al. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development. 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Pereira FA, Beasley D, Zheng H. Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development. 2003;130:5019–5029. doi: 10.1242/dev.00682. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HT, Kopan R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 2005;68:1951–1952. doi: 10.1111/j.1523-1755.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng HT, Kim M, Valerius MT, et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vooijs M, Ong CT, Hadland B, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134:535–544. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niranjan T, Murea M, Susztak K. The pathogenic role of notch activation in podocytes. Nephron Exp Nephrol. 2009;111:e73–e79. doi: 10.1159/000209207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21__.Waters AM, Wu MY, Onay T, et al. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. Excellent study showing that silencing of Notch in podocytes is necessary for maintenance of the glomerular filtration barrier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22_.Murea M, Park JK, Sharma S, et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 2010;78:514–522. doi: 10.1038/ki.2010.172. This current study describes the activation of the Notch pathway in different renal diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24__.Lin CL, Wang FS, Hsu YC, et al. Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes. 59:1915–1925. doi: 10.2337/db09-0663. Interesting study, describes the effect of Notch inhibitors in rat diabetic nephropathy model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25__.Lasagni L, Ballerini L, Angelotti ML, et al. Notch activation regulates differentially renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. doi: 10.1002/stem.492. Excellent study indicating the role of Notch in progenitor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong HW, Jeon US, Koo BK, et al. Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J Clin Invest. 2009;119:3290–3300. doi: 10.1172/JCI38416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi T, Terada Y, Kuwana H, et al. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 2008;73:1240–1250. doi: 10.1038/ki.2008.74. [DOI] [PubMed] [Google Scholar]
- 28.Morrissey J, Guo G, Moridaira K, et al. Transforming growth factor-beta induces renal epithelial jagged-1 expression in fibrotic disease. J Am Soc Nephrol. 2002;13:1499–1508. doi: 10.1097/01.asn.0000017905.77985.4a. [DOI] [PubMed] [Google Scholar]
- 29__.Bielesz B, Sirin YHS, Niranjan T, et al. Epithelial notch signaling regulates interstitial fibrosis development in kidneys of mice and man. J Clin Invest. 2010 doi: 10.1172/JCI43025. [Epub 2010 Oct 18]. New results describing the role of Notch in fibrosis development using transgenic and knock-out animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callahan R, Egan SE. Notch signaling in mammary development and oncogenesis. J Mammary Gland Biol Neoplasia. 2004;9:145–163. doi: 10.1023/B:JOMG.0000037159.63644.81. [DOI] [PubMed] [Google Scholar]
- 31.Ray WJ, Yao M, Mumm J, et al. Cell surface presenilin-1 participates in the gamma secretase-like proteolysis of Notch. J Biol Chem. 1999;274:36801–36807. doi: 10.1074/jbc.274.51.36801. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe MS. Therapeutic strategies for Alzheimer’s disease. Nat Rev Drug Discov. 2002;1:859–866. doi: 10.1038/nrd938. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe MS, Esler WP, Das C. Continuing strategies for inhibiting Alzheimer’s gamma-secretase. J Mol Neurosci. 2002;19:83–87. doi: 10.1007/s12031-002-0015-5. [DOI] [PubMed] [Google Scholar]
- 34.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 35.Real PJ, Tosello V, Palomero T, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36_.Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 464:1052–1057. doi: 10.1038/nature08878. The most recent study showing if Notch1 or Notch2 is targeted alone the animals do not develop goblet cell hyperplasia. [DOI] [PubMed] [Google Scholar]