Abstract
Nucleolar dominance is an epigenetic phenomenon that describes nucleolus formation around rRNA genes inherited from only one progenitor of an interspecific hybrid or allopolyploid. The phenomenon is widespread, occurring in plants, insects, amphibians, and mammals, yet its molecular basis remains unclear. We have demonstrated nucleolar dominance in three allotetraploids of the plant genus Brassica. In Brassica napus, accurately initiated pre-rRNA transcripts from one progenitor, Brassica rapa are detected readily, whereas transcripts from the ∼3000 rRNA genes inherited from the other progenitor, Brassica oleracea, are undetectable. Nuclear run-on confirmed that dominance is controlled at the level of transcription. Growth of B. napus seedlings on 5-aza-2′-deoxycytidine to inhibit cytosine methylation caused the normally silent, under-dominant B. oleracea rRNA genes to become expressed to high levels. The histone deacetylase inhibitors sodium butyrate and trichostatin A also de-epressed silent rRNA genes. These results reveal an enforcement mechanism for nucleolar dominance in which DNA methylation and histone modifications combine to regulate rRNA gene loci spanning tens of megabase pairs of DNA.
Keywords: rDNA, rRNA gene, nucleolus, Brassica, gene expression
Differential states of gene expression that do not result from changes in DNA sequence yet are clonally or meiotically heritable are known as epigenetic phenomena (Bestor et al. 1994; Holliday 1994). Among the earliest recognized examples is nucleolar dominance. Chromosomal sites where nucleoli form during interphase appear as secondary constrictions at metaphase, the primary constriction being the centromere (McClintock 1934). Navashin (1934) noted that in F1 hybrids of certain Crepis species, a secondary constriction was formed on the nucleolus-forming chromosome of only one parent. This was true regardless of which species was the maternal parent in the cross. Importantly, the repressed nucleolus could be recovered in an appropriate backcross, demonstrating that the nucleolus-forming chromosome had not been altered or lost in the hybrid. Decades later, it became clear that nucleolus organizer regions (NORs) are sites where hundreds to thousands of rRNA genes are tandemly arrayed, spanning several million base pairs of DNA (Wallace and Birnstiel 1966; Pardue et al. 1970; Gerbi 1971; Phillips et al. 1971). RNA polymerase I (Pol I) transcribes these genes to produce primary transcripts that are processed extensively to form the 18S, 5.8S, and 28S rRNAs that form the core of the ribosome (Reeder 1992; Paule 1994; Moss and Stefanovsky 1995). Nucleoli form only at rRNA gene loci where rRNAs are being synthesized. Therefore, Navashin’s phenomenon was ultimately interpreted as the cytological manifestation of failing to produce rRNA from one parental set of rRNA genes (Honjo and Reeder 1973).
Nucleolar dominance occurs in interspecific hybrids and allopolyploids of numerous genera (for review, see Reeder 1985; Pikaard and Chen 1997). In the early developmental stages of Xenopus laevis × Xenopus borealis hybrids, X. laevis rRNA is detected but X. borealis rRNA is not (Honjo and Reeder 1973; Cassidy and Blackler 1974). Reeder and Roan (1984) showed that dominance of X. laevis over X. borealis rRNA genes could be mimicked with plasmid-borne minigenes injected into oocytes. Their results suggested that X. laevis rRNA genes are transcriptionally dominant because of an increased number of enhancers relative to X. borealis rRNA genes in the region adjacent to the rRNA gene promoter (Boseley et al. 1979; Busby and Reeder 1983; Moss 1983; Labhart and Reeder 1984). This enhancer imbalance was proposed to allow dominant genes to titrate one or more limiting transcription factors, thereby making the factors unavailable to the under-dominant promoters.
Another hypothesis put forward to explain nucleolar dominance stems from the observation that rRNA gene promoters and RNA Pol I transcription systems evolve rapidly (Reeder 1974; Dover and Flavell 1984; Gerbi 1985; Flavell 1986). Consequently, an rRNA gene promoter from one species is often not recognized in an unrelated species because of the incompatibility of the Pol I transcription factors (Grummt et al. 1982; Mishima et al. 1982; Miesfeld and Arnheim 1984). Failure to express a species-specific Pol I transcription factor could, in turn, cause the failure to express one set of rRNA genes. This scenario presumably explains nucleolar dominance in human–mouse somatic cell hybrids (Elicieri and Green 1969; Miller et al. 1976; Perry et al. 1976; Croce et al. 1977; Onishi et al. 1984).
The species-specific transcription factor hypothesis seems unlikely to account for nucleolar dominance in closely related species capable of interbreeding (Reeder 1985). The latter prediction appears to hold true for the Brassica diploids (B. nigra, B. rapa, and B. oleracea) and their derived allotetraploids (B. napus, B. carinata, B. juncea) we are studying (Chen and Pikaard 1997). In each of the allotetraploids, transcripts from only one progenitor species’ rRNA genes are detected using S1 nuclease protection or primer extension assays (Chen and Pikaard 1997). However, Brassica rRNA gene promoters share ∼80% identity with one another and with the promoter of Arabidopsis thaliana, a related species within the Cruciferae. Both transient expression (Doelling and Pikaard 1996) and in vitro transcription (J. Saez-Vasquez and C. Pikaard, unpubl.) experiments have shown that Brassica and Arabidopsis rRNA gene promoters can function with the Pol I transcription machinery of the other species. Therefore, failure to express a transcription factor from one progenitor genome is unlikely to cause the inability to express one set of rRNA genes in Brassica allotetraploids.
In this paper, we present evidence that nucleolar dominance in the allotetraploid B. napus involves selective repression of rRNA genes derived from the progenitor species B. oleracea. Cytosine methylation (Chomet 1991; Bird 1992; Li et al. 1993; Bestor et al. 1994; Eden and Cedar 1994; Holliday 1994; Rainier and Feinberg 1994; Razin and Cedar 1994; Martienssen and Richard 1995) and histone deacetylation (Turner 1991; Lee et al. 1993; Loidl 1994; Owen-Hughes and Workman 1994) are both implicated in rRNA gene silencing, as is also the case in other epigenetic phenomena typically involving genes transcribed by RNA polymerase II (Pol II). These results suggest that chromatin modifications exert similar epigenetic effects throughout the genome.
Results
Nucleolar dominance in Brassica
Figure 1 summarizes our current knowledge of nucleolar dominance in the plant genus Brassica (Chen and Pikaard 1997). The six species represented in Figure 1A are all cultivated crops. The corners of the triangle (U 1935) are represented by diploids whose genomes are historically denoted aa (B. rapa, e.g., Chinese cabbage, turnip rape), bb (B. nigra, black mustard), or cc (B. oleracea, e.g., broccoli, cauliflower), and whose haploid chromosome number (n) varies from 8–10. The diploids combine in three ways to form the allotetraploids (amphidiploids) B. juncea (brown mustard), B. carinata (Abyssinian mustard), and B. napus (canola, oilseed rape). Using S1 nuclease protection and/or primer extension probes specific for rRNA genes of the three progenitor diploids, we have shown that in each tetraploid only rRNA genes inherited from one parent yield detectable steady-state transcripts initiated from the normal transcription start site (Chen and Pikaard 1997). These findings are recapitulated in Figure 1B. Using the B. nigra-specific S1 probe and RNA purified from appropriate species (Fig. 1B, lanes 3–7), B. nigra transcripts are detected readily in the B. nigra control (Fig. 1B, lane 5) and in the tetraploids B. carinata (Fig. 1B, lane 4), and B. juncea (Fig. 1B, lane 6). In contrast, using the B. oleracea probe (Fig. 1B, lanes 8–12), transcripts can only be detected in the B. oleracea control (Fig. 1B, lane 10) but not in tetraploids for which B. oleracea is a progenitor (Fig. 1B, lanes 9 and 11). Using the B. rapa probe, transcripts are detected in both B. rapa (Fig. 1B, lane 15) and B. napus (Fig. 1B, lane 16). Together, these data reveal that B. nigra rRNA genes are dominant over those of B. oleracea (in B. carinata) or B. rapa (in B. juncea), and B. rapa genes are dominant over those of B. oleracea (in B. napus). To investigate the basis for nucleolar dominance, B. napus was chosen for further study, in part because of the larger literature concerning this crop species.
Figure 1.
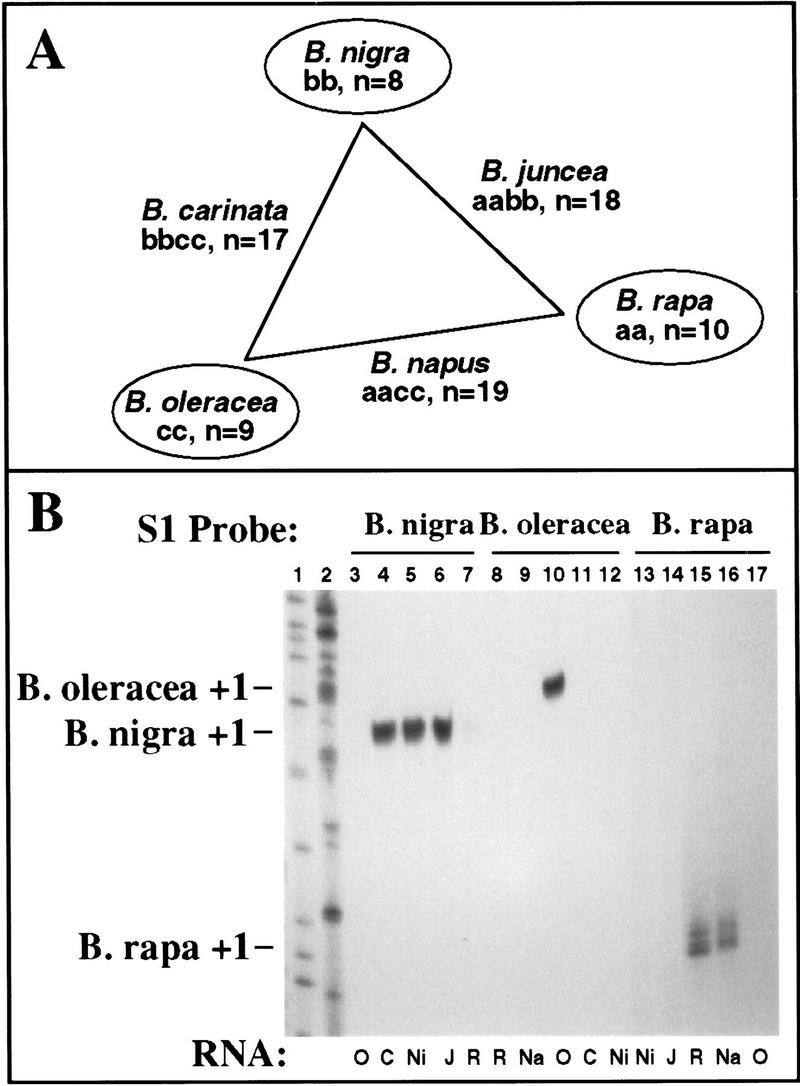
(A) Brassica diploids and their derived allotetraploids. The triangle illustrates the genomic relationships among six major Brassica crop species. B. nigra is placed at the top and B. rapa is placed higher than B. oleracea to summarize our finding that the hierarchy of nucleolar dominance among the diploid species is B. nigra → B. rapa → B. oleracea. Diploid genome compositions are denoted as aa, bb, or cc. (n) Chromosome number. (B) S1 nuclease protection results revealing the dominance hierarchy depicted in A. Total RNA (20 μg) isolated from leaves of B. oleracea (O); B. carinata (C); B. nigra (Ni); B. juncea (J); B. rapa (R); and B. napus (Na) was hybridized to 5′ end-labeled probes specific for B. nigra (lanes 3–7); B. oleracea (lanes 8–12); or B. rapa (lanes 13–17) rRNA transcripts. Following S1 digestion, protected probe fragments were resolved on a sequencing gel and visualized by autoradiography. Nigra transcripts are detected in nigra, carinata, and juncea; rapa transcripts are detected in rapa and napus; oleracea transcripts are detected only in oleracea. Sequencing reactions served as size markers in lanes 1 and 2.
Nucleolar dominance occurs at the level of transcription
The S1 probes used in Figure 1B hybridize to the extreme 5′ ends of the pre-rRNA transcripts. These sequences do not encode any of the structural rRNA incorporated into ribosomes and are thought to be processed rapidly and turned over (Warner 1989; Sollner-Webb et al. 1991; Mougey et al. 1993). Therefore, measurement of their steady-state levels, as in Figure 1B, is expected to provide a good estimate of actual transcription levels. In fact, close correlation between steady-state pre-rRNA levels, detected by S1 nuclease protection, and nascent transcript abundance, detected by nuclear run-on, has been demonstrated in response to the plant hormone cytokinin (Gaudino and Pikaard 1997). Nonetheless, one could argue that nucleolar dominance involves a mechanism by which one set of pre-rRNA transcripts is degraded preferentially.
To test definitively whether nucleolar dominance is controlled at the level of transcription, we devised an assay involving both nuclear run-on and S1 nuclease protection, an assay capable of discriminating nascent transcripts initiated from rRNA genes derived from either progenitor species (Fig. 2). B. napus nuclei were isolated and allowed to elongate nascent transcripts in the presence of [α-32P]UTP and α-amanitin. Radioactive RNA transcripts were then hybridized to nonradioactive B. rapa or B. oleracea promoter probes (Fig. 2, lanes 3 and 4). Labeled RNAs were also added to hybridization reactions including unrelated plasmid DNA (Fig. 2, lane 5) or no DNA (Fig. 2, lane 6). Following S1 digestion of RNA–DNA hybrids, protected RNAs were resolved on a denaturing polyacrylamide sequencing gel beside control reactions (Fig. 2, lanes 1 and 2) in which conventional S1 protection assays were performed using unlabeled B. oleracea or B. rapa total RNA and the appropriate end-labeled DNA probe. Using this approach, radioactive B. rapa RNA transcripts of the appropriate size were detected among the nuclear run-on products in B. napus (Fig. 2, lane 3), but B. oleracea transcripts were not detected (Fig. 2, lane 4). We conclude that nucleolar dominance is controlled at the level of transcription rather than RNA turnover.
Figure 2.
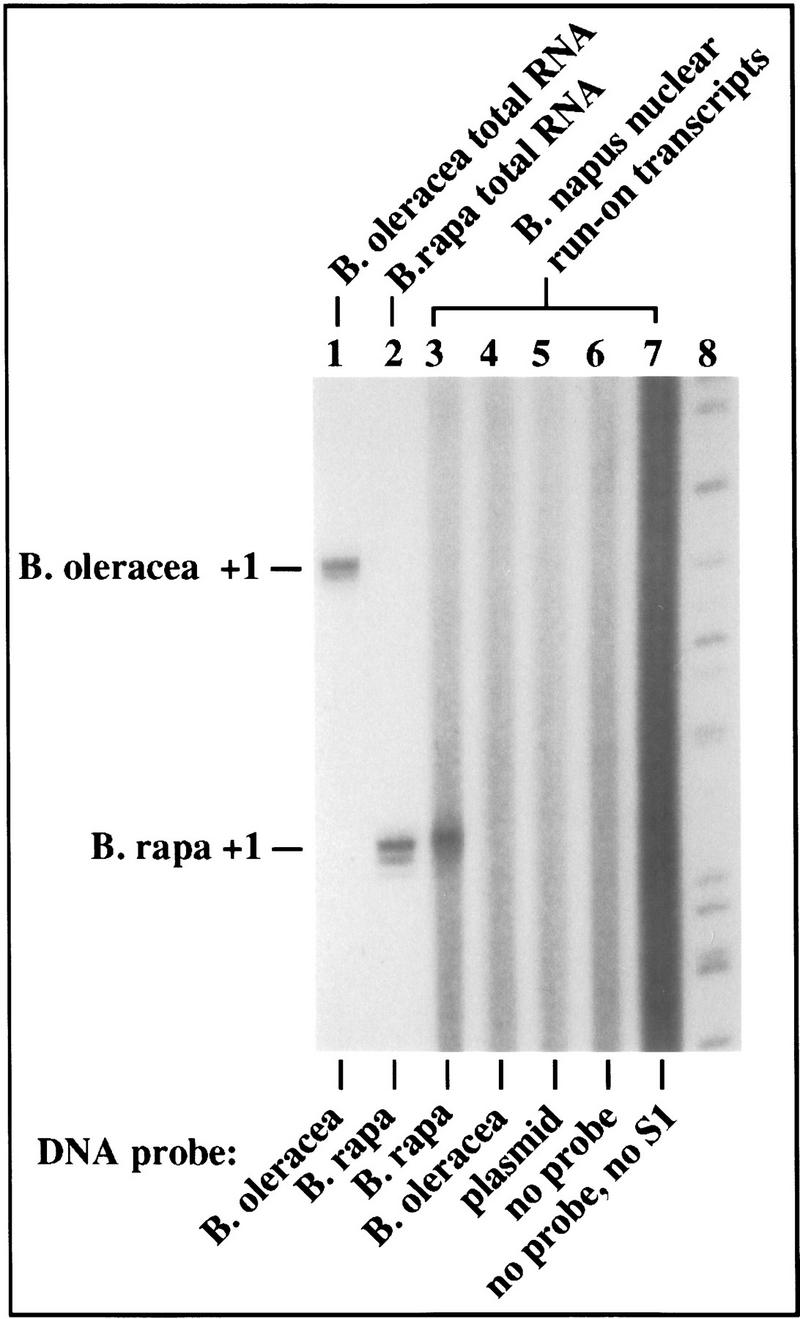
Nuclear run-on analysis confirms that nucleolar dominance is controlled at the level of transcription. (Lanes 1,2) Controls in which conventional S1 nuclease protection assays were performed using unlabeled B. oleracea and B. rapa RNA and end-labeled species-specific probes (as in Fig. 1B). For the remaining reactions (lanes 3–7), B. napus nuclei were incubated with ATP, CTP, GTP, and 32P[UTP] in the presence of 100 μg/ml α-amanitin to allow elongation of template-engaged RNA Pol I. (Lanes 3–5) The protected RNA fragments following hybridization of radioactive nascent RNA transcripts to unlabeled B. rapa, B. oleracea, or pBluescript plasmid DNA, respectively, followed by S1 nuclease digestion, electrophoresis and autoradiography. (Lane 6) Control in which run-on RNA was not hybridized to DNA before S1 digestion. (Lane 7) Control showing the continuous size distribution of labeled RNAs following nuclear run-on. Size markers were run in lane 8. Note that an S1-resistant RNA fragment of the correct size was obtained using the B. rapa probe (lane 3), but no protected products corresponding to nascent B. oleracea transcripts could be detected (lane 4).
Derepression of silenced rRNA genes by 5-aza-2′-deoxycytidine
In plants, as in other higher eukaryotes that methylate their genomic DNA, the vast majority of methylation occurs on cytosines present in CpG or CpNpG (plants only) motifs (Gruenbaum et al. 1981; Bird 1992; Martienssen and Richards 1995). In wheat, both active and inactive rRNA genes subjected to nucleolar dominance are methylated heavily within their intergenic spacers, but restriction endonuclease accessibility studies suggest that inactive genes are methylated somewhat more heavily (Flavell et al. 1988). However, the role of cytosine methylation in nucleolar dominance remains controversial: In Xenopus hybrids displaying nucleolar dominance, no correlation between DNA methylation status and gene activity could be demonstrated (Macleod and Bird 1982).
To test the role of cytosine methylation in Brassica, B. napus seeds were germinated on sterile media containing 5-aza-2′-deoxycytidine (aza-dC), a nucleotide analog that inhibits cytosine methylation (Jones 1985; Haaf 1995). Levels of deoxycytidine and methyldeoxycytidine in control and treated plants were then measured using a thin layer chromatography (TLC) assay (Kakutani et al. 1995). Quantitation of TLC plates by phosphorimaging revealed that methyldeoxycytidine accounts for ∼83% of the total cytidines present in TaqI sites (and presumably other CpG sites) in untreated B. napus plants (Fig. 3A). Treatment with aza-dC caused a reduction in methyldeoxycytidine content of ∼30%.
Figure 3.
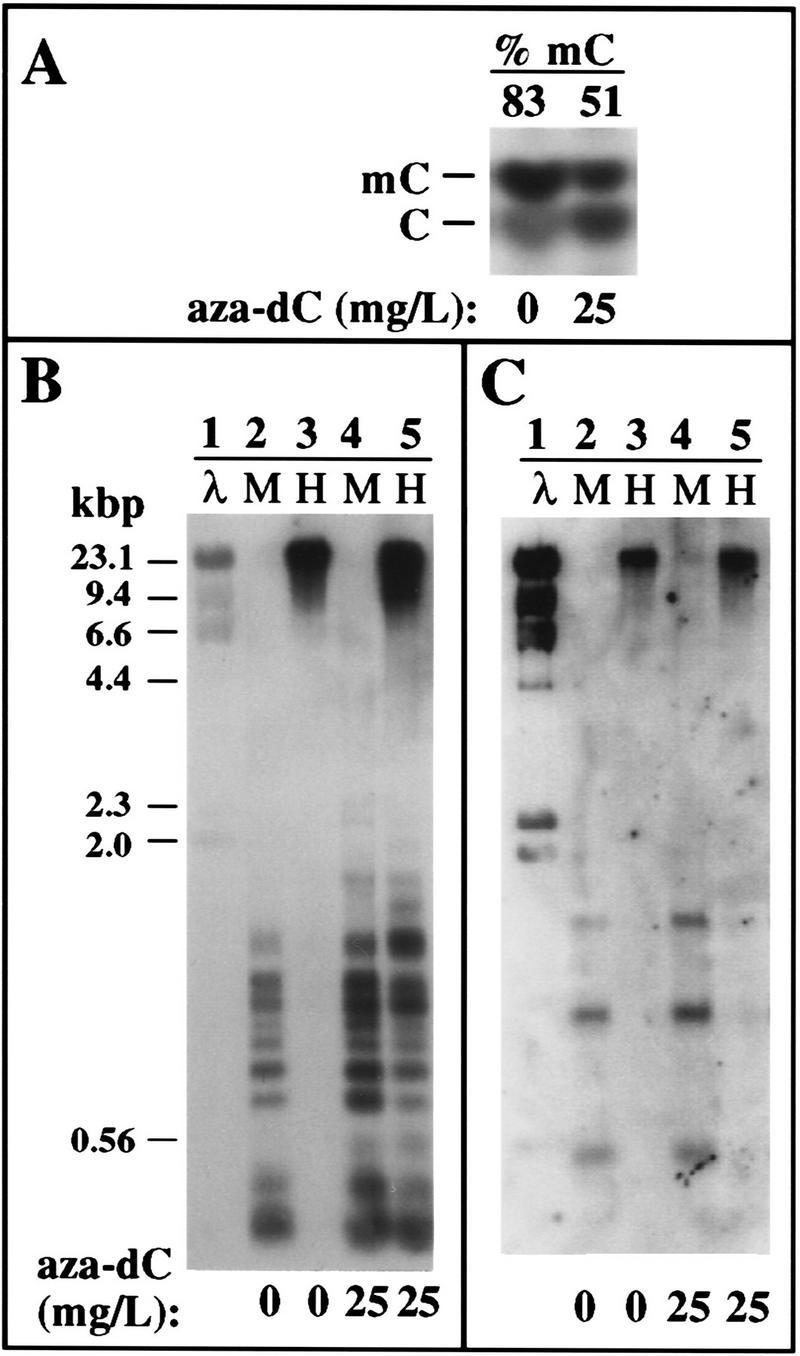
Effects of aza-dC on global and rRNA gene methylation. (A) Aza-dC treatment reduces genomic methyldeoxycytidine levels ∼30%. Genomic DNA isolated from control or aza-dC treated B. napus plants was cleaved to completion with TaqI. TaqI is insensitive to cytosine methylation and cleaves at the recognition sequence TCGA to leave a 5′ overhanging cytosine that can be end-labeled with T4 kinase and γ-labeled 32P[ATP]. Following complete DNase digestion, samples were subjected to TLC and autoradiography. Numbers at the top indicate the amount of methyldeoxycytidine (% of total deoxycytidine) in control and treated plants based on quantitation by phosphorimaging. (B,C) Aza-dC treatment reduces cytosine methylation in the vicinity of the rRNA gene promoter. DNA from control and aza-dC-treated B. napus plants was digested with the isoschizomers MspI (M) or HpaII (H), then subjected to electrophoresis, Southern blotting, and hybridization to a probe spanning −166 (relative to the transcription start site, +1) to the beginning of the 18S rRNA coding region (B). Aza-dC caused increased susceptibility to the methylation-sensitive enzyme Hpa II (cf. lanes 3 and 5). (C) Same blot was stripped and reprobed with a labeled 5S RNA gene fragment to verify that similar amounts of DNA were loaded in all lanes. HindIII-digested λ DNA markers were run in lane 1 and are visible on the autoradiogram because a small amount of λ DNA was mixed with the rDNA probe fragments in the labeling reactions.
The effect of aza-dC treatment on rRNA gene methylation was estimated by digesting DNA from control and treated plants with HpaII or MspI, followed by Southern blotting and hybridization with a probe covering the region from the promoter to the beginning of the 18S rRNA-coding sequences. Both enzymes cleave at the recognition sequence CCGG. However, HpaII will not cut if the inner C is methylated and cuts very poorly if the outer C is methylated such that, for all practical purposes, methylation at either C prevents HpaII cleavage (McClelland et al. 1994). In contrast, MspI will cut the DNA if the inner C is methylated, but will not cut if the outer C is methylated. Therefore, this enzyme pair allows one to evaluate the methylation status of the inner C of a CCGG sequence. Treatment with aza-dC increased the accessibility of B. napus rRNA genes to HpaII (Fig. 3B, cf. lanes 3 and 5), consistent with the TLC results showing a decrease in genomic 5-methyldeoxycytidine content. As a control to test whether similar amounts of DNA were loaded in all lanes, the same blot was stripped and probed with a labeled 5S rRNA gene fragment (Fig. 3C). No change in HpaII accessibility was detected among 5S genes following aza-dC treatment (Fig. 3C).
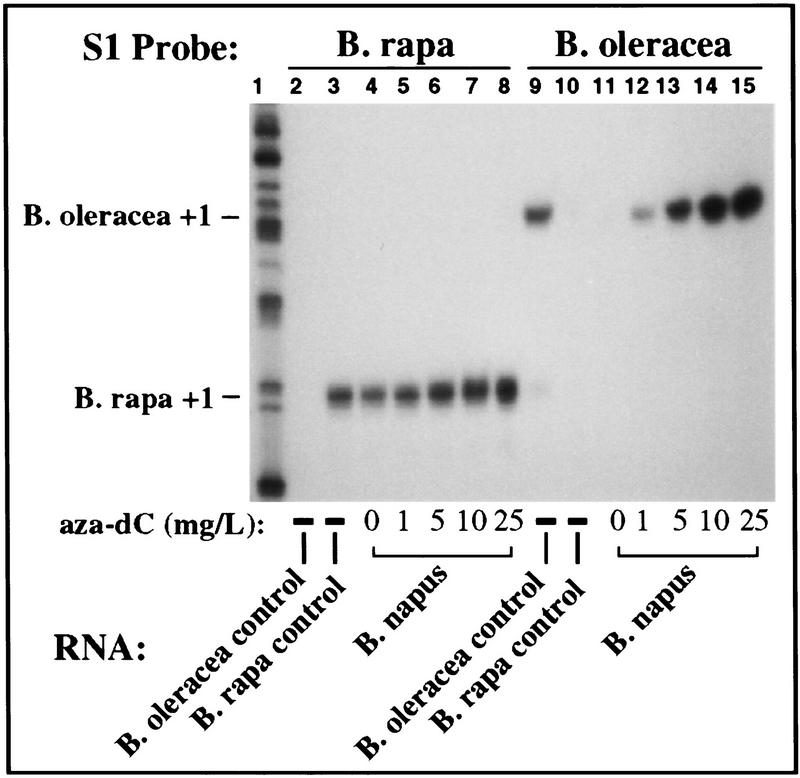
Next, rRNA transcripts in aza-dC-treated and untreated plants were analyzed using the S1 nuclease protection assay. As expected, transcripts from the dominant B. rapa rRNA genes were detected in untreated B. napus plants (Fig. 4, lane 4) and in plants treated with four different levels of aza-dC (Fig. 4, lanes 5–8). However, the B. oleracea rRNA genes that were silent in untreated plants (Fig. 4, lane 11) were derepressed in a dosage-dependent manner by aza-dC (Fig. 4, lanes 12–15) to an expression level similar to that of the dominant B. rapa rRNA genes. Interestingly, rRNA transcript levels from the dominant rRNA genes were also more abundant following aza-dC treatment (Fig. 4, cf. lanes 4–8), suggesting that reduced cytosine methylation leads to the derepression of rRNA genes in both the dominant and the silent classes.
Figure 4.
Aza-dC-induced derepression of B. oleracea rRNA genes in B. napus. Seeds were germinated on media containing varying concentrations of aza-dC, as indicated. After three weeks in culture, RNA (20 μg) was purified from leaves and subjected to S1 nuclease protection analysis using B. rapa (lanes 2–8) or B. oleracea (lanes 9–15) specific probes. B. oleracea and B. rapa RNA controls are in lanes 2,3 and 9,10; all other lanes used B. napus RNA. Note that B. oleracea rRNA genes were derepressed in B. napus (lanes 12–15) in an aza-dC-dosage-dependent manner. Dominant B. rapa genes are also up-regulated approximately threefold by aza-dC (cf. lanes 4 and 8). Labeled size markers were run in lane 1.
In other experiments not shown, derepression of under-dominant rRNA genes by aza-dC was also observed in the tetraploids B. carinata and B. juncea, suggesting the generality of the response demonstrated for B. napus in Figure 4.
Derepression of B. oleracea rRNA genes in B. napus by inhibitors of histone deacetylation
The ability of aza-dC to derepress rRNA genes subjected to nucleolar dominance implicates a role for cytosine methylation in the phenomenon in plants. However, nucleolar dominance also occurs in Drosophila (Durica and Krider 1977, 1978), a genus in which cytosine methylation is not detected (Rae and Steele 1979; Bird and Taggart 1980). This suggested that other chromatin modifications should also be considered.
Cytosine methylation and histone hyperacetylation status of specific genes are often correlated inversely. Active genes tend to be hypomethylated and associated with nucleosomes whose core histones are hyperacetylated (Csordas 1990; Turner 1991; Grunstein 1992; Laurenson and Rine 1992; Loidl 1994). Conversely, silent genes tend to be methylated heavily and wrapped in chromatin containing relatively hypoacetylated core histones. Importantly, correlations between gene activity and histone acetylation also occur in organisms that do not methylate their DNA, such as Drosophila and yeast. Therefore, we tested whether inhibitors of histone deacetylation might also depress rRNA genes subjected to nucleolar dominance. B. napus seeds were germinated on sterile media containing 0, 2, or 10 mm sodium butyrate (Kruh 1982) and RNA was isolated from seedlings after two weeks of growth. Equal amounts of RNA were then probed for B. rapa and B. oleracea rRNA transcripts using the S1 nuclease protection assay (Fig. 5A). In the absence of sodium butyrate, B. rapa rRNA genes were expressed (Fig. 5, lane 3) and B. oleracea transcripts were undetectable (Fig. 5, lane 6), as expected. Inclusion of 2 or 10 mm sodium butyrate in the media up-regulated the dominant B. rapa rRNA genes several fold (Fig. 5, lanes 4 and 5, respectively) and derepressed the B. oleracea genes (Fig. 5, lanes 7,8), though not quite to the level of the B. rapa genes (Fig. 5A, cf. lanes 5 and 8). Note that the probes had similar specific activities based on the signals obtained in the control reactions with B. oleracea and B. rapa RNA (Fig. 5A, lanes 9,10).
Figure 5.
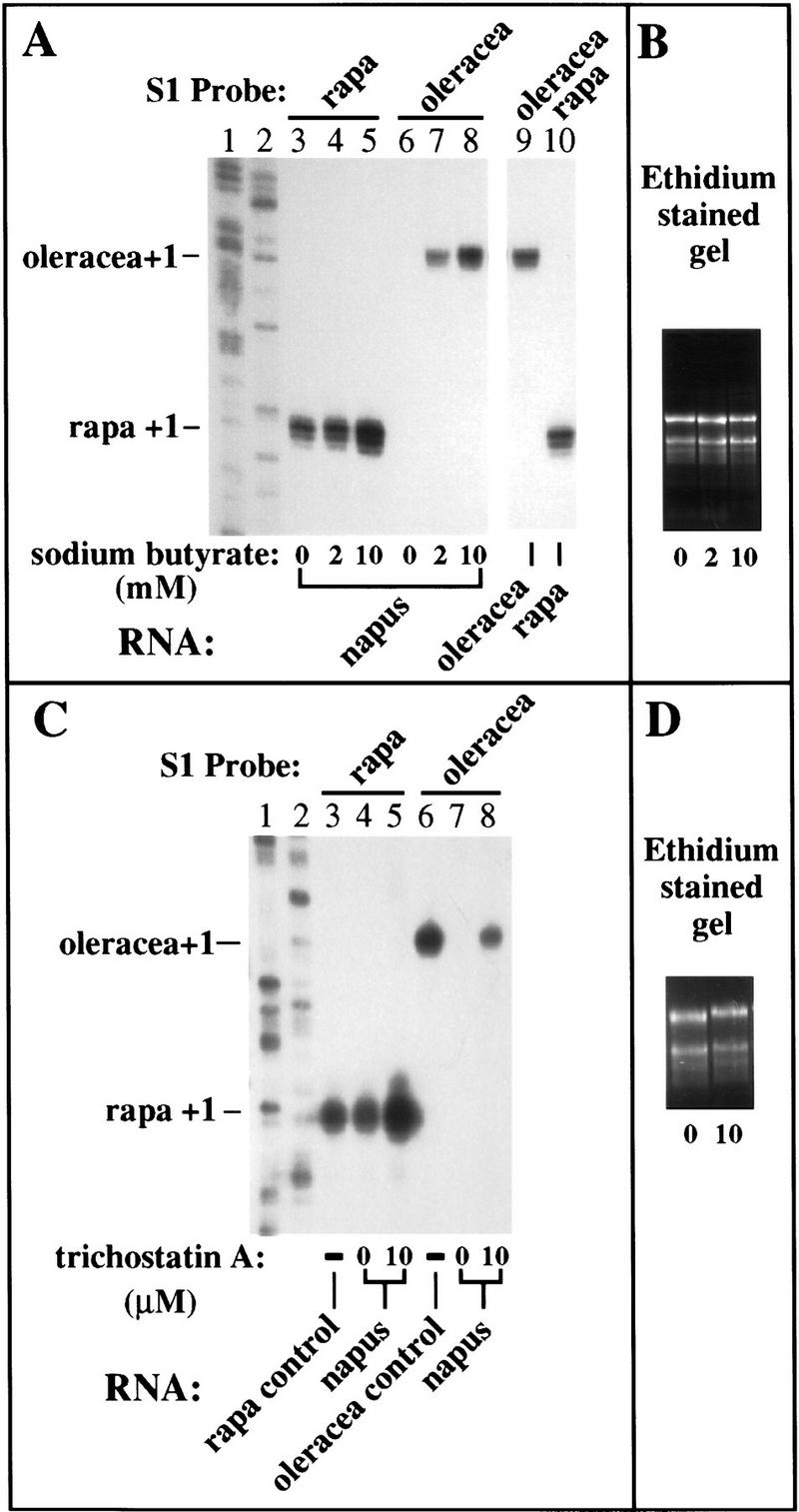
Inhibitors of histone deacetylation derepress silenced rRNA genes. (A,B) B. napus seeds were germinated on medium containing 0, 2, or 10 mm sodium butyrate and total RNA (10 μg) from two-week-old plants was subjected to S1 nuclease protection using the B. rapa (lanes 3–5,10) and B. oleracea-specific (lanes 6–9) probes. S1 protected products using RNA from the diploids B. oleracea and B. rapa were run on the same gel as controls to show that the two probes had equivalent specific activity (lanes 9,10). (Lanes 1,2) Sequencing ladders used as size markers. Note that the normally silent B. oleracea rRNA genes were activated by sodium butyrate treatment (cf. lanes 6 and 8) and dominant B. rapa rRNA gene transcript levels were also up-regulated two- to threefold (cf. lanes 3 and 5). (B) An ethidium bromide-stained agarose gel of RNA aliquots from plants subjected to the various treatments to verify that equivalent amounts of RNA were probed to yield the results in A. (C,D) In an experiment similar to that shown in A, B. napus seeds were germinated on medium containing 0 or 10 μm trichostatin A and RNA from two-week-old plants was subjected to S1 nuclease protection analysis using B. rapa and B. oleracea-specific probes. RNA from the diploid progenitors served as controls in lanes 3 and 6. Sequencing ladders served as size markers in lanes 1 and 2. Like sodium butyrate and aza-dC, trichostatin A also derepressed the normally silent B. oleracea genes (cf. lanes 7 and 8) and up-regulated B. rapa rRNA transcript levels (cf. lanes 4 and 5). (D) An ethidium-stained gel to confirm that equivalent amounts of RNA were tested to yield the results shown in C.
We also tested trichostatin A (Fig. 5C), a specific inhibitor of histone deacetylase active at much lower (1000-fold) concentration than sodium butyrate (Yoshida et al. 1990, 1995). As with aza-dC and butyrate treatments, under-dominant B. oleracea rRNA genes were derepressed by treatment with trichostatin A (Fig. 5C, cf. lanes 7 and 8) and dominant B. rapa rRNA genes were also up-regulated severalfold (Fig. 5C, cf. lanes 4 and 5).
Though sodium butyrate and trichostatin A have been shown to alter histone acetylation in yeast and animal cells, less is known about their effects in plants. Therefore, we purified core histones from control and treated B. napus plants and subjected them to electrophoresis on Triton–acid-urea (TAU) gels to resolve the acetylated isoforms. Both sodium butyrate and trichostatin A treatment caused the appearance on stained SDS-polycrylamide gels of several new protein bands when compared with histone profiles of control plants (data not shown). Identification of some of these new bands as isoforms of specific histones was obtained by Western blotting. Antisera raised against acetylated Tetrahymena histone H3 (provided by Dr. David Allis, University of Rochester, NY) yielded the clearest result, shown in Figure 6. One isoform of the correct mobility relative to HeLa core histones was shared by control and treated plants (isoform indicated by an asterisk). A lower-mobility isoform, labeled b/t in Figure 6, was induced by both sodium butyrate and trichostatin. Two higher-mobility isoforms were induced only by trichostatin (Fig. 6, lane 4, labeled t). Other high-mobility protein bands visible on stained gels and present only in the histone preparations of treated plants are most likely acetylated H4 variants but could not be identified definitively because of poor cross-reaction between antibodies raised against Tetrahymena H4 and plant histones (data not shown).
Figure 6.
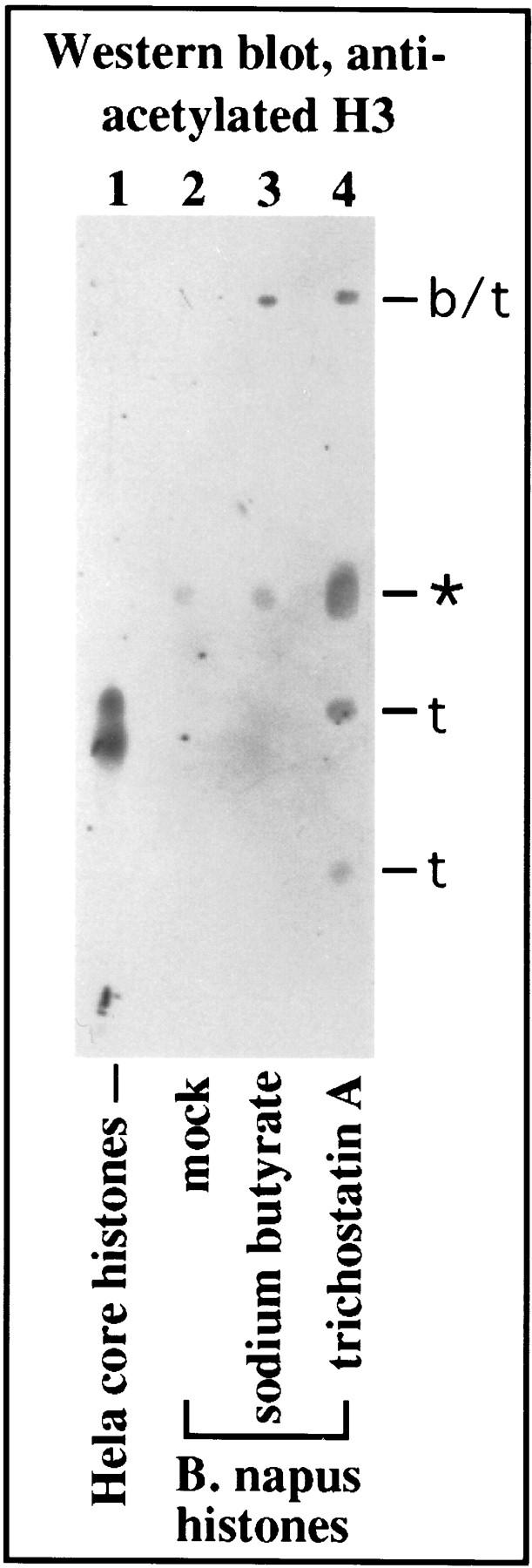
Sodium butyrate and trichostatin A alter histone isoform profiles in B. napus. Core histones from purified HeLa chromatin (lane 1; generously provided by Dr. Jerry Workman, The Pennsylvania State University, University Park) and partially purified histones from control (lane 2), butyrate (lane 3) and trichostatin A-treated (lane 4) plants were subjected to electrophoresis on triton–acid–urea gels to resolve histone isoforms. Proteins were then blotted to nitrocellulose and probed with antibodies raised against hyperacetylated histone H3 of Tetrahymena (kindly provided by Dr. David Allis). HeLa and plant H3 both cross-react with the antibody (lanes 1,2) and are located at gel positions expected based on previous studies (Waterborg et al. 1987; Waterborg 1992). Additional isoforms appear as a result of sodium butyrate and trichostatin A treatment (lanes 3,4). (b/t) Isoforms common to both treatments are denoted; (t) isoforms unique to trichostatin treatment are denoted. An asterisk denotes the only isoform present in control and chemically treated plants.
Taken together, the data of Figures 5 and 6 suggest a direct correlation between chemically-induced derepression of B. oleracea rRNA genes and alterations in B. napus histone modifications.
aza-dC and histone deacetylase inhibitors are not synergistic
We reasoned that if rRNA genes were only partially derepressed by aza-dC or histone deacetylase inhibitors, treatment with both types of compounds might dramatically increase rRNA transcripts levels beyond what is observed with either compound alone. Therefore, we compared rRNA transcript levels in B. napus seedlings germinated on medium containing aza-dC, trichostatin A, or both compounds (Fig. 7). Again, in the absence of either compound, B. rapa transcripts were detected but B. oleracea transcripts were not (Fig. 7, cf. lanes 3 and 7). Treatment with aza-dC caused B. oleracea genes to be derepressed to an expression level similar to that of the dominant B. rapa genes (Fig. 7, cf. lanes 4 and 8). Trichostatin A alone, at the concentration tested, was somewhat less effective than aza-dC in derepressing the B. oleracea rRNA genes, but increased expression of the dominant B. rapa genes to a similar extent as aza-dC (Fig. 7, lanes 5,9). The presence in the medium of both aza-dC and trichostatin A resulted in somewhat higher expression of both dominant and under-dominant rRNA genes than with either compound alone, but no dramatic synergism or strong additive effect was observed (Fig. 7, lanes 6,10). These data suggest that cytosine methylation and histone deacetylation collaborate in the repression of under-dominant rRNA genes and are likely to act on the same target loci or pathways.
Figure 7.
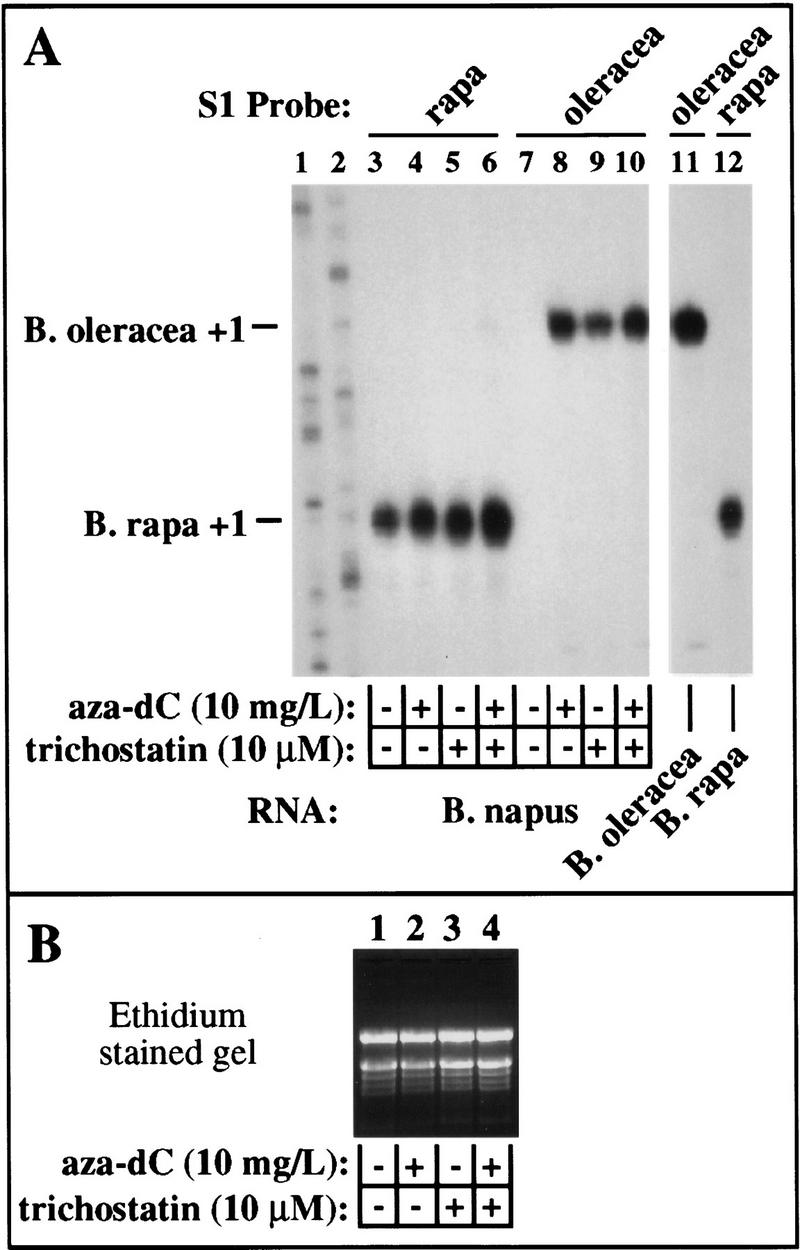
Aza-dC and trichostatin A are not synergistic in derepressing silent rRNA genes. (A) B. napus seeds were germinated on medium containing no additions (lanes 3,7), or on medium containing aza-dC (lanes 4,8), trichostatin A (lanes 5,9), or both chemicals (lanes 6,10). Plants were harvested after two weeks in culture and an equal aliquot of RNA from each treatment (see B) was hybridized to B. rapa (lanes 3–6,12) or B. oleracea-specific (lanes 7–11) probes and subjected to S1 nuclease protection analysis. RNA isolated from B. oleracea and B. rapa (lanes 11,12) served as controls. (Lanes 1,2) Sequencing ladders used as size markers. The data of lanes 3–12 are from the same exposure of the same gel; lanes 11 and 12 are separated from other lanes for clarity. Note that aza-dC and trichostatin together are only slightly more effective than either chemical alone.
The derepressed state of under-dominant rRNA genes is unstable
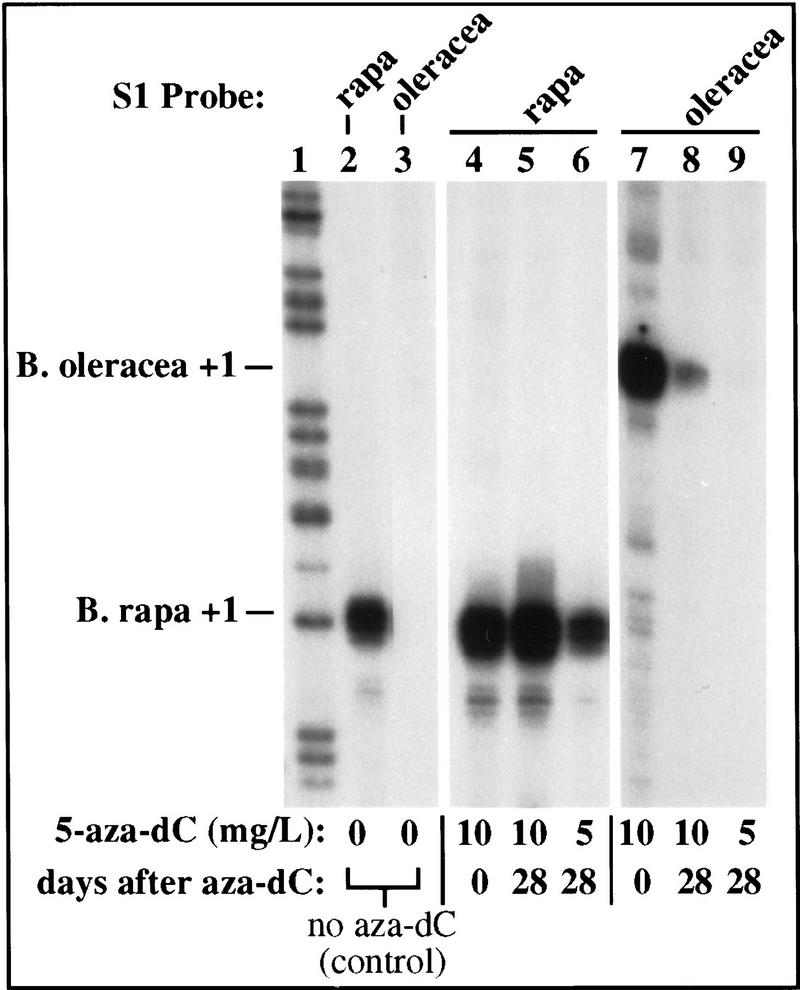
In triticale (wheat × rye) azacytidine treatment of developing seeds reportedly leads to an increased number of visible nucleoli in cells of plants grown from these seeds (Neves et al. 1995). We performed a similar experiment to determine whether rRNA genes derepressed in germinating seedlings by aza-dC treatment remained transcriptionally active in mature plants. B. napus seeds were again germinated on aza-dC. After 2 weeks in culture, seedlings were harvested (defined as day 0) or transferred to media lacking aza-dC and grown for an additional 28 days. RNA was isolated from total leaf tissue of day 0 and day 28 plants and transcripts were detected by S1 nuclease protection. As can be seen in Figure 8, rapa genes are expressed and oleracea transcripts are undetectable in B. napus plants not treated with aza-dC, as expected (Fig. 8, lanes 2,3). Germination and growth on 10 mg/liter aza-dC resulted in high expression of both rapa and oleracea genes in plants harvested on day 0 (Fig. 8, lanes 4 and 7). At day 28, the dominant rapa genes remained expressed at high levels (Fig. 8, lane 5) , but the under-dominant oleracea genes were expressed at 10-fold lower levels than at day 0 (Fig. 8, lane 8). Seedlings germinated on a lower amount of aza-dC (5 mg/liter) showed reduced expression of dominant rapa genes at day 28 (Fig. 8, cf. lanes 5 and 6), and oleracea transcripts in these plants were barely detectable (Fig. 8, lane 9). Therefore, under-dominant genes are not derepressed stably throughout vegetative growth by aza-dC treatment during germination and early growth.
Figure 8.
rRNA genes are not stably derepressed by aza-dC, suggesting that nucleolar dominance can be established throughout vegetative growth. B. napus seeds were germinated on medium containing 0, 5, or 10 mg/liter of aza-dC. After two weeks, half of the seedlings were harvested (defined as day 0) and RNA was purified. Remaining plants were grown 28 more days in the absence of aza-dC before RNA isolation. Total RNA (10 μg) was subjected to S1 nuclease protection analysis using B. rapa- or B. oleracea-specific probes. In untreated B. napus plants, B. rapa transcripts are detected (lane 2), but B. oleracea transcripts are not (lane 3), as expected. Aza-dC caused equivalent expression of B. oleracea and B. rapa rRNA genes (lanes 4,7) in plants harvested on day 0, also as expected. Twenty-eight days after aza-dC treatment, dominant B. rapa rRNA genes remained up-regulated in plants that had been grown on 10 mg/liter of aza-dC (cf. lanes 2,4,5) but not in plants grown on 5 mg/liter (cf. lanes 2 and 6). By day 28, B. oleracea transcript levels had dropped and were barely detectable in plants treated at 5 mg/liter of aza-dC (lanes 7–9). Lanes shown are all from the same exposure of the same gel but are subgrouped for clarity.
To test whether the recurrence of nucleolar dominance observed in Figure 8 was correlated with changes in cytosine methylation, we used the TLC assay and Southern blotting (as in Fig. 3) to examine DNA purified from day-28 whole plants. Methylation in plants 28 days after removal from aza-dC-containing medium was indistinguishable from control plants of the same age that had not been treated with aza-dC (data not shown). One possible explanation is that de novo methylation of aza-dC-demethylated DNA occurred to re-establish dominance. However, an alternative possibility is that a small number of meristem cells, possibly the slowly dividing cells in the central zone of the shoot apical meristem (Medford 1992), were not affected by aza-dC. Over time, clonal descendants of these unaffected cells might fully occupy the central and peripheral zones of the meristems in older plants (Dawe and Freeling 1991), giving rise to vegetative organs whose cells contain fully methylated nuclear DNA. At present we are unable to distinguish between these possibilities. DNA hypomethylation mutants (Vongs et al. 1993) or transgenic plants deficient for cytosine methyltransferase activity in (presumably) all cells (Finnegan et al. 1996; Ronemus et al. 1996) may prove useful in future studies.
Discussion
A role for gene silencing in nucleolar dominance
Our results suggest that inactive rRNA genes subjected to nucleolar dominance are silenced reversibly by a mechanism operating, at least in part, at the level of chromatin structure. Inhibiting either cytosine methylation or histone deacetylation is sufficient for the derepression of the inactive set of rRNA genes, suggesting that these processes are partners required together for rRNA gene silencing. These results agree well with what is known about the roles of DNA methylation and/or histone acetylation in other epigenetic phenomena such as suppression of silent mating type and telomere-proximal genes in yeast (Aparicio et al. 1991; Grunstein 1992), X-inactivation and gametic imprinting in mammals (Rainier and Feinberg 1994; Rastan 1994; Razin and Cedar 1994; Penny et al. 1996; Willard 1996), and suppression/activation of transposition and homology-dependent gene silencing in plants (Flavell 1994; Martienssen and Baron 1994; Matzke et al. 1994; Federoff et al. 1995; Jorgensen 1995). Specific genes analyzed in regard to these other epigenetic phenomena are typically protein-coding genes transcribed by RNA Pol II. Our data provide evidence that epigenetic regulation of genes transcribed by RNA Pol I also involves changes in DNA methylation and histone acetylation suggesting that these processes exert similar effects on distinct transcription systems.
There has long been evidence that changes in rRNA gene chromatin structure are correlated with rRNA gene activity in nucleolar dominance, though the role of cytosine methylation has remained uncertain. For instance, repressed rRNA genes subjected to nucleolar dominance in both Xenopus and wheat have been shown to be less susceptible to nuclease digestion than active genes (Macleod and Bird 1982; Flavell et al. 1988). In wheat, inactive rRNA genes were found to be methylated somewhat more heavily than the active genes, suggesting that methylation is correlated directly with nucleolar dominance (Flavell et al. 1988). However, no significant difference in the methylation status of dominant and under-dominant genes was found in Xenopus (Macleod and Bird 1982). In the latter study, Macleod and Bird (1982) concluded that DNA methylation status alone could not explain the expression state of active and inactive rRNA genes nor their different nuclease hypersensitivities (Macleod and Bird 1982; Bird 1992). However, these investigators noted that cytosine hypomethylation of specific sequences might still be necessary for gene expression, though apparently not sufficient. Our results, suggesting that both cytosine methylation and histone acetylation have a role in nucleolar dominance, can be taken to support the conclusions of both the Flavell and Bird laboratories and therefore may begin to offer a unifying explanation.
Possible target loci affected by aza-dC and histone deacetylase inhibitors
A question we are unable to answer at present is whether changes in cytosine methylation and histone acetylation act directly on the rRNA genes or act indirectly through another regulatory locus. For instance, loss of cytosine methylation or increased histone acetylation of rRNA genes organized in chromatin may be all that is needed for the Pol I transcription machinery to gain access to the promoters. Alternatively, a locus distinct from the rRNA genes may be regulated by changes in cytosine methylation or histone acetylation, leading to the downstream regulation of rRNA genes. The known differences in chromatin accessibility of active rRNA genes could be an indirect consequence of regulating the expression of an activator binding preferentially to the dominant genes to induce a more DNase-accessible state.
One reason for suspecting that a locus other than the rDNA might be the primary target for aza-dC and histone deacetylase inhibitors stems from our analyses of rDNA methylation in B. napus. Interestingly, the rDNA in B. napus, which includes both dominant B. rapa and under-dominant B. oleracea rRNA genes, was virtually insensitive to digestion with HpaII in the absence of aza-dC. In Figure 3B, most of the hybridization signal in lane 3 is at a position comigrating with the 23-kb size marker (which is close to the exclusion limit for this gel, therefore, many fragments could be much larger). Each rRNA gene is ∼10 kb in length, suggesting that HpaII cuts no more than once every two genes. This is surprising because there are at least 10 HpaII sites in the intergenic spacers of B. oleracea and B. rapa and additional HpaII sites are present within rRNA-coding sequences (Brassica-coding regions have not been sequenced, but the equivalent region in A. thaliana includes 41 HpaII sites). Therefore, essentially every HpaII site is methylated in virtually every rRNA gene of B. napus. Nonetheless, dominant genes are still expressed. Aza-dC treatment causes an increase in the susceptibility of rRNA genes to HpaII digestion (Fig. 3B, lane 5), but most of the hybridization signal still corresponds to fragments of 10–20+ kb. Based on these considerations, it is not clear that the amount of rDNA demethylation detected in Figure 3 can explain the derepression of under-dominant rRNA genes to the same expression level as for dominant genes, as shown in Figure 4. Perhaps CpG methylation at non-HpaII sites is more important for rRNA gene expression and is not detected in our assay. However, another possibility is that aza-dC is affecting another locus.
As discussed in the introduction, controlling the expression of a species-specific transcription factor could, in principle, control nucleolar dominance. However, this seems unlikely in Brassica. In transient expression experiments, we have found that the B. oleracea and B. rapa promoters are expressed at nearly identical levels whether transfected into protoplasts of B. oleracea or B. rapa. The oleracea and rapa genes and are also expressed at similar levels when cotransfected into B. napus protoplasts despite the fact that chromosomally encoded B. oleracea rRNA genes in these latter cells are repressed (M. Frieman, Z.C. Chen, and C.S. Pikaard, unpubl.). These results suggest that Brassica transcription factors are virtually identical across species boundaries. Furthermore, the transient expression results suggest that nucleolar dominance may only operate on endogenous chromosomal copies of the rRNA genes. Unlike transfected genes on plasmids, chromosomal genes have a history that includes multiple rounds of replication and chromatin mofifications and passage through multiple developmental transitions.
There is intriguing evidence that chromosomal loci outside the NORs influence rRNA gene expression and nucleolar dominance in Drosophila. Based on cytogenetic analyses of chromosome deletion and inversion stocks, a region flanking the NORs on both the X and Y chromosomes in Drosophila melanogaster appears to be required for their dominance over the single X-linked NOR of Drosophila simulans (Durica and Krider 1978). Rearrangements that delete these regions have no effect on expression of the cis-linked melanogaster NORs, but nucleolus formation at the D. simulans NOR is no longer suppressed. The size and identity of these deleted regions flanking the melanogaster NORs and their mechanism of action has not been defined. However, the fact that these loci act in trans suggests that they might encode or regulate a repressor.
The evidence for controlling loci linked to NORs in Drosophila is reminiscent of X chromosome inactivation in female mammals, an epigenetic mechanism for equalizing the effective dosage of X-linked genes in males and females. Treatment with 5-azacytidine was shown to reactivate many suppressed X-linked genes, an early indication that cytosine methylation was involved (Mohandas et al. 1981; Gartler and Goldman 1994; Haaf 1995). Inactive X chromatin also appears to be devoid of acetylated histone H4, a marker for active genes (Jeppesen and Turner 1993). Therefore changes in cytosine methylation and histone acetylation are shared by X inactivation and nucleolar dominance and both operate on a multimegabase scale [the ∼3000 B. oleracea rRNA genes in B. napus (Bennett and Smith 1991) span ∼30 Mb, whereas the human X chromosome is ∼184 Mb]. X inactivation is controlled by a locus that contains an essential transcription unit, Xist, that is expressed only on the inactive X (Rastan 1994; Tilghman and Willard 1995; Penny et al. 1995). Available evidence suggests that controlling the methylation status of the relatively small Xist promoter controls Xist expression and the subsequent inactivation of the X chromosome (Ariel et al. 1995; Beard et al. 1995; Zucotti and Monk 1995). It is conceivable that the methylation or histone acetylation status of an external locus analogous to Xist could affect the activity of an NOR. However, unlike X inactivation in somatic cells of females, the choice of which rRNA genes to inactivate in nucleolar dominance is not random. Instead, the rRNA genes of the same species are always inactivated when brought together in a hybrid with no apparent maternal or paternal effects. This implies the existence of a discrimination mechanism in nucleolar dominance as opposed to a chromosome counting mechanism as proposed for X inactivation (Tilghman and Willard 1995).
Establishment and enforcement mechanisms may work together
Our working model for nucleolar dominance involves two sets of mechanisms: those that initially discriminate dominant from under-dominant rRNA genes in a hybrid genome and those that enforce dominance and silencing. Our experiments provide insight into the likely enforcement mechanism. As for a likely discrimination mechanism, the enhancer imbalance hypothesis remains a viable possibility. The latter (Reeder 1985) can be combined with rRNA gene silencing into a scenario similar to one proposed by Flavell (1986). Perhaps a limiting transcription factor is titrated initially by the dominant genes, and the quiescent genes become packaged into a repressive chromatin structure that maintains their silence. Cytosine methylation patterns could be perpetuated at every round of division by maintenance methylation. Later in development, transcription factors may be available in excess over the number of active rRNA genes, yet the genes remain silent attributable to their chromatin structure. This may explain why counteracting the silencing mechanisms by aza-dC, butyrate, or trichostatin A allows under-dominant rRNA genes to be expressed, at least transiently. However, the transient burst of rRNA gene expression may deplete the transcription factor pool such that the competitive advantage of dominant genes again becomes apparent and dominance is re-established. Testing such speculations awaits the detailed characterization of the Pol I transcription system in Brassica and measurements of the levels of transcription factors throughout development.
Materials and Methods
Plant material and plant growth conditions
Brassica seed stocks were obtained from the Crucifer Genetics Cooperative (CrGC, Madison, WI). The stock numbers were: B. rapa (aa, CrGC 1-1), B. nigra (bb, CrGC 2-1), B. oleracea (cc, CrGC 3-1), B. juncea (aabb, CrGC 4-1), B. napus (aacc, CrGC 5-1), and B. carinata (bbcc, CrGC 6-1). Seeds were sterilized with 10% commercial bleach and plants were grown in Terra-Lite soil mix (Grace Horticultural Products) in a greenhouse or in Conviron PGW35 growth chambers. 5-aza-2′-deoxycytidine, sodium butyrate, and trichostatin A were all purchased from Sigma Chemical Co. (St. Louis, MO). Aza-dC and sodium butyrate stock solutions were made in water; trichostatin was dissolved in methanol. Appropriate volumes were added to partially cooled (∼45°C) germination media (Valvekens et al. 1988) that had been sterilized by autoclaving. The warm medium was then poured into sterile Magenta boxes (Sigma), which were quick-cooled at 4°C. Sterilized seeds were sown, 6–9 per Magenta box, as soon as the media had cooled completely and solidified. Seedlings were sprouted and grown in a growth chamber for 2–3 weeks using a 16-hr light/8-hr dark growth regime.
Nucleic acid purification
Genomic DNA was purified as described by Devey and Hart (1993). For Southern blotting experiments, 1 μg of total genomic DNA was digested with restriction endonucleases and subjected to agarose gel electrophoresis. RNA was extracted using either 4.5 m guanidinium thiocyanate followed by lithium chloride precipitation (Chomczynski and Sacchi 1987) or extracted in a buffer containing sodium chloride and SDS according to the method of Rapp et al. (1992).
S1 protection assays
S1 nuclease protection assays were performed as described previously (Doelling et al. 1993; Doelling and Pikaard 1995). Briefly, a DNA restriction fragment spanning the transcription start site was 5′ end-labeled with T4 kinase and γ-labeled 32P[ATP]. The labeled end upstream of the transcription start site was then preferentially cut off with another restriction enzyme and the desired probe fragment was gel-purified. The DNA probe was denatured and hybridized with purified cellular RNA (15 mg). Resulting DNA:RNA hybrids were digested with 150 units/ml S1 nuclease for 30 min at 37°C to digest the single-stranded nucleic acid (unhybridized DNA and RNA) and the products were resolved on a 6% sequencing gel. The size of the protected probe fragment corresponds to the distance from the transcription start site of the RNA to the labeled end of the probe. Probes were used in excess over RNA so that the amount of protected product was proportional to the amount of RNA transcript. To detect B. rapa transcripts the probe was the SphI (−110) to AvaII (+76) fragment; B. oleracea and B. nigra probes were AccI (−39) to AvaII (+103) fragments.
Nucleolar run-on assays
B. napus plants used for nuclear isolation were grown in the growth chamber for 3 weeks. The plants were transferred to a growth chamber with no lighting for ∼16 hr (overnight) before harvesting leaves. Nuclear isolation was according to Gaudino and Pikaard (1997) except that a three-step Percoll gradient was used. Briefly, fresh leaves were frozen in liquid nitrogen and ground in liquid nitrogen to a fine powder using a mortar and pestle. Fifty milliliters of nuclear isolation buffer (NIB) [0.4 m Mannitol, 50 mm Tris-HCl (pH 7.2), 0.2 mm EDTA, 0.1% BSA, 0.1% β-mercaptoethanol, and 5 mm MgCl2] was added per 10 g of leaf tissue. The resulting homogenate was centrifuged 10 min at 3000g, 4°C, and the pellet was resuspended gently (using a paintbrush) in 10%–20% of the original volume of NIB. After a second centrifugation step for 5 min at 3900g, the homogenate was loaded onto a 35%/50%/80% Percoll step gradient. The nuclei were collected from the interface between the 50% and 80% layers, adjusted to 10 ml in NIB and collected by centrifugation at 3900g, 5 min. Nuclei were then resuspended at ∼106–107 nuclei/ml in nuclei storage buffer [50 mm Tris-HCl (pH 7.2), 5 mm MgCl2, 75 mm KCl, 0.2 mm EDTA, 2 mm DTT, and 25% glycerol] and stored at −80°C. Quantitative estimates were based on counting DAPI-stained nuclei in a hemacytometer using fluorescence microscopy.
Approximately 1 × 107 nuclei were used for each run-on transcription reaction. Frozen nuclei were thawed, pelleted in a microcentrifuge, and resuspended in 300 μl of reaction mix that included 40 mm Tris-HCl (pH 7.5), 6 mm MgCl2, 2.5 mm spermidine, and 10 mm NaCl, 0.5 mm each ATP, CTP, and GTP, 100 μg/ml α-amanitin (Sigma), 600 U/ml RNasin (Promega), and 250 μCi α-32P[UTP]. The reaction mixture was incubated at 30°C for 45 min before it was terminated by addition of RNase-free DNase I (100 U/ml; Promega), SDS/Tris/EDTA buffer, and protease K (0.1 mg/ml). The run-on transcripts were extracted with phenol/chloroform, purified through a Sephadex G-50 spin column, and quantified by scintillation counting. Accurately initiated labeled transcripts were then detected by hybridizing radioactive RNA transcripts to unlabeled DNA probes using our standard S1 nuclease protection assay conditions. RNA/DNA hybrids were digested with S1 nuclease (200 U/ml), precipitated with ethanol, then digested further with a mixture of 1 mg/ml of RNase A and 20 U/ml RNase T1 to reduce background from other labeled RNA fragments. Protected products were then resolved in a 6% polyacrylamide sequencing gel and visualized by autoradiography.
Measurement of methyldeoxycytidine and deoxycytidine by TLC
TLC analysis was performed according to Kakutani et al. (1995). Briefly, 500 ng of genomic DNA purified from untreated or aza-dC-treated plants was digested to completion at 65°C with TaqI (recognition site T/CGA; cleavage is insensitive to cytosine methylation). Digested DNA fragments were treated with shrimp alkaline phosphatase, then end-labeled with γ-32P[ATP] and T4 polynucleotide kinase. Unincorporated nucleotides were removed by filtration on a Sephadex G-50 column. Fifty nanograms of TaqI-digested, end-labeled DNA fragments were digested in a 20-μl reaction containing 5 units of DNase I, 2 mg of phosphodiesterase I, 50 mm Tris-HCl at pH 8, and 5 mm MgCl2 for 2 hr at 37°C. The digestion products were then separated by TLC on cellulose plates (Aldrich Chemical Co.) developed in isobutyric acid:water:ammonium hydroxide (66:33:1). The positions of dCMP and 5-methyl-dCMP standards (10 mg each; Sigma Chemical Company) were visualized by UV shadowing and their positions marked with phosphorescent dye before autoradiography. Quantitation of radioactively labeled deoxycytidine and methyldeoxycytidine was determined using a Molecular Dynamics PhosphorImager.
Histone purification and analysis
B. napus seeds were germinated in 10 mm sodium butyrate or 10 μm trichostatin A as described above. Histones were isolated from whole plant tissues and purified extensively according to Waterborg and colleagues using differential salt/acid solubility and Bio-Rex 70 column chromatography (Mende et al. 1983; Waterborg et al. 1987). Histone fractions were dialyzed against 0.5% acetic acid (in water) and lyophilized.
To resolve histone isoforms, protein fractions were subjected to Triton–acid–urea gel electrophoresis (Cousens et al. 1979; Arts et al. 1995). Gels were composed of 15% polyacrylamide (30:0.2, acrylamide:bis-acrylamide), 5% acetic acid, 6 m urea, and 10 mm Triton X-100, and measured 21 cm wide × 30 cm long. Loading buffer (6 m urea, 0.02% pyronin, 5% acetic acid, 12.5 mg/ml of protamine sulfate) was added to the wells and the gel was pre-run at 300 V overnight (∼16 hr) in a buffer containing 5% acetic acid and 0.2 m glycine. The following day, 20 μg of protein from plants subjected to the various treatments (in loading buffer) was loaded in each well and subjected to electrophoresis at 400 V for 24 hr. Gels were Coomassie or silver-stained or were electroblotted to nitrocellulose membrane at 0.5 A for 15 min in 0.7% acetic acid using a Trans-Blot Cell (Bio-Rad). Transfer of proteins to the membrane was verified by reversible staining with Ponceau S (Sigma). Western blot analysis (Towbin et al. 1979) was performed using primary antibodies raised against Tetrahymena histones provided by Dr. C. David Allis (University of Rochester, NY). Histone–antibody complexes were detected using a secondary antibody and enhanced chemiluminescence according to the instructions of the supplier (Amersham).
Acknowledgments
We thank our Washington University colleagues Jeffrey Jeddeloh and Dr. Eric Richards for providing help and materials for the TLC assays. We thank Dr. C. David Allis for graciously providing anti-histone antibodies and Dr. Jerry Workman (The Pennsylvania State University) for providing purified HeLa histones as controls. We also thank Dr. Jakob Waterborg (University of Missouri, Kansas City) for advice concerning the purification and analysis of plant histones and appreciate Jenny Zhou’s (University of Rochester) advice and protocols for triton–acid–urea gel electrophoresis and electrophoretic transfer of histones for Western blot analysis. This work was supported by grants to C.S.P. from the U.S. Department of Agriculture, National Research Initiative Competitive Grants Program (grant 94-373012-0658) and the National Science Foundation (DMB-9617471). Z.J.C. was supported, in part, by a Monsanto Postdoctoral Fellowship in Plant Biology.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL pikaard@biodec.wustl.edu; FAX (314) 935-4432.
References
- Aparicio OM, Billington BL, Gottschling DE. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- Ariel M, Robinson E, McCarrey JR, Cedar H. Gamete-specific methylation correlates with imprinting of the murine Xist gene. Nature Genet. 1995;9:312–315. doi: 10.1038/ng0395-312. [DOI] [PubMed] [Google Scholar]
- Arts J, Lansink M, Grimbergen J, Toet KH, Kooistra T. Stimulation of tissue-type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. Biochem J. 1995;310:171–176. doi: 10.1042/bj3100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, Li E, Jaenisch R. Loss of methylation activates Xist in somatic but not in embryonic cells. Genes & Dev. 1995;9:2325–2334. doi: 10.1101/gad.9.19.2325. [DOI] [PubMed] [Google Scholar]
- Bennett RI, Smith AG. The complete nucleotide sequence of the intergenic spacer region of an rDNA operon from Brassica oleracea and its comparison with other crucifers. Plant Mol Biol. 1991;16:1095–1098. doi: 10.1007/BF00016085. [DOI] [PubMed] [Google Scholar]
- Bestor TH, Chandler VL, Feinberg AP. Epigenetic effects in eukaryotic gene expression. Dev Genet. 1994;15:458–462. doi: 10.1002/dvg.1020150603. [DOI] [PubMed] [Google Scholar]
- Bird A. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- Bird AP, Taggart MH. Variable patterns of total DNA and rDNA methylation in animals. Nucleic Acids Res. 1980;8:1485–1497. doi: 10.1093/nar/8.7.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseley P, Moss T, Machler M, Portmann R, Birnstiel M. Sequence organization of the spacer DNA in a ribosomal gene unit of X. laevis. Cell. 1979;17:19–31. doi: 10.1016/0092-8674(79)90291-5. [DOI] [PubMed] [Google Scholar]
- Busby SJ, Reeder RH. Spacer sequences regulate transcription of ribosomal gene plasmids injected into Xenopus embryos. Cell. 1983;34:989–996. doi: 10.1016/0092-8674(83)90556-1. [DOI] [PubMed] [Google Scholar]
- Cassidy DM, Blackler AW. Repression of nucleolar organizer activity in an interspecific hybrid of the genus Xenopus. Dev Biol. 1974;41:84–96. doi: 10.1016/0012-1606(74)90285-1. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Pikaard CS. Transcriptional analysis of nucleolar dominance in polyploid plants: Biased expression/silencing of progenitor rRNA genes is developmentally regulated in Brassica. Proc Natl Acad Sci. 1997;94:3442–3447. doi: 10.1073/pnas.94.7.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chomet PS. Cytosine methylation in gene-silencing mechanisms. Curr Opin Cell Biol. 1991;3:438–443. doi: 10.1016/0955-0674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Cousens LS, Gallwitz D, Alberts BM. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979;254:1716–1723. [PubMed] [Google Scholar]
- Croce CM, Talavera A, Basilico C, Miller OJ. Suppression of production of mouse 28S rRNA in mouse-human hybrids segregating chromosomes. Proc Natl Acad Sci. 1977;74:694–697. doi: 10.1073/pnas.74.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas A. On the biological role of histone acetylation. Biochem J. 1990;265:23–38. doi: 10.1042/bj2650023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RK, Freeling M. Cell lineage and its consequences in higher plants. Plant J. 1991;1:3–8. [Google Scholar]
- Devey ME, Hart GE. Chromosomal localization of intergenomic RFLP loci in hexaploid wheat. Genome. 1993;36:913–918. doi: 10.1139/g93-120. [DOI] [PubMed] [Google Scholar]
- Doelling JH, Pikaard CS. The minimal ribosomal RNA gene promoter of Arabidopsis thaliana includes a critical element at the transcription initiation site. Plant J. 1995;8:683–692. doi: 10.1046/j.1365-313x.1995.08050683.x. [DOI] [PubMed] [Google Scholar]
- ————— Species-specificity of rRNA gene transcription in plants manifested as a switch in polymerase-specificity. Nucleic Acids Res. 1996;24:4725–4732. doi: 10.1093/nar/24.23.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelling JH, Gaudino RJ, Pikaard CS. Functional analysis of Arabidopsis thaliana rRNA gene and spacer promoters in vivo and by transient expression. Proc Natl Acad Sci. 1993;90:7528–7532. doi: 10.1073/pnas.90.16.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover GA, Flavell RB. Molecular co-evolution: rDNA divergence and the maintenance of function. Cell. 1984;38:622–623. doi: 10.1016/0092-8674(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Durica DS, Krider HM. Studies on the ribosomal RNA cistrons in interspecific Drosophila hybrids. Dev Biol. 1977;59:62–74. doi: 10.1016/0012-1606(77)90240-8. [DOI] [PubMed] [Google Scholar]
- ————— Studies on the ribosomal RNA cistrons in Drosophila hybrids. II. Heterochromatic regions mediating nucleolar dominance. Genetics. 1978;89:37–64. doi: 10.1093/genetics/89.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden S, Cedar H. Role of DNA methylation in the regulation of transcription. Curr Opin Genet Dev. 1994;4:255–259. doi: 10.1016/s0959-437x(05)80052-8. [DOI] [PubMed] [Google Scholar]
- Elicieri GL, Green H. Ribosomal RNA synthesis in human-mouse hybrid cells. J Mol Biol. 1969;41:253–260. doi: 10.1016/0022-2836(69)90390-8. [DOI] [PubMed] [Google Scholar]
- Federoff N, Sclappi M, Raina R. Epigenetic regulation of the maize Spm transposon. BioEssays. 1995;17:291–297. doi: 10.1002/bies.950170405. [DOI] [PubMed] [Google Scholar]
- Finnegan EJ, Peacock WJ, Dennis ES. Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc Natl Acad Sci. 1996;93:8449–8454. doi: 10.1073/pnas.93.16.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell RB. The structure and control of expression of ribosomal RNA genes. Oxford Surv Plant Mol Cell Biol. 1986;3:252–274. [Google Scholar]
- ————— Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc Natl Acad Sci. 1994;91:3490–3496. doi: 10.1073/pnas.91.9.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell RB, O’Dell M, Thompson WF. Regulation of cytosine methylation in ribosomal DNA and nucleolus organizer expression in wheat. J Mol Biol. 1988;204:523–534. doi: 10.1016/0022-2836(88)90352-x. [DOI] [PubMed] [Google Scholar]
- Gartler SM, Goldman MA. Reactivation of inactive X-linked genes. Dev Genet. 1994;15:504–514. doi: 10.1002/dvg.1020150609. [DOI] [PubMed] [Google Scholar]
- Gaudino RJ, Pikaard CS. Cytokinin induction of RNA polymerase I transcription in Arabidopsis thaliana. J Biol Chem. 1997;272:6799–6804. doi: 10.1074/jbc.272.10.6799. [DOI] [PubMed] [Google Scholar]
- Gerbi SA. Localization and characterization of the ribosomal RNA cistrons in Sciara coprophila. J Mol Biol. 1971;58:499–511. doi: 10.1016/0022-2836(71)90367-6. [DOI] [PubMed] [Google Scholar]
- ————— . Evolution of ribosomal DNA. In: McIntyre RJ, editor. Molecular evolutionary genetics. New York, NY: Plenum Press; 1985. pp. 419–517. [Google Scholar]
- Gruenbaum Y, Naveh-Many T, Cedar H, Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981;292:860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- Grummt I, Roth E, Paule MR. rRNA transcription in vitro is species-specific. Nature. 1982;296:173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histones as regulators of genes. Sci Am. 1992;267:68–74. doi: 10.1038/scientificamerican1092-68. [DOI] [PubMed] [Google Scholar]
- Haaf T. The effects of 5-azacytidine and 5-azadeoxycytidine on chromosome structure and function: Implications for methylation-associated cellular processes. Pharmacol Ther. 1995;65:19–46. doi: 10.1016/0163-7258(94)00053-6. [DOI] [PubMed] [Google Scholar]
- Holliday R. Epigenetics: An overview. Dev Genetics. 1994;15:453–457. doi: 10.1002/dvg.1020150602. [DOI] [PubMed] [Google Scholar]
- Honjo T, Reeder RH. Preferential transcription of Xenopus laevis ribosomal RNA in interspecies hybrids between Xenopus laevis and Xenopus mulleri. J Mol Biol. 1973;80:217–228. doi: 10.1016/0022-2836(73)90168-x. [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Turner BM. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation—a cytogenetic marker for gene expression. Cell. 1993;74:281–289. doi: 10.1016/0092-8674(93)90419-q. [DOI] [PubMed] [Google Scholar]
- Jones PA. Altering gene expression with 5-azacytidine. Cell. 1985;40:485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen RA. Cosuppression, flower color patterns, and metastable gene expression states. Science. 1995;268:686–691. doi: 10.1126/science.268.5211.686. [DOI] [PubMed] [Google Scholar]
- Kakutani T, Jeddeloh JA, Richards EJ. Characterization of an Arabidopsis thaliana DNA hypomethylatiuon mutant. Nucleic Acids Res. 1995;23:130–137. doi: 10.1093/nar/23.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982;42:65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Labhart P, Reeder RH. Enhancer-like properties of the 60/81 bp elements in the ribosomal gene spacer of Xenopus laevis. Cell. 1984;37:285–289. doi: 10.1016/0092-8674(84)90324-6. [DOI] [PubMed] [Google Scholar]
- Laurenson P, Rine J. Silencers, silencing, and heritable transcription states. Microbiol Rev. 1992;56:543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Loidl P. Histone acetylation: Facts and questions. Chromosoma. 1994;103:441–449. doi: 10.1007/BF00337382. [DOI] [PubMed] [Google Scholar]
- Macleod D, Bird A. DNase I sensitivity and methylation of active versus inactive rRNA genes in Xenopus species hybrids. Cell. 1982;29:211–218. doi: 10.1016/0092-8674(82)90105-2. [DOI] [PubMed] [Google Scholar]
- Martienssen R, Baron A. Coordinate suppression of mutation caused by Robertson’s Mutator transposons in maize. Genetics. 1994;136:1157–1170. doi: 10.1093/genetics/136.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen RA, Richards EJ. DNA methylation in eukaryotes. Curr Opin Genet Dev. 1995;5:234–242. doi: 10.1016/0959-437x(95)80014-x. [DOI] [PubMed] [Google Scholar]
- Matzke AJM, Neuhuber F, Park Y-D, Ambros PF, Matzke MA. Homology-dependent gene silencing in transgenic plants: Epistatic silencing loci contain multiple copies of methylated transgenes. Mol & Gen Genet. 1994;244:219–229. doi: 10.1007/BF00285449. [DOI] [PubMed] [Google Scholar]
- McClelland M, Nelson M, Raschke E. Effect of site-specific methylation on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res. 1994;22:3640–3659. doi: 10.1093/nar/22.17.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The relationship of a particular chromosomal element to the development of the nucleoli in Zea mays. Z Zellforsch Mikrosk Anat. 1934;21:294–328. [Google Scholar]
- Medford JI. Vegetative apical meristems. Plant Cell. 1992;4:1029–1039. doi: 10.1105/tpc.4.9.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende LM, Waterborg JH, Mueller RD, Matthews HR. Isolation, identification, and characterization of histones from plasmodia of the true slime mold Physarum polycephalum using extraction with guanidine hydrochloride. Biochemistry. 1983;22:38–51. doi: 10.1021/bi00270a006. [DOI] [PubMed] [Google Scholar]
- Miesfeld R, Arnheim N. Species-specific rDNA transcription is due to promoter-specific binding factors. Mol Cell Biol. 1984;4:221–227. doi: 10.1128/mcb.4.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OJ, Miller DA, Dev VG, Tantravahi R, Croce CM. Expression of human and suppression of mouse nucleolus organizer activity in mouse-human somatic cell hybrids. Proc Natl Acad Sci. 1976;73:4531–4535. doi: 10.1073/pnas.73.12.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Financsek I, Kominami R, Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: Identification of a species-dependent initiation factor. Nucleic Acids Res. 1982;10:6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T, Sparkes RS, Shapiro LJ. Reactivation of an inactive human X chromosome: Evidence for X-inactivation by DNA methylation. Science. 1981;211:393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Moss T. A transcriptional function for the repetitive ribosomal spacer in Xenopus laevis. Nature. 1983;302:223–228. doi: 10.1038/302223a0. [DOI] [PubMed] [Google Scholar]
- Moss T, Stefanovsky VY. Promotion and regulation of ribosomal transcription in eukaryotes by RNA polymerase I. Prog Nucleic Acids Res Mol Biol. 1995;50:25–66. doi: 10.1016/s0079-6603(08)60810-7. [DOI] [PubMed] [Google Scholar]
- Mougey EB, O’Reilly M, Miller OL, Beyer A, Sollner-Webb B. The terminal balls characteristic of eukaryotic rRNA transcription units in chromatin spreads are rRNA processing complexes. Genes & Dev. 1993;7:1609–1620. doi: 10.1101/gad.7.8.1609. [DOI] [PubMed] [Google Scholar]
- Navashin M. Chromosomal alterations caused by hybridization and their bearing upon certain general genetic problems. Cytologia. 1934;5:169–203. [Google Scholar]
- Neves N, Heslop-Harrison JS, Viegas W. rRNA gene activity and control of expression mediated by methylation and imprinting during embryo development in wheat × rye hybrids. Theor Appl Genet. 1995;91:529–533. doi: 10.1007/BF00222984. [DOI] [PubMed] [Google Scholar]
- Onishi T, Berglund C, Reeder RH. On the mechanism of nucleolar dominance in mouse-human somatic cell hybrids. Proc Natl Acad Sci. 1984;81:484–487. doi: 10.1073/pnas.81.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Hughes T, Workman JL. Experimental analysis of chromatin function in transcription control. Crit Rev Eukaryot Gene Expr. 1994;4:403–441. [PubMed] [Google Scholar]
- Pardue ML, Gerbi SA, Eckhardt RA, Gall JC. Cytological localization of DNA complementary to ribosomal RNA in polytene chromosomes of Diptera. Chromosoma. 1970;29:268–290. doi: 10.1007/BF00325943. [DOI] [PubMed] [Google Scholar]
- Paule MR. Transcription of ribosomal RNA by eukaryotic RNA polymerase I. In: Conaway RC, Conaway JW, editors. Transcription: Mechanisms and regulation. New York, NY: Raven Press, Ltd.; 1994. pp. 83–106. [Google Scholar]
- Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- Perry RP, Kelley DE, Schibler U, Huebner K, Croce CM. Selective suppression of the transcription of ribosomal genes in mouse-human hybrid cells. J Cell Physiol. 1976;98:553–560. doi: 10.1002/jcp.1040980313. [DOI] [PubMed] [Google Scholar]
- Phillips RL, Kleese RA, Wang SS. The nucleolus organizer region of maize (Zea mays L.): Chromosomal site of DNA complementary to ribosomal RNA. Chromosoma. 1971;36:79–88. [Google Scholar]
- Pikaard CS, Chen ZJ. Nucleolar dominance. In: Paule MR, editor. RNA polymerase I: Transcription of eukaryotic ribosomal RNA. Austin, TX: R.G. Landes; 1997. . (In press.) [Google Scholar]
- Rae PMM, Steele RE. Absence of cytosine methylation at CCGG and GCGC sites in the rDNA coding regions and intervening sequences of Drosophila and the rDNA of other higher insects. Nucleic Acids Res. 1979;6:2987–2995. doi: 10.1093/nar/6.9.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainier S, Feinberg AP. Genomic imprinting, DNA methylation and cancer. J Natl Cancer Inst. 1994;86:753–759. doi: 10.1093/jnci/86.10.753. [DOI] [PubMed] [Google Scholar]
- Rapp JC, Baumgartner BJ, Mullet JE. Quantitative analysis of transcription and RNA levels of 15 barley chloroplast genes. J Biol Chem. 1992;267:21404–21411. [PubMed] [Google Scholar]
- Rastan S. X chromosome inactivation and the Xist gene. Curr Opin Genet Dev. 1994;4:292–297. doi: 10.1016/s0959-437x(05)80056-5. [DOI] [PubMed] [Google Scholar]
- Razin A, Cedar H. DNA methylation and genomic imprinting. Cell. 1994;77:473–476. doi: 10.1016/0092-8674(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Reeder RH. Ribosomes from eukaryotes: Genetics. In: Nomura M, editor. Ribosomes. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1974. pp. 489–519. [Google Scholar]
- ————— Mechanisms of nucleolar dominance in animals and plants. J Cell Biol. 1985;101:2013–2016. doi: 10.1083/jcb.101.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— . Regulation of transcription by RNA polymerase I. In: McKnight SL, Yamamoto KR, editors. Transcriptional regulation. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 315–347. [Google Scholar]
- Reeder RH, Roan JG. The mechanism of nucleolar dominance in Xenopus hybrids. Cell. 1984;38:39–44. doi: 10.1016/0092-8674(84)90524-5. [DOI] [PubMed] [Google Scholar]
- Ronemus MJ, Galbiati M, Ticknor C, Chen J, Dellaporta SL. Demethylation-induced developmental pleiotropy in Arabidopsis. Science. 1996;273:654–657. doi: 10.1126/science.273.5275.654. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B, Pape L, Ryan K, Mougey EB, Poretta R, Nikolov E, Paalman MH, Lazdins I, Martin C. Expression of mouse and frog rRNA genes: Transcription and processing. Mol Cell Biochem. 1991;104:149–154. doi: 10.1007/BF00229814. [DOI] [PubMed] [Google Scholar]
- Tilghman SM, Willard HF. Epigenetic regulation in mammals. In: Elgin SCR, editor. Chromatin structure and gene expression. Oxford, UK: Oxford University Press; 1995. pp. 197–222. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM. Histone acetylation and control of gene expression. J Cell Science. 1991;99:13–20. doi: 10.1242/jcs.99.1.13. [DOI] [PubMed] [Google Scholar]
- U N. Genome analysis in Brassica with special references to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Genet. 1935;7:389–452. [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongs A, Kakutani T, Martienssen R, Richards E. Arabidopsis thaliana DNA methylation mutants. Science. 1993;260:1926–1928. doi: 10.1126/science.8316832. [DOI] [PubMed] [Google Scholar]
- Wallace H, Birnstiel ML. Ribosomal cistrons and the nucleolar organizer. Biochem Biophys Acta. 1966;114:296–310. doi: 10.1016/0005-2787(66)90311-x. [DOI] [PubMed] [Google Scholar]
- Warner J. Synthesis of ribosomes in Saccharomyces cerevisiae. Microbiol Rev. 1989;53:256–271. doi: 10.1128/mr.53.2.256-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterborg JH. Existence of two histone H3 variants in dicotyledonous plants and correlation between their acetylation and plant genome size. Plant Mol Biol. 1992;18:181–187. doi: 10.1007/BF00034947. [DOI] [PubMed] [Google Scholar]
- Waterborg JH, Winico I, Harrington RE. Histone variants and acetylated species from the alfalfa plant Medicago sativa. Arch Biochem Biophys. 1987;256:167–178. doi: 10.1016/0003-9861(87)90435-8. [DOI] [PubMed] [Google Scholar]
- Willard HF. X chromosome inactivation, XIST, and the pursuit of the X-inactivation center. Cell. 1996;86:5–7. doi: 10.1016/s0092-8674(00)80071-9. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- Yoshida M, Horinouchi S, Beppu T. Trichostatin A and trapoxin: Novel chemical probes for the role of histone acetylation in chromatin structure and function. BioEssays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- Zucotti M, Monk M. Methylation of the mouse Xist gene in sperm and eggs correlates with imprinted Xist expression and paternal X-inactivation. Nature Genet. 1995;9:316–320. doi: 10.1038/ng0395-316. [DOI] [PubMed] [Google Scholar]