Abstract
The Hsp90 chaperone machine is required for the folding, activation and/or stabilization of more than 50 proteins directly related to malignant progression. Hsp90 contains small molecule binding sites at both its N- and C-terminal domains, however, limited structural and biochemical data regarding the C-terminal binding site is available. In this report, the small molecule binding site in the Hsp90 C-terminal domain was revealed by protease fingerprinting and photoaffinity labeling utilizing LC-MS/MS. The identified site was characterized by generation of a homology model for hHsp90α using the SAXS open structure of HtpG and docking the bioactive conformation of NB into the generated model. The resulting model for the bioactive conformation of NB bound to Hsp90α is presented herein.
Hsp90 is the core component of a chaperone machine that modulates the folding, activation, and stability of more than 200 substrates. Hsp90 functions by undergoing a series of conformational changes that are driven by the binding and hydrolysis of ATP, which are modulated through Hsp90's interactions with a variety of co-chaperones and partner proteins (reviewed in(1–2)). Because Hsp90-dependent clients are directly associated with all six hallmarks of cancer(3), Hsp90 is under intense investigation as a pharmacological target for the treatment of cancer (4–5).
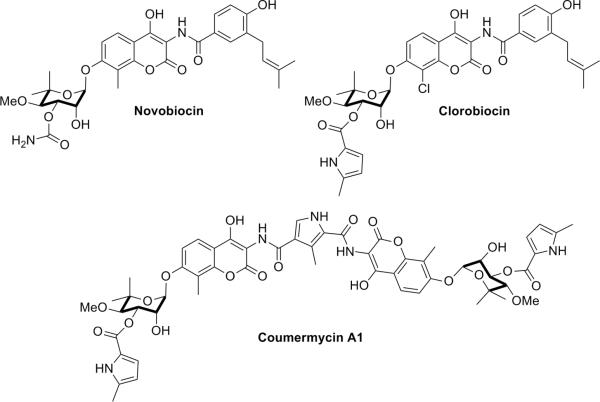
Hsp90 contains drugable sites at both its N- and C-terminal domains. High affinity Hsp90 inhibitors that bind the Hsp90 N-terminal nucleotide binding site are well characterized, as they have been co-crystallized with this domain (i.e., geldanamycin and radicicol (6–7)). In fact, several N-terminal inhibitors are currently in clinical trials for the treatment of cancer (8). In 2000, Neckers and co-workers identified the first C-terminal inhibitor of Hsp90 (9–10) by demonstrating the ability of the Hsp90 C-terminus to bind novobiocin (NB) (Fig. 1) and proposed this domain to represent a nucleotide–binding site that allosterically regulates nucleotide binding at the N-terminus.
Figure 1. Representative Hsp90 C-terminal inhibitors.
Not only does NB inhibit Hsp90 function by binding to the C-terminus of Hsp90, but related family members chlorobiocin (CB) and coumermycin A1 also display Hsp90 inhibitory profiles that are different from those manifested by N-terminal inhibitors (Fig. 1). In addition, C-terminal inhibitors exhibit unique effects on Hsp90's conformation, activity, and interactions with co-chaperones and clients (9–12), highlighting this site as a potential target for Hsp90 modulation. Unfortunately, the mechanism of action for Hsp90 “C-terminal inhibitors” has not been adequately pursued in large part due to their poor pharmacological potency (100–700 μM)(9–10, 12–13). Although analogues of NB that exhibit improved Hsp90-inhibitory and anti-cancer activity (14–19) have been reported, the inability to obtain co-crystal structures with these molecules bound to the chaperone has hampered further development.
Crystal structures of yeast Hsp90, its human ER homologue (Grp94), and E. coli homologue (HtpG) have provided insights into the conformational changes Hsp90 undergoes during the substrate folding process. In addition, low resolution small-angle X-ray scattering (SAXS) (20–21) and cryo-electron microscopy studies (22–23) have provided additional evidence in support of the multiple conformations necessary for folding client substrates. While SAXS (20–21) and cryo-electron microscopy (22–23) studies have clearly demonstrated the Hsp90 C-terminus to adopt distinct conformations, these structures have not provided the resolution necessary for structure-based drug design of improved inhibitors. Unfortunately, the available structures of Hsp90's C-terminal domain and its homologues are similar, and represent the closed, clamped conformation, in which the apparent binding site is inaccessible. In addition, as first suggested by Agard and co-workers, Hugel and co-workers have recently confirmed that the Hsp90 C-terminus undergoes significant conformational changes and opens across the dimerization domain when the Hsp90 N-terminal ATP binding site is occupied, providing a potential mechanism for client protein release (24).
To circumvent limitations imposed upon the rational development of NB analogues through a structure-based approach, the NB binding site located in the Hsp90 C-terminus was sought after via photolabile NB derivatives, which upon covalent attachment to Hsp90 could aid in elucidation of the Hsp90 C-terminal binding site. Subsequent refinement of the biologically active conformation NB bound to Hsp90 could then be derived from the SAXS structure of HtpG in its open conformation, which allows occupancy of the C-terminus. As revealed by co-crystal structures of NB bound to closely related enzymes (e.g., DNA gyrase/topoisomerase(25–26)), the active conformation of NB could then be docked, and subjected to a ligand-supported refinement followed by a systematic molecular dynamics (MD) based methodology to identify the binding site for NB and its analogues. Herein, we present our approach towards elucidation of the Hsp90 C-terminal binding site following this protocol.
RESULTS AND DISCUSSION
Identification of the Hsp90 C-terminal Protease Resistant Core
Binding of NB and chlorobiocin (CB) to the Hsp90 C-terminus protects this domain from proteolysis by trypsin (12), which is not the case for N-terminal inhibitors, suggesting that C-terminal occupancy plays a significant role in protein conformation. Surface plasmon resonance spectroscopy analysis of the binding of chlorobiocin and coumerimycin A1 to full length Hsp90 and Hsp90CT indicates that the C-terminal domain of Hsp90 binds the compounds with affinities comparable to the intact, full length protein, suggesting that truncation does not compromise the structure of the Hsp90's C-terminal binding site (R.L. Matts, J. R. Manjarrez and K. Szalba, unpublished results). To determine the amino acid residues that define the CB-bound protease resistant core of Hsp90, a His-tagged Hsp90 C-terminal construct was digested with V8 protease in the presence and absence of chlorobiocin. V8 was chosen because the N- and C-terminal regions of Hsp90 are deficient in Lys and Arg residues, but rich in Glu and Asp.
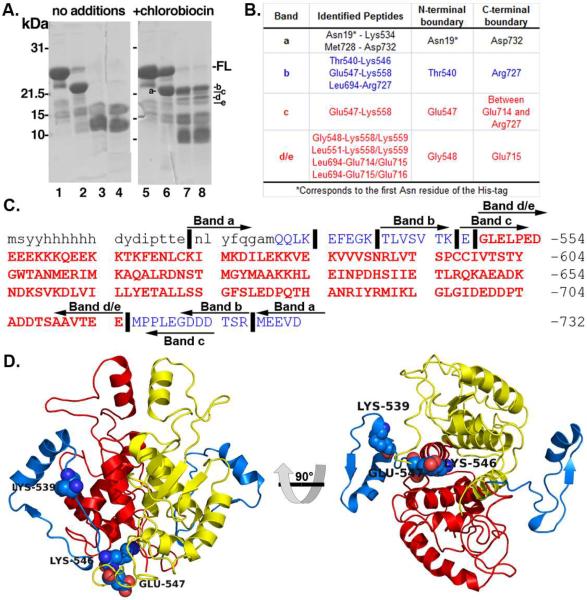
Upon SDS-PAGE analysis of Hsp90 C-terminal proteolysis products, four bands were found resistant to V8 cleavage in samples containing CB that were absent from the control (Fig. 2A). Analysis of the in-gel tryptic digests of each band by nanospray-MS/MS is summarized in Figure 2. Band-a was generated in both the control and CB-treated samples on ice upon treatment with V8 protease, which corresponds to cleavage at the first Glu residue in the N-terminus of the Hsp90CT construct and removes the His-tag (Fig. 2 and Supplemental Fig. 1). The tryptic peptides identified in each band are described in Figure 2B. Band-c contained the tryptic peptide spanning residues Glu547-Lys588, but lacked the tryptic/V8 peptides corresponding to the Leu694-Glu714/Glu715 peptides present in bands-d and -e. Band c also lacked the tryptic peptide Leu694-Arg727 generated from the C-terminal end of band-b, suggesting that band-c had a V8 cut between residue Glu714 and Arg727. Regardless of the exact position of the C-terminal V8 cleavage of band-c, the analysis of the two lower MW bands (d/e) localizes the CB-bound protease resistant core of Hsp90CT to residues Gly548-Glu714 (Fig. 2), which is consistent with Neckers earlier studies in support of residues 538–728. Overall, the data suggests that the binding of chlorobiocin to the Hsp90CT is within a region that connects the middle domain of Hsp90 to its C-terminal domain, and protects this region from proteolysis as highlighted in yellow and red in Figure 2D, corresponding to each monomer of the Hsp90 homodimer.
Figure 2.
V8 protease resistant core of Hsp90's C-terminal Domain. a) Protease-resistance of chlorobiocin bound Hsp90CT. Hsp90CT was incubated in the presence (lanes 2–4 & 6–8) of V8 protease with the addition of DMSO (vehicle control: lanes 1–4) or 800 mM chlorobiocin (lanes 5–8) for 0 min (lanes 2 & 6), 15 min (lanes 3 & 7) or 30 min (lanes 4 & 8). Lanes 1 and 5: Undigested full length Hsp90CT (-FL). Protease resistant bands a–e as indicated. b) Table including the sequences identified and boundaries for each band identified after proteolysis and SDS-PAGE. Red and blue rows correspond to the protease resistant core and flanking regions, respectively. c) Sequence of the Hsp90 C-terminus. Lower and upper case letters indicate residues from the His-tag and Hsp90 C-terminus, respectively. Vertical lines (∣) indicate important cleavage sites that define the boundaries of bands a–e. Peptide bands a–e are depicted by left and right arrows denoting N- and C- termini of these bands. Bold and red residues comprise the chlorobiocin induced Hsp90 CT protease resistant core. d) Hsp90 C-terminus, important cleavage sites labeled (spheres); red and yellow cartoons provide the protease resistant core from each Hsp90 monomer, while blue cartoon indicates upstream residues not part of protease resistant core.
Identification of the NB binding site in Hsp90's C-terminal Domain
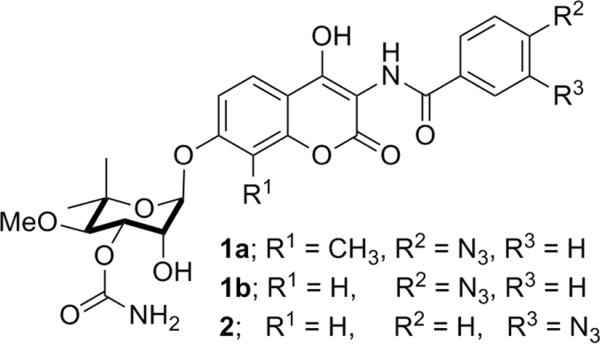
Photolabile derivatives of novobiocin were synthesized for the purpose of identifying the Hsp90 C-terminal binding site. As noted earlier, the Hsp90 C-terminus is resistant to proteolysis by trypsin in the presence of chlorobiocin. Similarly, compound 1a also protected the Hsp90 C-terminus from trypsin proteolysis (Fig. 3), indicating that the photolabile compound bound Hsp90 in a manner similar to novobiocin and chlorobiocin (12) (See Supplemental Fig. 1C).
Figure 3. Novobiocin derivatives used in photoaffinity labeling studies.
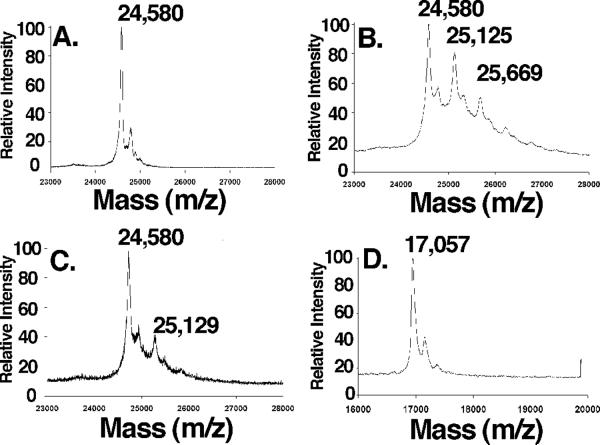
Novobiocin analogues containing a photo-reactive azide moiety placed in the p- (1a/1b) or m-position (2) were used in lieu of the phenol and prenyl side chain present in NB, respectively. A recombinant His-tagged Hsp90 C-terminal construct was incubated in the presence or absence of 1a (27) followed by UV-irradiation. No change in the m/z of Hsp90CT was detected upon MALDI-TOF analysis of the Hsp90 C-terminus upon UV-irradiation in the absence of 1a (Fig. 4A), however, in the presence of 1a a new polypeptide peak (m/z= 25,125) with a mass approximately 545 Da greater than the parental peak (m/z= 24,580) was detected (Fig. 4B), which corresponds to the molecular weight of compound 1a minus one molecule of N2. To confirm that both the azide-containing molecule and the non-labeled novobiocin bind to the same location, crosslinking studies were carried out in the presence of an equimolar concentration of NB and 1a, resulting in a >80% decrease in the intensity of the 25,125 peak (Fig. 4C), indicating the inhibitors bind competitively. A small portion of the Hsp90CT was found to be modified by two molecules of 1a, therefore an experiment was performed with 10-fold lower concentrations of the photolabile inhibitor (2). A significant reduction in the amount of doubly modified Hsp90CT was observed, indicating that the first binding event is most specific (Supplemental Fig. 2). To demonstrate crosslinking specificity, apomyoglobin was irradiated in the presence of 1a, and no crosslinking was observed, confirming selectivity of these compounds for Hsp90 (Fig. 4D). Identical crosslinking peaks were detected when 2 was irradiated in the presence of Hsp90, confirming that azide placement on the benzamide side chain did not affect binding.
Figure 4. Crosslinking of 1 to Hsp90CT is specific.
Hsp90CT (a–c) or apomyogobin (d) were UV irradiated as described under “Materials and Methods” in the presence of a) DMSO, b & d) 5 mM 1a, c) 5 mM 1a plus 5 mM NB and analyzed by MALDI-TOF mass spectrometry.
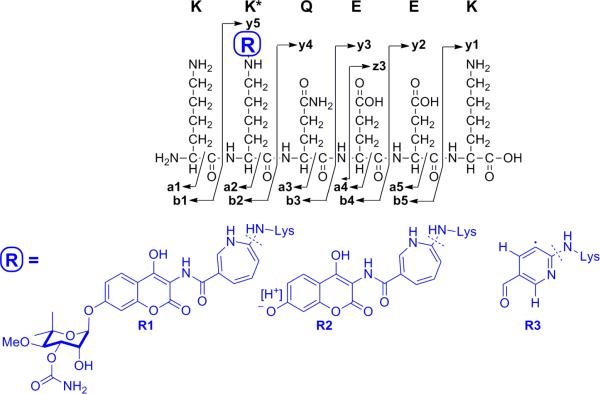
To determine the peptide sequence to which the photoactivatable derivative was crosslinked, control and 1b-crosslinked and 2-crosslinked Hsp90 were digested with trypsin, and the masses of the tryptic peptides were analyzed by ultra-high mass accuracy Fourier transform ion cyclotron resonance (FTICR) mass spectroscopy (mass accuracy of 10 ppm). Crosslinking of 2 is calculated to increase the theoretical mass of a crosslinked peptide by 527.1613 Da. Analysis of the data identified a peptide corresponding to a mass of ~527 amu greater than that predicted for an unlabeled peptide sequence, namely: 559-KKQEEK-564 or 560-KQEEKK-565 (1316.6093/~MH1+ 789.4465+527.1540).
As the 2-linked peptide was present as both a +1 (m/z 1316.60) and +2 (m/z 658.80) ion, MS2 fragmentation of the MH2+ 658.80 and MH1+ 1316.60 ions was carried out to identity the labeled amino acid (Supplementary Fig. 3, Supplementary Scheme 1A and Supplementary Table 1B). In addition, the fragmentation pattern of NB and the underivatized KKQEEK peptide (m/z 789) were analyzed for comparison to the labeled peptide (Supplementary Fig. 4A and B). Consistent with the fragmentation of novobiocin, the MS2 spectrum of the 658.80 and 1316.60 ions contained one dominant ion at 1099.47 corresponding to the loss of noviose and the addition of a proton to maintain the ions positively charged state (Supplementary Fig. 3 and Table 1). An ion at 218.12 corresponding to positively charged noviose in the MS2 spectrum of the 658.80 ion reiterated the presence of crosslinked 2 (Supplementary Fig. 5 and Supplementary Table 1C). A peak with an m/z of 907 amu was predicted for loss of the aminocoumarin ring, however, this was not observed. In contrast, prominent peaks with m/z of 892.55 (MH1+) and 446.70 (MH2+) were present and are consistent with the loss of the aminocoumarin ring and a CH3 radical, which occurs upon rearrangement of the aminocycloheptatriene ring to a pyridine ring (Fig. 5, Supplementary Scheme 1, Supplementary Fig. 3 and Supplementary Table 1B – See supplementary materials and methods for a full detailed discussion of the analysis of the MS data).
Figure 5. Laddering ions generated from the 2-crosslinked peptides.
R1, R2, and R3 represent the major fragmentation products generated from crosslinking with 2.
Because of the small number of laddering ions observed in the MS2 spectra of the 1312.60 and 658.8 ions, which is common for basic peptides (28), MS2 peaks were selected and analyzed by MS3 to elucidate the exact peptide sequence. The MS3 spectra of the 1099 peak resulting from the parental 658.80 MH2+ (Supplementary Fig. 6 and 7 and Supplemental Table 2) and 1316.60 MH1+ ions (Supplemental Fig. 3 and Supplemental Table 2) confirmed fragmentation of the 1099 (KK-R2QEEK, Fig.5) and 892 (KK-R3QEEK) ions. The presence of y4 and y3 ions minus one or two NH3 in the fragmentation pattern of the 1099 ion was consistent with a KKQEEK sequence rather than KQEEKK. The MS3 spectra of the 389 ion that was present in the MS2 spectra of the 658.80 MH2+ ion indicated a z3 EEK ion (Fig. 5 and Supplemental Table 2), further confirming the peptide sequence.
Together, these results place the photolabile novobiocin derivatives and accordingly, novobiocin, alongside the peptide 559-KKQEEK-564, and attached directly to K560. This segment is located in the Hsp90 C-terminus, within the region of the C-terminal fragment that is protected from proteolysis upon binding to chlorobiocin and novobiocin.
Modeling of NB's conformation
To further refine the NB binding site in Hsp90, conformations of NB co-crystallized with DNA gyrase/topoisomerase (PDB IDs: 1S14, 1AJ6, 1KIJ) were analyzed. NB was found to adopt a partially folded conformation in each of these structures. This conformation of NB was extracted and used to dock into the site of Hsp90 identified via photoaffinity labeling.
Modeling of the Hsp90 NB binding site
The data generated from the photoaffinity labeling studies with NB provides information regarding the putative binding site of NB. After careful analysis of the homologous peptide in the full length crystal structure of yeast Hsp90 (538-AEREKEIK-545), it was not clear whether there was a pocket large enough to accommodate NB in the regions flanking this peptide. Initial attempts to dock novobiocin with this crystal structure were unsuccessful. However in 2008, the solution structure of the bacterial Hsp90 homologue, HtpG, was determined by Agard and co-workers using SAXS in concert with molecular modeling (21). As the full length yeast crystal structure depicts Hsp90 in its closed, clamped conformation, it was postulated that the extended form, as found in solution, would unveil the C-terminal binding site. Generation of this model allowed for identification of the C-terminal inhibitor binding site using the information garnered from the photoaffinity and proteolytic studies.
In an effort to correlate the binding site identified by photoaffinity studies with the open structure of Hsp90α, a homology model of hHsp90α was generated using the SAXS structure of HtpG as the template. The homology model of hHsp90α was constructed using software “Modeller” (29). The solution structure of HtpG (dimer) was used as a template for building of the model. The modeled structure was subsequently subjected to a molecular dynamics protocol for further refinement.
Docking of NB into the hHsp90α homology was guided by the following constraints: 1) Binding of NB to the C-terminal domain protects residues in the N-terminal region of the construct from proteolysis by V8 protease; 2) proteolytic fingerprinting identified the peptide KKQEEK in human Hsp90α as the site of crosslinking for photo-reactive NB derivative; and 3) the bioactive conformation of NB. NB was docked rigidly in the hHsp90α C-terminus by maintaining a distance constraint of 3.5Å between the hydroxyl of the noviose and the amide nitrogen of Asn686. A multiple sequence alignment of related proteins from different organisms indicated two conserved residues adjacent to the photoaffinity–labeled peptide fragment, Lys538 and Glu562 in hHsp90α. Another residue that is highly conserved is Lys558 in hHsp90α. Minimization of protein side chains with a distance constraint of 3.5 Å between NB and residues Asn686, Lys538, and Glu562 and was carried out while keeping NB and the protein backbone rigid to allow relaxation of the side chains and minimization of negative interactions. The binding site was subsequently defined as residues that reside within 8Å of NB and were subjected to minimization while keeping the rest of the protein and NB fixed to remove unfavorable steric and hydrophobic contacts and to allow side chains to relax and to facilitate NB alignment in the cavity. The resulting complex was further refined by a short MD simulation (100ps). This Hsp90α-NB complex was found to be stable after simulation. (RMSD<2Å) The interactions manifested between NB and Hsp90α are depicted in Figure 6.
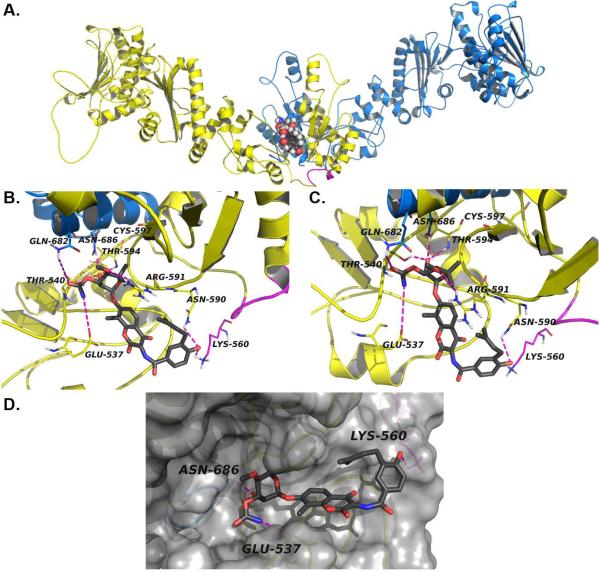
Figure 6. Modeled structure of the Novobiocin binding site in hHsp90α.
a) The Hsp90α homology model homodimer and the C-terminal binding site with NB (spheres) docked at the interface of two monomers (blue and yellow) b) close-up of NB (grey sticks) docked in Hsp90α homology model, the crosslinked fragment (lines) and predicted hydrogen bonds (dashes) are depicted in magenta and c) close-up of NB noviose (grey sticks) docked in Hsp90α homology model, predicted hydrogen bonds (dashes) are represented in magenta, d) surface representation of Hsp90α homology model CT binding site with NB (grey sticks) docked. Only one molecule of NB is shown to be bound to Hsp90 homodimer, an equivalent binding site contained on the other monomer has not been shown for clarity.
It is evident from Figure 6 B and C that NB is responsible for providing important interactions with several of the residues present in the binding site. These interactions can be summarized as follows: 1) A HB interaction between the noviose sugar hydroxyl of novobiocin and Asn686 and Thr540 of hHsp90α; 2) HB interactions between the oxygen of the prenylated hydroxylphenyl ring of novobiocin and both Asn590 and Lys560 of hHsp90α, a residue identified via photoaffinity labeling studies; 3) the CO and -NH of the noviose carbamate of novobiocin is making a HB interactions with Glu537 and Gln682; 4) Thr594 provides HB interactions with the oxygen of the glycosidic bond linking the noviose and coumarin ring; and 5) HB interaction between the noviose methoxy group and Arg591.
Biochemically–guided modeling based on the bacterial homologue of Hsp90, HtpG, produced a human Hsp90 model that upon further refinement, unveiled the binding mode for Hsp90 C-terminal inhibitors. This mode of binding explains many of the previously observed biological and biochemical consequences that result upon Hsp90 C-terminal inhibition.
Although there have been previous attempts to model the NB binding site, the results were not consistent with available biochemical data. Prior models developed for the Hsp90 C-terminal inhibitor binding site were generated using homology modeling, MD simulations, and pocket-finder algorithms(30–31). However, using biochemical analyses, affinity labeling, and homology modeling, we have identified a binding site for C-terminal Hsp90 inhibitors that supports prior biochemical studies.
For example, Neckers and co-workers originally proposed the existence of a binding site in the Hsp90 C-terminus and determined that NB binding was localized to residues 542–732 (hHsp90α) (9–10). Additionally, it was demonstrated that addition of the C-terminal peptide 667–680 (hHsp90α) reduced Hsp90's ability to bind immobilized NB, and Hsp90 binding to immobilized NB could be competed with this peptide (9). In the model proposed, this peptide sequence is located in the α-helix proximal to the sugar binding pocket of NB, and is important for interactions with the noviose moiety on NB. Consistent with this pocket being adjacent to the NB binding site, Hartson and coworkers have shown that NB blocks the AC88 antibody (a monoclonal antibody that recognizes an epitope within the amino acids 668–684 in hHsp90α (32)) from binding Hsp90 (12), thus providing two key pieces of previously reported biochemical data that further supports the location of this binding site.
Furthermore, ATP binding plays an important role in the conformational reorganization of Hsp90, in a manner complementary to the dimerization of two Hsp90 monomers at the C-terminus, which is essential for Hsp90 activity. Once dimerization occurs at the C-terminus, Hsp90 undergoes a complex conformational cycle that facilitates reorganization and folding of client proteins(33–34). The C-terminal inhibitor, Coumermycin A1, was shown to disrupt C-terminal dimerization, and halt the conformational cycle (11). In addition, the protein folding process involves a multitude of cochaperones and partner proteins. Novobiocin and related antibiotics have been shown to disrupt interactions with partner proteins that bind Hsp90 to both the C- and N-terminus, i.e. Cdc37, p23, Hsc70, FKBP52 and PP5(9, 11–12). C-terminal inhibitors also prevent the binding of small molecules to the N-terminus (10). Additionally, prior proteolysis experiments and those presented herein with NB and related family members demonstrate that C-terminal occupation protects Hsp90 from proteolysis by altering its conformational state. Specifically, NB shields Lys615 and Arg620 from cleavage. This result can be explained by the examination of Hsp90 in its various conformational states. Lys615 and Arg620 are solvent exposed on the surface of an alpha helix in the semi-closed and closed states. However, in the extended form, Lys615 and Arg620 are shielded by surrounding residues, and hence, are not accessible to proteases. Interestingly, the pivot point of Hsp90's movement between the middle and C-terminal domains of Hsp90 (hHsp90α residue 550) is located within close proximity to this binding site (20–21, 23). Taken together, these data indicate that conformational changes within the C-terminus occur upon inhibitor binding and cause global conformational changes within the entire homodimer.
Additionally, Retzlaff and co-workers have demonstrated through mutational analyses that Hsp90 is regulated by a switch point in its C-terminal domain(35). Mutation of the residue equivalent to Cys597 in hHsp90α (Ile538 in HtpG) altered Hsp90's ATPase and chaperone activity, modified N-terminal and C-terminal domain associations, and shifted the conformational equilibrium of Hsp90 within its ATPase cycle(35). In our model, Cys597 forms part of the NB binding pocket (Fig. 8B/C). Thus, the model suggests that C-terminal inhibitors have the ability to interact with and stabilize the region around this pivot point, and prohibiting the global conformational changes required for chaperone activity. Consequently, upon C-terminal occupation, the Hsp90 machinery is stalled in the open conformation, which results in protection of Lys615 and Arg620 from proteolysis, hinders N-terminal ATP hydrolysis, and prevents the binding of N-terminal inhibitors. Once again, occupation of this putative binding pocket in the Hsp90 C-terminus can finally explain previously observed biochemical observations.
In addition, Hugel and coworkers recently determined the dynamics and kinetics of C-terminal dimerization, whereby the C-terminus is capable of opening in the presence of N-terminal ligands (i.e. ATP and ATP analogues). Additionally, it was observed that N- and C-terminal dimerization is intimately related, and that C-terminal dimerization is not required for N-terminal dimerization. These findings suggest that the C-terminus is in constant flux, whereby a multitude of conformations exist (24). In the model presented herein, novobiocin predominantly interacts with one monomer of the Hsp90 homodimer. Together with the data presented by Hugel, it can be reasoned that small molecule binding of the Hsp90 C-terminus may occur while this domain is open, preventing dimerization and continuation through the catalytic cycle. Alternatively, C-terminal inhibitors may bind after dimerization, preventing occupation of the N-terminal binding pocket by ATP, which is supported by prior studies by Marcu et. Al. whom demonstrated that novobiocin binding to the C-terminus prevented N-terminal occupation (10).
Hsp90 C-terminal inhibitors have garnered significant attention during the past decade, specifically due to problems associated with N-terminal inhibition as observed in the clinic (36). Structural information regarding the C-terminus, especially the NB binding site, has been scarce, and attempts at co-crystallization have been unsuccessful. Accordingly, effective analogue design and data analysis has been hindered. Therefore, we have utilized photoaffinity labeling and proteolytic studies to identify residues that form the NB binding site. Through elucidation of the cross-linked peptide and molecular modeling alongside the reported solution structure of Hsp90, potential binding sites were carefully evaluated. As a result of these studies and those before us, we are finally able to provide a binding site for NB and other C-terminal inhibitors that accounts for the biological activities manifested by NB. This model provides a new paradigm for the development of future Hsp90 inhibitors that target the C-terminal binding pocket.
METHODS
Characterization of the protease resistant core of Hsp90CT
Recombinant His-tagged Hsp90CT (amino acids 531–732 human Hsp90α) was incubated with V8 protease at 37 °C in the presence of 800 µM Chlorobiocin or an equivalent volume of DMSO (vehicle control). Protease resistant bands were separated by SDS-PAGE, excised, digested with trypsin and analyzed by LC-MS/MS as described in the “Supplementary Methods”.
Identification of the NB binding site in Hsp90CT
After optimization of crosslinking conditions with 1 (Fig. 3), recombinant His-tagged Hsp90CT was exposed to four flashes of 184 μJ of UV radiation in the presence of 0.5 mM of either 1, 2 or DMSO (vehicle control), followed by digestion of the samples with trypsin. The tryptic peptides were subjected to capillary LC-MS/MS experiments using tandem LTQ-FT Mass Spectrometer (ThermoFinnigan) under conditions described previously(37), followed by data analysis as described in “Supplementary Methods”.
Refinement of Hsp90α Structure
The sequence of hHsp90α was used to search similar sequences using NCBI blast.(38) A multiple sequence alignment was performed using ClustalW with default parameters to align these sequences and to identify structurally conserved regions and important residues.(39) The minimization of the side chains was performed using Sybyl molecular modeling software.(40) Initially, the protein was held fixed except for the binding site residues (residues falling within 8Å of novobiocin molecule). Thereafter, all side chains and the binding site were kept free and minimization was done. All the minimization was done until the RMS gradient of 0.05 Kcal/MolÅ was obtained using AMBER9 force field.
Molecular Dynamics Protocol
Hsp90α-NB was prepared for simulation in AMBER9 package while the simulation was done using NAMD software.(41) The force field parameters for NB were calculated by antechamber module of AMBER9. The HtpG-NB complex was solvated in a box of water with buffering distance of 10 Å; assuming normal charge states of ionizable groups corresponding to pH 7, sodium (Na+) and chloride (Cl−) counter-ions were added to achieve charge neutrality and to mimic biological environment more closely. All Na+ and Cl− ions were placed at least 8 Å away from any protein atoms and from each other. The system was subjected to initial minimization for 20000 steps (40ps) keeping protein backbone fixed which was followed by 20000 steps (40ps) of minimization without fixing anything (to allow system to relax freely). Further details of the molecular dynamics protocol used to compute the final model and Homology modeling of the NB binding site in Hsp90α are presented in “Supplemental Materials and Methods”
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of this project by NIH CA120458 (B.S.J.B), the Oklahoma Agricultural Experiment Station (Project No. 1975), NIH CA125392 (R.L.M), NIH Training Grant (T32 GM008545) on Dynamic Aspects in Chemical Biology (L.B.P.), ACS Division of Medicinal Chemistry Predoctoral Fellowship (L.B.P.), the Concern Foundation (CF0406, S.D.H.) and NSF MRI and EPSCoR programs (award #0722494: S.D.H.). We would also like to thank D. Agard for the coordinates of the open HtpG SAX structure.
Footnotes
Supporting Information Available This material is free of charge via the Internet
REFERENCES
- 1.Pearl LH, Prodromou C. Structure and Mechanism of the Hsp90 Molecular Chaperone Machinery. Annu. Rev. Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 2.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem. J. 2008;410:439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Bishop SC, Burlison JA, Blagg BSJ. Hsp90: a novel target for the disruption of multiple signaling cascades. Curr. Cancer Drug Tar. 2007;7:369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- 5.McDonald E, Workman P, Jones K. Inhibitors of the Hsp90 molecular chaperone: attacking the master regulator in cancer. Curr. Top. Med. Chem. 2006;6:1091–1107. doi: 10.2174/156802606777812004. [DOI] [PubMed] [Google Scholar]
- 6.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 7.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: Targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 8.Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee W. Heat shock protein 90: inhibitors in clinical trials. J. Med. Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 9.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 10.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl. Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 11.Allan RK, Mok D, Ward BK, Ratajczak T. Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J. Biol. Chem. 2006;281:7161–7171. doi: 10.1074/jbc.M512406200. [DOI] [PubMed] [Google Scholar]
- 12.Yun B-G, Huang W, Leach N, Hartson SD, Matts RL. Novobiocin induces a distinct conformation of Hsp90 and alters Hsp90-cochaperone-client interactions. Biochemistry. 2004;43:8217–8229. doi: 10.1021/bi0497998. [DOI] [PubMed] [Google Scholar]
- 13.Galam L, Hadden MK, Ma Z, Ye QZ, Yun BG, Blagg BS, Matts RL. High-throughput assay for the identification of Hsp90 inhibitors based on Hsp90-dependent refolding of firefly luciferase. Bioorg. Med. Chem. 2007;15:1939–1946. doi: 10.1016/j.bmc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burlison JA, Avila C, Vielhauer G, Lubbers DJ, Holzbeierlein J, Blagg BS. Development of novobiocin analogues that manifest anti-proliferative activity against several cancer cell lines. J. Org. Chem. 2008;73:2130–2137. doi: 10.1021/jo702191a. [DOI] [PubMed] [Google Scholar]
- 15.Burlison JA, Blagg BS. Synthesis and Evaluation of Coumermycin A1 Analogues that Inhibit the Hsp90 Protein Folding Machinery. Org. Lett. 2006;8:4855–4858. doi: 10.1021/ol061918j. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly A, Blagg BS. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr. Med. Chem. 2008;15:2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews SB, Vielhauer GA, Manthe CA, Chaguturu VK, Szabla K, Matts RL, Donnelly AC, Blagg BS, Holzbeierlein JM. Characterization of a novel novobiocin analogue as a putative C-terminal inhibitor of heat shock protein 90 in prostate cancer cells. Prostate. 2010;70:27–36. doi: 10.1002/pros.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelton SN, Shawgo ME, Matthews SB, Lu Y, Donnelly AC, Szabla K, Tanol M, Vielhauer GA, Rajewski RA, Matts RL, Blagg BS, Robertson JD. KU135, a novel novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells. Mol. Pharmacol. 2009;76:1314–1322. doi: 10.1124/mol.109.058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu XM, Shen G, Neckers L, Blake H, Holzbeierlein J, Cronk B, Blagg BS. Hsp90 inhibitors identified from a library of novobiocin analogues. J. Am. Chem. Soc. 2005;127:12778–12779. doi: 10.1021/ja0535864. [DOI] [PubMed] [Google Scholar]
- 20.Krukenberg KA, Bottcher UM, Southworth DR, Agard DA. Grp94, the endoplasmic reticulum Hsp90, has a similar solution conformation to cytosolic Hsp90 in the absence of nucleotide. Protein Sci. 2009;18:1815–1827. doi: 10.1002/pro.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krukenberg KA, Forster F, Rice LM, Sali A, Agard DA. Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure. 2008;16:755–765. doi: 10.1016/j.str.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bron P, Giudice E, Rolland JP, Buey RM, Barbier P, Diaz JF, Peyrot V, Thomas D, Garnier C. Apo-Hsp90 coexists in two open conformational states in solution. Biol. Cell. 2008;100:413–425. doi: 10.1042/BC20070149. [DOI] [PubMed] [Google Scholar]
- 23.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol. Cell. 2008;32:631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratzke C, Mickler M, Hellenkamp B, Buchner J, Hugel T. Dynamics of heat shock protein 90 C-terminal dimerization is an important part of its conformational cycle. P. Natl. Acad. Sci. 2010;107:16101–16106. doi: 10.1073/pnas.1000916107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellon S, Parsons JD, Wei Y, Hayakawa K, Swenson LL, Charifson PS, Lippke JA, Aldape R, Gross CH. Crystal structures of Escherichia coli topoisomerase IV ParE subunit (24 and 43 kilodaltons): a single residue dictates differences in novobiocin potency against topoisomerase IV and DNA gyrase. Antimicrob. Agents Ch. 2004;48:1856–1864. doi: 10.1128/AAC.48.5.1856-1864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holdgate GA, Tunnicliffe A, Ward WH, Weston SA, Rosenbrock G, Barth PT, Taylor IW, Pauptit RA, Timms D. The entropic penalty of ordered water accounts for weaker binding of the antibiotic novobiocin to a resistant mutant of DNA gyrase: a thermodynamic and crystallographic study. Biochemistry. 1997;36:9663–9673. doi: 10.1021/bi970294+. [DOI] [PubMed] [Google Scholar]
- 27.Shen G, Yu X. m., Blagg BSJ. Syntheses of photolabile novobiocin analogues. Bioorg. Med. Chem. Lett. 2004;14:5903–5906. doi: 10.1016/j.bmcl.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Tang XJ, Thibault P, Boyd RK. Fragmentation reactions of multiply-protonated peptides and implications for sequencing by tandem mass spectrometry with low-energy collision-induced dissociation. Anal. Chem. 1993;65:2824–2834. doi: 10.1021/ac00068a020. [DOI] [PubMed] [Google Scholar]
- 29.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 30.Sgobba M, Degliesposti G, Ferrari AM, Rastelli G. Structural models and binding site prediction of the C-terminal domain of human Hsp90: a new target for anticancer drugs. Chem. Biol. Drug Des. 2008;71:420–433. doi: 10.1111/j.1747-0285.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- 31.Sgobba M, Forestiero R, Degliesposti G, Rastelli G. Exploring the Binding Site of C-Terminal Hsp90 Inhibitors. J Chem. Inf. Comp. Sci. 2010;50:1522–1528. doi: 10.1021/ci1001857. [DOI] [PubMed] [Google Scholar]
- 32.Hartson SD, Thulasiraman V, Huang W, Whitesell L, Matts RL. Molybdate Inhibits Hsp90, Induces Structural Changes in Its C-Terminal Domain, and Alters Its Interactions with Substrates†. Biochemistry. 1999;38:3837–3849. doi: 10.1021/bi983027s. [DOI] [PubMed] [Google Scholar]
- 33.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter K, Soroka J, Skalniak L, Leskovar A, Hessling M, Reinstein J, Buchner J. Conserved Conformational Changes in the ATPase Cycle of Human Hsp90. J. Biol. Chem. 2008;283:17757–17765. doi: 10.1074/jbc.M800540200. [DOI] [PubMed] [Google Scholar]
- 35.Retzlaff M, Stahl M, Eberl HC, Lagleder S, Beck J, Kessler H, Buchner J. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep. 2009;10:1147–1153. doi: 10.1038/embor.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duerfeldt AS, Blagg BSJ. Hsp90 inhibition: Elimination of shock and stress. Bioorg. Med. Chem. Lett. 2010;20:4983–4987. doi: 10.1016/j.bmcl.2010.06.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikehata K, Duzhak TG, Galeva NA, Ji T, Koen YM, Hanzlik RP. Protein Targets of Reactive Metabolites of Thiobenzamide in Rat Liver in Vivo. Chem. Res. Toxicol. 2008;21:1432–1442. doi: 10.1021/tx800093k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 39.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 40.SYBYL p 7.3, Tripos International, 1699 South Hanley Rd., St. Louis, Missouri, 63144, USA.
- 41.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.