Abstract
Rationale
The molecular correlate of the calcium release-activated calcium current (ICRAC), the channel protein Orai1, is upregulated in proliferative vascular smooth muscle cells (VSMC). However, the role of Orai1 in vascular disease remains largely unknown.
Objective
The goal of this study was to determine the role of Orai1 in neointima formation after balloon-injury of rat carotid arteries and its potential upregulation in a mouse model of VSMC remodeling.
Methods and Results
Lentiviral particles encoding short-hairpin RNA (shRNA) targeting either Orai1 (shOrai1) or STIM1 (shSTIM1) caused knockdown of their respective target mRNA and proteins and abrogated store-operated calcium entry and ICRAC in VSMC; control shRNA was targeted to luciferase (shLuciferase). Balloon-injury of rat carotid arteries upregulated protein expression of Orai1, STIM1 and calcium-calmodulin kinase IIdelta2 (CamKIIδ2); increased proliferation assessed by Ki67 and PCNA and decreased protein expression of myosin heavy chain in medial and neointimal VSMC. Incubation of the injured vessel with shOrai1 prevented Orai1, STIM1 and CamKIIδ2 upregulation in the media and neointima; inhibited cell proliferation and markedly reduced neointima formation 14 days post injury; similar results were obtained with shSTIM1. VSMC Orai1 and STIM1 knockdown inhibited nuclear factor for activated T-cells (NFAT) nuclear translocation and activity. Furthermore, Orai1 and STIM1 were upregulated in mice carotid arteries subjected to ligation.
Conclusions
Orai1 is upregulated in VSMC during vascular injury and is required for NFAT activity, VSMC proliferation and neointima formation following balloon-injury of rat carotids. Orai1 provides a novel target for control of VSMC remodeling during vascular injury or disease.
Keywords: Calcium channels, CRAC channels, vascular smooth muscle proliferation, neointima formation
Introduction
Vascular smooth muscle cell (VSMC) proliferation is a major contributor to vascular occlusive diseases such as atherosclerosis, hypertension and neointimal hyperplasia after angioplasty. Damage to blood vessel walls triggers pathological changes which include the switch of medial VSMC from a contractile to a synthetic phenotype. In response to injury, VSMCs proliferate and migrate across the internal elastic lamina towards the vascular lumen, produce extracellular matrix, form the structure called neointima, and result in narrowing of vessel lumen with devastating consequences on cardiovascular function.
Calcium (Ca2+) signaling plays an essential role in VSMC phenotypic changes1. A major Ca2+ influx pathway activated in response to extracellular agonits is the store-operated Ca2+ entry (SOCE) pathway which is initiated by phospholipase C (PLC) activation and subsequent IP3-mediated discharge of internal Ca2+ Stores2-5. SOCE is associated with a highly Ca2+ selective conductance called Ca2+ release-activated Ca2+ current (ICRAC) first identified in mast cells6 and subsequently in other cell types, including VSMC7-9. The molecular components of ICRAC have been recently discovered and belong to two protein families: The Ca2+ sensor STIM1 located mainly in the endoplasmic reticulum (ER)10, 11 and the Ca2+ channel Orai1 expressed at the plasma membrane (PM)12-14. Decrease of Ca2+ concentration within the lumen of the ER causes STIM1 to reorganize into punctate structures and relocate to discrete regions of close ER/PM contacts to activate Orai1 through binding of a minimal 107 amino acids C-terminal region of STIM1 (called SOAR/CAD)15, 16 to the C- and N-termini of Orai1. Mammals have another STIM protein, STIM2 and two Orai proteins, Orai2 and Orai3 that are also activated by STIM proteins when ectopically co-expressed in the HEK293 system. We have recently provided evidence for an endogenous ICRAC-like current encoded by Orai317 while Orai2 contribution to native Ca2+ conductances remains unknown. Members of the transient receptor potential canonical (TRPC) family of cation channels have also been suggested to be the molecular correlates of non-selective SOCE conductances that are distinct from ICRAC18, 19.
We and others have demonstrated that Orai1 and STIM1 are upregulated in synthetic proliferative VSMCs9, 20. Data from our laboratory showed that either STIM1 or Orai1 knockdown using silencing RNA (siRNA) inhibited SOCE, ICRAC and synthetic VSMC proliferation and migration in response to serum and platelet-derived growth factor (PDGF)9, 21. We also showed that Orai1 and STIM1 were upregulated in medial and neointimal VSMC from rat carotids after balloon injury21, suggesting that these proteins are involved in vascular pathophysiology. Interestingly, two recent reports have specifically addressed the role of STIM1 in VSMC remodeling in vivo and independently reported that STIM1 in vivo knockdown inhibited neointima formation after balloon injury in rat carotids22, 23. However, the mechanism of action of STIM1 and the exact PM ion channel that mediates STIM1 function during VSMC remodeling in vivo is unclear. In addition to its role in activating Orai1-mediated ICRAC, STIM1 positively regulates the function of Orai2, Orai3 and Orai1/Orai3 heteromultimeric channels in their store-operated mode24-27 and that of the store-independent Orai1/Orai3 arachidonate-regulated Ca2+ (ARC) channel28 as well as that of most TRPC channels 18, 29, 30. Further, STIM1 has recently been shown to regulate the function of L-type Ca2+ channels in different cell types, including VSMCs31, 32.
The significance of Orai1 upregulation during vascular injury, its interdependence on STIM1 and its role in VSMC remodeling and neointima formation in vivo is an important question that has remained unanswered. Therefore, in this study we comparatively tested the role of Orai1 and STIM1 in VSMC proliferation and neointima formation in vivo using the rat carotid artery balloon injury model. We found that either Orai1 or STIM1 in vivo knockdown is sufficient to inhibit VSMC proliferation and neointima formation to a similar extent. We show that Orai1 is required for nuclear translocation and activation of nuclear factor for activated T-cells (NFAT) in VSMCs. Furthermore, we report that Orai1 and STIM1 upregulation is also a feature of another model of vascular remodeling: the carotid ligation in mice, highlighting the importance of Orai1-mediated ICRAC in smooth muscle phenotypic switching and unraveling Orai1 as a potential target for vascular occlusive diseases.
Materials and Methods
All antibodies/reagents concentrations, dilutions and origins are described in detail in online supplement. Cell culture, generation of lentiviruses and surgeries were conducted using standard procedures. An expanded material and methods section is available in online supplement.
Results
VSMC Orai1 and STIM1 knockdown using lentiviral particles-encoding shRNA abrogates SOCE and ICRAC
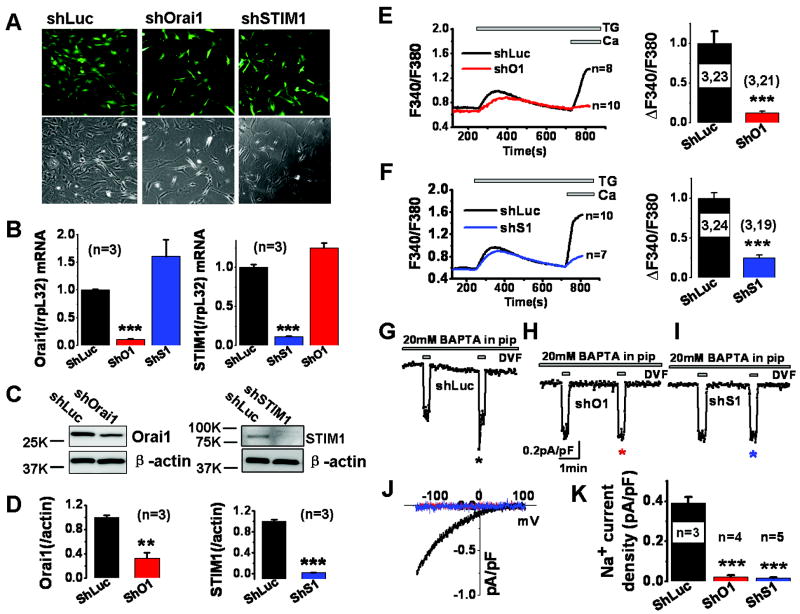
ShRNA sequences targeting either Orai1 or STIM1 were cloned under the control of the H1 promoter in the lentiviral vector pFUGW-GFP which separately encodes green fluorescent protein (GFP) under the control of the Ub-c promoter. Lentiviral particles were produced and used to infect synthetic cultured rat aortic VSMCs. These viral particles were efficient at infecting 90-95% of VSMCs, as visualized by GFP fluorescence (Figure 1A). Infection of VSMC with either shOrai1 or shSTIM1 viruses caused marked decrease in cell number (Figure 1A), consistent with previous studies demonstrating a role for Orai1/STIM1 in VSMC proliferation9, 21. Real-time PCR showed that shOrai1 viruses reduced their respective mRNA levels with no significant effect on STIM1 mRNA levels; similar results were obtained with shSTIM1 viruses (Figure 1B). Orai1 and STIM1 protein knockdown after shRNA treatment was documented by western blotting (Figure 1C, D). ShOrai1 viruses reduced Orai1 mRNA and protein levels by 88.7± 0.76% and 67.4± 9.34% respectively while shSTIM1 viruses reduced STIM1 mRNA and protein levels by 88.8± 0.53% and 98.1± 0.66% respectively; n=3 (Figure 1B, D). Furthermore, shOrai1 had no significant effect on expression of Orai2 and Orai3 homologs as measured using real-time PCR (Supplemental Figure I-A), ruling out potential cross-effects of shOrai1 on Orai2 and Orai3. Ca2+ imaging was performed on selected VSMCs displaying strong GFP fluorescence, thus selecting only cells with almost complete knockdown. ShOrai1 and shSTIM1 viruses inhibited the extent of SOCE activated by thapsigargin in VSMC by 87.7± 2.38% and 74.8± 2.98% n=21 and 19 respectively as compared to cells infected with shLuciferase lentivirus (Figure 1E, F); the remaining SOCE in cells infected with shSTIM1 viruses despite almost complete STIM1 protein downregulation is likely due to native STIM2, consistent with previous studies10. The inhibition of SOCE upon Orai1 knockdown could be effectively rescued by expressing siRNA-resistant cyan fluorescent protein (CFP)-Orai1clone in VSMC (Supplemental Figure I-B, C). Previous studies from our lab and others have shown that Orai2 and Orai3 do not contribute to SOCE in rat and human VSMCs9, 21, 33. Importantly, shOrai1 and shSTIM1 viruses dramatically inhibited ICRAC (by comparison to shLuciferase) measured by whole cell patch clamp after passive store depletion with dialysis of 20mM of the chelator BAPTA through the patch pipette (Figure 1G-K). ICRAC was measured in a bath solution containing 20mM Ca2+ (Ca2+ ICRAC and was amplified using short pulses (30 seconds) of standard divalent free (DVF; Na+ ICRAC) solutions as described previously8, 9. ICRAC showed typical current depotentiation in DVF solutions (Figure 1G). ShOrai1 inhibited Ca2+ ICRAC and Na+ ICRAC by 90% and 95% respectively [Na+ ICRAC values are reported as Ca2+ ICRAC values are too small to be reliable: control, 0.39± 0.02 pA/pF, n=3; shOrai1, 0.02± 0.01 pA/pF, n=4 at -100mV. ShSTIM1 inhibited Ca2+ ICRAC and Na+ ICRAC by 92% and 96% respectively [shSTIM1, 0.01±0.004 pA/pF (Na+ ICRAC) at -100mV; n=5 (Figure 1K)]. Current-voltage (I/V) relationships for Na+ ICRAC are shown in Figure 1J; statistical analyses on Na+ ICRAC recordings from different cells are shown in Figure 1K.
Figure 1. Effect of shOrai1 and shSTIM1 lentiviruses on SOCE and ICRAC.
A. Cultured VSMCs were infected with either shLuciferase, shOrai1 or shSTIM1 lentiviruses and infected cells are visualized by GFP fluorescence. Phase pictures are shown to gauge the infection efficiency. Note the reduced cell number in shOrai1 and shSTIM1 conditions compared to shLuciferase control. B. quantitative PCR shows that shOrai1 and shSTIM1 lentiviruses specifically and efficiently reduced their target mRNA (normalized to rpl32 mRNA) 5 days post infection. ShOrai1and shSTIM1 infection of VSMCs caused a small (not statistically significant) increase in STIM1 and Orai1 mRNA respectively. C. Western blotting shows that shOrai1 and shSTIM1 lentiviruses caused efficient protein knockdown of their respective proteins 5 days post infection. D. Statistical analysis of Orai1 and STIM1 band densitometry normalized to β-actin is shown. E. shOrai1 lentiviral infection essentially abrogated SOCE activated by thapsigargin (2μM) compared to shLuciferase lentivirus control in VSMCs; statistical analysis from 3 independent experiments totaling 21 cells is depicted (control shluciferase, 3 experiments, n=23). F. shSTIM1 lentiviral infection also caused substantial reduction in SOCE activated by thapsigargin (2μM) compared to shLuciferase lentivirus control in VSMCs; statistical analysis from 3 independent experiments totaling 19 cells is depicted (control shluciferase, 3 experiments, n=24). G-I. Whole cell patch clamp recordings of ICRAC activated in VSMCs by dialysis of 20mM BAPTA through the patch pipette and showing substantial decrease of Ca2+ ICRAC currents (at -100mV) recorded in 20mM external Ca2+ and Na+ ICRAC currents recorded in divalent free (DVF) external solutions, from shOrai1- (n=4) and shSTIM1-infected VSMCs (n=5) compared to shLuciferase-infected VSMCs (n=3). A DVF pulse was applied immediately after break-in to gauge leak current, which was subsequently subtracted from the DVF current obtained after store depletion. J. I/V relationships of Na+ ICRAC from VSMCs infected with shLuciferase, shOrai1 or shSTIM1 lentiviruses were taken where indicated by the color-coded asterisks. K. Statistical analyses on Ca2+ ICRAC and Na+ ICRAC currents taken at -100mV from several independent VSMCs infected with shLuciferase, shOrai1 or shSTIM1 lentiviruses.
Rat carotid balloon injury causes increased Orai1/STIM1 protein expression and VSMC proliferation
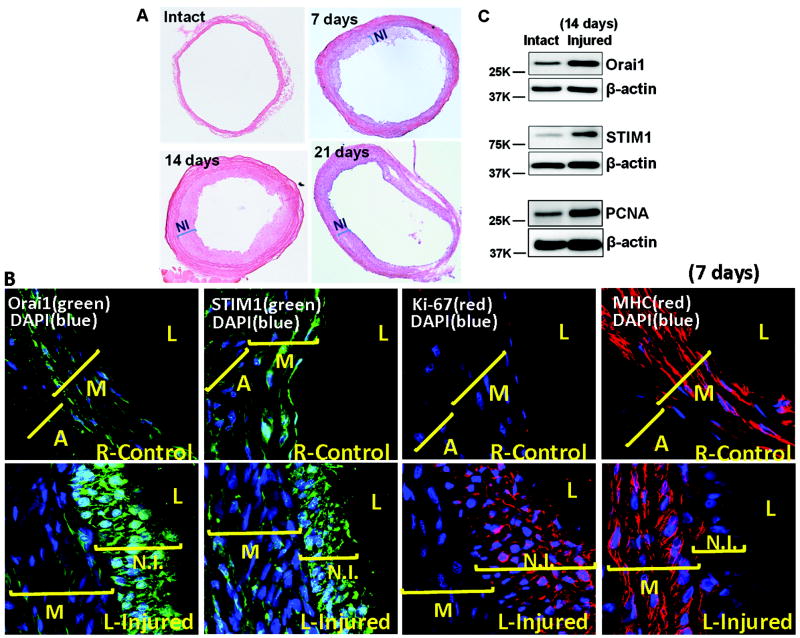
We performed balloon injury on rat left carotids as described in methods; right carotids were used as internal controls. Carotid injury causes significant neointima formation at 7 day post injury with peak neointima observed at 14 day post injury (Figure 2 A), consistent with previous studies34. Left injured and right control carotid arteries were isolated, sectioned and subjected to immunofluorescence (IF) staining using Orai1, STIM1, Ki67 and myosin heavy chain (MHC) specific antibodies, as described in the methods. Figure 2B shows IF staining on carotid arteries 7 days post injury where Orai1 and STIM1 proteins are clearly upregulated in neointimal VSMCs from left injured carotids arteries compared to right control carotids. Orai1/STIM1 protein upregulation in neointimal VSMCs from injured left carotids compared to medial VSMCs from right carotids was confirmed by Western Blotting 14 day post injury (Figure 2C). Western blots also show increased expression of proliferating cell nuclear antigen (PCNA) in injured carotids at day 14 post-injury, suggesting increased cell proliferation (Figure 2C). Ki67 staining was used as an additional marker of cell proliferation at 7 day post injury and clearly shows positive staining of neointimal VSMCs from injured left carotids while right carotids display no significant Ki67 expression. While right control carotids show robust staining of the contractile VSMC marker MHC in the media layer, injured left carotids show reduced MHC staining in medial VSMCs and dramatic decrease in the neointimal layer (Figure 2B).
Figure 2. Orai1/STIM1 proteins and balloon carotid injury in rats.
A. H&E staining of balloon-injured left carotid artery sections showing increased neointima as early as 7 day after injury compared to sham-operated arteries (intact). Neointima formation peaks at 14 days post injury and is still prominent 21 day post-injury. B. IF staining with specific antibodies shows increased protein expression of Orai1 and STIM1in neointima of left injured carotid arteries 7 day post injury in comparison with right non-injured control carotids. IF staining also show increased expression of the proliferative marker Ki67 and decreased expression of VSMC contractile marker myosin heavy chain (MHC) in neointimal layers of balloon-injured left carotid artery sections compared to right non-injured control carotids. C. Western blotting on medial and neointimal VSMC from balloon-injured left carotid arteries compared with the medial layer from sham-operated arteries showing increased protein expression of Orai1, STIM1 and the proliferative marker PCNA in balloon-injured left carotid arteries 14 day post-injury. A, adventitia; M, media; NI, Neointima; L, leumen.
Effects of in vivo infection with shOrai1 and shSTIM1 lentiviruses on VSMC proliferation and protein expression
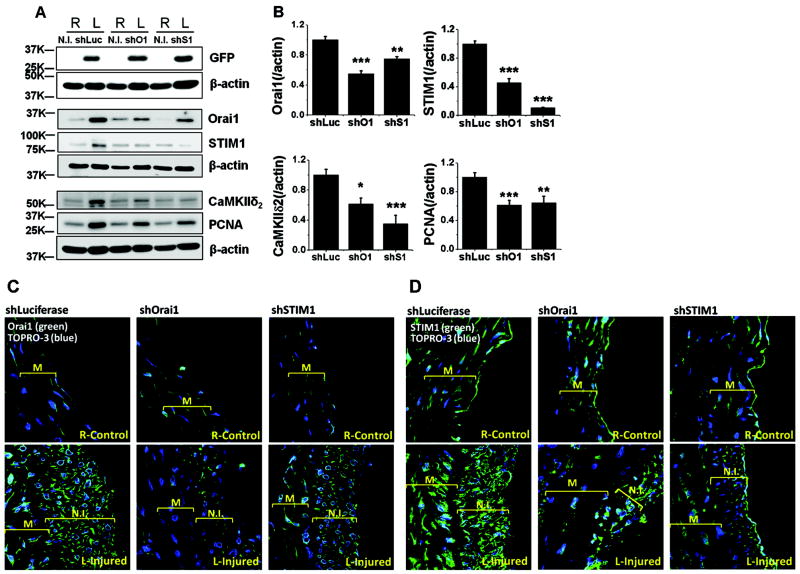
A series of vascular injury experiments were conducted on left carotids of rats to evaluate the effect of in vivo knockdown of Orai1 and STIM1 on VSMC proliferation and protein expression. Left carotids were injured and treated with either shLuciferase, shOrai1 or shSTIM1 lentiviruses for 30 minutes as outlined in the methods; right carotids were neither injured nor treated with lentiviruses (N.T) and were used throughout as internal controls. As shown in Figure 3A, left carotid arteries were successfully infected by lentiviruses as demonstrated by positive expression of GFP; right non-injured and non-infected show no GFP expression (Figure 3A). Immunohisto-chemistry (IHC) showed positive GFP staining in vessel sections injured and treated with shOrai1 lentiviruses (Supplemental Figure II). Injured left carotid arteries treated with shLuciferase show a dramatic increase in protein expression of Orai1 and STIM1. These injured shLuciferase-treated carotids also showed upregulation of the CamKIIδ2 isoform, a major downstream effector of Ca2+ signaling in synthetic VSMCs and an important determinant in neointima formation34,35. Importantly, injured shLuciferase-treated left carotids showed increased PCNA expression indicating increased VSMC proliferation after injury. Treatment of injured left carotids with shOrai1 lentiviral particles substantially prevented the upregulation of Orai1 protein and the proliferative marker PCNA 14 day post-injury (Figure 3A, B). Interestingly, shOrai1 also prevented upregulation of STIM1 and CamKIIδ2 proteins. This is in contrast to in vitro knockdown of Orai1 in proliferative VSMCs which had no effect on STIM1 or Orai2/3 expression (Figure 1B & Supplemental Figure I-A), suggesting that chronic in vivo Orai1 knockdown is complex and prevents VSMC from injured carotids to acquire markers associated with switch to proliferative phenotypes (Figure 3A, B). In a manner similar to shOrai1, shSTIM1 was able to substantially prevent STIM1, Orai1, PCNA and CamKIIδ2 upregulation (Figure 3A, B). Figure 3B shows statistical analysis on western blotting densitometry of Orai1, STIM1, CamKIIδ2 and PCNA proteins normalized to corresponding β-actin from 6 rats per condition.
Figure 3. Orai1/STIM1 ShRNA lentiviral infection, VSMC proliferation and protein expression.
A. ShLuciferase, shOrai1 and shSTIM1 lentiviruses efficiently infected carotid vessels as evidenced with expression of GFP in medial and neointimal protein extracts from left carotid arteries that are balloon-injured (14 day after injury) and infected with viral particles; right non-infected carotids show no GFP expression. ShOrai1 inhibited upregulation of Orai1 protein as well as that of STIM1 and CamKIIδ2, 14 day post-injury. Similarly, shSTIM1 inhibited upregulation of STIM1 protein as well as that of Orai1 and CamKIIδ2. ShOrai1 and shSTIM1 inhibited VSMC proliferation as evidenced by reduced protein expression of PCNA in medial and neointimal protein extracts from balloon-injured left carotids treated with shOrai1 and shSTIM1 lentiviruses compared to injured left carotids treated with shLuciferase lentiviruses. B. Statistical analyses on Orai1, STIM1, CamKIIδ2 and PCNA protein expression data on extracts of medial and neointimal VSMC from ballon-injured left carotids and treated with shLuciferase, shOrai1 or shSTIM1 lentiviruses. Data represent densitometry on protein bands with average ± SEM from 6 rats per condition, determined using Image J and normalized to βactin expression. C-D. IF using specific anti-Orai1 (C) and anti-STIM1 (D) antibodies on left carotid sections (14 day after injury) injured and treated with shLuciferase, shOrai1 or shSTIM1 lentiviruses (bottom) and corresponding right non-injured non-treated control carotids (top).
IF experiments using antibodies against Orai1, STIM1 and CamKIIδ2 were performed on right (control) and left carotids (injured and treated with shRNA), 14 days post-injury (Figure 3C, D). Orai1 (C), STIM1 (D) and CamKIIδ2 (supplemental figure III) protein expression are clearly increased in medial and neointimal VSMC from injured and shLuciferase-treated left carotids. Treatment with shOrai1 caused decrease protein expression of Orai1 (Figure 3C; See also supplemental figure IV), STIM1 (Figure 3D) and CamKIIδ2 (supplemental figure III) in medial and neointimal VSMC compared to shLuciferase-treated carotids; similar results were obtained when injured left carotids were treated with shSTIM1 viruses (Figure 3C, D). Secondary antibodies controls are shown in supplemental figure IV.
Orai1 and STIM1 in vivo knockdown prevents neointima formation
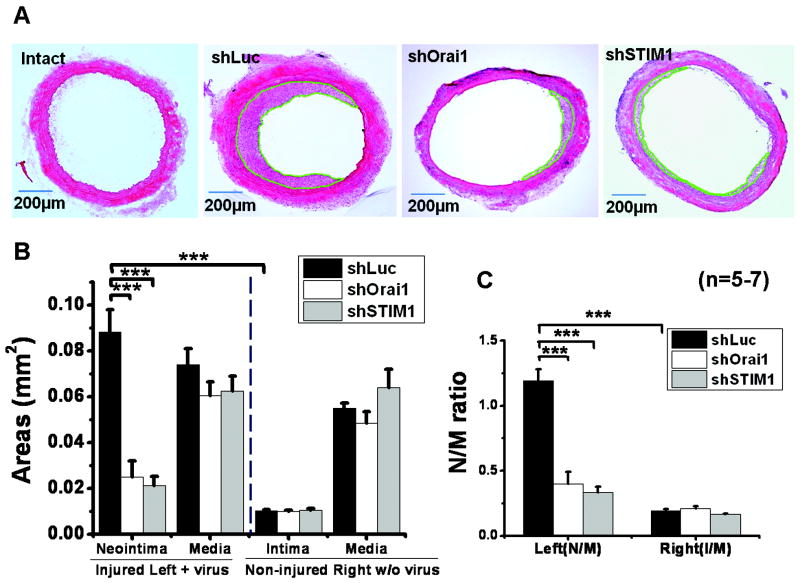
Left carotid injury followed by in vivo lentiviral infection with shLuciferase, shOrai1 and shSTIM1 were performed as described above. Figure 4A shows H&E staining of carotid artery cross sections from sham-operated control non-injured (intact) and balloon-injured and treated with lentiviral particles encoding either shLuciferase (shLuc; n=7), shOrai1 (n=5) or shSTIM1 (n=5) 14 days after injury. Treatment with shOrai1 or shSTIM1 dramatically attenuated neointima formation as compared to shLuciferase (Figure 4A). Figure 4B shows statistical analysis of neointima and media layer areas (in mm2) from left injured carotids and intima and media layer areas from right non-injured carotids obtained from 5-7 independent rats per condition and determined using the Image J Software. The size of neointima is dramatically increased 14 day after injury and this increase is largely attenuated by shOrai1 and shSTIM1. However, the medial layer area from injured carotids treated with shLuciferase is not significantly different from that of carotids injured and treated with shOrai1 or shSTIM1 or that of non-injured right carotid controls (Figure 4B). Figure 4C represents statistical analysis of area ratios of neointima/media (N/M) and clearly shows increased N/M ratios in injured and shLuciferase-treated left carotids; these N/M ratios were substantially reduced in left carotid arteries that were injured and treated with either shOrai1 or shSTIM1 lentiviruses (Figure 4C). Supplemental Table I depicts data on vessel and lumen size measurements from conditions.
Figure 4. ShOrai1 and shSTIM1 lentiviral infection after balloon-injury inhibited neointima formation.
A. H&E staining on left carotid artery sections from either sham-operated (intact) or balloon-injured rats treated with shLuciferase (shLuc), shOrai1 or shSTIM1 lentiviruses 14 day post-injury and lentiviral treatment. B,C. Statistical analyses on medial and neointimal areas (mm2; B) and media/neointima (M/N) ratios (C) from 5-7 independent rats per condition determined using the Image J software from left injured and treated with lentiviruses and right non injured non-treated controls. Neointimal regions are highlighted in green.
We next sought to evaluate whether STIM1 and Orai1 contribution to VSMC proliferation is mediated by constitutive STIM1/Orai1-mediated SOCE due to pre-coupling of STIM1/Orai1 in synthetic proliferative VSMCs. We failed to detect any basal (in absence of store depletion) SOCE activity in synthetic VSMCs using either Ca2+ imaging or patch clamp electrophysiology (Supplemental Figure V). Confocal microscopy on ectopically expressed eYFP-STIM1 in VSMCs showed no STIM1 puncta under resting conditions; STIM1 puncta was observed only after thapsigargin treatment (Supplemental Figure VI-A). Similarly, co-expression of eYFP-STIM1 and CFP-Orai1 showed co-localization only after store depletion (Supplemental Figure VI-B), suggesting that the contribution of STIM1/Orai1 to VSMC remodeling is likely regulated by growth/vasoactive factors.
Orai1/STIM1 knockdown inhibits NFAT nuclear translocation and activity
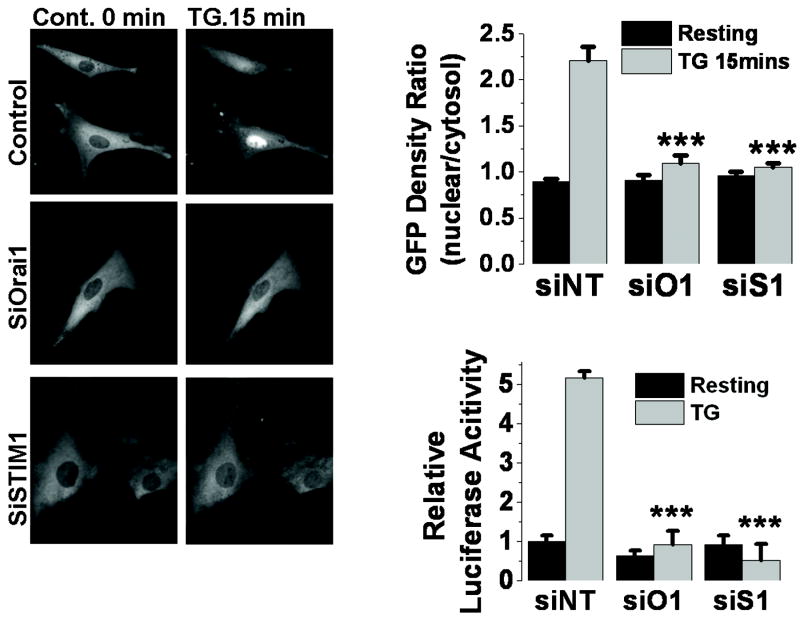
To delineate downstream pathways involved in Orai1- and ICRAC-mediated increase in VSMC proliferation, we sought to evaluate the contribution of VSMC Orai1-mediated ICRAC to the activation of the transcription factor, nuclear factor for activated T cells (NFAT), a known downstream target of ICRAC in lymphocytes. Synthetic VSMC were transfected with a plasmid encoding NFAT-GFP fusion protein and either siOrai1, siSTIM1 or non-targeting control siRNA and NFAT nuclear translocation was monitored by following GFP fluorescence before and after activation of SOCE by 2μM thapsigargin for 15 minutes. Knockdown of either Orai1 (n=15) or STIM1 (n=11) drastically inhibited NFAT nuclear translocation compared to siRNA control (n=28; Figure 5A, B). The average % of inhibition of NFAT nuclear translocation was 85.4% for siOrai1 and 92.3% for siSTIM1.
Figure 5. Orai1 and STIM1 knockdown prevents NFAT nuclear translocation and activity.
A. VSMCs were transfected with a plasmid encoding NFAT-GFP after transfection with either siRNA against Orai1 (siO1), STIM1 (siS1) or non-targeting siRNA control (siNT). NFAT-GFP nuclear translocation in response to thapsigargin (2μM for 15 minutes) was monitored under fluorescence microscope. B. Data was quantified using Image J and efficiency of NFAT-GFP translocation was represented as a ratio of GFP fluorescence in nucleus/cytosol. C. Luciferase reporter assays with and without 2μM thapsigargin treatment of VSMCs transfected with siOrai1 (siO1), siSTIM1 (siS1) or non-targeting siRNA (siNT).
We also used a luciferase reporter assay to directly measure NFAT activity upon VSMC transfection with siOrai1, siSTIM1 or control siRNA as described in methods. NFAT activity was determined using the NFAT-driven luciferase construct (pIL2-Luc) co-transfected with the Renilla-luciferase vector (as an internal control) in VSMC previously transfected with different siRNA. SiOrai1- (n=6) and siSTIM1-transfected VSMCs (n=4) displayed essentially abrogated luciferase activities compared to control siRNA-transfected cells (n=6; Figure 5C).
Orai1/STIM1 are upregulated in carotids from mice subjected to ligation injury
To determine a potential broader role of Orai1/STIM1, we investigated the upregulation of Orai1 and STIM1 in carotid artery sections from mice subjected to disturbed flow or increased shear stress due to carotid ligation. IHC staining with specific antibodies against Orai1 and STIM1 in ligation-injured carotids (at day 21 post-ligation) from mice shows marked upregulation of Orai1 and STIM1 proteins in media and neointima from ligation-injured carotids compared to control (Figure 6); Orai1 and STIM1 staining was correlated with that of the VSMC marker Smooth muscle alpha-actin (SMα-actin) on contiguous sections, indicating that cells with upregulated Orai1 and STIM1 are VSMCs (Figure 6). STIM1 staining in intact vessels was almost undetectable at the dilution of primary antibody used for injured sections (1:2000) and became visible at a dilution of primary antibody of 1:1200 (see inset). These data suggest that Orai1 and STIM1 upregulation is a more general feature of VSMC remodeling shared by other models of vascular occlusive diseases.
Figure 6. Orai1/STIM1 proteins are upregulated in carotid arteries from mice subjected to ligation.
IHC staining with specific antibodies against Orai1 and STIM1 in ligation-injured carotids from mice shows a marked upregulation of Orai1 and STIM1 proteins in media and neointima from ligation-injured carotids 21 days post-ligation compared to their respective control vessels. Contiguous sections from the same vessels were also stained with anti-SMα-actin antibody as a VSMC marker. Dilutions used for primary antibodies are: anti-Orai1, 1:400; anti-STIM1, 1:2000 (inset section dilution was 1:1200 for control vessel); anti- SMα-actin, 1:800.
Discussion
Orai1 and STIM1 have recently emerged as central molecular players for SOCE and ICRAC as well as store-independent ARC channels5, 36. Both Orai1 and STIM1 are upregulated in synthetic proliferative VSMC compared to quiescent freshly isolated VSMC9, 20. STIM1 is clearly regulating an increasing number of ion channels and future research is likely to extend further the list of channels, transporters and pumps regulated by STIM1. Indeed, in addition to Orai1-mediated ICRAC, STIM1 was shown to regulate Orai2 and Orai3 channels in their store-operated mode24, 26 as well as the store-independent ARC channels mediated by Orai1/Orai3 heteromers28, 37. STIM1 also regulates the function of all TRPC channels, with the exception of TRPC718 and has recently been shown by two independent groups to control the function of L-type Ca2+ channels31, 32.
Therefore, this study set out to specifically test the involvement of Orai1 in neointima formation by comparison with STIM1 and determine the significance of Orai1 upregulation in synthetic proliferative VSMC from injured arteries. Inhibition of Orai1 upregulation during carotid injury using lentiviral particles encoding shRNA prevented Orai1 protein increase 14 day post injury. Interestingly, Orai1 in vivo knockdown also prevented upregulation of STIM1 and CamKIIδ2. Importantly, Orai1 in vivo knockdown inhibited VSMC proliferation assessed by PCNA and dramatically reduced neointima formation. Both Orai1 and STIM1 knockdown inhibited NFAT nuclear translocation and transcriptional activity, providing a transcriptional pathway downstream of Orai1 and ICRAC-mediated Ca2+ entry for the control of VSMC proliferation. The upregulation of Orai1 and STIM1 appears to be a feature of proliferative VSMC in other models of vascular diseases such as carotid ligation model in mice, highlighting the universality of ICRAC Ca2+ entry pathway in VSMC remolding regardless whether the trigger for this remodeling is acute mechanical injury with removal of endothelium or chronic disturbance of flow or shear stress-induced injury.
The fact that Orai1 knockdown is as efficient as STIM1 in preventing neointima formation in vivo, suggests that Orai1 can be a potential target for treatment of VSMC remodeling during vascular diseases. Conceptually, Orai1 would offer a far better target than STIM1 for the following reasons: 1) by its virtue of being an ion channel expressed at the PM, Orai1 is more accessible than STIM1 that is mainly expressed in the ER and 2) Targeting STIM1 could be associated with side effects since STIM1 is involved in controlling a plethora of ion channels in addition to Orai1-mediated ICRAC. However, Orai1 and ICRAC are prominently functional in the immune system; the main feature of Orai1 deficient patients and mice is severe immunodeficiency38-40. Thus, while it is logical to presume that any potential drug against Orai1 and ICRAC in the treatment of VSMC remodeling is likely to be immunosuppressive, this might not be the case. Orai1 and ICRAC clearly mediate SOCE in both leukocytes (T-cells, B-Cells and mast cells) and VSMC, yet there is evidence suggesting that the molecular organization of Orai1 at the PM is distinct between VSMC and other cell types. Indeed, the non specific drug 2-Aminoethoxydiphenyl borate (2-APB) completely abrogates SOCE in VSMC at low μM concentrations (2-5μM)9 while higher concentrations (30-50μM) are required to inhibit SOCE in leukocytes and other cell types41. The reason for this difference is unknown but might be due to differences in posttranslational modifications of Orai1 or differential requirement of regulatory molecules between VSMC and other cell types. A recent report by Li et al, showed that a SOCE inhibitor, S66 is more potent in endothelial cells than immune cells42,despite the fact that Orai1 encodes SOCE and ICRAC in both cell types8, 12. This suggests that Orai1 and ICRAC in the vasculature might have subtle differences that would endow them with different sensitivities to drugs compared to other tissues.
In summary, this study provides evidence that Orai1 could be used as a target for vascular occlusive diseases. Future studies aimed at better understanding Orai1 molecular organization and its mechanisms of regulation in VSMC are likely to realize the use of Orai1 as an efficient target for treatment of vascular occlusive diseases.
Supplementary Material
Novelty and Significance.
What is known?
Orai1 calcium (Ca2+) channels are expressed in vascular smooth muscle cells (VSMC) and their expression is increased in proliferative migratory “synthetic” VSMC.
Orai1 mediates calcium release-activated calcium (CRAC) current in VSMC that is required for VSMC proliferation and migration in vitro.
What new information does this article contribute?
Balloon injury in rat carotids and carotid ligation in mice causes increased expression of Orai1 protein in medial and neointimal VSMC.
Knockdown of Orai1 expression in vivo by transduction of lentiviruses-encoding shRNA into balloon-injured carotid arteries prevent Orai1, STIM1 and CamKIIδ2 upregulation, VSMC proliferation in vivo and neointima formation.
Orai1 knockdown in VSMC inhibits the nuclear translocation and activity of the transcription factor, nuclear factor for activated T-cells (NFAT).
Orai1 and STIM1 are two critical components of the CRAC channel (ICRAC) that regulate Ca2+ entry in non-excitable cells. The role of Orai1 in vascular function and disease is only beginning to emerge. Our work shows that Ca2+ entry through Orai1 and ICRAC is essential for driving VSMC remodeling in vivo during vascular disease such as restenosis. In vivo knockdown of Orai1 by shRNA in a rat balloon injury model prevents upregulation of Orai1, STIM1 and CamKIIδ2 in VSMC; inhibits VSMC proliferation and neointima formation. Orai1 knockdown in VSMC inhibits NFAT nuclear translocation and activation, providing a potential mechanism for Orai1-dependent regulation of VSMC remodeling. These findings reveal a role for Orai1 and ICRAC in driving VSMC proliferation during vascular disease. Orai1 targeting with specific drugs might have the potential for treatment of vascular occlusive diseases.
Acknowledgments
Sources of Funding This work was supported by the National Institutes of Health grant 5R01HL097111 to Mohamed Trebak and in part by grant 5R01HL095566 to Khalid Matrougui.
Non-standard Abbreviations and Acronyms
- CRAC
calcium release-activated calcium
- NFAT
nuclear factor for activated T-cells
- STIM1
Stromal interacting molecule1
- SOAR/CAD
STIM Orai activating region/CRAC activating domain
- PLC
Phospholipase C
- VSMC
vascular smooth muscle cell
- SOCE
store-operated calcium entry
- TRPC channels
transient receptor potential canonical channels
- MHC
myosin heavy chain
- SMα-actin
smooth muscle alpha actin
- PCNA
proliferating cell nuclear antigen
- CamKIIδ2
calcium/calmodulin kinase II delta2
- IHC
Immunohistochemistry
- IF
Immunofluorescence
Footnotes
Disclosures None.
References
- 1.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: Calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frischauf I, Schindl R, Derler I, Bergsmann J, Fahrner M, Romanin C. The stim/orai coupling machinery. Channels (Austin, Tex. 2008;2:261–268. doi: 10.4161/chan.2.4.6705. [DOI] [PubMed] [Google Scholar]
- 3.Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflugers Arch. 2008;457:405–415. doi: 10.1007/s00424-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 5.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 6.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 7.Zweifach A, Lewis RS. Mitogen-regulated ca2+ current of t lymphocytes is activated by depletion of intracellular ca2+ stores. Proc Natl Acad Sci U S A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and orai1 mediate crac currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for stim1-and orai1-dependent store-operated calcium influx through icrac in vascular smooth muscle cells: Role in proliferation and migration. Faseb J. 2009 doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. Stim is a ca2+ sensor essential for ca2+-store-depletion-triggered ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. Stim1, an essential and conserved component of store-operated ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in orai1 causes immune deficiency by abrogating crac channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 13.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. Cracm1 multimers form the ion-selective pore of the crac channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide rnai screen of ca(2+) influx identifies genes that regulate ca(2+) release-activated ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. Stim1 clusters and activates crac channels via direct binding of a cytosolic domain to orai1. Cell. 2009 doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. Soar and the polybasic stim1 domains gate and regulate orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by orai3: Selective requirement of orai3 versus orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285:19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. Trpc channels as stim1-regulated socs. Channels (Austin, Tex. 2009;3:221–225. doi: 10.4161/chan.3.4.9198. [DOI] [PubMed] [Google Scholar]
- 19.Ambudkar IS. Trpc1: A core component of store-operated calcium channels. Biochem Soc Trans. 2007;35:96–100. doi: 10.1042/BST0350096. [DOI] [PubMed] [Google Scholar]
- 20.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M. Essential role for stim1/orai1-mediated calcium influx in pdgf-induced smooth muscle migration. Am J Physiol Cell Physiol. 2010;298:C993–1005. doi: 10.1152/ajpcell.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompre AM, Hulot JS. Rna interference targeting stim1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther. 2008 doi: 10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo RW, Wang H, Gao P, Li MQ, Zeng CY, Yu Y, Chen JF, Song MB, Shi YK, Huang L. An essential role for stim1 in neointima formation following arterial injury. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn338. [DOI] [PubMed] [Google Scholar]
- 24.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of orai1, orai2, and orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 25.Schindl R, Frischauf I, Bergsmann J, Muik M, Derler I, Lackner B, Groschner K, Romanin C. Plasticity in ca2+ selectivity of orai1/orai3 heteromeric channel. Proc Natl Acad Sci U S A. 2009;106:19623–19628. doi: 10.1073/pnas.0907714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindl R, Muik M, Fahrner M, Derler I, Fritsch R, Bergsmann J, Romanin C. Recent progress on stim1 domains controlling orai activation. Cell Calcium. 2009;46:227–232. doi: 10.1016/j.ceca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Frischauf I, Muik M, Derler I, Bergsmann J, Fahrner M, Schindl R, Groschner K, Romanin C. Molecular determinants of the coupling between stim1 and orai channels: Differential activation of orai1-3 channels by a stim1 coiled-coil mutant. J Biol Chem. 2009;284:21696–21706. doi: 10.1074/jbc.M109.018408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignen O, Thompson JL, Shuttleworth TJ. Stim1 regulates ca2+ entry via arachidonate-regulated ca2+-selective (arc) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. Stim1 heteromultimerizes trpc channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. Stim1 gates trpc channels, but not orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, stim1, reciprocally controls orai and cav1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park CY, Shcheglovitov A, Dolmetsch R. The crac channel activator stim1 binds and inhibits l-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 33.Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated ca2+ entry, is functionally associated with na+/ca2+ exchanger and plasma membrane ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol. 2009;297:C1103–1112. doi: 10.1152/ajpcell.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.House SJ, Singer HA. Camkii-delta isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:441–447. doi: 10.1161/ATVBAHA.107.156810. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The multifunctional ca2+/calmodulin-dependent kinase ii {delta} (camkii{delta}) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J Biol Chem. 2011;286:7990–7999. doi: 10.1074/jbc.M110.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuttleworth TJ. Arachidonic acid, arc channels, and orai proteins. Cell Calcium. 2009 doi: 10.1016/j.ceca.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mignen O, Thompson JL, Shuttleworth TJ. Both orai1 and orai3 are essential components of the arachidonate-regulated ca2+-selective (arc) channels. J Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S. Orai1 deficiency and lack of store-operated ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. The Journal of allergy and clinical immunology. 2009;124:1311–1318 e1317. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feske S. Crac channelopathies. Pflugers Arch. 2010;460:417–435. doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feske S, Picard C, Fischer A. Immunodeficiency due to mutations in orai1 and stim1. Clin Immunol. 2010;135:169–182. doi: 10.1016/j.clim.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakriya M, Lewis RS. Potentiation and inhibition of ca(2+) release-activated ca(2+) channels by 2-aminoethyldiphenyl borate (2-apb) occurs independently of ip(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, Majeed Y, Tumova S, Seymour VA, Taylor H, Stacey M, O’Regan D, Foster R, Porter KE, Kearney MT, Beech DJ. Orai1 and crac channel dependence of vegf-activated ca2+ entry and endothelial tube formation. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.