Abstract
Current forays into tissue engineering of articular cartilage in vitro using the self-assembling method have produced constructs possessing significant extracellular matrix and resulting mechanical properties. However, large numbers of native articular chondrocytes are necessary to produce functional engineered cartilage; all previous work with the self-assembling process has used 5.5×106 cells/construct. In this study, the effects of initial cell seeding (0.25 – 11×106 cells/construct) on tissue quality were investigated. Results showed that tissue engineered articular cartilage was formed, when using at least 2 million cells/construct, possessing dimensional, compositional, and compressive properties approaching those of native tissue. It was noted that higher seeding contributed to thicker constructs with larger diameters and had a significant effect on resulting biochemical and biomechanical properties. It was further observed that aggregate modulus increased with increased seeding. By combining gross morphological, histological, biochemical, and biomechanical results, an optimal initial seeding for the self-assembling process of 3.75×106 cells/construct was identified. This finding enhances the translatability of this tissue engineering process by reducing the number of cells needed for tissue engineering of articular cartilage by 32% while maintaining essential tissue properties.
Keywords: tissue engineering, aggregate modulus, biochemical functionality, seeding density
INTRODUCTION
Articular cartilage functions to lubricate and transmit loads in diarthrodial joints.11 This mechanical functionality can be attributed to the dense ECM components and their corresponding unique organization within the tissue,2 specifically, collagen type II and glycosaminoglycans (GAG). Bovine articular cartilage has been shown to have an aggregate modulus under creep indentation of ~140 kPa for immature tissue,21 increasing to ~800 kPa as tissue matures.35 The biochemical content of healthy cartilage typically correlates well with biomechanical properties and has been shown to be 10–30% collagen and 3–10% GAG when normalized to wet weight of tissue.22,31 Cartilage’s structural content and organization can be adversely affected by acute injury or by osteoarthritis, a degenerative process that leads to intense pain and subsequent joint instability and mechanical inferiority.24 Due to tissue breakdown and its subsequent poor natural healing response, tissue engineering has been investigated as a viable alternative for tissue replacement using a multitude of treatment modalities and culture methodologies.9,10,15,18,19,26,30,39
Prior studies have used one such modality, known as the self-assembling process, as a means of engineering articular cartilage constructs that contained one-third the total collagen and two-thirds more sulfated glycosaminoglycans (sGAGs) per dry weight than native tissue, in addition to an aggregate modulus which approached one-third native values.21 The preclusion of a scaffold in this process is one step in the translational approach toward the production of tissue engineered articular cartilage constructs fit for clinical use. A scaffoldless approach may eliminate potential problems, including stress-shielding, toxicity of degradation products, and an inflammatory response.5,6 Recently, this process has been further optimized by increasing its translatability and functionality via the elimination of fetal bovine serum from the culture medium, as well as the application of confinement-induced stress.12 These advancements have led to the production of tissue engineered constructs with an aggregate modulus of 225 kPa and containing 6% GAG and 4% collagen normalized to tissue wet weight.
The ability to produce cartilaginous constructs with the self-assembling process depends upon having a sufficient supply of chondrogenic cells for seeding (5.5×106/construct). Various options for obtaining an adequate supply include collecting and expanding chondrocytes from non load-bearing areas of articular cartilage,8,28 in addition to the use of differentiated mesenchymal7,37,41 and/or embryonic stem cells.27 Independent of which chondrogenic cell source is used, an adequate number of cells must be obtained to form functional constructs. Previous work has demonstrated in other cartilage engineering systems that cell seeding density has a dramatic effect on tissue matrix enrichment1,17,20,40 and subsequent response to mechanical stimuli.29,31,34 Specifically, a 90% and 75% increase in GAG and collagen concentrations, respectively, was exhibited when increasing chondrocyte seeding from 10×106 cells/mL scaffold to 60×106 cells/mL scaffold after 4 wks in culture.29,31 These increases were accompanied by a 3.3-fold increase in aggregate modulus. Similar effects on biochemical properties have been observed in scaffold models, most notably using a PGA-based scaffold system. Increasing the seeding density of chondrocytes from 10×106 cells/mL scaffold to 25×106 cells/mL scaffold contributed to a 47% and 25% increase in GAG and collagen concentrations, respectively. Furthermore, lower cell seeding densities have been shown to deposit insufficient extracellular matrix,23,38 leading to a deleterious effect on mechanical properties.23,31,38
To address the issue of initial cell seeding in the self-assembling process, this study investigated a wide range of initial chondrocyte seeding values and their subsequent effect on the biochemical and biomechanical properties of self-assembled articular cartilage constructs. The goals of the study were to 1) identify the lowest number of cells required to assemble and form functional constructs, 2) reduce the number of cells needed to form constructs without compromising the resulting tissue’s salient properties, and 3) identify an optimal seed at which constructs have properties most resembling native tissue. The hypotheses of this study were: 1) There exists a lower seed limit below which cells cannot assemble into functional constructs. 2) Increasing initial cell seeding will correlate with increasing biochemical content and resulting biomechanical properties eventually reaching a plateau. These hypotheses were assessed in two sequential studies. The first study identified the lowest seed limit by self-assembling chondrocytes at various low cell numbers and assessing their ability to assemble at 24 hrs post-seeding. The second hypothesis was assessed by increasing the number of cells per construct to twice that of previous studies (11×106 cells/construct) and evaluating neotissue content and functionality at 4 wks.
MATERIALS AND METHODS
Chondrocyte isolation and seeding
Chondrocytes were isolated from the distal femur of four 1-wk-old male calves (Research 87, Boston, MA) less than 36 hrs post-slaughter with 470 units/mL collagenase type II (Worthington Biochemical Corp., Lakewood, NJ) in digest medium. Digest medium was DMEM supplemented with 4.5 g/L D-glucose (Invitrogen, Carlsbad, CA), 10% FBS (Gemini Bioproducts, Woodland, CA), 1% penicillin/streptomycin/fungizone (PSF) (Cambrex, Walkersville, MD), 1% non-essential amino acids (Invitrogen), 10 mM HEPES (Sigma, St. Louis, MO), 0.4 mM L-proline (Acros Organics, Geel, Belgium), and 50 µg/mL L-ascorbic acid (Acros). Cells from each donor leg were counted on a hemocytometer and viability was assessed using the trypan blue exclusion test. Approximately 150 million cells were isolated from each leg and viability was greater than 96%. After isolation and counting, chondrocytes from four donor legs were pooled together and frozen in culture medium supplemented with 20% FBS and 10% DMSO (Fisher Scientific, Pittsburgh, PA) at −80°C. Cell viability was again assessed after thawing prior to seeding and found to be greater than 92%.
Chondrocyte self-assembly
Each well of a 48-well plate was coated with 1 mL of 2% molecular biology grade agarose (Fisher). A sterilized mold-making device was inserted into each well to form agarose wells 5 mm in diameter and 10 mm tall. Agarose was allowed to cool and set for 1 hr, at which point the mold-making device was removed. Over the next 48 hrs, four changes of 500 µL culture medium were performed to each well, allowing the media to replace the PBS in the agarose wells. Culture medium was DMEM supplemented with 4.5 g/L D-glucose, 1% PSF, 1% insulin-transferrin-selenous acid (ITS+) (BD Scientific, Franklin Lakes, NJ), 100 nM dexamethasone (Sigma), 50 µg/mL ascorbate-2-phosphate (Sigma), 40 µg/mL L-proline, and 100 µg/mL sodium pyruvate (Fisher). Following medium replacement, frozen passage 0 (P0) chondrocytes were thawed, counted, and seeded in 150 µL culture medium into their respective groups at the following initial cell numbers: 1) Lowest cell number required: 0.25, 0.5, 1.0, and 2.0 million cells per construct and 2) Expanded initial cell seeding study: 2.0, 3.75, 5.5, 8.25, and 11 million cells per construct. After 4 hrs of culture, an additional 350 µL of culture medium was added to each construct, being careful not to disrupt the forming construct. Each construct was allowed to sit undisturbed for the next 20 hrs, which allowed the cells to coalesce into free-floating constructs, at which point a full media change was performed. Media changes continued for all treatment groups every 24 hrs. After 2 wks of culture, the constructs were unconfined from their wells and placed into untreated, agarose-bottomed wells for the duration of culture, as illustrated previously.12
Histology
Following culture, samples were frozen and cryosectioned at a thickness of 14 µm. GAG and collagen distributions throughout the constructs were analyzed via Safranin-O/fast green and picrosirius red staining, respectively.
Quantitative biochemistry
Biochemical samples were lyophilized, minced, and resuspended in 0.8 mL of 0.05 M acetic acid containing 0.5 M sodium chloride. To this suspension, 0.1 mL of a 10 mg/mL pepsin (Sigma) solution in 0.05 M acetic acid was added, and the suspension was mixed at 4°C for 96 hrs. Next, 0.1 mL of 10x Tris-buffered saline (TBS) buffer was added along with 0.1 mL pancreatic elastase (1 mg/mL dissolved in 1x TBS buffer). This suspension was mixed at 4°C overnight. From this digest, total DNA content was assessed by the Picogreen Cell Proliferation Assay kit (Molecular Probes, Eugene, OR). Total sGAG content was measured using the Blyscan Glycosaminoglycan Assay kit (Biocolor, Belfast, Northern Ireland), derived from 1,9-dimethyl-methylene blue binding.33 Post hydrolyzation by 2 N NaOH for 20 min at 110°C, samples were assayed for total collagen content using a chloramine-T hydroxyproline assay.42 The amount of type II collagen was quantified by a sandwich ELISA (Chondrex, Redmond, WA). A similar protocol was modified to detect the amount of collagen type I using a monoclonal mouse anti-human capture antibody (U.S. Biological, Swampscott, MA) and polyclonal rabbit anti-human detection antibody (U.S. Biological).
Creep indentation analysis
Biomechanical analysis was performed by evaluating samples with an indentation apparatus.3 Each specimen was adhered to the sample platen with cyanoacrylate glue and fully submerged in saline solution. The specimen was aligned so that the construct surface was perpendicular to the load shaft of the apparatus. Following alignment, a tare mass of 0.2 g (0.002 N) was loaded onto the specimen using a 1.00 mm diameter rigid, flat-ended, porous indenter tip. Each sample was allowed to equilibrate under the tare load until the deformation rate fell below 10−6 mm/sec. Upon reaching tare equilibrium, a step test load of 0.7 g (0.007 N) was automatically applied to the specimen, and displacement was measured until equilibrium was reached. At this time, the step load was removed and recovery displacement recorded until equilibrium was reached for the final time. The sample thickness along with indentation displacement data were used to calculate the intrinsic biomechanical properties based on the linear biphasic theory to include aggregate modulus (HA), permeability, and Poisson’s ratio.3,4
Statistical analysis
Six samples were assessed biochemically and biomechanically for each treatment group. A single factor ANOVA was used to analyze the samples and a Tukey’s post-hoc test was conducted when warranted. Significance was defined as p < 0.05.
RESULTS
Study 1: Lowest cell number required
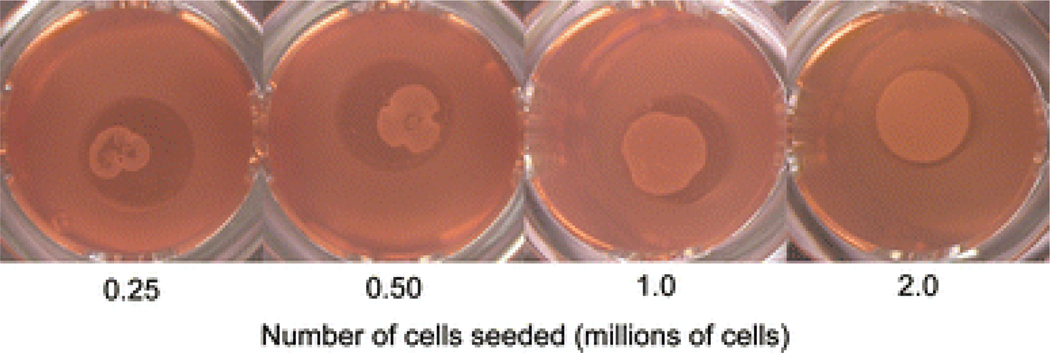
Constructs were visually assessed 24 hrs post seeding to determine the lowest initial cell seeding needed to assemble and form cartilage constructs. Of the four groups, only the 2.0×106 cells/construct group assembled homogeneously and filled out the entire 5 mm well (Fig. 1). The other three groups assembled into smaller aggregates of cells, but were amorphously shaped, nonhomogeneous in nature, and did not fill the 5 mm well.
Figure 1.
Bottom view of self-assembled constructs in culture for study 1. Cells seeded in 5 mm wells at respective initial cell seedings aggregated and coalesced into constructs at 24 hrs. A minimum seed of 2 million cells was required to form a uniform, homogeneous construct.
Study 2: Expanded initial cell seeding investigation
Gross appearance and histology
The gross appearance of all treatment groups is shown in Fig. 2. After 4 wks in culture, the thickness of the constructs increased as the initial cell seeding increased. The thickness of constructs with 2, 3.75, 5.5, 8.25, and 11 million cells was 0.21 ± 0.02 mm, 0.35 ±0.07 mm, 0.50 ± 0.12 mm, 0.61 ± 0.06 mm, and 0.62 ± 0.07 mm, respectively. Constructs with 2 million cells were significantly thinner than all other constructs, and constructs with 3.75 million cells were significantly thinner than those seeded with 5.5, 8.25, 11 million cells. The differences between the latter three treatment groups were not significant.
Figure 2.
Gross morphology (row 1) and histological sections (rows 2 and 3) of constructs cultured for 4 wks at respective seedings. Each ruler marking denotes 1 mm. Increases in diameter and thickness were observed with increased seeding. Intense staining of Safranin-O/fast green (sGAGs) and picrosirius red (collagen) was observed for all treatment groups. Scale bar denotes 200 µm.
In addition to thickness, diameter also increased with increased seeding. The constructs had diameters of 4.78 ± 0.11 mm, 5.22 ± 0.22 mm, 5.52 ± 0.32 mm, 5.80 ± 0.08 mm, and 5.58 ± 0.19 mm for seeds of 2, 3.75, 5.5, 8.75, and 11 million cells, respectively. Constructs assembled with 2 million cells proved to have a significantly smaller diameter than all other constructs, followed by the constructs with 3.75 million having a significantly smaller diameter than those seeded with 5.5, 8.25, and 11 million cells. No significant differences were observed between the diameters of constructs seeded with 5.5, 8.25, and 11 million cells.
Histological staining revealed homogeneous Safranin-O staining, indicating constructs with abundant GAG. Picrosirius red staining showed homogeneous staining for collagen, excluding the rim of the constructs, where a collection of collagen was observed to have formed a shell around the construct. This ring of collagen along the outside of the constructs was most noticeably observed in groups with 8.75 and 11 million cells.
Quantitative biochemistry
An increase in seeding was shown to have a significant effect on the wet weights of self-assembled constructs. A significant difference was observed between the wet weights of groups seeded with 2, 3.75, and 5.5 million cells, which reached wet weights of 3.89 ± 0.57 mg, 8.00 ± 1.44 mg, and 11.90 ± 1.01 mg, respectively. In addition, the groups seeded with 8.75 and 11 million cells were significantly greater than all of the other groups with wet weights of 17.05 ± 1.94 mg and 18.37 ± 1.93 mg, though these differences were not significant from one another.
The number of cells per construct was assayed, demonstrating that all treatments were significantly different in their seeding, excluding groups with 3.75 and 5.5 million cells, which contained 4.7 ± 0.8 and 5.8 ± 0.5 million cells, respectively. Groups with 2, 8.25, and 11 million cells were significantly different from each other and from all other groups with 2.3 ± 1.0, 8.9 ± 1.9, and 13.0 ± 0.9 million cells, respectively.
Increased seeding was also shown to have a significant effect on biochemical content per wet weight, including GAG and total collagen. The group seeded with 2 million cells produced significantly less GAG than all other groups, producing 3.4 ± 2.2% while groups with 3.75, 5.5, 8.25, and 11 million cells produced 8.2 ± 1.5%, 7.6 ± 0.9%, 7.0 ± 0.2%, and 6.8 ± 0.7% GAG per wet weight, respectively (Fig. 3A, white bars). The differences in the four highest seeding groups were not significant. It is worth noting that a similar increasing trend was observed for GAG per construct with increasing seeding (Fig. 3A, shaded bars). Increasing seeding was shown to have a significant inverse relationship with total collagen content per wet weight (Fig. 3B, white bars). Constructs seeded with 2 million cells produced a significantly greater amount of collagen per wet weight than all other constructs, with 24 ± 7%. The group with the next highest collagen production was 3.75 million cells, which produced 11 ± 2% collagen per wet weight. Following the inverse trend, groups with 5.5, 8.25, and 11 million cells produced total normalized collagen of 7.7 ± 0.9%, 5.1 ± 1.3%, and 4.5 ± 0.7%, respectively, though these differences were not significant. However, total collagen per construct was not significantly different for any treatment group (Fig. 3B, shaded bars).
Figure 3.
Biochemical properties of constructs normalized to wet weight (white bars) and per construct (shaded bars). A) GAG. Increased seeding led to significant increases in GAG/construct, whereas all constructs seeded with 3.75×106 cells or greater had a significant increase in GAG production over the lowest seeding group when normalized to wet weight. B) Total collagen. No significant differences were observed between any treatment group for collagen/construct, while significant decreases in collagen/ww were observed with increased seeding. Data are shown mean ± standard deviation and significance defined as p < 0.05. Groups not connected by the same letter are deemed significantly different and capital letters denote differences between biochemical content normalized to wet weight and lowercase letters denote differences when normalized per construct.
An increase in cell seeding from 2 million cells to 3.75 cells contributed to a significant increase in the production of collagen II. Constructs seeded with 2 million cells had a significantly smaller production of collagen II compared to all other treatment groups, containing 40 ± 24 µg/construct. No significant differences were observed between the treatment groups with 3.75, 5.5, 8.25, and 11 million cells, as they produced 207 ± 46 µg, 262 ± 46 µg, 231 ± 43 µg, 221 ± 46 µg per construct, respectively. Production of collagen type I was also assayed but none of the treatment groups produced a detectable amount of the protein.
Biomechanical evaluation
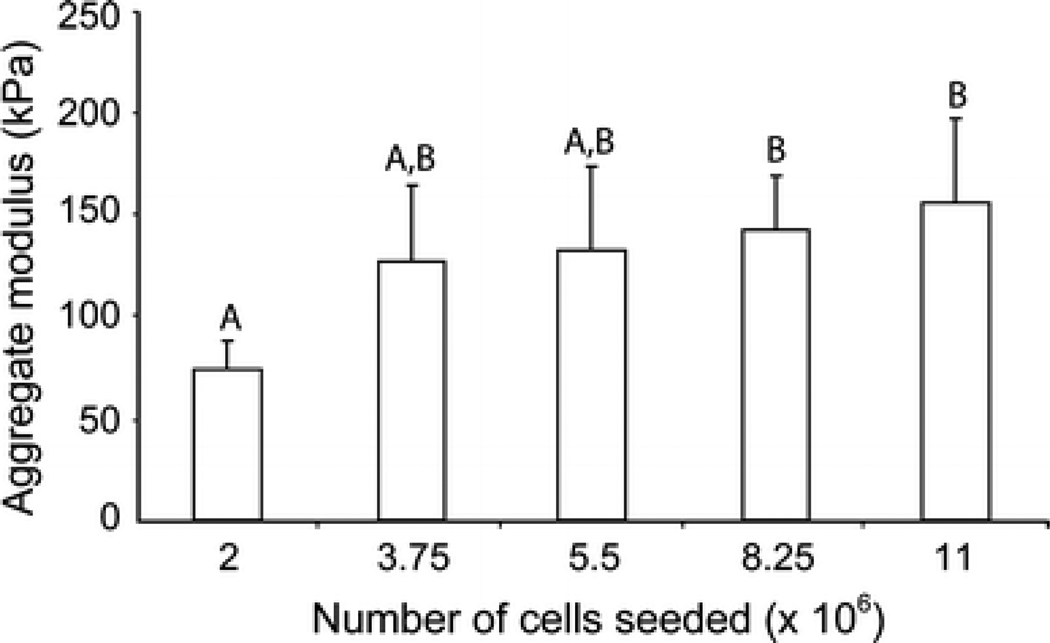
Using creep indentation, initial seeding was found to have an impact on the aggregate modulus of constructs (Fig. 4). Constructs seeded with 2 million cells were found to have the lowest aggregate modulus at 74 ± 14 kPa, which was significantly lower than the aggregate modulus of 8.25 and 11 million cells at 143 ± 27 kPa and 156 ± 41 kPa, respectively. The groups with 3.75 and 5.5 million cells had a greater aggregate modulus than the constructs with 2 million cells, at 127 ± 37 kPa and 132 ± 31 kPa, although this difference was not significant.
Figure 4.
Aggregate modulus of constructs. Mechanical properties increase with increased initial seeding. Data are shown mean ± standard deviation and significance defined as p < 0.05. Groups not connected by the same letter are deemed significantly different.
No significant differences were observed for Poisson’s ratio or permeability of tissue engineered constructs at different seeds. The values for Poisson’s ratio were 0.15 ± 0.13, 0.24 ± 0.14, 0.17 ± 0.08, 0.26 ± 0.05, and 0.21 ± 0.05 for constructs seeded with 2, 3.75, 5.5, 8.25, and 11 million cells respectively. Corresponding construct permeabilities were 8.15 ± 5.47×10−14 m4 N−1 s−1, 11.2 ± 8.2×10−14 m4 N−1 s−1, 5.86 ± 7.35×10−14 m4 N−1 s−1, 9.03 ± 9.41×10−14 m4 N−1 s−1, and 9.15 ± 6.00×10−14 m4 N−1 s−1.
DISCUSSION
In this study, a self-assembling process was utilized to assess the effects of initial cell seeding for constructs produced using bovine articular chondrocytes. The gross and histological appearances, biochemical, and biomechanical properties were analyzed for each seeding to determine its effect on neocartilage functionality. The results revealed that a minimum of 2 million cells were necessary to assemble and form tissue engineered constructs, and furthermore, demonstrated that increased seeding caused constructs to increase in diameter and thickness. Additionally, increasing seeding significantly affected the biochemical and biomechanical properties of all constructs. In this study, no dosing effects were observed; instead, a plateau of biomechanical and biochemical properties was exhibited at or above 3.75 million cells, below which decreases in the construct’s salient functional properties were noted. These data confirm the two hypotheses that there exists an initial cell seeding threshold below which chondrocytes cannot assemble into uniform constructs, and increasing seeding leads to an increase in biochemical and biomechanical properties up to a plateau.
The self-assembling process is a unique tissue engineering modality which produces functional neocartilage tissue without the use of a scaffold, allowing chondrocytes to coalesce with one another instead of adhering to a substratum. This process seems to follow work by Steinberg et al.13,14,36 on the differential adhesion hypothesis, which states that monodispersed cells aggregate and coalesce by maximizing intracellular adhesion and minimizing total free energy. Additionally, it has been shown that seeding density, and its varying adhesion forces, is an important determining factor of resulting tissue properties.32 Specifically, a critical seeding density is required such that the energy minimization and adhesion forces allow the formation of a construct with desired dimensions. Data from study 1 are consistent with these results, indicating that the minimum seeding required to form constructs from articular chondrocytes for a 5 mm well is 2 million cells/construct. Furthermore, results of study 2 show that increasing this seeding contributes to changes in neotissue architectural and functional properties.
Our data suggest that an initial seeding threshold was observed at 3.75 million cells, above which there were no considerable changes in construct extracellular matrix deposition. As higher initial seeding was achieved, an inverse relationship between normalized total collagen and sGAG content was observed, indicating that higher cell numbers have a greater positive effect on GAG deposition and retention than on collagen. These data agree with a number of other studies using chondrocytes which show an increase in GAG content with increased seeding density,29,31 reaching native values (~5% ww) for all treatment groups at or above 3.75 million cells/construct. Collagen data in this study differ from other chondrocyte studies as collagen concentration has historically been shown to increase with increased seeding density. This difference is most likely associated with increased cell-cell communication in the self-assembling process versus other cell culture systems including agarose encapsulation20,29,31 and cell-scaffold systems16,38 in addition to differences in media composition, most notably the lack of serum. However, it is of note that the concentration of GAG and collagen deposition reported in the literature at 4 wks of culture for agarose encapsulation (1.2% and 3% ww)31 and cell-scaffold systems (1.1% and 2% ww)38 fall below the lowest values obtained in this study (3.4% and 4.5% ww). Furthermore, constructs assembled with 3.75 million cells, the optimal initial seeding, contain a 6.8-fold increase in GAG density (8.2%) and 3.7-fold increase in collagen density (11%) compared to other cartilage tissue engineering systems.
Aggregate modulus values of tissue engineered constructs correlated well with normalized sGAG content and increased with increasing seeding. Analogous to biochemical composition, compressive data exhibited a plateau effect at 3.75 million cells/construct, above which there were slight but not significant increases. These data mirror previous work illustrating an increase in compressive stiffness as cell density is increased in an agarose hydrogel culture system,29 and is an exciting finding in that the correlation of increase in stiffness and increase in GAG density are indicative of the structure-function relationship present in native articular cartilage. Additionally, the aggregate moduli of functional tissue engineered articular cartilage produced with at least 3.75 million cells (≥ 127 kPa) meet or exceed those of native immature bovine articular cartilage (139 ± 41 kPa)21 or other cell systems (~80 kPa),29 further illustrating the translational potential of the self-assembling process.
Native articular cartilage is heterogeneous in cellularity, varying with depth from surface as well as age. Cellularity has been shown to decrease from 290 to 150 million cells/ml tissue near the articular surface for fetal and adult bovine tissue, respectively, to 120 to 50 million cells/ml tissue at a depth of 1.0 mm.25 Data for self-assembled constructs assessed in this study are exciting as they fall between 485 and 850 million cells/ml, approximately 3-fold increased over native tissue values. However, as constructs are cultured temporally in vitro, they grow in thickness while no differences are found in cellular content (data not shown); thus, cellularity normalized to construct volume is decreased temporally. One can expect, therefore, that in vitro culture beyond 4 wks will lead to cellular content approaching native values
In summary, this study’s results are exciting as they advance previous work with the self-assembling process by demonstrating the ability to reduce the number of cells seeded per construct by 32% without compromising the biochemical and biomechanical properties, thus increasing the potential translatability of the self-assembling process. Furthermore, the identification of a minimum initial number of cells to form articular cartilage constructs is a significant enhancement of the self-assembling process of articular cartilage. One deficiency that remains for tissue engineered constructs is the reduced collagen content compared to native tissue values. In future work, external stimuli such as growth factors or mechanical stimulation can be studied to further enhance the self-assembly process to increase the collagen density to more closely align with native tissue.
ACKNOWLEDGEMENTS
The authors would like to gratefully acknowledge funding support from NIAMS RO1 AR053286.
REFERENCES
- 1.Almarza AJ, Athanasiou KA. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomed Eng. 2005;33:943–950. doi: 10.1007/s10439-005-3311-8. [DOI] [PubMed] [Google Scholar]
- 2.Ateshian GA, Hung CT. Functional properties of native articular cartilage. In: Guilak F, Butler DL, Goldstein SA, editors. Functional tissue engineering. New York: Springer-Verlag; 2003. pp. 46–68. [Google Scholar]
- 3.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12:340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 4.Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop. 1995:254–266. [published erratum appears in Clin Orthop 1995 Nov;(320):283] [PubMed] [Google Scholar]
- 5.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 6.Athanasiou KA, Korvick D, Schenck RC. Biodegradable implants for the treatment of osteochondral defects in a goat model. Tissue Eng. 1997;3:363–373. [Google Scholar]
- 7.Bernardo ME, Emons JA, Karperien M, Nauta AJ, Willemze R, Roelofs H, Romeo S, Marchini A, Rappold GA, Vukicevic S, Locatelli F, Fibbe WE. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res. 2007;48:132–140. doi: 10.1080/03008200701228464. [DOI] [PubMed] [Google Scholar]
- 8.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 9.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 10.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 11.Darling EM, Athanasiou KA. Biomechanical strategies for articular cartilage regeneration. Ann Biomed Eng. 2003;31:1114–1124. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- 12.Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26:238–246. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–1620. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 14.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Freed LE, Vunjak-Novakovic G, Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cell Biochem. 1993;51:257–264. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- 16.Freed LE, Hollander AP, Martin I, Barry JR, Langer R, Vunjak-Novakovic G. Chondrogenesis in a cell-polymer-bioreactor system. Exp Cell Res. 1998;240:58–65. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 17.Freed LE, Martin I, Vunjak-Novakovic G. Frontiers in tissue engineering. In vitro modulation of chondrogenesis. Clin Orthop. 1999:S46–S58. [PubMed] [Google Scholar]
- 18.Glowacki J, Yates KE, Maclean R, Mizuno S. In vitro engineering of cartilage: effects of serum substitutes, TGF-beta, and IL-1alpha. Orthod Craniofac Res. 2005;8:200–208. doi: 10.1111/j.1601-6343.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 19.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, Freed LE. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun. 2001;286:909–915. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 20.Heywood HK, Sembi PK, Lee DA, Bader DL. Cellular utilization determines viability and matrix distribution profiles in chondrocyte-seeded alginate constructs. Tissue Eng. 2004;10:1467–1479. doi: 10.1089/ten.2004.10.1467. [DOI] [PubMed] [Google Scholar]
- 21.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 22.Hu JCY, Athanasiou KA. Chapter 4: Structure and function of articular cartilage. In: An YH, Martin KL, editors. Handbook of histology methods for bone and cartilage. Totowa, NJ: Humana Press; 2003. pp. 73–95. [Google Scholar]
- 23.Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 24.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 25.Jadin KD, Wong BL, Bae WC, Li KW, Williamson AK, Schumacher BL, Price JH, Sah RL. Depth-varying density and organization of chondrocytes in immature and mature bovine articular cartilage assessed by 3d imaging and analysis. J Histochem Cytochem. 2005;53:1109–1119. doi: 10.1369/jhc.4A6511.2005. [DOI] [PubMed] [Google Scholar]
- 26.Klein TJ, Schumacher BL, Schmidt TA, Li KW, Voegtline MS, Masuda K, Thonar EJ, Sah RL. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 27.Koay EJ, Hoben GM, Athanasiou KA. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem Cells. 2007;25:2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 28.Litzke LE, Wagner E, Baumgaertner W, Hetzel U, Josimovic-Alasevic O, Libera J. Repair of extensive articular cartilage defects in horses by autologous chondrocyte transplantation. Ann Biomed Eng. 2004;32:57–69. doi: 10.1023/b:abme.0000007791.81433.1a. [DOI] [PubMed] [Google Scholar]
- 29.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 30.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 31.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Napolitano AP, Chai P, Dean DM, Morgan JR. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;13:2087–2094. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 33.Pietila K, Kantomaa T, Pirttiniemi P, Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164:30–36. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 34.Saini S, Wick TM. Concentric cylinder bioreactor for production of tissue engineered cartilage: effect of seeding density and hydrodynamic loading on construct development. Biotechnol Prog. 2003;19:510–521. doi: 10.1021/bp0256519. [DOI] [PubMed] [Google Scholar]
- 35.Scott CC, Athanasiou KA. Design, validation, and utilization of an articular cartilage impact instrument. Proc Inst Mech Eng [H] 2006;220:845–855. doi: 10.1243/09544119JEIM97. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg MS. Mechanism of tissue reconstruction by dissociated cells. II. Time-course of events. Science. 1962;137:762–763. doi: 10.1126/science.137.3532.762. [DOI] [PubMed] [Google Scholar]
- 37.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 39.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27:4434–4442. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 41.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 42.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]