Abstract
The function, efficacy, and safety of protein biopharmaceuticals are tied to their three-dimensional structure. The analysis and verification of this higher-order structure are critical in demonstrating manufacturing consistency and in establishing the absence of structural changes in response to changes in production. It is, therefore, essential to have reliable, high-resolution and high sensitivity biophysical tools capable of interrogating protein structure and conformation. Here, we demonstrate the use of hydrogen/deuterium exchange mass spectrometry (H/DX-MS) in biopharmaceutical comparability studies. H/DX-MS measurements can be conducted with good precision, consume only picomoles of protein, interrogate nearly the entire molecule with peptide level resolution, and can be completed in a few days. Structural comparability or lack of comparability was monitored for different preparations of interferon-β-1a. We present specific graphical formats for the display of H/DX-MS data that aid in rapidly making both the qualitative (visual) and quantitative assessment of comparability. H/DX-MS is capable of making significant contributions in biopharmaceutical characterization by providing more informative and confident comparability assessments of protein higher order structure than are currently available within the biopharmaceutical industry.
Keywords: biotherapeutic, interferon, protein conformation, biosimilar, protein drug, structure
INTRODUCTION
The term “comparability” is a critical concept in the biopharmaceutical industry that is linked to the development and commercialization a of therapeutic protein (also referred to as protein drug, protein pharmaceutical, protein biopharmaceutical or just biopharmaceutical)1. Comparability studies establish consistency of drug manufacturing, the absence of drug product changes when modifications to the manufacturing process are made, and aid in assessing biosimilars2–5. Hence comparability studies are essential for maintaining an effective linkage of all the data acquired during all phases of biopharmaceutical development6–7 as well as establishing biopharmaceutical equivalence.
The unique association of comparability with biopharmaceuticals stems from the fact that proteins are large complicated molecules, containing thousands of atoms that are connected to form long chains (polypeptides) that fold in very complex patterns giving rise to a unique three-dimensional structure. This three-dimensional structure (also referred to as its higher-order structure or conformation) plays a critical role in the biology/function and in the solution physico-chemical properties of a protein8–11. So important was the relationship between protein structure and protein function that the pioneering researchers who championed this area (Anfinsen, Stein and Moore12–14) were awarded the Nobel Prize in Chemistry in 1972. Over time, the initial static picture of protein structure has given way to the realization that these macromolecules have significant flexibility or dynamic motion, due to fluctuations in the forces (e.g., hydrogen bonds, electrostatic forces) that are critical for maintaining higher order structure15–17. This temporal component was soon realized to play a significant role in the behavior of proteins, especially for protein-protein interactions and protein stability (e.g., aggregation)18–22. Consequently, the three-dimensional structure of a protein has in addition to a spatial component, a temporal component that enables these molecules to existent in solution as an ensemble of different molecular conformations23–24.
A further complexity of biopharmaceuticals that links them to comparability studies is that the production of protein drugs relies on the use of cells. As a result, control over their production is not as precise as synthesis of a small molecule drug, for example. During their biological synthesis, biopharmaceuticals may undergo in vivo chemical modifications [post-translational modifications (PTMs)] that may proceed to various levels of completion. These changes, which increase the chemical micro-heterogeneity of the biopharmaceutical, can lead to changes three-dimensional structure and therefore function/behavior. In vivo PTMs can be easily altered (either knowingly or unknowingly) during biopharmaceutical manufacturing by using different cells lines, expression systems, and/or growth conditions. In addition, further PTMs can occur in vitro through changes in purification strategies as well as in filling, vialing, and storage steps. Even variations in material contact surfaces (including container closure) and site(s) of manufacture can lead to changes in a biopharmaceutical and its three-dimensional structure25, which can ultimately change the final biopharmaceutical product.
Manufacturers of biopharmaceuticals must demonstrate to regulatory agencies consistency of the structural complexity of the biopharmaceutical they are making. To achieve this, comprehensive comparability assessment, as discussed in regulatory documents such as ICH Q5E5, is conducted including biochemical, biophysical, biological, and if necessary non-clinical and/or clinical studies. To assess the comparability [FOOTNOTE: ICH Q5E states that the conclusion of comparability means that the products are “highly similar”; we also use the term comparability/comparable when referring to data (i.e., mass spectra and summaries of HX MS data) that are highly similar] of the three-dimensional structure of a biopharmaceutical, classical biophysical techniques such as CD, fluorescence, UV, DSC, ITC, AUC, FTIR and dye binding have been commonly used1,26. The information provided by these methods generally corresponds to a global or average readout of all the elements of a protein that contribute to the measured signal. Hence these methods are significantly challenged when faced with the task of detecting small, but significant changes between two nearly identical proteins, which effectively amounts to finding a small difference between two very large signals. In addition, the signal from different reporter elements (on a protein that is only slightly different in conformation from another protein) may even offset one another, masking the presence of real differences. With some methods, signal may come from only a very limited number of reporter elements (e.g., aromatic amino acid residues) in the protein and the absence of a reporter element in the area where a change has occurred may allow the actual change to go undetected.
Other more sophisticated structural tools (that are capable of detecting very small structural changes), such as NMR or X-ray crystallography, are either too time consuming and complex to use, or not applicable for routine biopharmaceutical analysis due to large size of the biopharmaceutical (for NMR) or an inability to be crystallized (for x-ray crystallography). Thus, there is a strong need to develop other informative, sensitive, and practical methods that can rapidly survey the structure of a biopharmaceutical to detect small, local differences. One such method that may fulfill this need is hydrogen/deuterium exchange mass spectrometry (H/DX-MS).
Hydrogen/deuterium exchange (H/DX) was first described by Hvidt and Linderstrom-Lang 27, and has been detected with various spectroscopic techniques including NMR and FTIR to understand protein structure and dynamics28–29. The coupling of H/DX to mass spectrometry (MS) came in 199130 and was further enhanced by pairing the experiment with proteolytic digestion31 enabling structural changes to be resolved to the peptide level (typically 5–10 amino acids). This method is sensitive to changes in protein conformation and has steadily gained popularity, even within the biopharmaceutical industry32–36. The advantages of H/DX-MS include very low sample requirements (a few hundred picomoles), the ability to make the measurements on any protein regardless of its size, and to do so in native or formulated buffer conditions. We note that protein structures cannot be determined with H/DX MS and that for this reason, the method is limited to comparisons of conformational states that influence the exchange process. H/DX-MS also offers a way of assessing some of the dynamic properties of a protein on a practical routine basis with relatively good sample throughput. Therefore, H/DX-MS seems to be an ideal method for conducting the conformational comparisons of biopharmaceuticals.
The goal of the present study is to further expand earlier work37 and discussions on the utility of H/DX-MS in the biopharmaceutical industry with specific focus on its use in biophysical comparability studies. We have used H/DX-MS to compare the higher-order structure and structural dynamics of several different preparations/forms of interferon-β-1a (IFN). While some changes to the manufacturing of this protein did not result in any detectable conformational changes, some in vitro induced PTMs altered the conformation of IFN in undesirable ways that were easily detected with H/DX-MS. In situations where IFN’s conformation was changed, the regions of change were identified and localized to short stretches of the polypeptide backbone.
To make the analysis of H/DX-MS in comparability studies more attractive to the biopharmaceutical industry, we propose some modes of data display and data analysis that should allow for easier assessment of differences in the spatial and temporal aspects of biopharmaceutical three-dimensional structure. Such graphical displays and analysis also produce a more quantifiable assessment of protein drug comparability. Finally, we speculate on future developments that will further enable this tool to play a more mainstream role in comparability studies and potentially provide new insights into the understanding of protein biopharmaceutical behavior, thereby enabling more rapid and cost effective biopharmaceutical development.
EXPERIMENTAL SECTION
All chemicals were purchased from Sigma-Aldrich/Fluka (St. Louis, MO, USA) unless otherwise noted. The IFN samples were produced in CHO cells and purified by Biogen Idec, Inc. Guanidine HCl, Tris (2-Carboxyethyl) phosphine Hydrochloride (TCEP) and trifluoroacetic acid (TFA) were obtained from Pierce (Rockford, IL, USA). Hydrogen peroxide (30 %) was acquired from Acros (Morris Plains, NJ, USA).
IFN Samples
A total of five different samples of IFN were used in this work. A representative and qualified IFN reference material was compared to four other IFN samples: a) IFN material stored at −70 °C for over eight years, b) IFN material that was pegylated, c) IFN material made using different tissue culture media (serum-free) and growth conditions, and d) IFN that was oxidized. IFN material from source “a” (frozen at −70 °C for over eight years) was used to make oxidized IFN by incubating IFN with 0.06% H2O2 for 6 hours at ambient temperature followed by SEC purification (see Supporting Information and Figure S1). The four methionine residues and the one free (non-disulfide bonded) cysteine residue were completely oxidized, as verified by MS and MS/MS experiments (data not shown). Purified pegylated IFN (PEG-IFN) was produced by coupling a 20 kDa PEG-aldehyde to the N-terminus of IFN38. For H/DX-MS experiments, all IFN samples were formulated to 0.25 mg/mL in 100 mM sodium phosphate, 200 mM NaCl, pH 7.2.
Peptide-level H/DX- MS
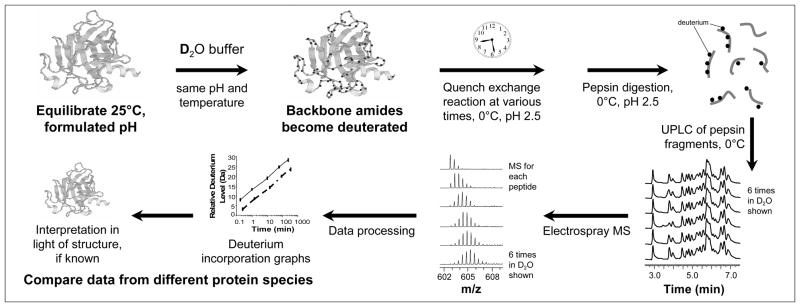
Peptide-level H/DX-MS was performed as previously described32, see also Figure 1. Briefly, at the start of an IFN H/DX reaction, time zero (t = 0), 1.5 μL of IFN (13 μM) was diluted into 18.5 μL of the identical buffer (100 mM sodium phosphate, 200 mM NaCl, pD 7.2) containing D2O rather than H2O, which constituted an approximate 13-fold dilution. The labeling mixtures were incubated at 20 °C and deuterium exchange was quenched at the following time points: 10 seconds, 1, 10, 60 and 240 minutes. Labeling was quenched by making a 1:1 dilution with 200 mM sodium phosphate, 0.1 M TCEP and 4 M guanidine HCl, pH 2.3. The quench dilution dropped the pH of the labeling reaction to 2.6 while simultaneously unfolding the protein and reducing disulfides33,39. Quenched samples were digested, desalted and separated online using a Waters UPLC system designed for H/DX-MS40. Approximately 16 pmoles of exchanged/quenched IFN were injected onto an immobilized pepsin column41 where the digestion and peptide trapping were performed for 3 minutes with a flow rate of 0.1 mL/min in 0.05% formic acid at 15 °C. The peptic peptides were trapped on an ACQUITY BEH C18 1.7 μm peptide precolumn trap (Waters Corp. Milford, MA) maintained at 0 °C. Flow was diverted by a switching valve and the trapped peptides flushed from the trap onto an ACQUITY BEH C18 1.7 μm, 1mm × 100mm column (Waters Corp. Milford, MA) for separation at 40 μL/min and 0 °C (average back-pressure was approximately 8500 psi). An 8 minute linear acetonitrile gradient (5–55%) with 0.05 % formic acid was used to separate the peptides. Eluate from the C18 column was directed into a Waters QToF premier or Synapt HD mass spectrometer with electrospray ionization and lock-mass correction (using Glu-fibrinogen peptide). Peptide recovery is not a consideration in these experiments as the amount of deuterium in each peptide is not a function of how much peptide is ionized and ultimately detected. The entire experiment was optimized to achieve a strong MS signal on as many peptides as possible; peptides with weak signals were not processed. Blank injections (data not shown) were used between each sample injection to confirm that there was no carryover of peptides between runs; carryover can be a problem for some proteins (as we have described elsewhere42) and is highly sequence dependent. Mass spectra were acquired from m/z 255 to 1800. Peptic peptides were identified using a combination of exact mass and MSE, aided by Waters IdentityE software 43. Peptide deuterium levels were determined as described by Weis et al. 44 using the Excel based program HX-Express. No adjustment was made for deuterium back-exchange during analysis and therefore all results are reported as relative deuterium exchange levels expressed in either mass unit (Da) or relative fractional exchange45. The latter was obtained by dividing the deuterium level (in Da) for each peptide by the maximum number of deuterium that could theoretically be incorporated into each peptide (see also below).
Figure 1.
Representative workflow of a H/DX-MS experiment (used with permission, from Ref 49). The protein sample is equilibrated to the desired temperature and diluted at least 10-fold into deuterated buffer (same composition as formulation buffer). The deuteration is allowed to progress and aliquots are taken at various times and quenched by lowering the pH to 2.5 and the temperature to 0 °C. These quenched samples are digested on-line with immobilized pepsin, the peptic peptides captured and separated with reversed-phase chromatography at 0 °C, and the mass of each peptide determined by mass spectrometry. The relative level of deuteration is plotted vs. time and samples are compared.
H/DX data display and analysis
The analysis and display of IFN H/DX-MS data involved creating several data arrays “S” for each of the “n” IFN samples and for each IFN comparison set studied. A raw data array [Sn(Mi,t)] contains the relative mass increase data, Mi,t, (expressed in Da, resulting from deuterium incorporation) as a function of time, t, for each IFN peptide, i, identified and common to all IFN samples in that array. The peptic peptide “i” value for each IFN peptide was based on its computed sequence mid point. The IFN peptide with the lowest sequence mid point number was assigned an “i” value of 1. The peptide with the next lowest sequence mid point number was then assigned an “i” value of 2 and so on, until all peptic IFN peptides were assigned a unique “i” value (see Supporting Information and Figure S2 for further details). In cases where two (or more) peptides had sequence midpoint numbers that were the same, the peptide with the lower N-terminus sequence position was given the lower “i” value. For most of the IFN samples, as many as 73 peptides were identified; however, only 67 of these peptides were common to all IFN H/DX-MS samples that were compared.
Once an “i” value was assigned to each IFN peptide and the Sn(Mi,t) arrays are created, the Sn(Mi,t) data arrays were transformed into a second data array [Sn(Fi,t) arrays] containing the relative fractional exchange data, Fi,t, for the same “i” peptides as a function of “t”. This transformation was achieved by dividing the Mi,t data values for each peptide by the theoretical maximum number of exchangeable backbone amide hydrogen atoms that each peptide “i” could exchange. For an H/DX experiment, this corresponds to the number of amino acid residues in the peptide minus one for the N-terminus (which rapidly back exchanges46) and minus the number of proline residues in the peptide, since prolines do not contain an amide hydrogen.
The generation of Sn(Fi,t) data arrays was particularly useful since it normalized the exchange data for each peptide “i” enabling the relative exchange properties for each peptide to be compared to each other. The plotting of Sn(Fi,t) data for each sample in a graphical format, where x = i and y = relative fractional exchange generated five separate traces for each sample. Each of these traces corresponded to a specific time point “t” at which H/DX-MS data were collected for all IFN peptides. When plotting data this way, the x-axis represents the sequentially ordered peptides of IFN as equally spaced “x” values. This arrangement allows the x-axis to act as a quasi-sequence representation of the IFN molecule starting from the peptide closest to the N-terminus at x = 1 to the peptide closest to the C-terminus, which corresponds to the largest “i” value (which here corresponds to i = 67). Since the x-axis does not provide an exact 1 to 1 physical linear representation of the sample molecule’s sequence, the term quasi-sequence is applied to the x-axis.
Using the above plotting format, all the IFN H/DX-MS data from two IFN samples that were being compared could be plotted on one graph to allow direct visual comparison. This was achieved using a mirror or butterfly plot. In this work, we have taken a standard approach in forming these mirror plots by having the reference IFN data, Sref(Fi,t), plotted in the positive “y” direction while the experimental IFN data, Sexp(Fi,t), plotted in the negative “y” direction (negative values are generated simply by multiplying the Sexp(Fi,t) values by −1). Such a data display format provides an informative picture of the relative physical dynamic behavior of the different regions of each IFN molecule and at the same time provides an initial qualitative visual assessment of H/DX-MS data between the two IFN samples that are being compared.
In addition to the qualitative analysis, a quantitative assessment of H/DX-MS comparison data was obtained by calculating the difference between the two raw data arrays, reference and experimental. To generate the main difference array, D(ΔMi,t), the difference between the appropriate Sn(Mi,t) array values was computed as indicated in Eq.1:
| Eq. 1 |
Differences were only calculated between array elements with the same “i” and same “t” value. A second difference array, Ds(i), was generated by calculating the sum of the D(ΔMi,t) data for each peptide over all five time points, as indicated by Eq. 2,:
| Eq. 2 |
The values for both D(ΔMi,t) and Ds(i) can be either positive or negative, and are in the same units (Da) as the value of Mi,t itself. Positive results indicate that the reference material exchanges more rapidly than the experimental sample and therefore that the reference material has a more open or flexible structure. Negative values mean the reverse where the experimental sample exchanges more rapidly than the reference, indicating that the experimental sample had a more open or flexible structure. Ds(i) essentially correlates with the difference in the area under the H/DX graph between the reference and experimental IFN samples for the same peptide ‘i” for deuterium incorporation graphs (such as those shown in Figure 3). Ds(i) values thus provides a quick and useful summary of H/DX differences to assess comparability of each peptide using all of the data acquired during the H/DX-MS experiment.
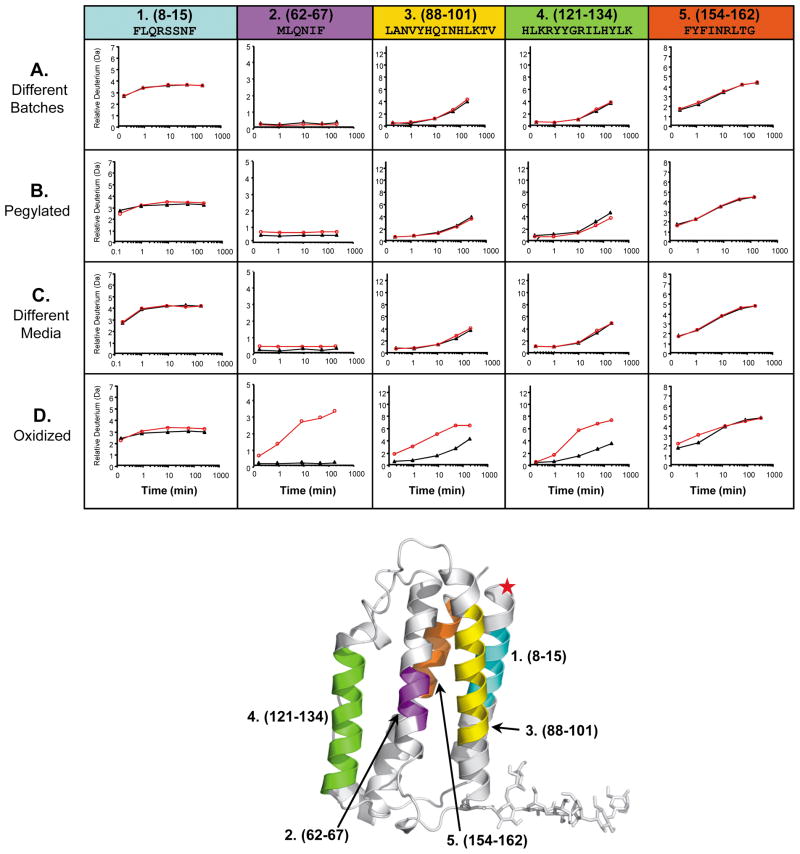
Figure 3.
A small subset of deuterium incorporation graphs generated for five IFN peptic peptides from four different IFN H/DX-MS comparability experiments. In each graph, the reference IFN data is the black line with closed triangles while the experimental IFN data, to which it is being compared, is the red line with open circles. Row A: comparison of two different large scale IFN batches prepared over 8 years apart; Row B: comparison of IFN versus N-terminally pegylated IFN; Row C: comparison of IFN produced using different cell culture media and growth conditions; Row D: comparison of IFN versus oxidized IFN (oxidation of Met and Cys residues C17, M1, M36, M62, M117 was 100% as indicated by LC/MS peptide mapping, data not shown). Columns 1–5 represent five (of the 67) different IFN peptic peptides that were identified and monitored in all IFN H/DX-MS comparison experiments. Each column (peptide) is designated with a specific color, shown at the top. The actual position of these peptides in the three-dimensional structure of IFN is shown with the same color code on the x-ray crystal structure of human IFN (PDB:1AU1 67) located at the bottom of the figure. A red star indicates the position of the pegylation.
Assessing uncertainty in the measurements
Data collected in this work and our prior reported work37 that involved replicate (involving at least 3 or more) experiments over different days, has allowed us to calculate [see Supporting Information concerning the calculation of uncertainty in D(ΔMi,t) and Ds(i)] the error or uncertainty (expressed at one standard deviation) in D(ΔMi,t) to be about ± 0.14 Da. This uncertainty in D(ΔMi,t) appears to be independent of the magnitude of peptide size, reaction time or the magnitude of the mass difference (see Supporting Information, Figure S3). Using this estimate of uncertainty, which is in reasonable agreement with that reported in the literature 47, a 98% confidence limit (or interval) for D(ΔMi,t) of about ± 0.5 Da has been calculated, which if exceeded at any time point during the course of deuterium incorporation, would statistically be considered a significant difference in deuterium levels. In other words, if the two H/DX curves for a given peptide “i” that are being compared are in fact highly similar (or the same within the variability limits of method), then no single D(ΔMi,t) data point should exceed the ± 0.5 Da limits. Exceeding the ±0.5 Da threshold would imply a difference in the physicochemical environment of the same peptide from the two IFN samples, which would be consistent with a difference in the higher-order structure between the two IFN molecules being compared. Using the standard deviation for D(ΔMi,t), a calculated 98% confident limit (or interval) for Ds(i) of ± 1.1 Da was also determined.
The overall expression of significant differences between two proteins or forms of a protein can be put on a quantitative basis by formulating two Difference Indices, DI(1) and DI(2), using Equations 3 and 4 that are essentially a summation of any differences found across all peptides in a protein:
| Eq. 3 |
| Eq. 4 |
The larger the DI values, the more differences exist between the two things being compared. In calculating these DI values, any values for (abs[Ds(i)] − 1.1) or (abs[D(ΔMi,t)] − 0.5) that were negative would be assigned a value of zero since these values for Ds(i) and D(ΔMi,t) would indicate that these data points were within acceptable specified confidence limits (Note: in situations where the calculation of Ds(i)abs values, as defined by Equation 5 below, was required they would replace Ds(i) in Eq. 3).
The individual experimental mass differences observed for all D(ΔMi,t) data points for a given peptide “i” between the two samples that are identical will ideally represent just random experimental noise in which D(ΔMi,t) values are positive and negative such that when summed, cancel giving a small Ds(i) value. In situations where a real difference exists, it is very likely that the difference in the two H/DX graphs will exist over a number of different time points and these differences will most likely be in the same direction. When such D(ΔMi,t) values are summed, as shown above in Eq. 2, they will reinforce each other yielding a much larger Ds(i) value. However, in some cases large differences may actually exist that are both positive and negative, as indicated in Figure 17.6.8 in reference48. In this case the H/DX curve for the reference and experimental samples will have very different slopes and intersect during some point in the exchange process. Ds(i) values calculated for this situation will be misleadingly small due to the cancellation effect resulting from real significant differences that are opposite in sign. To avoid these missed positive differences and potentially false positives, which could arise from Ds(i) values actually exceeding the 98% confidence limits while no D(ΔMi,t) exceeds ± 0.5 Da, the following minimum non-comparability (or not “highly similar”, see footnote above) criteria were established that must be met before any two peptides can be considered potentially not comparable (or not “highly similar”):
For a given peptide “i” at least one D(ΔMi,t) data point must exceed the ± 0.5 Da limit and the Ds(i) for the that peptide must also exceed ± 1.1 Da.
-
For a given peptide “i” if one D(ΔMi,t) data point exceeds ± 0.5 Da, but the Ds(i) value for the that peptide does not exceed ± 1.1 Da, recalculate the value for Ds(i) by summing the absolute (abs) differences, where the sum is taken over all time points, t, as shown in Eq 5:
Eq. 5 If Ds(i)abs exceeds the limit value of ± 1.1 Da, the two peptides are not comparable (not highly similar). However, if this new sum does not exceed the 1.1 Da limit, flag this peptide pair for manual review and assessment.
RESULTS AND DISCUSSION
We conducted the following four IFN H/DX-MS comparisons: 1) reference IFN material versus IFN frozen for eight years, 2) reference IFN material versus pegylated IFN (PEG-IFN), 3) reference IFN material made using standard tissue culture growth media and conditions versus IFN material made using different tissue culture media and growth conditions, and 4) reference IFN material versus oxidized IFN. We started by comparing all these samples with traditional biophysical tools including: SEC, LC/MS, AUC, CD, disulfide mapping, fluorescence, and UV characterization. The data collected from these techniques indicated that all of the IFN samples were comparable (data not shown), with the exception of oxidized IFN.
In the case of oxidized IFN, differences were detected by SEC, CD and fluorescence measurements. SEC results showed that oxidized IFN eluted at a slightly shorter retention time (see Supporting Information, Figure S1A), CD showed an approximate 9% loss of α-helical content in the far-UV region (Figure S1B), and fluorescence measurements revealed a blue shift of roughly 3 nm upon oxidation (Figure S1C). Taken together, these data indicated that the conformation of oxidized IFN was not comparable to reference IFN material. While these techniques all revealed a difference, they did not indicate where differences were located in the IFN molecule or how wide-spread or localized the differences were. In general, such problems are common to all PTMs on protein drugs, even for those that are known to have a significant effect on the biopharmaceutical. Although MS and MS peptide mapping provide localization information in terms of primary structure changes, they do not answer questions about the extent or nature of conformational changes resulting from such modifications. For all practical purposes, these questions remain unanswered for PTMs due to the lack of appropriate biophysical tools. The oxidized IFN sample therefore represented an excellent positive control for conducting comparability analyses using H/DX-MS.
Preliminary assessment of comparability with H/DX-MS
Each form of IFN used in the above four comparisons was subjected to analysis by H/DX-MS as outlined in the Experimental Section, graphically illustrated in Figure 149. In each IFN H/DX comparison, data were acquired for both reference and experimental IFN samples (i.e., oxidized, pegylated, etc.), on the same day. Such comparisons were repeated anywhere from two to four times, with the exception of comparison “a” (see Experimental Section), which was only conducted once.
In each comparison experiment, at least 67 peptic peptides were unambiguously identified. These 67 peptides represented approximately 95% of the IFN amino acid sequence, as shown in Figure 2. Many of the peptides overlap (Figure 2A) and the chromatographic reproducibility of a typical IFN H/DX-MS experiment was very high (Figure 2B, left) as was the quality of the mass spectra (Figure 2B, right). Different lots of C18 peptide traps, and C18 columns were tested throughout the analysis, with very minimal differences found (data not shown).
Figure 2.
(A) Pepsin digestion coverage map of IFN. Each red bar under the sequence indicates an identified IFN peptic peptide that was monitored during all H/DX-MS experiments. These peptides cover 157 of the total 167 amino acid residues in IFN yielding a linear sequence coverage of 95%. (B) Left: Representative UPLC chromatography (TIC is shown) of a typical IFN H/DX-MS experiment with H/DX time points of 0, 10 seconds, 1, 10, 60 and 240 minutes. Right: Mass spectra of the peptide covering residues 8–15 (sequence FLQRSSNF) at the same time points, top to bottom, as shown in the left panel. The red arrow at ~5.6 minutes in the left panel indicates the retention time of this peptide.
With the 67 unique IFN peptic peptides that were identified, and in combination with the 4 different IFN comparisons, a total of 268 individual “deuterium incorporation graphs” (such as that shown in Figure 1) were generated. Such a large amount of data is hard to show all at once in a quickly interpretable form. This point is illustrated in Figure 3, which shows a small subset of the deuterium incorporation graphs (20 out of the total 268) for five peptic fragments (labeled 1–5), from each of the four IFN comparisons. Comparison deuterium incorporation data for the different batches of IFN (Figure 3, row A), the pegylated IFN (Figure 3, row B), and the IFN made using different tissue culture media and growth conditions (Figure 3, row C) show qualitatively (visually) good comparability to the reference IFN comparator in all cases for the peptides shown. These data indicate, within the sequences covered by these five peptides, that the conformations of the IFN sample pairs were comparable.
However, H/DX-MS data for oxidized-IFN were not found to be comparable to the reference IFN. Peptides 2, 3, and 4 (Figure 3, row D) illustrate just a few of the peptides where deuterium incorporation differences were observed. These three peptides correspond to residues 62–67, 88–101, and 121–134, in the IFN molecule respectively. In these three peptides, more deuterium was incorporated into the oxidized form of IFN implying that the oxidized-IFN was more solvent exposed and/or less hydrogen bonded. The observed difference between these two IFN samples with H/DX-MS is consistent with differences also found by SEC, CD, and fluorescence measurements mentioned above and shown in Figure S1.
H/DX-MS data display, analysis approaches, and associated issues
In conducting comparability studies on biopharmaceuticals using H/DX-MS, several areas exist where further opportunities for improvement would be very helpful. Some of these areas are concerned with data analysis, data display, and the automation of these activities. As illustrated in the above section, the task of processing and presenting all of the data in H/DX-MS experiments to allow for quick and easy data interpretation is by no means trivial and has traditionally relied greatly on the manual and visual assessment of the experimenter. Unfortunately investigators must spend a significant amount of time manually processing and reviewing large and complex data sets (which can amount to hundreds of peptides when much larger proteins such as monoclonal antibodies are studied32–33) to make interpretations and conclusions. Clearly this is a highly labor intensive and potentially error prone process. In attempts to condense the data into smaller presentations, some investigators have made use of only a single exchange time point (or have actually collected only one time point) for each peptide50. In other cases, a more complex approach is employed to show the kinetic, magnitude, as well as the spatial information of an H/DX-MS experiment33 using just non-overlapping peptides. Such approaches do not provide a quick, simple, and robust way to simultaneously use and analyze all of the data and inherent data redundancy in H/DX-MS experiments. Hence display formats that use multiple time points over a wide span of time and that maximize the usage of all of the H/DX-MS data available should provide a better understanding of the structural and solution behavioral properties of a biopharmaceutical, thereby producing better, quicker, and more reliable comparisons. To achieve this goal we have explored an alternative approach for displaying and conducting data analysis of H/DX-MS comparability data, as illustrated in the following sections.
Alternative modes to display and analyze H/DX-MS data
Comparable Samples
Graphical display of H/DX-MS data obtained for both a reference IFN material, Sref(Fi,t), made using standard tissue culture media and growth conditions versus H/DX-MS data obtained for experimental IFN material made using a different tissue culture media and growth conditions, Sexp(Fi,t), are shown in Figure 4A. The standard we have adopted is to have the reference material plotted on the top and the experimental data plotted on the bottom of the mirror plot. These data represent the relative fractional exchange, averaged from four independent comparison experiments obtained on the same day. This plot allows for a quick and simple qualitative (visual) comparison of all the H/DX data acquired in this experiment in terms of the spatial and temporal H/DX properties of the two IFN samples, which in this case showed good comparability. It should also be noted that this form of data presentation allows one to immediately recognize regions of the IFN molecule that undergo very rapid exchange (e.g., peptides with x-axis values between 38–42), slow exchange (e.g., peptides with x-axis values between 6–7 & 32–36) or no exchange at all (e.g., peptides with x-axis values between 26–28).
Figure 4.
Comparability profile of reference IFN versus experimental lot of IFN produced using different cell culture media and growth conditions. (A) Mirror plot of the average relative fractional exchange data for reference IFN (top) versus the experimental IFN (bottom), as a function of peptide “i” - note that the standard plotting scheme we have adopted is to have reference on top and experiment on the bottom of the mirror plot. Each point is an average of four separate and independent H/DX-MS comparison experiments. The orange, red, cyan, blue and black lines correspond to data acquired at 10 seconds, 1, 10, 60 and 240 minutes of deuteration, respectively, for both samples. The x-axis is the calculated peptide midpoint, i, position of each of the 67 peptides compared (see also Supporting Information and Figure S2). The y-axis is the average calculated relative fractional exchange, as described in the Experimental Section. (B) Plot of the H/DX-MS difference data described in the Experimental Section and calculated from the average data shown in Figure 4A, between reference IFN and experimental IFN. The orange, red, cyan, blue and black lines correspond to the average D(ΔMi,t) values calculated for H/DX-MS data acquired at 10 seconds, 1, 10, 60 and 240 minute time points, respectively (Each point is an average of the four separate and independent H/DX-MS comparison experiments from Fig 4A). The black vertical bar at each peptide “i” position along the x-axis is the average Ds(i) value for that peptide. The blue dotted lines at y-axis values ±0.5 Da represent the 98% confidence limit for D(ΔMi,t) data, while the black dotted lines at y-axis values ±1.1 Da represent the 98% confidence limit for Ds(i) data. DI(1) and DI(2) were calculated as described in the text.
More critical analysis of the data in Figure 4A was achieved by plotting the corresponding differences, D(ΔMi,t) and Ds(i), as shown in Figure 4B. Note that in this figure, the differences are the average of four calculations, one for each replicate analysis. Also plotted with this difference data are two blue horizontal dotted lines (at y = ± 0.5 Da) and two black horizontal dotted lines (at y = ± 1.1 Da) that correspond to the 98% confidence limits for D(ΔMi,t) and Ds(i) values, respectively. These lines represent the limits that need to be exceeded by D(ΔMi,t) and Ds(i) to signify difference between the two samples being compared (see explanation in the Experimental Section). The results shown in Figure 4B clearly indicate that none of the D(ΔMi,t) and Ds(i) values exceed either of their corresponding 98% confidence limits, hence both IFN samples have been assessed to be comparable via H/DX-MS analysis. Using Eqs. 3 and 4, the comparison in Figure 4 gave DI(1) and DI(2) values (expressed as a whole number) that equaled zero. These two samples of IFN, therefore, were not conformationally different by any of our metrics.
Comparisons using only one or two H/DX-MS experiments
In Figure 4, four separate H/DX-MS experiments were conducted and averaged. If only a single H/DX-MS experiment was performed to assess comparability, our initial established criteria for comparability (as outlined in the Experimental Section) would not be rigorously met in most cases. Figure 5A-D shows the D(ΔMi,t) and Ds(i) values obtained for each of the four individual H/DX-MS comparison experiments used to generate data shown in Figure 4. In each of these separate plots there are D(ΔMi,t) and/or Ds(i) values (although at very low level and low frequency) that exceeded the corresponding 98% confidence limits that we have established. As a result, values for DI(1) and DI(2) were generated that were small, but not equal to zero (although in both cases these values just barely exceeded or missed exceeding their respected threshold). Difference index values in excess of zero indicate the potential for non-comparability or at least provide grounds for a lack of comparability, which must be investigated further. However, the smaller the difference index values, the small the difference between two samples. In the case of comparison “a” (reference IFN versus IFN material that was stored at −70 °C for over eight years, see Experimental Section) where only one H/DX-MS experiment was conducted, plots of D(ΔMi,t) and Ds(i) data showed more variability and in a few areas data actually just exceeded the 98% confidence limits especially for Ds(i) data (see Figure S4A). Nevertheless, DI values still equaled zero. Similar results were also seen in the case of comparison “b” (reference IFN compared to pegylated IFN) where two H/DX-MS experiments were collected and averaged (Figure S4B). In order to deal with the experimental variability of H/DX-MS experiments, as obtained with our present H/DX-MS instrumental system, and adhere to our comparability acceptance criteria, we conclude that we need to conduct at least three or more separate H/DX-MS experiments and average them before making critical assessments. Less critical analyses may only require one or two replicate comparison experiments.
Figure 5.
The four individual H/DX-MS experiments (panels A-D) all acquired on the same day and used to calculate the average difference data in Figure 4B. See the legend to Figure 4 for descriptions of symbols, lines, and colors.
Another consideration is the type of comparison that is being performed. What we have just illustrated (Figure 5) is the reproducibility for comparisons with data obtained from repetitive experiments within the same vial or pools of the same vial. Vial-to-vial comparisons, or pooled vial comparisons are a slightly different case which we have not illustrated here. The theoretical expectation is that analytical variability in those circumstances, as is the case for any analytical experiment in which sample heterogeneity is being tested, would be slightly higher than the variability described in Figure 5. The H/DX MS method would be equally suited to analyze vial-to-vial comparisons to determine overall product heterogeneity.
Interday comparisons
One of the other issues noted in conducting H/DX-MS comparability studies was increased variability when the data for two identical samples were acquired on different days versus both on the same day. Figure 6 shows the D(ΔMi,t) and Ds(i) values that were measured when the reference and experimental samples corresponded to same IFN material, but analyzed on two different days. The interday comparison data (Figure 6) is significantly more variable than intraday analysis (Figure 5), yielding values for DI(1) and DI(2) of 17 and 6, respectively. Such results point to the existence of potential experimental day-to-day errors that need to be better controlled before interday comparisons can be reliably conducted.
Figure 6.
The effects of conducting H/DX-MS comparison experiments using data acquired on different days. These H/DX-MS difference data were obtained in the comparison of the IFN reference sample to itself, but analyzed on two different days (not consecutive). Comparing this plot with each of the single H/DX-MS experiments shown in Figure 5 indicates the quality of H/DX-MS inter-day data vs. intra-day data. See the legend to Figure 4 for descriptions of symbols, lines, and colors.
Non-comparable samples
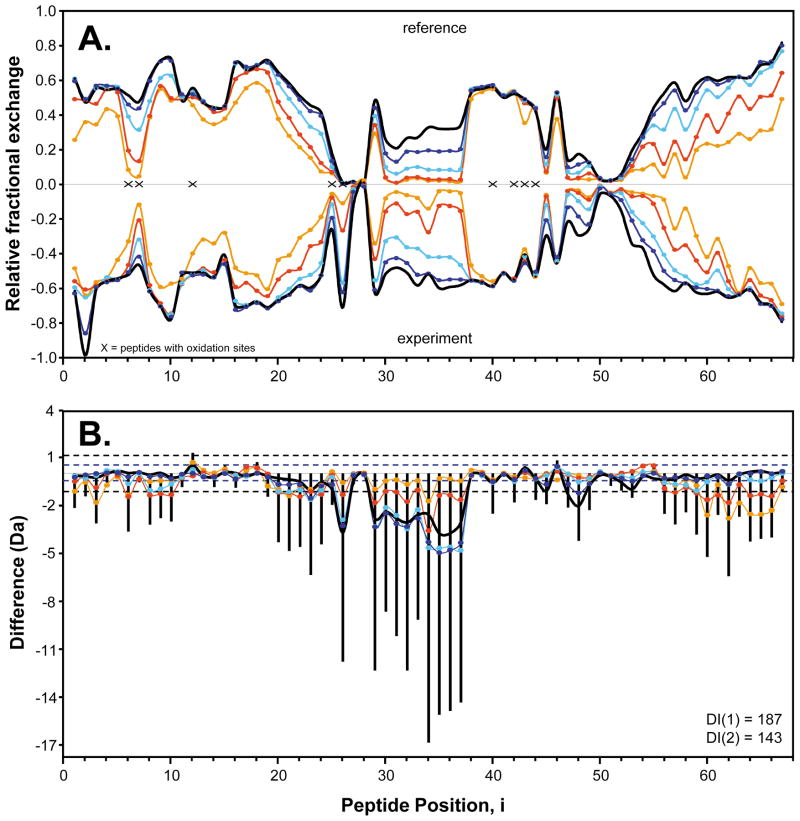
Finally, and most importantly, one IFN comparability experiment conducted in this work involved the comparison of reference IFN versus the oxidized IFN, see Figure 7. These results represent the average of three separate, independent comparison H/DX-MS experiments. The relative fractional exchange mirror plot, Figure 7A, for these two IFN samples immediately shows visual differences in the areas where x = 6–7 and 30–35. The difference plot in Figure 7B shows much more readily where deuteration differences exist along IFN’s sequence and clearly indicate that the oxidized IFN is significantly different from the non-oxidized reference IFN material. Not only can we say that these samples are not comparable, but with the aid of Figure 7B we can map and localized these changes to a cluster of regions on the IFN molecule, with the most intense changes occurring in the region of peptides associated with peptides numbered 29–38 and peptide number 48. Other smaller changes appear in three clustered areas located in different parts of the sequence of IFN. Overall, H/DX-MS data indicate wide-spread structural perturbation to IFN upon oxidation. Such results are not that surprising given that there are five sites of chemical modification that are dispersed over the entire IFN sequence and structure. Again as in Figure 4 we can quantify this comparison by calculating the difference indices [DI(1) and DI(2)] for this comparability set using equations 3 and 4. Values obtained for DI(1) and DI(2) were 187 and 143, respectively, indicating a high degree of difference. These two versions of IFN, therefore, are not structurally comparable. By starting with a DI, then a mirror plot (Figure 7A for example), and then drilling down into the raw H/DX-MS data, analysis becomes much more efficient and targeted. The exact location and nature of any differences revealed could be determined by locating the peptic peptides with difference in deuteration (Figure 7B), analysis of the deuterium incorporation graphs for just those peptides, and rationalization of the differences in light of the structure of the protein.
Figure 7.
Comparability profile of reference IFN reference versus oxidized IFN. (A) Mirror plot of average relative fractional exchange data for IFN reference (top), versus oxidized IFN (bottom). These data are the average of three separate, independent comparison experiments. (B) Plot of the average H/DX-MS difference data calculated from the data shown in part A. See the legend to Figure 4 for descriptions of symbols, lines, and colors.
Additional considerations
In detecting differences between two or more molecules using H/DX-MS we would like to point out that H/DX-MS is not capable, at present, of analyzing conformational differences in co-existing, low level components of the population. While it has been known since very early on51 that co-existing populations can easily be revealed by H/DX MS, conformations that constitute below about 10–20% of the population are not easily analyzed. We point out therefore, that H/DX MS cannot make measurements of conformational differences in subpopulations that represent <1% of the total. In fact, we are not aware of any method that can make this measurement.
CONCLUSIONS
The idea of biopharmaceutical comparability arises from the concept of “Well Characterized Biological Product”3,52–54. The underpinning of this concept rests upon the notion that modern analytical chemistry, biochemistry, biophysics and biology (via bioassay) can adequately prove whether comparability exist between any two or more samples of the same biopharmaceutical without the need to conduct copious and lengthy clinical trials. Nevertheless, the ability of establishing comparability 1,5,53,55–56 between two or more biopharmaceuticals depends on the criteria, which unfortunately is not that straightforward. As pointed out in the introduction, the adoption of certain unique three-dimensional structure (which in reality is an ensemble of conformations) is essential for proteins to accomplish their function, such as catalyzing an enzymatic reaction or binding to a target molecule. If changes to a biopharmaceutical’s higher-order structure occur (or if the ensemble of conformations is allowed to increase or decrease beyond its normal range of conformations) it can compromise a biopharmaceutical’s function or cause adverse effects, such as trigging immune responses, etc. 1,57. Therefore, consistent production of protein biopharmaceuticals that have the same, or comparable, folded structure is essential.
Bringing improved scientific information to bear quickly and reliably to demonstrate protein comparability offers significant opportunities to reduce biopharmaceutical drug development time and cost, and offers the ability to make more informed decisions for developing better and more effective drugs. Unfortunately, practical, high resolution, and routine analytical tools capable of providing the critical assessment of a protein’s spatial structure are significantly lacking, while similar tools for assessing the temporal component of a protein’s three-dimensional structure are nearly non-existent. The H/DX-MS results that we have presented in this paper, along with our earlier work32–33,37,58 and the work of others34–36,47,50, highlight the potential for H/DX-MS to fill this void. While we have chosen a small protein (IFN) to illustrate comparability, the methods described here are also highly suitable and completely transferrable to much larger protein such as antibodies, which we have already tackled with H/DX MS32–33. Much wider implementation of H/DX-MS into the biopharmaceutical industry will require the continued improvement and correction of the main weaknesses of the method: eventual automation and seamless coupling of high performance separation40, robotic sample handling59, better MS methods and instrumental systems, and computer software60–62 (aimed at H/DX-MS peptide identification, data analysis and display) to develop a complete commercial turn-key H/DX-MS system. The development of such an instrumental system is important to provide optimal and consistent performance in terms of sample throughput, reproducibility, and sensitivity. At present, the need to develop more effective data processing software to further shorten data analysis and improve data presentation is of particular importance. In this work we have introduced some ideas that address this latter general area. Clearly, additional future developments in H/DX-MS, such as ETD63–65 to improve spatial resolution, and the use of gas phase H/DX to obtain information on amino acid side chain behavior66, hold even greater capability for this technology. Such capability should find its way into the toolbox of the biopharmaceutical industry not only for conducting comparability studies, but also for assessing stability, formulation development and structure analysis/drug design.
Supplementary Material
Acknowledgments
We thank Rohin Mhatre and Helena Madden for their continued support and encouragement, and Thomas E. Wales and Marek Kloczewiak for their technical assistance. This work was support in part by the National Institutes of Health (R01-GM070590 and R01-GM086507) and a research collaboration with the Waters Corporation. This is contribution 973 from the Barnett Institute.
ABBREVIATIONS
- IFN
Interferon-β-1a
- H/DX-MS
Hydrogen/Deuterium Exchange Mass Spectrometry
- PTM
Post-Translational Modification
- CD
Circular Dichroism
- DSC
Differential Scanning Calorimetry
- ITC
Isothermal Titration Calorimetry
- AUC
Analytical Ultracentrifugation
- NMR
Nuclear Magnetic Resonance Spectroscopy
- FTIR
Fourier Transform Infrared Spectroscopy
- UV
ultraviolet
Footnotes
Electronic Supporting Information is available. This information includes details of the characterization of oxidized IFN, examples of how to determine the “i” values for peptic peptides and additional figures illustrating other comparisons. This information is available via the Internet at http://wiley.interscience.com.
References
- 1.Lundblad RL. Approaches to the Conformational Analysis of Biopharmaceuticals. Boca Raton, FL: Chapman & Hall/CRC Press; 2009. Dec 15, p. 336. [Google Scholar]
- 2.Rader RA. (Re)defining biopharmaceutical. Nat Biotechnol. 2008;26(7):743–751. doi: 10.1038/nbt0708-743. [DOI] [PubMed] [Google Scholar]
- 3.Zuñiga L, Calvo B. Biosimilars approval process. Regul Toxicol Pharmacol. 2010;56(3):374–377. doi: 10.1016/j.yrtph.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Schellekens H, Moors E. Clinical comparability and European biosimilar regulations. Nat Biotechnol. 2010;28(1):28–31. doi: 10.1038/nbt0110-28. [DOI] [PubMed] [Google Scholar]
- 5.ICH Q5E guidelines. http://www.ich.org/LOB/media/MEDIA1196.pdf.
- 6.Chirino AJ, Mire-Sluis A. Characterizing biological products and assessing comparability following manufacturing changes. Nat Biotechnol. 2004;22(11):1383–1391. doi: 10.1038/nbt1030. [DOI] [PubMed] [Google Scholar]
- 7.Chirino AJ, Mire-Sluis AR. State of the art analytical comparability: a review. Dev Biol (Basel) 2005;122:3–26. [PubMed] [Google Scholar]
- 8.Hegyi H, Gerstein M. The relationship between protein structure and function: a comprehensive survey with application to the yeast genome. J Mol Biol. 1999;288(1):147–164. doi: 10.1006/jmbi.1999.2661. [DOI] [PubMed] [Google Scholar]
- 9.Sadowski MI, Jones DT. The sequence-structure relationship and protein function prediction. Curr Opin Struct Biol. 2009;19(3):357–362. doi: 10.1016/j.sbi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Ecroyd H, Carver JA. Unraveling the mysteries of protein folding and misfolding. IUBMB Life. 2008;60(12):769–774. doi: 10.1002/iub.117. [DOI] [PubMed] [Google Scholar]
- 11.Bolen DW, Rose GD. Structure and Energetics of the Hydrogen-Bonded Backbone in Protein Folding. Annu Rev Biochem. 2008;77(1):339–362. doi: 10.1146/annurev.biochem.77.061306.131357. [DOI] [PubMed] [Google Scholar]
- 12.Moore S, Stein WH. Chemical structures of pancreatic ribonuclease and deoxyribonuclease. Science. 1973;180(85):458–464. doi: 10.1126/science.180.4085.458. [DOI] [PubMed] [Google Scholar]
- 13.Bartholeyns J, Moore S, Stein WH. Ribonuclease activity in the pancreas. Arch Int Physiol Biochim. 1974;82(5):966–967. [PubMed] [Google Scholar]
- 14.Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181(96):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- 15.Teilum K, Olsen J, Kragelund B. Functional aspects of protein flexibility. Cell Mol Life Sci. 2009;66(14):2231–2247. doi: 10.1007/s00018-009-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper A. Thermodynamic fluctuations in protein molecules. Proc Natl Acad Sci U S A. 1976;73(8):2740–2741. doi: 10.1073/pnas.73.8.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henzler-Wildman K, Kern D. Dynamic personalities of proteins. Nature. 2007;450(7172):964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 18.Travaglini-Allocatelli C, Ivarsson Y, Jemth P, Gianni S. Folding and stability of globular proteins and implications for function. Curr Opin Struct Biol. 2009;19(1):3–7. doi: 10.1016/j.sbi.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Wright PE, Dyson HJ. Linking folding and binding. Curr Opin Struct Biol. 2009;19(1):31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang KE, Dill KA. Native protein fluctuations: the conformational-motion temperature and the inverse correlation of protein flexibility with protein stability. J Biomol Struct Dyn. 1998;16(2):397–411. doi: 10.1080/07391102.1998.10508256. [DOI] [PubMed] [Google Scholar]
- 21.Kamerzell TJ, Middaugh CR. The complex inter-relationships between protein flexibility and stability. J Pharm Sci. 2008;97(9):3494–3517. doi: 10.1002/jps.21269. [DOI] [PubMed] [Google Scholar]
- 22.van der Kamp MW, Schaeffer RD, Jonsson AL, Scouras AD, Simms AM, Toofanny RD, Benson NC, Anderson PC, Merkley ED, Rysavy S, Bromley D, Beck DA, Daggett V. Dynameomics: a comprehensive database of protein dynamics. Structure. 2010;18(4):423–435. doi: 10.1016/j.str.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portman JJ. Cooperativity and protein folding rates. Curr Opin Struct Biol. 2010;20(1):11–15. doi: 10.1016/j.sbi.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Boehr DD, Nussinov R, Wright PE. The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol. 2009;5(11):789–796. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kueltzo LA, Wang W, Randolph TW, Carpenter JF. Effects of solution conditions, processing parameters, and container materials on aggregation of a monoclonal antibody during freeze-thawing. J Pharm Sci. 2008;97(5):1801–1812. doi: 10.1002/jps.21110. [DOI] [PubMed] [Google Scholar]
- 26.Deechongkit S, Aoki KH, Park SS, Kerwin BA. Biophysical comparability of the same protein from different manufacturers: A case study using Epoetin alfa from Epogen® and Eprex®. J Pharm Sci. 2006;95(9):1931–1943. doi: 10.1002/jps.20649. [DOI] [PubMed] [Google Scholar]
- 27.Hvidt A, Linderstrom-Lang K. Exchange of hydrogen atoms in insulin with deuterium atoms in aqueous solutions. Biochim Biophys Acta. 1954;14(4):574–575. doi: 10.1016/0006-3002(54)90241-3. [DOI] [PubMed] [Google Scholar]
- 28.Englander SW, Mayne L. Protein folding studied using hydrogen-exchange labeling and two-dimensional NMR. Annu Rev Biophys Biomol Struct. 1992;21:243–265. doi: 10.1146/annurev.bb.21.060192.001331. [DOI] [PubMed] [Google Scholar]
- 29.Haris PI, Chapman D. The conformational analysis of peptides using Fourier transform IR spectroscopy. Biopolymers. 1995;37(4):251–263. doi: 10.1002/bip.360370404. [DOI] [PubMed] [Google Scholar]
- 30.Katta V, Chait BT. Conformational changes in proteins probed by hydrogen-exchange electrospray-ionization mass spectrometry. Rapid Commun Mass Spectrom. 1991;5(4):214–217. doi: 10.1002/rcm.1290050415. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Smith DL. Determination of amide hydrogen exchange by mass spectrometry: a new tool for protein structure elucidation. Protein Sci. 1993;2(4):522–531. doi: 10.1002/pro.5560020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010;9(8):1716–1728. doi: 10.1074/mcp.M900540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houde D, Arndt J, Domeier W, Berkowitz S, Engen JR. Characterization of IgG1 conformation and conformational dynamics by hydrogen/deuterium exchange mass spectrometry. Anal Chem. 2009;81(7):2644–2651. doi: 10.1021/ac802575y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rand KD, Jorgensen TJD, Olsen OH, Persson E, Jensen ON, Stennicke HR, Andersen MD. Allosteric Activation of Coagulation Factor VIIa Visualized by Hydrogen Exchange. J Biol Chem. 2006;281(32):23018–23024. doi: 10.1074/jbc.M602968200. [DOI] [PubMed] [Google Scholar]
- 35.Olsen OH, Rand KD, Østergaard H, Persson E. A combined structural dynamics approach identifies a putative switch in factor VIIa employed by tissue factor to initiate blood coagulation. Protein Sci. 2007;16(4):671–682. doi: 10.1110/ps.062504907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkitt W, Domann P, O’Connor G. Conformational changes in oxidatively stressed monoclonal antibodies studied by hydrogen exchange mass spectrometry. Protein Sci. 2010;19(4):826–835. doi: 10.1002/pro.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaltashov IA, Bobst CE, Abzalimov RR, Berkowitz SA, Houde D. Conformation and dynamics of biopharmaceuticals: transition of mass spectrometry-based tools from academe to industry. J Am Soc Mass Spectrom. 2010;21(3):323–337. doi: 10.1016/j.jasms.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker DP, Lin EY, Lin K, Pellegrini M, Petter RC, Chen LL, Arduini RM, Brickelmaier M, Wen D, Hess DM, Chen L, Grant D, Whitty A, Gill A, Lindner DJ, Pepinsky RB. N-Terminally PEGylated Human Interferon-beta-1a with Improved Pharmacokinetic Properties and in Vivo Efficacy in a Melanoma Angiogenesis Model. Bioconjugate Chemistry. 2005;17(1):179–188. doi: 10.1021/bc050237q. [DOI] [PubMed] [Google Scholar]
- 39.Zhang HM, McLoughlin SM, Frausto SD, Tang H, Emmett MR, Marshall AG. Simultaneous reduction and digestion of proteins with disulfide bonds for hydrogen/deuterium exchange monitored by mass spectrometry. Anal Chem. 2010;82(4):1450–1454. doi: 10.1021/ac902550n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wales TE, Fadgen KE, Gerhardt GC, Engen JR. High-speed and high-resolution UPLC separation at zero degrees Celsius. Anal Chem. 2008;80(17):6815–6820. doi: 10.1021/ac8008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Pan H, Smith DL. Hydrogen exchange-mass spectrometry: optimization of digestion conditions. Mol Cell Proteomics. 2002;1(2):132–138. doi: 10.1074/mcp.m100009-mcp200. [DOI] [PubMed] [Google Scholar]
- 42.Fang J, Rand KD, Beuning PJ, Engen JR. False EX1 signatures caused by sample carryover during HX MS analyses. Int J Mass Spectrom. 2010 doi: 10.1016/j.ijms.2010.1006.1039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ. Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Mol Cell Proteomics. 2006;5(1):144–156. doi: 10.1074/mcp.M500230-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Weis DD, Engen JR, Kass IJ. Semi-automated data processing of hydrogen exchange mass spectra using HX-Express. J Am Soc Mass Spectrom. 2006;17(12):1700–1703. doi: 10.1016/j.jasms.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Wales TE, Engen JR. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom Rev. 2006;25(1):158–170. doi: 10.1002/mas.20064. [DOI] [PubMed] [Google Scholar]
- 46.Englander SW, Kallenbach NR. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys. 1983;16(4):134. doi: 10.1017/s0033583500005217. [DOI] [PubMed] [Google Scholar]
- 47.Burkitt W, O’Connor G. Assessment of the repeatability and reproducibility of hydrogen/deuterium exchange mass spectrometry measurements. Rapid Commun Mass Spectrom. 2008;22(23):3893–3901. doi: 10.1002/rcm.3794. [DOI] [PubMed] [Google Scholar]
- 48.Morgan CR, Engen JR. Investigating solution-phase protein structure and dynamics by hydrogen exchange mass spectrometry. Curr Protoc Protein Sci Chapter. 2009;17(Unit 17.6):1–17. doi: 10.1002/0471140864.ps1706s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houde D, Engen JR. Conformational dynamics of proteins by mass spectrometry. Hydrogen/deuterium exchange and covalent labeling approaches. Chimica oggi (Chemistry Today) 2009;27(2):20–23. [Google Scholar]
- 50.Graf C, Stankiewicz M, Kramer G, Mayer MP. Spatially and kinetically resolved changes in the conformational dynamics of the Hsp90 chaperone machine. EMBO J. 2009;28(5):602–613. doi: 10.1038/emboj.2008.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miranker A, Robinson C, Radford S, Aplin R, Dobson C. Detection of transient protein folding populations by mass spectrometry. Science. 1993;262(5135):896–900. doi: 10.1126/science.8235611. [DOI] [PubMed] [Google Scholar]
- 52.Covic A, Kuhlmann M. Biosimilars: recent developments. Int Urol Nephrol. 2007;39(1):261–266. doi: 10.1007/s11255-006-9167-5. [DOI] [PubMed] [Google Scholar]
- 53.Wrotnowski C. Well-characterized biologicals: Trends and views - Compounds can provide framework for research into biological products. Gen Eng News. 2000;20(17):21. [Google Scholar]
- 54.Bohlega S, Al-Shammri S, Sharoqi IA, Dahdaleh M, Gebeily S, Inshasi J, Khalifa A, Pakdaman H, SzA3lics M, Yamout B. Biosimilars: opinion of an expert panel in the Middle East. Curr Med Res Opin. 2008;24(10):2897–2903. doi: 10.1185/03007990802381554. [DOI] [PubMed] [Google Scholar]
- 55.Davis GC, Beals JM, Johnson C, Mayer MH, Meiklejohn BI, Mitlak BH, Roth JL, Towns JK, Veenhuizen M. Recommendations regarding technical standards for follow-on biologics: comparability, similarity, interchangeability. Curr Med Res Opin. 2009;25(7):1655–1661. doi: 10.1185/03007990903017313. [DOI] [PubMed] [Google Scholar]
- 56.Reingold SC, Steiner JP, Polman CH, Cohen JA, Freedman MS, Kappos L, Thompson AJ, Wolinsky JS. The challenge of follow-on biologics for treatment of multiple sclerosis. Neurology. 2009;73(7):552–559. doi: 10.1212/WNL.0b013e3181b2a6ce. [DOI] [PubMed] [Google Scholar]
- 57.Chirino AJ, Ary ML, Marshall SA. Minimizing the immunogenicity of protein therapeutics. Drug Discov Today. 2004;9(2):82–90. doi: 10.1016/S1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- 58.Bobst CE, Abzalimov RR, Houde D, Kloczewiak M, Mhatre R, Berkowitz SA, Kaltashov IA. Detection and characterization of altered conformations of protein pharmaceuticals using complementary mass spectrometry-based approaches. Anal Chem. 2008;80(19):7473–7481. doi: 10.1021/ac801214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chalmers M, Busby S, Pascal B, He Y, Hendrickson C, Marshall A, Griffin P. Probing protein ligand interactions by automated hydrogen/deuterium exchange mass spectrometry. Anal Chem. 2006;78(4):1005–1014. doi: 10.1021/ac051294f. [DOI] [PubMed] [Google Scholar]
- 60.Pascal BD, Chalmers MJ, Busby SA, Griffin PR. HD Desktop: An Integrated Platform for the Analysis and Visualization of H/D Exchange Data. J Am Soc Mass Spectom. 2009;20(4):601–610. doi: 10.1016/j.jasms.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slysz G, Baker C, Bozsa B, Dang A, Percy A, Bennett M, Schriemer D. Hydra: software for tailored processing of H/D exchange data from MS or tandem MS analyses. BMC Bioinformatics. 2009;10(1):162. doi: 10.1186/1471-2105-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lou X, Kirchner M, Renard BY, Kothe U, Boppel S, Graf C, Lee CT, Steen JA, Steen H, Mayer MP, Hamprecht FA. Deuteration distribution estimation with improved sequence coverage for HX/MS experiments. Bioinformatics. 2010;26(12):1535–1541. doi: 10.1093/bioinformatics/btq165. [DOI] [PubMed] [Google Scholar]
- 63.Rand KD, Zehl M, Jensen ON, Jorgensen TJ. Protein hydrogen exchange measured at single-residue resolution by electron transfer dissociation mass spectrometry. Anal Chem. 2009;81(14):5577–5584. doi: 10.1021/ac9008447. [DOI] [PubMed] [Google Scholar]
- 64.Zehl M, Rand KD, Jensen ON, Jorgensen TJ. Electron transfer dissociation facilitates the measurement of deuterium incorporation into selectively labeled peptides with single residue resolution. J Am Chem Soc. 2008;130(51):17453–17459. doi: 10.1021/ja805573h. [DOI] [PubMed] [Google Scholar]
- 65.Rand KD, Adams CM, Zubarev RA, Jorgensen TJ. Electron capture dissociation proceeds with a low degree of intramolecular migration of peptide amide hydrogens. J Am Chem Soc. 2008;130(4):1341–1349. doi: 10.1021/ja076448i. [DOI] [PubMed] [Google Scholar]
- 66.Rand KD, Pringle SD, Murphy JP, 3rd, Fadgen KE, Brown J, Engen JR. Gas-phase hydrogen/deuterium exchange in a traveling wave ion guide for the examination of protein conformations. Anal Chem. 2009;81(24):10019–10028. doi: 10.1021/ac901897x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karpusas M, Nolte M, Benton CB, Meier W, Lipscomb WN, Goelz S. The crystal structure of human interferon beta at 2.2-A resolution. Proc Natl Acad Sci U S A. 1997;94(22):11813–11818. doi: 10.1073/pnas.94.22.11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.