SUMMARY
In the vertebrate retina, neurites from distinct neuronal cell types are constrained within the plexiform layers, allowing for establishment of retinal lamination. However, the mechanisms by which retinal neurites are segregated within the inner or outer plexiform layers are not known. We find that the transmembrane semaphorins Sema5A and Sema5B constrain neurites from multiple retinal neuron subtypes within the inner plexiform layer (IPL). In Sema5A−/−; Sema5B−/− mice, retinal ganglion cells (RGCs), amacrine and bipolar cells exhibit severe defects leading to neurite mistargeting into the outer portions of the retina. These targeting abnormalities are more prominent in the outer (OFF) layers of the IPL and result in functional defects in select RGC response properties. Sema5A and Sema5B inhibit retinal neurite outgrowth through PlexinA1 and PlexinA3 receptors both in vitro and in vivo. These findings define a set of ligands and receptors required for the establishment of inner retinal lamination and function.
INTRODUCTION
The organization of neural circuits into laminae provides a mechanism for generating specific patterns of neuronal connectivity within many regions of the nervous system. In the vertebrate retina, neuronal circuitry is primarily organized in two separate synaptic regions: the outer and inner plexiform layers (OPL and IPL, respectively), which reside at the boundaries of the three retinal cell body layers (Masland, 2001; Wassle, 2004). Six major neuronal cell types located in three cell body layers elaborate neuronal processes in a stereotypic fashion within the two plexiform layers (Masland, 2001; Sanes and Zipursky, 2010). In the IPL, the two main retinal pathways that respond to an increment (ON pathway) or a decrement (OFF pathway) in illumination are organized in spatially segregated layers. Over the past two decades, our knowledge of the genetic programs controlling neuronal cell type specification in the vertebrate retina has advanced greatly (Livesey and Cepko, 2001; Ohsawa and Kageyama, 2008). However, the cellular and molecular events required for the development of laminar organization in the retina are largely unknown. Further, the establishment of retinal lamination and its relationship to visual system function remain to be investigated.
Current evidence implicates both adhesive and repulsive molecules in directing retinal neurite stratification and targeting in the IPL. For example, Dscams and Sidekicks, homophilic cell adhesion molecules, participate in lamina-specific neurite arborization within the chicken IPL (Yamagata and Sanes, 2008; Yamagata et al., 2002). DSCAMs in the mouse also regulate retinal neurite self-avoidance (Fuerst et al., 2009; Fuerst et al., 2008), and two separate point mutations in DSCAM disturb process stratification of select neuronal subtypes in the murine retina (Fuerst et al., 2010). In addition, the transmembrane semaphorin Sema6A signals through the PlexinA4 (PlexA4) receptor to direct processes from select subtypes of murine retinal neurons to specific sublaminae within the IPL (Matsuoka et al., 2011). However, molecular cues that direct the targeting of the vast majority of neuronal subtypes to specific sublaminae within the IPL, or that serve more generally to segregate retinal neurites to either the inner or outer plexiform layers, have yet to be identified.
Here, we show that the murine transmembrane repellents Sema5A and Sema5B together constrain the neurites of many inner retinal neurons to the IPL. In the absence of Sema5A and Sema5B, or the PlexinA1 and PlexinA3 receptors that mediate their function in the IPL, RGC, amacrine and bipolar cell neurites that normally stratify in the IPL instead extend toward the outer retina, resulting in functional deficits in retinal responses to visual stimuli.
RESULTS
Transmembrane class5 semaphorins Sema5A and Sema5B are expressed in the developing retina
To define cues that regulate the lamination of neuronal processes during retinal development, we first determined the expression patterns of Class5 and Class6 transmembrane semaphorins (Sema5A, 5B, and Sema6B, 6C, 6D) in the developing mouse retina (Figure 1; data not shown). We observed strong expression of Sema5A and Sema5B mRNA in the embryonic and early postnatal retina (Figures 1A–1H; data not shown; none of our own or commercially available Sema5A or Sema5B antibodies specifically stained mouse retinas). Sema5A and Sema5B exhibit very similar expression patterns, and both are expressed throughout early postnatal development when RGC dendrites, amacrine cell neurites, and bipolar cell axons arborize and make synaptic connections within the IPL. At P0 and P3, robust Sema5A and Sema5B mRNA expression is observed in the outer neuroblastic layer (ONBL) (Figures 1A–1F), directly adjacent to the inner neuroblastic layer (INBL), which we labelled with anti-Pax6, a marker for most RGCs and amacrine cells (Figure 1E and 1F). At P7, P10, and P14, the expression of both Sema5A and Sema5B becomes more restricted and is observed in the middle to outer part of inner nuclear layer (INL) (Figures 1G and 1H; data not shown). Sema5A and Sema5B transcripts are not detectable at P21, a time when retinal development is almost complete.
Figure 1. Sema5A and Sema5B are Expressed in the ONBL During Retinal Development and Cooperate to Direct Distinct Amacrine Cell Neurite Targeting to the IPL in vivo.
(A–D, G and H) In situ hybridization for Sema5A (A, C and G) and Sema5B (B, D and H) on retinal sections from P0 (A and B), P3 (C and D), and P7 (G and H) wild-type mice. At P0 and P3, strong expression of both Sema5A and Sema5B is observed in the ONBL (A–D). At P7, strong expression of Sema5B is observed in the middle to outer portions of the INL (H), whereas robust Sema5A expression is found in the middle portion of the INL (G).
(E and F) In situ hybridization for Sema5A (E, green) and Sema5B (F, green) on retinal sections from P3 mice followed by immunohistochemistry using anti-Pax6 (red), which labels most RGCs and amacrine cells in the INBL of the retina. Prominent expression of Sema5A and Sema5B is observed in the ONBL adjacent and complementary to the Pax6-positive INBL.
(I–P) Wild-type (I and M), Sema5A−/− (J and N), Sema5B−/− (K and O), and Sema5A−/−; Sema5B−/− (L and P) adult retina sections were immunostained with antibodies against tyrosine hydroxylase (TH, green, I–L) or vesicular glutamate transporter type 3 (vGlut3, green, M–P). TO-PRO-3 dye (blue) was used to visualize organization of nuclear layers within the retina. In Sema5B−/− and Sema5A−/−; Sema5B−/− retinas, TH-positive dopaminergic amacrine cells and also vGlut3-positive amacrine cells exhibit defects in stereotypic neurite arborization (K, L, O and P; n=6 Sema5B−/− mice, n=12 Sema5A−/−; Sema5B−/− mice). In wild-type and Sema5A−/− retinas, dopaminergic amacrine cell neurites are observed predominantly within the S1 sublamina of the IPL (I and J). In contrast, aberrant TH-positive neurites are observed in the INL of Sema5B−/− and Sema5A−/−; Sema5B−/− retinas (yellow arrows, K and L). Similarly, vGlut3-positive amacrine cell neurites stratify within the S2/S3 sublaminae in wild-type and Sema5A−/− retinas (M and N), however, this laminar stratification is disrupted, leading to an aberrant neurite targeting within the S1 sublamina in Sema5B−/− retinas (yellow arrow, O), and the S1 sublamina and INL in Sema5A−/−; Sema5B−/− retinas (yellow arrows, P).
Scale bar: 100 μm in H for A–H, 50 μm in L for I–L, and in P for M–P.
Transmembrane Class5 semaphorins regulate inner retinal neurite arborization in vivo
The two class5 semaphorins, Sema5A and Sema5B, are phylogenetically conserved membrane-bound semaphorin proteins and share an identical arrangement of semaphorin domains and thrombospondin type-1 repeats (TSRs) with an overall amino acid similarity of 72% (Adams et al., 1996; Tran et al., 2007; Yazdani and Terman, 2006). Previous work has shown that Sema5A and Sema5B can act as guidance cues to either attract or repel processes belonging to different neuronal populations (Goldberg et al., 2004; Hilario et al., 2009; Kantor et al., 2004; Lett et al., 2009; Oster et al., 2003).
We generated mice harboring knock-out alleles of Sema5A and Sema5B by targeting exon 6 of Sema5A and exon 2 of Sema5B, each of which encode the first 41 or 51 amino acids, respectively, of these proteins (Figure S1). Our Sema5A and Sema5B mutant mice lack full-length Sema5A and Sema5B proteins (Figures S1G and S1H). Unlike the early embryonic lethality observed in previously generated Sema5A null mice (in a mixed 129/NMRI genetic background (Fiore et al., 2005)), we found that in a 129/C57BL/6 mixed genetic background our Sema5A−/−, Sema5B−/−, and Sema5A−/−; Sema5B−/− mice are viable and fertile. This difference could be due to either the utilization of different targeting strategies and/or mouse genetic backgrounds. These results strongly suggest that our Sema5A and Sema5B mutant mice are null mutants.
Sema5A−/−; Sema5B−/− mice exhibit severe defects in the stereotypic neurite arborization of multiple amacrine cell types. In Sema5A−/−; Sema5B−/− mice, tyrosine hydroxylase (TH)-expressing dopaminergic amacrine cells, which predominantly stratify within the S1 sublamina of the IPL in wild-type (WT) retinas (Figure 1I), exhibit dramatic mistargeting within both the INL and OPL (Figure 1L). Similarly, vGlut3-expressing amacrine cells, which mostly stratify within the S2/S3 sublaminae of the IPL in WT retinas (Figure 1M), show severe neurite mistargeting within both the IPL and INL in Sema5A−/−; Sema5B−/− mice (Figure 1P). In addition, AII amacrine cells (labelled with Disabled-1 (Dab-1)), cholinergic amacrine cells (labeled with choline acetyltransferase (ChAT)), calretinin-positive cells, and calbindin-positive cells all exhibit pronounced ectopic neurite extension toward the outer retina in these mutant mice (Figures 2A–2H). Importantly, these defects are observed with full penetrance and expressivity in Sema5A−/−; Sema5B−/− animals (n=12 Sema5A−/−; Sema5B−/− mice, n=12 WT mice). Sema5B−/− mice also exhibit neurite arborization defects involving these same neuronal subtypes (Figures 1K and 1O; data not shown), although these phenotypes are less severe than those seen in Sema5A−/−; Sema5B−/− mice. Sema5A−/− mice, and also Sema5A+/−; Sema5B+/− mice, did not show defects in these same classes of retinal neurons (Figures 1J and 1N; Figure S2; data not shown). These results suggest that Sema5A and Sema5B play redundant roles in regulating multiple amacrine cell neurite arborization events in vivo. This is in contrast to our observations showing that Sema6A/PlexA signalling regulates neurite stratification of only a very small number of amacrine cell types, and serves to guide these projections toward, not away, from the inner retina within the IPL (Matsuoka et al., 2011). Therefore, Sema6A and Sema5A/Sema5B serve distinct roles in directing amacrine cell neurites to their appropriate retinal sublaminae.
Figure 2. Sema5A and Sema5B Cooperate During Retinal Development to Constrain Neurite Extension from Multiple Amacrine and RGC Subtypes Within the IPL in vivo.
(A–H) Wild-type (A, C, E and G) and Sema5A−/−; Sema5B−/− (B, D, F and H) adult retina sections were immunostained with antibodies against Dab-1 (A and B), ChAT (C and D), calretinin (E and F), and calbindin (G and H). Subtypes of amacrine cells and RGCs labeled by these markers exhibit characteristic neurite arborization within the IPL of wild-type retinas (A, C, E and G). However, all of these neuronal subtypes extend aberrant neurites (yellow arrows) toward the INL, and sometimes into the OPL, in Sema5A−/−; Sema5B−/− retinas (B, D, F and H).
(I–N) Wild-type (I, K and M) and Sema5A−/−; Sema5B−/− (J, L and N) postnatal retina sections from P2 (I and J), P4 (K and L), and P7 (M and N) mice were immunostained with anti-calbindin. At P2, neurite outgrowth from calbindin-positive cells does not apparently differ between wild-type and Sema5A−/−; Sema5B−/− retinas (I and J), however, at P4, calbindin-positive cells extend aberrant neurites toward the INL in Sema5A−/−; Sema5B−/− retinas (yellow arrows, L). At P7, ectopic stratification of aberrant neurites from calbindin-positive cells is prominent within the INL of Sema5A−/−; Sema5B−/− retinas (yellow arrow, N).
(O and P) Wild-type (O) and Sema5A−/−; Sema5B−/− (P) P7 retina were immunostained with anti-Pax6. In Sema5A−/−; Sema5B−/− retinas, misdirected neuronal processes, including calbindin-positive cell neurites, split Pax6-labeled cells into two layers in the inner region of the INL (yellow arrow, P).
Scale bars: 50 μm in H for A–H, and in P for I–P.
We next assessed Sema5A and Sema5B control of neurite targeting in the early postnatal IPL in vivo. Overall retinal structure, visualized by anti-calbindin and the nuclear marker TO-PRO3, is apparently equivalent between WT and Sema5A−/−; Sema5B−/− mice prior to P2 (Figures 2I and 2J; data not shown). Starting around P3~P4, when both Sema5A and Sema5B are strongly expressed in the ONBL (Figures 1C and 1D), amacrine cell and RGC subtypes labeled with anti-calbindin in Sema5A−/−; Sema5B−/− mice begin to extend neurites toward the ONBL (Figure 2L), a phenotype never observed in WT retinas (Figure 2K). This suggests that Sema5A and Sema5B prevent amacrine cell and RGC subtypes from extending neurites toward the ONBL. At P7, calbindin+ cell neurites in Sema5A−/−; Sema5B−/− retinas extend further within the INL, forming an ectopic plexiform layer that results in a discontinuity among the Pax6+ nuclei in the INL (Figures 2M, 2N, 2O and 2P). A similar discontinuity is also observed in the INL of adult Sema5A−/−; Sema5B−/− retinas, along with minor displacement of retinal cell nuclei within the IPL (Figures 2A–2H). Cholinergic amacrine cells and calretinin+ cells also extend aberrant neurites within the INL of Sema5A−/−; Sema5B−/− retinas at P7 (Figure S3; data not shown), and as early as P3–P4 (data not shown). Therefore, Sema5A and Sema5B direct lamination of multiple retinal neurites to the IPL during early postnatal retinal development.
Multiple RGC subtypes exhibit aberrant dendritic targeting in class5 semaphorin mutants
We next asked whether Sema5A and Sema5B affect RGC dendritic arborization within the IPL in vivo. We crossed Sema5A−/−, Sema5B−/−, and Sema5A−/−; Sema5B−/− mutant mice to a previously described transgenic mouse line in which green fluorescent protein (GFP) is expressed under the control of thy1 regulatory elements (Thy1::GFP–M mouse line), sparsely labeling a diverse set of RGCs, including ON and OFF RGCs, and thereby allowing us to trace single RGC dendritic arbors (Feng et al., 2000). In wild-type Thy1::GFP–M mice, nearly all RGCs exhibit dendritic arbors that are stratified within specific sublaminae (Figure 3A). In contrast, ~85% of GFP-labeled RGCs in Thy1::GFP–M; Sema5A−/−; Sema5B−/− mice have dendrites that arborize broadly within the IPL, extending into the INL, OPL, and, in a few cases, the ONL (Figures 3B and 3C; quantification in 3D). GFP-labeled RGCs in Thy1::GFP–M; Sema5A−/− and Thy1::GFP–M; Sema5B−/− mice show much milder dendritic arborization deficits compared to Thy1::GFP–M; Sema5A−/−; Sema5B−/− mice (Figure 3D).
Figure 3. Sema5A and Sema5B Constrain Dendritic Targeting of Multiple Thy1 M::GFP-Labeled RGC Subtypes, and also M1-type ipRGCs, to the IPL.
(A–C) Wild-type; Thy-1::GFP–M (A) and Sema5A−/−; Sema5B−/−; Thy-1::GFP–M (B and C) adult retina sections were immunostained with anti-GFP. Nearly all RGCs in wild-type; Thy-1::GFP–M retinas exhibit dendritic stratification within specific sublaminae (A). In contrast, GFP-labeled RGCs in Sema5A−/−; Sema5B−/−; Thy-1::GFP–M retinas show dendritic arbors that aberrantly extend toward outer regions of the retina (yellow arrows, B and C).
(D) Quantification of GFP-labeled RGC dendritic termination in adult retinas of wild-type, Sema5A−/−, Sema5B−/−, or Sema5A−/−; Sema5B−/− mice harboring the Thy-1::GFP–M transgene (n=3 animals for wild-type and Sema5A−/− mice, n=4 animals for Sema5B−/− and Sema5A−/−; Sema5B−/− mice). Each individual RGC was scored for the location of its dendritic termination within the retina (WT RGCs: 98.2% in IPL, 1.8% in INL, 0% in OPL/ONL; Sema5A−/− RGCs: 84.7% in IPL, 15.3% in INL, 0% OPL/ONL; Sema5B−/− RGCs: 76.7% IPL, 17.4% in INL, 5.9% in OPL/ONL; Sema5A−/−; Sema5B−/− RGCs: 15.4% in IPL, 53.6% in INL, 31% in OPL/ONL). The number of RGCs quantified for this analysis: n=57 for wild-type, n=59 for Sema5A−/−, n=86 for Sema5B−/−, n=84 for Sema5A−/−; Sema5B−/−.
(E–G) Wild-type (E) and Sema5A−/−; Sema5B−/− (F and G) adult retina sections were immunostained with an antibody against the N-terminus of melanopsin, which labels multiple subtypes of ipRGCs, including M1-type ipRGCs, which normally exhibit dendritic stratification within the S1 sublamina of the IPL in WT retinas. In contrast, M1-type ipRGC dendrites aberrantly extend toward the INL in Sema5A−/−; Sema5B−/− retinas (yellow arrows, F and G).
(H) Quantification of M1-type ipRGC dendritic stratification within the IPL in adult retinas of wild-type and Sema5A−/−; Sema5B−/− mice (n=6 animals for both WT and Sema5A−/−; Sema5B−/− mice). M1-type ipRGC dendritic termination was scored for normal (S1 sublamina) or abnormal laminar stratification location within the retina (WT RGCs: 95.1% in S1 of the IPL, 4.7% in INL, 0% in OPL/ONL; Sema5A−/−; Sema5B−/− RGCs: 17.6% in S1 of the IPL, 70.6% in INL, 11.8% in OPL/ONL). The number of M1-type ipRGCs quantified for this analysis: n=82 for wild-type, and n=85 for Sema5A−/−; Sema5B−/−.
(I–I″ and J–J″) Sema5A+/−; Sema5B+/− (I–I″) and Sema5A−/−; Sema5B−/− (J–J″) P14 retina sections were double-immunostained with the anti-melanopsin (I and J) and anti-calretinin (I′ and J′). In Sema5A+/−; Sema5B+/− retinas, multiple ipRGC subtypes labeled by the anti-melanopsin exhibit dendritic stratification within two distinct IPL domains: one resides within the S1 sublamina of the OFF layer (white arrow in I) and the other lies within S4/S5 sublaminae of the ON layer (white arrowhead in I). In Sema5A−/−; Sema5B−/− retinas, ipRGC dendritic stratification within the S4/S5 sublaminae (white arrowhead in J) is preserved, however, dendritic stratification within the S1 sublamina is severely disrupted, resulting in aberrant dendritic extension within the INL (yellow arrows in J). Anti-calretinin labels neuronal processes in three strata at the borders of S1 and S2, S2 and S3, and S3 and S4 within the IPL in the Sema5A+/−; Sema5B+/− retina (white bars in I′). In contrast, in the Sema5A−/−; Sema5B−/− retina, the outer stratification residing at the border of S1 and S2 within the OFF layer in the Sema5A+/−; Sema5B+/− retina was severely disturbed (yellow arrows in J′), whereas the two stratification bands closer to GCL were relatively less disturbed (white bars in J′).
(K–M) In vitro neurite outgrowth assay using dissociated retinal neurons derived from E14.5 retinas. Dissociated retinal neurons were cultured for 48hr on confluent monolayers of HEK293 cell lines stably transfected with either a control vector (K), or with vectors expressing Sema5A (L) or Sema5B (M). Retinal neurons cultured on Sema5A- or Sema5B-expressing HEK293 cells show reduced neurite length compared to those cultured on control cells, though a similar number of retinal neurons cultured on these different cell lines exhibit neurite outgrowth.
(N) Quantification of average neurite length per neuron from in vitro retinal neurite outgrowth assays (n=59 cells for control, n=48 cells for Sema5A, and n=57 cells for Sema5B). Both Sema5A and Sema5B significantly inhibit retinal neuron neurite outgrowth in vitro (average neurite length per neuron: 219.5 ± 20.3 μm for control, 91.7 ± 6.8 μm for Sema5A, and 106.1 ± 8.4 μm for Sema5B. Error bars are SEM (n=3 independent experiments). ** indicates P < 0.01 by one-way analysis of variance (ANOVA) followed by Tukey’s HSD test.
Scale bars: 50 μm in C for A–C, in G for E–G, 30 μm in J″ for I–I″ and J–J″, and 100 μm in M for K–M.
M1-type melanopsin-expressing intrinsically photosensitive ganglion cells (ipRGCs), visualized here with an antibody directed against the N-terminus of melanopsin, normally stratify within the outermost S1 sublamina of the IPL (Figure 3E) (Ecker et al., 2010; Schmidt and Kofuji, 2009). However, in Sema5A−/−; Sema5B−/− retinas M1-type ipRGC dendrites fail to stratify in the S1 sublamina and instead arborize in the INL and OPL (Figures 3F, 3G and 3H). This same antibody directed against melanopsin clearly labels dendritic stratification of distinct ipRGC subtypes within two discrete domains of the IPL in P14 retinas (Figures 3E and 3I) (Ecker et al., 2010; Fuerst et al., 2009). We found that dendritic stratification of ipRGC subtypes in distinct IPL sublaminae at P14 is selectively disrupted in Sema5A−/−; Sema5B−/− retinas; ipRGC dendritic stratification in the S1 sublamina (within the OFF layer) of the IPL is severely disrupted, similar to what we observed in adult Sema5A−/−; Sema5B−/− retinas (Figures 3I, 3J, 3F and 3G). However, ipRGC dendritic stratification within the inner (ON) layers is not apparently different from that observed in Sema5A+/−; Sema5B+/− retinas (arrowheads in Figures 3I and 3J). In addition, Figures 3I′ and 3J′ show that neurites from calretinin+ cells, normally confined to three strata in control retinas, are selectively disrupted within the outer (OFF) layers, but not within the inner (ON) layers, of the Sema5A−/−; Sema5B−/− IPL. We found that earlier in retinal development, at P7 (Figure S3), and even as early as P3–P4 (data not shown), ipRGCs dendrites found normally within the OFF layer are misdirected into the INL of Sema5A−/−; Sema5B−/− retinas. This is similar to the time course of certain amacrine cell neurite mistargeting events observed in Sema5A−/−; Sema5B−/− retinas (Figures 2 and S3). Therefore, Sema5A and Sema5B constrain dendritic targeting of RGCs to the IPL in vivo, playing a more prominent role in regulating stratification within the OFF relative to the ON layers of the IPL. RGC dendritic targeting abnormalities in Sema5A−/−; Sema5B−/− retinas are not correlated with axonal projection abnormalities to retinorecipient brain targets; we find that all RGC axon central trajectories, as assessed by anterogrande tracing, and ipRGC axonal projections to their major CNS targets, as assessed using a genetically encoded tracer (Hattar et al., 2006), reveal no defects in RGC axonal targeting to the brain (Figure S4).
To determine whether Sema5A and Sema5B directly regulate neurite development, we asked if these cues affect neurite outgrowth in dissociated embryonic retinal neurons. WT E14.5 retinal neurons were cultured on top of a confluent monolayer of stable HEK293 cell lines expressing Sema5A, Sema5B, or harboring an empty expression vector. Based on neuronal morphological characteristics and also the developmental timing of retinal neuronal cell type generation (Byerly and Blackshaw, 2009; Ohsawa and Kageyama, 2008), a majority of the neurons in our cultures included either RGCs or amacrine cells. The mean neurite length of retinal neurons cultured on the Sema5A- or Sema5B-expressing HEK293 cells was significantly shorter (~50–60%) that observed on control HEK293 cells (Figures 3K–3N). Thus, exogenous Sema5A and Sema5B inhibit retinal neurite outgrowth in vitro.
Bipolar cell axon targeting exhibits select defects in class5 semaphorin mutants
Bipolar cells form synaptic connections with both amacrine cells and RGCs within the IPL (Masland, 2001; Wassle, 2004). In contrast to the organized distribution of bipolar cell axon terminals within the IPL, observed as vGlut1+ puncta in WT retinas, we found an ectopic band of vGlut1+ puncta within the INL of Sema5A−/−; Sema5B−/− retinas (Figures 4A′ and 4B′). These ectopic vGlut1+ puncta co-localized with the aberrantly projecting axon terminals of cone OFF bipolar cells labelled with anti-synaptotagmin2 (Syt2) (Fox and Sanes, 2007) (Figures 4A, 4A″, 4B and 4B″). Similarly, cone OFF bipolar cells labelled by anti-neurokinin 3 receptor (NK3R) (Haverkamp et al., 2003), which normally establish axon terminals within the S1 sublamina of WT retinas (Figure 4C), also exhibit robust axon termination defects within the INL of Sema5A−/−; Sema5B−/− retinas (Figure 4D). In contrast, rod ON bipolar cells labelled by an antibody directed against protein kinase C alpha (PKCα) (Haverkamp et al., 2003) exhibit normal axonal terminations within the appropriate ON sublaminae of Sema5A−/−; Sema5B−/− retinas with the exception of a very few mislocalized axon terminals within the INL (Figures 4E and 4F). By immunolabelling with an antibody directed against calcium binding protein 5 (CaBP5), which labels three distinct types of bipolar cells, including type-5 cone ON, type-3 cone OFF, and rod bipolar cells (Ghosh et al., 2004), we observed in Sema5A−/−; Sema5B−/− retinas that the CaBP5+ rod and cone ON bipolar cell axonal terminations within IPL ON sublaminae show little or no difference compared to WT (Figures 4G and 4H). However, type-3 OFF cone bipolar cell axonal terminations, which stratify within the S2 sublamina of the WT IPL (Ghosh et al., 2004), are more disorganized, exhibiting ectopic axonal terminations within the S1 sublamina or the INL in Sema5A−/−; Sema5B−/− retinas (Figures 4G and 4H). These findings are consistent with the selective disruption of the OFF, but not ON, arbors of amacrine cells and RGCs in Sema5A−/−; Sema5B−/− retinas.
Figure 4. Bipolar Cell Axon Terminals are Selectively Mislocalized in Sema5A−/−; Sema5B−/− Mutant Mice.
(A–A″ and B–B″) Wild-type (A–A″) and Sema5A−/−; Sema5B−/− (B–B″) adult retina sections were double-immunostained with anti-synaptotagmin2 (Syt2, A and B) and anti-vGlut1 (A′ and B′) (merged in A″ and B″). Ectopic cone OFF bipolar cell axonal terminals (anti-Syt2yellow arrows in B) and pan-bipolar cell axonal terminals (anti-vGlut1, yellow arrows in B′) are co-localized in the INL (B″).
(C–H) Wild-type (C, E, G) and Sema5A−/−; Sema5B−/− (D, F, H) adult retina sections were immunostained with anti-NK3R (C and D), anti-PKCα (E and F), and anti-CaBP5 (G and H). In wild-type retinas, distinct bipolar cell types labeled by these markers show axon termination within specific sublaminae (indicated by orange brackets and white bars in C, E, G). In contrast, Sema5A−/−; Sema5B−/− retinas exhibit a severe defect in type-1 and-2 cone OFF bipolar cell axon termination (anti-NK3R, D), resulting in mislocalization of axonal terminals within the INL (yellow arrow in D). Type-3 cone OFF bipolar cells (anti-CaBP5, normal axonal terminals are indicated by an outer white bar in G also exhibit disorganized axonal terminations within the OFF layers of the IPL and the INL in Sema5A−/−; Sema5B−/− retinas (indicated by a white bracket and yellow arrows in H). We observed very few mislocalized rod ON bipolar cell axonal terminals within the INL of Sema5A−/−; Sema5B−/− retinas (white asterisk, F), however the vast majority of rod and cone ON bipolar cell axon terminals (anti-PKCα in F and anti-CaBP5 in H) is observed within the appropriate sublaminae of the IPL (orange brackets and a white bar in F and H).
(I–N) Wild-type (I, K and M) and Sema5A−/−; Sema5B−/− (J, L, and N) adult retina sections were immunostained with antibodies against calbindin (I and J), Goα (K and L), and vGlut1 (M and N). Normal stratification of horizontal cell neurites (anti-calbindin), ON bipolar cell dendrites (anti-Goα), and photoreceptor axon terminals (anti-vGlut1) was observed in the OPL of Sema5A−/−; Sema5B−/− retinas, as compared to WT retinas.
Scale bars: 50 μm in B″ for A–A″, B–B″, C–F, 30 μm in H for G and H, and in N for I–N.
We examined the OPL in Sema5A−/−; Sema5B−/− retinas using antibodies directed against calbindin (horizontal cells), vGlut1 (photoreceptor axon terminals), Goα (ON bipolar cells), and PKCα (rod bipolar cells), and observed normal OPL neurite stratification (Figures 4I–4N; data not shown). Although Sema5A and Sema5B can inhibit retinal neuron neurite extension, Sema5A−/−; Sema5B−/− retinas do not exhibit defects in mosaic patterning or neuronal tiling of dopaminergic amacrine cells, ipRGCs, or AII amacrine cells (data not shown). Sema5A−/−; Sema5B−/− retinas do not show significant differences in the abundance of retinal cell types as compared to control Sema5A+/−; Sema5B+/− retinas (Table S1). Therefore, the primary role of Sema5A and Sema5B is apparently to direct inner retinal neurite targeting.
Class5 semaphorin mutants exhibit visual function abnormalities
To address the functional consequences that might result from the neurite targeting defects in Sema5A−/−; Sema5B−/− mice, we recorded light responses ex vivo from neurons in the GCL of Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− retinas using a multi-electrode array (Meister et al., 1994; Ye et al., 2009). Consistent with previous studies (Renteria et al., 2006; Segev et al., 2004), the vast majority of spiking neurons that we recorded were RGCs based on their spike patterns. We found that the total number of RGCs that responded to wholefield increments or decrements in illumination (referred to hereafter as ON or OFF stimuli) was similar in Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− retinas (n=12 retinas from 6 animals for each genotype; n=222 RGCs for Sema5A+/−; Sema5B+/− and n=248 RGCs for Sema5A−/−; Sema5B−/−). However, Sema5A−/−; Sema5B−/− retinas had ~ 5 fold more RGCs that exhibit spontaneous neural activity but did not respond to wholefield stimuli (Figure 5A; n=103 RGCs for Sema5A+/−; Sema5B+/− and n=501 RGCs for Sema5A−/−; Sema5B−/−). To analyze RGC light responses in more detail, we focused on 97 Sema5A+/−; Sema5B+/− RGCs and 92 Sema5A−/−; Sema5B−/− RGCs that exhibited responses to all light stimuli presented, including wholefield, local spot, random noise, and direction selective stimuli (see Figures S5, S6 and Experimental Procedures for details). To determine if RGC ON or OFF light responses were differentially affected in Sema5A−/−; Sema5B−/− retinas, we distributed RGCs that responded to wholefield and local spot stimuli according to an ON-OFF index that quantifies RGC responses to these stimuli as a weighted difference between the maximal response amplitude following an increment or decrement in light intensity: RGCs that respond exclusively to the onset of illumination have an ON-OFF index of 1; RGCs that respond exclusively to the offset of illumination have an index of −1; RGCs that respond equally to both stimuli have an index of 0 (Figures 5B and S5; see Experimental Procedures for detailed description of ON-OFF index calculation). While Sema5A+/−; Sema5B+/− RGCs exhibit a relatively broad distribution of ON–OFF index values, Sema5A−/−; Sema5B−/− RGCs exhibit a distribution that is heavily skewed toward ON responses, with very few OFF responses recorded (Figures 5D–5G; median ON-OFF index values for Sema5A+/−; Sema5B+/−, 0 in wholefield and −0.15 in local spot experiments; for Sema5A−/−; Sema5B−/−, 0.5 in wholefield and 0.35 in local spot experiments; p=0.00042 for the wholefield response difference and p=1.18E-09 for the spot response difference (Student’s t-test)).
Figure 5. Visual Function Abnormalities Observed in the Sema5A−/−; Sema5B−/− Retina.
(A) Quantitative summary of the number of spiking RGCs assessed during wholefield ON or OFF stimulation. A total of 749 Sema5A−/−; Sema5B−/− (n=6 animals) and 325 control Sema5A+/−; Sema5B+/− (n=6 animals) spiking cells were identified using wholefield stimulus. Of these cells, 501 Sema5A−/−; Sema5B−/− (67 %) and 103 Sema5A+/−; Sema5B+/− (31 %) spiking cells did not exhibit light responsiveness following the wholefield stimulation. The total number of spiking cells in Sema5A−/−; Sema5B−/− retinas was increased ~2.3 times, as compared to control Sema5A+/−; Sema5B+/− retinas.
(B and D–G) Scatter plots of the ON-OFF index for Wholefield (x axis) and Spot (y axis) stimulus for Sema5A+/−; Sema5B+/− (a left panel in B) and Sema5A−/−; Sema5B−/− (a right panel in B). Control Sema5A+/−; Sema5B+/− RGCs show a wide range of responses, ranging from OFF to ON (ON-OFF index from −1 (OFF) to 1 (ON)). In contrast, Sema5A−/−; Sema5B−/− RGCs exhibit mostly ON responses and significantly fewer OFF type responses. (D–G) Quantification of data shown in B for Sema5A+/−; Sema5B+/− (D and F) and Sema5A−/−; Sema5B−/− (E and G) genotypes under wholefield (D and E) or local spot (F and G) stimulus condition. A significant shift in the ON-OFF index towards ON responses is observed in Sema5A−/−; Sema5B−/− retinas, as compared to the relatively broad distribution of responses in Sema5A+/−; Sema5B+/− retinas. Vertical red lines (in D–G) highlight the median values of the ON-OFF index (Median ON-OFF index values for Sema5A+/−; Sema5B+/− retinas: 0 in the wholefield experiment (D) and −0.15 in the local spot experiment (F); for Sema5A−/−; Sema5B−/− retinas: 0.5 in the wholefield experiment (E) and 0.35 in the local spot experiment (G). p=0.00042 for wholefield response difference, and p=1.18E-09 for spot response difference (Student’s t-test).
(C) Box plots for Direction Selectivity Indexes (DSi) computed for the 4 types of moving bars stimuli (see Experimental Procedures). Edges of the blue box represent the 25th and 75th percentile, whiskers extend to the most extreme data points, central mark represents the median, and red crosses are outliers. Both Sema5A−/−; Sema5B−/− and control Sema5A+/−; Sema5B+/− retinas exhibit strong correlations between DSi values when tested with narrow and wide bar stimuli.
(H and I) Representative dark-adapted ERGs (H) and intensity-response functions for the amplitude of the a-wave and b-wave of dark-adapted ERGs (I) obtained from Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− mice. Although response waveforms of Sema5A−/−; Sema5B−/− mice were comparable to those of Sema5A+/−; Sema5B+/− mice, the amplitudes of the b-waves in Sema5A−/−; Sema5B−/− mice were significantly smaller than those produced by Sema5A+/−; Sema5B+/− animals. * and ** indicate P < 0.05 and P < 0.01, respectively, by Student’s t-test. Data points are an average ± SEM for ≥3 mice.
(J and K) Representative light-adapted ERGs (J) and intensity-response functions for the amplitude of light-adapted ERGs (K) obtained from Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− mice. No significant differences in the light-adapted ERGs were observed between Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− mice.
To assess direction selectivity of the RGC responses, we presented stimuli using bars of different widths (100 or 240 μm widths) moving in 8 evenly spaced directions (Figure S6). We found that direction selective responses were not significantly different between Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− mice (Figures 5C and S6). Consistent with this observation, the optokinetic reflex (Cahill and Nathans, 2008) was also unaffected (Figures S7A and S7B). In addition, Sema5A−/−; Sema5B−/− retinas show no significant differences in RGC response implicit times and decay times following visual stimulation, or in receptive field sizes, as compared to Sema5A+/−; Sema5B+/− retinas (Figure S5, data not shown). Taken together, these findings demonstrate that the OFF pathway is specifically impaired in Sema5A−/−; Sema5B−/− mice, consistent with the selective disruption in OFF layer neuronal stratification in the Sema5A−/−; Sema5B−/− IPL.
To further assess visual function, we also measured rod- and cone-mediated full-field electroretinographic (ERG) responses in Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− mice. Strobe flash stimuli to mice dark-adapted overnight elicit the summed activity of rod photoreceptors (a-wave) (Penn and Hagins, 1969) and rod depolarizing bipolar cells (b-wave) (Kofuji et al., 2000; McCall and Gregg, 2008). Although the overall response waveforms of Sema5A−/−; Sema5B−/− mice were comparable to those of Sema5A+/−; Sema5B+/− mice, the amplitude of the b-wave, but not the a-wave, was significantly smaller in Sema5A−/−; Sema5B−/− mice compared to Sema5A+/−; Sema5B+/− mice (Figures 5H and 5I). This result is consistent with an intrinsic defect in inner retinal visual functions in Sema5A−/−; Sema5B−/− mice. Since the a-wave amplitude is not different between the control and Sema5A−/−; Sema5B−/− mice, the reduction in b-wave amplitude in Sema5A−/−; Sema5B−/− mice does not result from changes in rod photoreceptor activity (McCall and Gregg, 2008). The implicit time of the b-wave did not differ between these two genotypes for both dark and light-adapted conditions (data not shown), suggesting that synaptic connectivity between photoreceptors and bipolar cells is preserved in Sema5A−/−; Sema5B−/− mice. These data are consistent with our observation of normal photoreceptor axon terminals and bipolar cell dendrite stratification in the OPL of Sema5A−/−; Sema5B−/− retinas. The amplitudes of the high frequency oscillatory potentials of the b-wave, which are thought to reflect neuronal activity in the inhibitory feedback pathway initiated by amacrine cells (Wachtmeister, 1998; Wachtmeister and Dowling, 1978), were also reduced in Sema5A−/−; Sema5B−/− mice (data not shown). In addition, light adapted responses reflecting activity of the cone ON pathway (Sharma et al., 2005) did not differ between Sema5A+/−; Sema5B+/− and Sema5A−/−; Sema5B−/− mice (Figures 5J and 5K). Taken together, these ERG recording results reveal visual function abnormalities in the inner retinal pathway, possibly involving inhibitory feedback pathways, in Sema5A−/−; Sema5B−/− retinas.
Class5 semaphorins and PlexinA1 or PlexinA3 show complementary expression patterns in the developing postnatal retina
Mice harboring null mutations in the genes encoding conventional semaphorin receptors – the nine plexins (PlexA1–A4, B1–B3, C1, and D1) and two neuropilins (Npn-1 and Npn-2) – do not exhibit retinal lamination defects similar to those observed in Sema5A−/−; Sema5B−/− mice (Matsuoka et al., 2011). Although PlexB3 was previously shown to bind to Sema5A in vitro (Artigiani et al., 2004), we observed neither robust PlexB3 expression during early postnatal retinal development nor retinal defects in PlexB3−/− null mutant retinas (data not shown). We narrowed the field of candidate plexin and/or neuropilin class 5 sema receptors by conducting mRNA expression analyses for all plexins and neuropilins in the developing retina (data not shown). Based upon our observation of Sema5A and Sema5B expression and function, we assumed that Sema5A and Sema5B receptors should be expressed in the INBL. We observed strong PlexA1, PlexA2, and PlexA3 expression in the GCL and INL of the early postnatal retinas, as previously reported (Murakami et al., 2001), and nearly identical PlexA1 and PlexA3 expression patterns within the INBL beginning at E14.5 (Figures 6E, 6F, 6I and 6J). Immunolabelling using antibodies that specifically recognize PlexA1, PlexA2, and PlexA3 (Figures S8K–P) revealed that PlexA1 and PlexA3 proteins are broadly localized in the IPL, including in RGCs and the optic nerve (Figures 6A and 6B), throughout postnatal retinal development. PlexA2 protein is found in more restricted regions of the postnatal IPL and is not likely expressed in RGCs (Figures 6A–6D and S8A–S8J). These data suggest that PlexA1 and PlexA3 function within the INBL in multiple subtypes of amacrine cells and RGCs but not in bipolar cells, which are mostly localized in the ONBL (Figures 6E, 6F, 6I and 6J). Strikingly, Sema5A/5B and PlexA1/A3 exhibit complementary expression patterns in the developing postnatal retina (Figures 6G–6J), supporting the idea that Sema5A and Sema5B could serve as repulsive ligands for RGCs and amacrine cells that express PlexA1 and PlexA3.
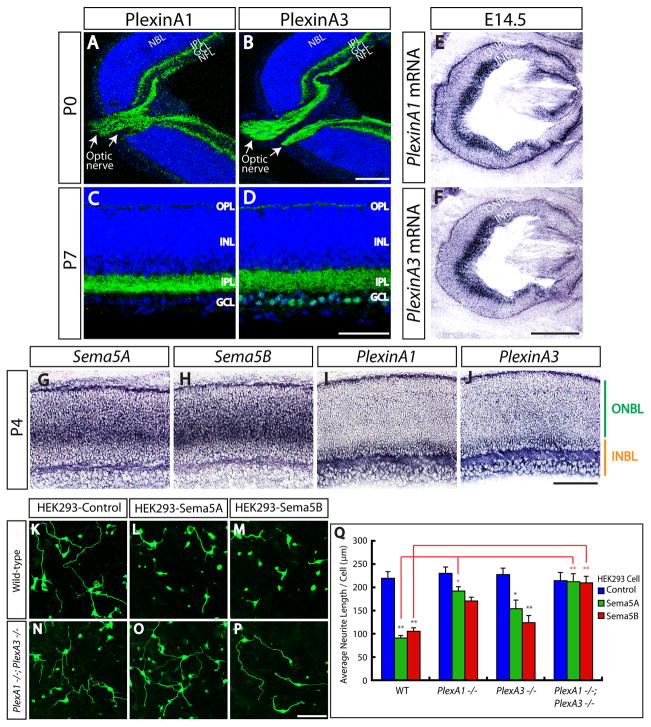
Figure 6. Sema5A/Sema5B and PlexinA1/PlexinA3 Exhibit Complementary mRNA Expression In the Developing Postnatal Retina, and PlexinA1 and PlexinA3 Mediate Inhibitory Responses of Retinal Neurons to Sema5A and Sema5B in vitro.
(A–D) Wild-type retina sections from postnatal P0 (A and B) and P7 (C and D) mice were immunostained with antibodies against PlexA1 (A and C) and PlexA3 (B and D). Both PlexA1 and PlexA3 are present in the nerve fiber layer (NFL) and the optic nerve (white arrows in A and B) at P0, revealing that PlexA1 and PlexA3 are expressed in RGCs. At P7, both PlexA1 and PlexA3 are present throughout the entire IPL. NBL, neuroblast layer.
(E and F) In situ hybridization for PlexA1 (E) or PlexA3 (F) on adjacent E14.5 embryonic retina sections. Both PlexA1 and PlexA3 are expressed in the INBL.
(G–J) In situ hybridization for Sema5A (G), Sema5B (H), PlexA1 (I), or PlexA3 (J) on P4 retina sections. Strong expression of Sema5A and Sema5B mRNA was detected in the ONBL, whereas strong PlexA1 and PlexA3 mRNA expression was restricted to the INBL.
(K–P) In vitro neurite outgrowth assay using dissociated retinal neurons from E14.5 wild-type (K–M) and PlexA1−/−; PlexA3−/− (N–P) embryos, cultured for 48hr on confluent monolayers of HEK293 cell lines expressing Sema5A, Sema5B, or transfected with a control expression vector. Wild-type retinal neurons cultured on Sema5A- or Sema5B-expressing cells exhibit reduced neurite extension (average neurite length per neuron) as compared to cells transfected with a control vector (K–M). However, inhibitory responses to Sema5A and Sema5B are not observed in PlexA1−/−; PlexA3−/− retinal neurons (N–P).
(Q) Quantification of average neurite length per neuron from in vitro neurite outgrowth assays (K–P), utilizing retinal neurons from E14.5 PlexA1−/−, PlexA3−/−, and PlexA1−/−; PlexA3−/− mutant embryos. Both Sema5A and Sema5B significantly inhibit neurite outgrowth from wild-type retinal neurons in vitro (see Figure 3K–3N). However, neurite outgrowth inhibition by Sema5A or Sema5B was abolished when PlexA1−/−; PlexA3−/− retinal neurons were used in this assay, and it was significantly attenuated for PlexA1−/− single mutant embryonic retinal neurons cultured on Sema5A-expressing HEK293 cells. The average neurite length per neuron: For PlexA1−/− embryos −231.1 ± 17.0 μm for control (n=56), 192.9 ± 15.0 μm for Sema5A (n=59), and 171.0 ± 12.4 μm for Sema5B (n=62); For PlexA3−/− embryos −227.3 ± 15.0 μm for control (n=58), 154.7 ± 14.2 μm for Sema5A (n=60), and 124.0 ± 10.5 μm for Sema5B (n=53); For PlexA1−/−; PlexA3−/− embryos −199.7 ± 16.2 μm for control (n=49), 195.2 ± 19.3 μm for Sema5A (n=57), and 196.1 ± 13.7 μm for Sema5B (n=46). Error bars are SEM (n=3 independent experiments). Black * indicates statistical significance of neurite outgrowth on ligand-expressing HEK293 cells as compared to control HEK293 cells within each genotype. Red * indicates statistical significance under the same treatment between wild-type and other retinal neuron genotypes. * and ** show P < 0.05 and P < 0.01, respectively, by multi-factorial ANOVA followed by Tukey’s HSD test.
Scale bars: 100 μm in B for A and B, 50 μm in D for C and D, 500 μm in F for E and F, 100 μm in J for G–J, and 100 μm in P for K–P.
Class5 semaphorin inhibition of retinal neurite outgrowth is mediated by PlexA1 and PlexA3 in vitro
To test if PlexA1 and PlexA3 are indeed functional receptors capable of mediating the inhibitory actions of Sema5A and Sema5B on retinal neurons, we conducted neurite outgrowth assays using retinal neurons obtained from E14.5 PlexA1−/−, PlexA3−/−, or PlexA1−/−; PlexA3−/− embryos. As noted above (Figures 3K–3N), we found that both Sema5A and Sema5B inhibit total neurite outgrowth from WT retinal neurons by ~50–60% (Figures 6K–6M and 6Q). However, there was no inhibition of neurite outgrowth by either Sema5A or Sema5B when PlexA1−/−; PlexA3−/− double mutant retinal neurons were used in this assay (Figures 6N–6P and 6Q). Inhibition of retinal neurite outgrowth by Sema5A, but not Sema5B, was partially attenuated when PlexA1−/− retinal neurons were assessed (Figure 6Q), and there was little or no attenuation of Sema5A or Sema5B inhibition when PlexA3−/− retinal neurons were similarly assayed (Figure 6Q). These results suggest that PlexA1 and PlexA3 function redundantly to mediate Sema5A and Sema5B inhibition of neurite outgrowth in vitro.
PlexinA1/PlexinA3 double null mutants phenocopy the inner retinal lamination defects observed in Sema5A−/−; Sema5B−/− mutants
PlexA1−/−; PlexA3−/− double mutant mice, in contrast to PlexA1−/− or PlexA3−/− single mutants, exhibit severe, fully penetrant and expressive, defects in IPL neurite targeting that closely match defects observed in Sema5A−/−; Sema5B−/− retinas (Figures 7A–7R). In PlexA1−/−; PlexA3−/− retinas, multiple RGC, amacrine and bipolar cell subtypes exhibit aberrant neurite extension into the INL and OPL (n=4 PlexA1−/−; PlexA3−/− animals). In addition, quantification of M1 type ipRGC neurites revealed very similar patterns of aberrant projections in Sema5A−/−; Sema5B−/− and PlexA1−/−; PlexA3−/− retinas (WT RGCs: 95.1% in S1 of the IPL, 4.7% in INL, 0% in OPL/ONL; Sema5A−/−; Sema5B−/− RGCs: 17.6% in S1 of the IPL, 70.6% in INL, 11.8% in OPL/ONL; PlexA1−/−; PlexA3−/− RGCs: 14.3% in S1 of the IPL, 73.8% in INL, 11.9% in OPL/ONL). These results demonstrate redundant roles in vivo for PlexA1 and PlexA3 in the regulation of RGC, amacrine and bipolar cell neurite targeting within the retina, and they strongly support the hypothesis that PlexA1 and PlexA3 function as Sema5A and Sema5B receptors in vivo.
Figure 7. PlexA1−/−; PlexA3−/− Double Mutant Retinas Phenocopy Sema5A−/−; Sema5B−/− Mutant Retinal Stratification Defects.
(A–D) Wild-type (A), PlexA1−/− (B), PlexA3−/− (C), and PlexA1−/−; PlexA3−/− (D) adult retina sections were immunostained with anti-TH (green). In PlexA1−/−; PlexA3−/− retinas, TH-positive dopaminergic amacrine cells exhibit defects in neurite arborization identical to those observed in Sema5A−/−; Sema5B−/− retinas (yellow arrows in D; n=4 for PlexA1−/−, PlexA3−/−, and PlexA1−/−; PlexA3−/− genotypes). In wild-type, PlexA1−/−, and PlexA3−/− retinas, dopaminergic amacrine cell neurites are observed predominantly in the S1 sublamina of the IPL (A–C). In contrast, aberrant TH-positive neurites are observed in the INL of PlexA1−/−; PlexA3−/− retinas (D), demonstrating that PlexA1 and PlexA3 play redundant roles in constraining the extension of these neurites within the IPL in vivo.
(E–R) Wild-type (E, G, I, K, M, O and Q) and PlexA1−/−; PlexA3−/− (F, H, J, L, N, P and R) adult retina sections were immunostained with antibodies against Dab-1 (E and F), vGlut3 (G and H), ChAT (I and J), calretinin (K and L), calbindin (M and N), vGlut1 (O and P), and melanopsin (Q and R). All of the RGC, amacrine and bipolar cell subtypes labeled by these markers phenocopy neurite stratification defects observed in Sema5A−/−; Sema5B−/− retinas. Similar to Sema5A−/−; Sema5B−/− mutants, PlexA1−/−; PlexA3−/− retinas exhibit multiple RGC, amacrine and bipolar subtype neurite mistargeting events directed toward the INL and OPL (F, H, J, L, N, P and R), never toward the GCL, and none of which are observed in wild-type retinas (E, G, I, K, M, O and Q). M1-type ipRGC dendritic termination (Q and R) was scored for normal (S1 sublamina) or abnormal laminar stratification location (n=3 animals for PlexA1−/−; PlexA3−/− mice; ipRGC dendritic termination: 14.3% in S1 of the IPL, 73.8% in INL, 11.9% in OPL/ONL). The number of M1-type ipRGCs quantified for this analysis: n=42 for PlexA1−/−; PlexA3−/− mice. See Figure 3H for comparison of this scoring obtained from PlexA1−/−; PlexA3−/− mutants to that obtained from wild-type and Sema5A−/−; Sema5B−/− mutants. The fraction of ipRGCs that exhibit abnormal laminar stratification, and the severity of this abnormal stratification, in Sema5A−/−; Sema5B−/− and PlexA1−/−; PlexA3−/− mutants is very similar.
Scale bars: 50 μm in D for A–F, and in P for G–R.
DISCUSSION
We identify here molecular cues that segregate neurites from RGCs, amacrine and bipolar cells within the IPL during retinal development (Figure 8). The transmembrane semaphorins Sema5A and Sema5B exhibit very similar expression patterns in the ONBL of the early postnatal retina and they inhibit neurite outgrowth from retinal neurons in vitro. Loss of both Sema5A and Sema5B in vivo leads to severe defects in the establishment of inner retinal lamination and also a selective defect in laminar stratifications of the OFF region of the IPL. These selective disruptions in the OFF circuit within the IPL of Sema5A−/−; Sema5B−/− retinas result in visual abnormalities, including greatly reduced RGC OFF responses and a reduction in the ERG b-wave amplitude. Sema5A and Sema5B do not regulate neurite stratification of OPL processes in these mutants, demonstrating that retinal neurite lamination within the IPL and OPL are controlled by distinct mechanisms. We find that the semaphorin receptors PlexA1 and PlexA3 exhibit broad expression in the developing postnatal INBL, complementary to the expression of Sema5A and Sema5B in the ONBL. PlexA1 and Plex3 together mediate inhibitory responses of retinal neurons to Sema5A and Sema5B in vitro, and PlexA1−/−; PlexA3−/− retinas completely phenocopy the retinal stratification defects observed in Sema5A−/−; Sema5B−/− retinas. Taken together, these findings show that the transmembrane cues Sema5A and Sema5B expressed in the ONBL provide repulsive guidance signals to extending neurites from amacrine cell and RGC subtypes that express PlexA1 and PlexA3 in the INBL, and also that these guidance events are critical for retinal neural circuit formation.
Figure 8. Class 5 Semaphorin Regulation Of Inner Retinal Lamination.
During early postnatal retinal development, Sema5A and Sema5B, which are expressed in the ONBL, direct neurite extension from inner retinal neuron cell types within the IPL through the combined action of the PlexA1 and PlexA3 receptors. Loss of Sema5A and Sema5B, or PlexA1 and PlexA3, results in neurite mistargeting into the outer retina. Sema5A and Sema5B are critical for maintaining the segregation of IPL and OPL neurites during retinal development, and they also play a crucial role in establishing IPL lamination, most prominently in the OFF layers.
Class 5 semaphorins regulate retinal laminar stratification by RGCs, amacrine cells, and bipolar cells
Cone OFF bipolar cells, which do not express high levels of PlexA1 and PlexA3 in the ONBL of the early postnatal retina, also exhibit axon terminal mislocalization in Sema5A−/−; Sema5B−/− and PlexA1−/−; PlexA3−/− retinas. PlexA1 and PlexA3 expression is restricted to the INBL, and these molecules are apparently not expressed in the ONBL or in bipolar cells throughout early postnatal retinal development. We observed that calbindin+ amacrine cell and RGC subtypes begin extending aberrant processes toward the ONBL in both central and peripheral regions of Sema5A−/−; Sema5B−/− retinas as early as P3 (data not shown), the peak of bipolar cell genesis in the mouse retina (Byerly and Blackshaw, 2009; Young, 1985). Most bipolar cell axon targeting and stratification within the IPL occurs later than P3 (Morgan et al., 2006), particularly in the peripheral retina, and bipolar cell axon branching begins well after subtypes of RGCs and amacrine cell projections stratify (Stacy and Wong, 2003). Taken together, these observations suggest that mislocalization of bipolar cell axon terminals within the INL of Sema5A−/−; Sema5B−/− and PlexA1−/−; PlexA3−/− retinas is a secondary consequence of the neurite targeting defects in multiple amacrine cell and RGC subtypes in these mutants.
The primary deficit in neurite targeting that affects multiple RGC and amacrine cell subtypes in Sema5A−/−; Sema5B−/− and PlexA1−/−; PlexA3−/− mutant retinas is difficult to precisely assign, since all of the RGC and specific amacrine subtypes we examined exhibit neurite targeting deficits. PlexA1 and PlexA3 receptors are expressed in the optic nerve and also broadly within the INBL throughout early postnatal development, suggesting that RGCs and amacrine cells express these two receptors. RGCs are dispensable for generating IPL sublamination by amacrine and bipolar cell neurites (Kay et al., 2004), and amacrine cells provide laminar stratification cues for RGCs within the IPL (Huberman et al., 2010; Matsuoka et al., 2011; Mumm et al., 2006; Stacy and Wong, 2003). Thus, class5 semaphorins could serve predominantly as repellents by forming either a gradient or a strict boundary for amacrine cells, requiring that the remaining neuronal cell types subsequently respond to different local cues associated with amacrine cells. However, it is also possible that class5 semaphorins act on multiple neuronal cell types independently, since both RGCs and amacrine cells express PlexA1 and PlexA3. Selectively removing the PlexA1 and PlexA3 genes in one or more subtypes of RGCs or amacrine cells will address this question.
Class 5 semaphorin regulation of retinal function
The ON and OFF visual system pathways are controlled by distinct circuits in the mammalian retina (Masland, 2001; Wassle, 2004). Understanding how the separation between ON and OFF visual circuits is established during development is fundamental in order to understand visual information processing. Distinct bipolar cell subtypes that contribute to the ON and OFF pathways stratify in spatially segregated ON and OFF layers of the IPL, forming synapses on RGC dendrites that are similarly segregated (Famiglietti and Kolb, 1976; Masland, 2001; Wassle, 2004). We show here through the use of multi-electrode RGC recordings following various types of visual stimuli that RGC responses to OFF, but not ON, visual stimuli are significantly affected in Sema5A−/−; Sema5B−/− retinas. This is consistent with our observation that the OFF circuit within the IPL is more severely disturbed than the ON circuit in Sema5A−/−; Sema5B−/− retinas. Therefore, these findings strongly support the idea that correct patterns of bipolar cell and RGC neurite stratification within the OFF layers of the IPL are required for RGC OFF responses to visual stimuli.
We speculate that the significant increase in the number of RGCs that exhibit spontaneous neuronal activity in the absence of visual stimuli in Sema5A−/−; Sema5B−/− retinas is caused by wiring abnormalities among amacrine cells, RGCs, and bipolar cells. RGCs receive input from either cone bipolar cells or amacrine cells (Masland, 2001). Amacrine cells typically provide inhibitory input onto either bipolar axon terminals or dendrites of RGCs, thereby modulating RGC firing responses. In Sema5A−/−; Sema5B−/− retinas, multiple subtypes of RGCs, amacrine cells, and bipolar cells exhibit dramatically misdirected neurites beyond the IPL. These defects likely result in synaptic connectivity deficits among these three neuronal cell types, leading to a lack of inhibitory control at the level of RGCs.
Sema5A−/−; Sema5B−/− retinas also exhibit ERG b-wave and oscillatory potential (OP) amplitude reduction. Inhibitory feedback pathways established by amacrine cells in the INL, including GABAergic and dopaminergic pathways, are thought to affect b-wave and OP amplitude (Dong and Hare, 2002; McCall et al., 2002; Naarendorp et al., 1993; Wachtmeister, 1998). In Sema5A−/−; Sema5B−/− retinas, multiple amacrine cells, including dopaminergic amacrine cells, exhibit severe neurite stratification defects. Therefore, it seems likely that synaptic connectivity among amacrine cells, RGCs, and bipolar cells is not preserved in Sema5A−/−; Sema5B−/− retinas. These wiring abnormalities, leading to disturbed inhibitory neuronal transmission pathways, may underlie the abnormal RGC and ERG responses in Sema5A−/−; Sema5B−/− retinas.
Mechanisms governing lamination in the mammalian CNS
Lamina-specific synaptic connectivity is a key feature of neuronal organization in both vertebrate and certain invertebrate nervous systems (Sanes and Zipursky, 2010). Previous studies on the function of cell adhesion molecules (CAMs) during retinal development, including Sidekick and Dscam CAMs in both the chicken and the mouse, demonstrate requirements for these CAMs in the generation of laminar targeting specificity among retinal neuronal subtypes expressing these adhesion molecules within the IPL (Fuerst et al., 2010; Yamagata and Sanes, 2008; Yamagata et al., 2002). In addition to adhesive interactions, however, recent observations show that repulsion plays a critical role in select neuronal stratification events in the IPL (Matsuoka et al., 2011). Taken together with our findings here on class 5 semaphorins and their role in regulating the establishment of neural connectivity within the retina, these observations suggest that inner retinal lamination is initially orchestrated through a combination of spatially distinct transmembrane semaphorin repellent cues. Sema6A, which is expressed in inner retinal neuron subtypes, directs a small subset of select laminar stratification events within the IPL; in contrast, Sema5A and Sema5B guidance cues present in outer retinal neurons control inner retinal lamination by constraining a major portion of inner retinal neurites to the IPL. It seems likely that a combination of transmembrane semaphorin short-range repulsive interactions and attractive interactions mediated by CAMs facilitates laminar stratification by regulating synapse formation among select neuronal subtypes that together participate in the formation of specific neural circuits in the retina. Outer retinal lamination events within the OPL are controlled by as yet unidentified repellents or attractants.
Our observations showing how transmembrane class5 semaphorins and their PlexA1 and PlexA3 receptors function during retinal development provide an example of repulsive guidance cue regulation of mammalian IPL lamination and segregation of inner retinal neurites from the outer retina. Correct development of inner retinal lamination is critical for appropriate physiological retinal responses, demonstrating that establishment of retinal laminar organization and retinal circuit function are intimately related. It will be of interest to determine whether similar molecular mechanisms facilitate the elaboration of laminar organization in other regions of the CNS, including the spinal cord and the cerebral cortex, and to understand how laminar organization in these regions of the CNS is related to function. Defining this relationship will advance our understanding of lamination as an organizing principle throughout the nervous system.
METHODS
Animals
The day of vaginal plug observation was designated as embryonic (E) 0.5 and the day of birth as postnatal (P) day 0. Genetically modified mouse lines and targeting strategies for the generation of Sema5A−/− and Sema5B−/− mice are described in Supplemental Experimental Procedures.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Matsuoka et al., 2011). The primary antibodies used in this study are listed in Supplemental Experimental Procedures.
In situ hybridization
In situ hybridization was performed on either fresh frozen or PFA-fixed retina sections (20 μm thickness) as described previously (Matsuoka et al., 2011). Digoxigenin-labeled antisense riboprobes specific for the coding sequences of Sema5A (3353–3860 bp), Sema5B (2808–3366 bp), PlexA1 (381–1314 bp), and PlexA3 (4977–5616 bp) were used for in situ hybridization.
Neurite outgrowth assay
The dissociation of mouse retinas was performed as previously described with some modifications (Maxeiner et al., 2005). The detailed dissociation method is described in Supplemental Experimental Procedures. Dissociated E14.5 retinal neurons (1 × 105 cells) were plated on top of a confluent monolayer of control HEK293 cells, or a stable HEK293 cell line expressing either Sema5A or Sema5B, in 24 well plates and then cultured for 48 hr in culture medium, (containing B-27 supplement, 2 mM L-Glutamine, 10 ng/mL ciliary neurotrophic factor (CNTF, R&D Systems), 50 ng/mL brain-derived neurotrophic factor (BDNF), 5 mM forskolin, 5 mg/mL insulin, 50 units/mL penicillin, and 50 ug/mL streptomycin), fixed in 4% PFA for 15 min, incubated with anti-βIII-tubulin (promega at 1:1000) followed by incubation with goat anti-mouse IgG conjugated with Alexa488 (Invitrogen at 1:500), and then imaged for neurite outgrowth analysis. Neurite lengths of retinal neurons were quantified using ImageJ plugin.
Electroretinogram (ERG) recording
ERG measurements were performed as previously described (Samuels et al., 2010). The amplitude of the a-wave was measured at 8 ms after flash presentation from the prestimulus baseline. The amplitude of the b-wave was measured to the b-wave peak from the a-wave trough or, if no a-wave was present, from the baseline. The amplitude of individual oscillatory potentials was measured from the negative trough to the subsequent peak. The implicit times of the b-wave and individual oscillatory potentials were measured at the positive peak.
Multi-electrode array recordings
RGC responses to a variety of light stimuli were recorded using a Multielectrode Array System (Multi Channel Systems, ALA Scientific). Dissections and recording conditions were previously described (Meister et al., 1994; Ye et al., 2009). Visual stimuli were generated and presented using Matlab software (Natick, MA, http://www.mathworks.com/), and the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). See Supplemental Experimental Procedures for a detailed description of the recordings and data analysis.
Optokinetic reflex (OKR) recordings
OKR measurements were performed as previously described (Cahill and Nathans, 2008).
Statistical analysis
The statistical significance of the differences between mean values among two or more groups was determined using Student’s t-test or ANOVA followed by Tukey’s HSD test, respectively. The criterion for statistical significance was set at p < 0.05. Error bars are SEM.
Supplementary Material
Highlights.
Sema5A/5B constrain neurites from multiple retinal neuron subtypes within the IPL
Sema5A/5B direct anatomical and functional properties of the OFF retinal pathway
PlexinA1 and PlexinA3 mediate Sema5A and Sema5B inhibition both in vitro and in vivo
Class5 semaphorin signaling separates the IPL from the outer retina
Acknowledgments
We thank K.-W. Yau and T. Xue for the Opn4Tau-LacZ/Tau-LacZ mice, M. Tessier-Lavigne for the PlexA3−/− mice, B. Howell for the Dab-1 antibody, and F. Haeseleer for the CaBP5 antibody. We also thank D. Kantor, S. Kozlov, C. Hawkins, and K. Takamiya for their assistance with the generation of the Sema5A−/− and Sema5B−/− mice. We thank M. Riccomagno, K. Mandai, S.-H. Wang, Y. Duan, and T. Tran for helpful suggestions and discussions throughout this project; D. Johnson for assistance with mouse experiments; and members of Kolodkin laboratory for assistance. This work was supported by R01 NS35165 to A.L.K.; a predoctoral fellowship from the Nakajima Foundation to R.L.M.; NRSA F31NS056558-01A1 to O.C.; the Veterans Administration to I.S.S. and N.S.P.; the Foundation Fighting Blindness to N.S.P.; R01 NS065048 to Y.Y.; R01 NS047333 to R.J.G. A.L.K. and J.N. are investigators of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Betz H, Puschel AW. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev. 1996;57:33–45. doi: 10.1016/0925-4773(96)00525-4. [DOI] [PubMed] [Google Scholar]
- Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Byerly MS, Blackshaw S. Vertebrate retina and hypothalamus development. Wiley Interdiscip Rev Syst Biol Med. 2009;1:380–389. doi: 10.1002/wsbm.22. [DOI] [PubMed] [Google Scholar]
- Cahill H, Nathans J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: application to genetic and drug-induced variation. PLoS One. 2008;3:e2055. doi: 10.1371/journal.pone.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, Hare WA. GABAc feedback pathway modulates the amplitude and kinetics of ERG b-wave in a mammalian retina in vivo. Vision Res. 2002;42:1081–1087. doi: 10.1016/s0042-6989(02)00032-9. [DOI] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fiore R, Rahim B, Christoffels VM, Moorman AF, Puschel AW. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol Cell Biol. 2005;25:2310–2319. doi: 10.1128/MCB.25.6.2310-2319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503:280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Harris BS, Johnson KR, Burgess RW. A novel null allele of mouse DSCAM survives to adulthood on an inbred C3H background with reduced phenotypic variability. Genesis. 2010;48:578–584. doi: 10.1002/dvg.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Ghosh KK, Hirano AA, Wassle H. Immunocytochemical description of five bipolar cell types of the mouse retina. J Comp Neurol. 2003;455:463–476. doi: 10.1002/cne.10491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario JD, Rodino-Klapac LR, Wang C, Beattie CE. Semaphorin 5A is a bifunctional axon guidance cue for axial motoneurons in vivo. Dev Biol. 2009;326:190–200. doi: 10.1016/j.ydbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Clandinin TR, Baier H. Molecular and cellular mechanisms of lamina-specific axon targeting. Cold Spring Harb Perspect Biol. 2010;2:a001743. doi: 10.1101/cshperspect.a001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Kay JN, Roeser T, Mumm JS, Godinho L, Mrejeru A, Wong RO, Baier H. Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development. 2004;131:1331–1342. doi: 10.1242/dev.01040. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett RL, Wang W, O’Connor TP. Semaphorin 5B is a novel inhibitory cue for corticofugal axons. Cereb Cortex. 2009;19:1408–1421. doi: 10.1093/cercor/bhn179. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermuller J, Brune H, Kirsch T, Pieper M, Degen J, Kruger O, Willecke K, et al. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci. 2005;25:566–576. doi: 10.1523/JNEUROSCI.3232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall MA, Gregg RG. Comparisons of structural and functional abnormalities in mouse b-wave mutants. J Physiol. 2008;586:4385–4392. doi: 10.1113/jphysiol.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the rho1 subunit abolishes GABA(C) receptor expression and alters visual processing in the mouse retina. J Neurosci. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Pine J, Baylor DA. Multi-neuronal signals from the retina: acquisition and analysis. J Neurosci Methods. 1994;51:95–106. doi: 10.1016/0165-0270(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Baier H, Wong RO. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn. 2001;220:246–258. doi: 10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Hitchock PF, Sieving PA. Dopaminergic modulation of rod pathway signals does not affect the scotopic ERG of cat at dark-adapted threshold. J Neurophysiol. 1993;70:1681–1691. doi: 10.1152/jn.1993.70.4.1681. [DOI] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Oster SF, Bodeker MO, He F, Sretavan DW. Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development. 2003;130:775–784. doi: 10.1242/dev.00299. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Renteria RC, Tian N, Cang J, Nakanishi S, Stryker MP, Copenhagen DR. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci. 2006;26:11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Sturgill GM, Grossman GH, Rayborn ME, Hollyfield JG, Peachey NS. Light-evoked responses of the retinal pigment epithelium: changes accompanying photoreceptor loss in the mouse. J Neurophysiol. 2010;104:391–402. doi: 10.1152/jn.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev R, Goodhouse J, Puchalla J, Berry MJ., 2nd Recording spikes from a large fraction of the ganglion cells in a retinal patch. Nat Neurosci. 2004;7:1154–1161. doi: 10.1038/nn1323. [DOI] [PubMed] [Google Scholar]
- Sharma S, Ball SL, Peachey NS. Pharmacological studies of the mouse cone electroretinogram. Vis Neurosci. 2005;22:631–636. doi: 10.1017/S0952523805225129. [DOI] [PubMed] [Google Scholar]
- Stacy RC, Wong RO. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol. 2003;456:154–166. doi: 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Wachtmeister L, Dowling JE. The oscillatory potentials of the mudpuppy retina. Invest Ophthalmol Vis Sci. 1978;17:1176–1188. [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.