Abstract
Functional neuroimaging studies of ADHD have focused on the neural correlates of cognitive control. However, for many youths with ADHD, emotional lability is an important clinical feature of the disorder. We aimed to identify the neural substrates associated with emotional lability that were distinct from impairments in cognitive control and to assess the effects that stimulants have on those substrates. We used functional magnetic resonance imaging (fMRI) to assess neural activity in adolescents with (N=15) and without (N=15) ADHD while they performed cognitive and emotional versions of the Stroop task that engage cognitive control and emotional processing, respectively. The participants with ADHD were scanned both on and off stimulant medication in a counterbalanced fashion. Controlling for differences in cognitive control, we found that during the emotional Stroop task, adolescents with ADHD as compared with controls demonstrated atypical activity in the medial prefrontal cortex (mPFC). Stimulants attenuated activity in the mPFC to levels comparable with controls.
Keywords: Stroop, Medial Prefrontal Cortex, Cognitive Control, Emotional Lability
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is among the most common diagnoses in pediatric psychiatry with 3–10% of school age children affected by the disorder(Barkley 2005). Although ADHD is characterized by inattention, hyperactivity, and impulsivity, one of the most challenging aspect of the disorder is the heightened emotional lability (EL) that is highly prevalent in children with ADHD(Barkley 1997a). Emotional lability indicates a tendency for intense, or strong, emotional reactions(Conners 2008; Maedgen & Carlson 2000; Sobanski et al 2010) and has been described in youths with ADHD in both the clinical and research literature(Barkley & Fischer 2010) for several decades, beginning as early as 1798 with Alexander Crichton’s description of hyperactive children demonstrating a “morbid exaggeration of emotional excitability” (Crichton 1798). In addition, epidemiological studies demonstrate that youths with ADHD have rates of depression and anxiety disorders far beyond those expected by chance alone(Biederman et al 1991).
Despite the clinical significance of EL in ADHD, its neurobiological substrates are unknown. There are two main, competing hypotheses. The first maintains that EL in ADHD patients stems primarily from impairments in cognitive control and this manifests itself as an impaired capacity to suppress responses elicited by emotional stimuli(Barkley 1997b). The competing hypothesis maintains that it is emotional processing itself that is dysfunctional with emotional stimuli generating unusually, strong emotional responses in youth with ADHD(Sonuga-Barke et al 1992). Neuroimaging studies that have examined emotional processing in ADHD subjects have not fully tested these two hypotheses because they did not attempt to disentangle emotional processing from more general deficits in cognitive control(Peterson 2003). To overcome this, we used functional magnetic resonance imaging (fMRI) to examine whether adolescents with ADHD have altered task-related activation during an emotional processing task and whether these atypical task-related activations could be dissociated from activations associated with cognitive control.
To examine emotional processing, we used an emotional Stroop task(Passarotti et al 2009; Whalen et al 2006). In this task, we presented participants with neutral and emotionally salient words (i.e., words such as ‘month’ and ‘death,’ respectively). The emotionally salient words had either positive or negative valence (e.g., ‘happy’ or ‘hate,’ respectively). On each trial, the same word was written several times (Figure 1A). The task was to indicate on a keypad the number of times the word was written. Participants are often slower and less accurate in counting the number of times that a word is presented during trials with emotion-denoting words than during the neutral trials (i.e. trials with words without emotional salience)(Williams et al 1996). This effect of emotion-denoting words on task performance is referred to as an attentional bias, or distraction, and it is thought to reflect participants’ difficulty in diverting attention away from the emotion evoked by the emotional words.
Figure 1. Examples of the trial types for the emotional and cognitive Stroop tasks.
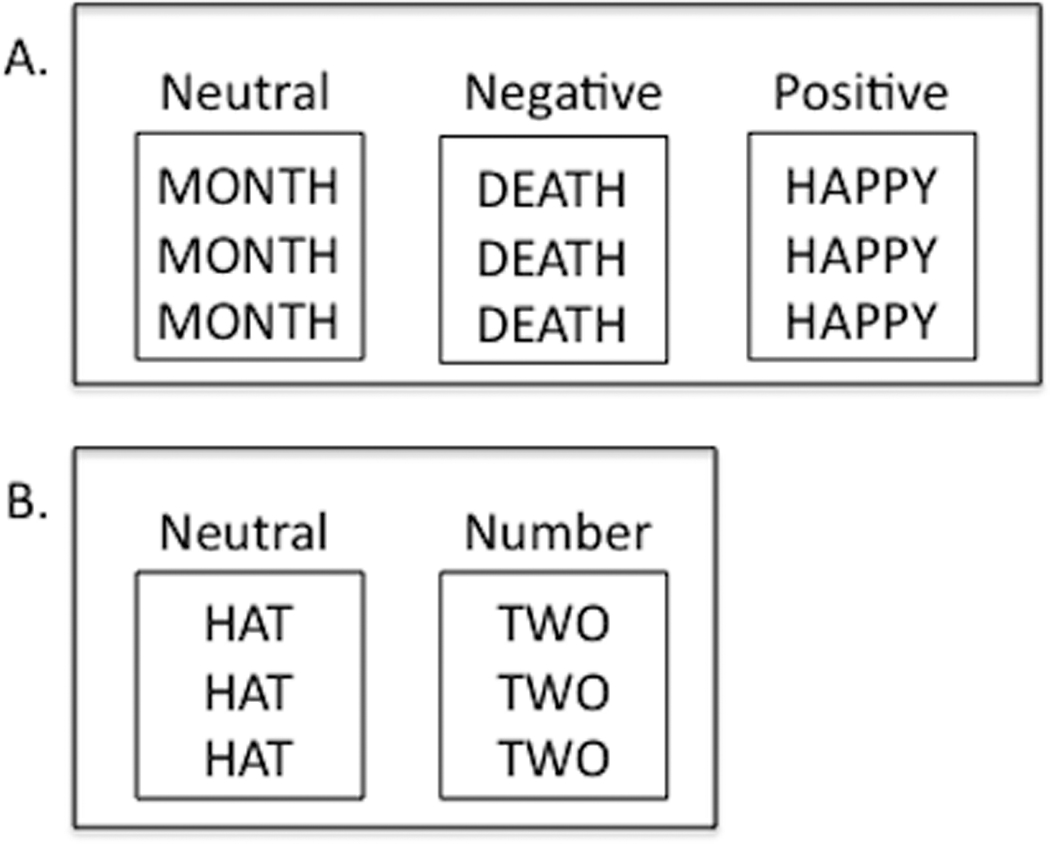
(A) In the emotional Stroop, there were 3 conditions: neutral words, negatively valenced words, and positively valenced words. During the neutral word trials, subjects were presented with words lacking emotional content; during the negatively and positively valenced word trials, subjects were presented with words associated with high emotional valence. The subject’s task was to indicate on a keypad the number of times that the word was written. For each of the examples provided, the correct response is “3.” (B) In the cognitive Stroop, there were 2 conditions: neutral words and number words. During the neutral word trials, subjects were presented with words describing an article of clothing; during the number word trials, subjects were presented with number words that were incongruent with the number of times the word was presented (e.g., “two” written 3 times, in which the correct response would be “3”). As with the emotional Stroop, the subject’s task was to indicate on a keypad the number of times that the word was written.
To dissociate emotional processing from cognitive control, we also used a cognitive Stroop task(Bush et al 2006) that was analogous to the emotional Stroop. As in the emotional Stroop, the subject’s task was to indicate the number of times that a word was written. The difference, however, was that with the cognitive Stroop, the distracter trials consisted of number words that conflicted with the number of presentations (e.g., the word “two” presented three times, in which case the correct answer is three; Figure 1B). Conversely, during neutral trials, participants were shown words denoting an article of clothing. Like other cognitive versions of the Stroop, this task requires cognitive control, because during the distracter trials participants automatically read the number word and then have to inhibit the tendency to answer with the number indicated by the word(Bush et al 2006; MacLeod 1991).
By using both cognitive and emotional versions of the Stroop, we aimed to test the following hypotheses: During the cognitive Stroop, we hypothesized that activation would be detected in the neural substrates associated with cognitive control. These substrates include the dorsal striatum, dorsal anterior cingulate, lateral prefrontal cortex (PFC), and thalamus(Casey et al 2007). (Prior research has addressed group level differences between ADHD subjects and controls during the cognitive Stroop; this was not a focus of this study.) Having identified brain region associated with cognitive control, we then aimed to covary for the effects of cognitive control and identify brain regions that differed between ADHD participants and controls in their responses during the emotional Stroop. In doing so, we intended to tease apart the effects of cognitive control (as determined by the cognitive Stroop) from the effects of emotional processing (as determined by the emotional Stroop). We hypothesized that the ADHD participants would differ from controls in emotional processing even after covarying for their impaired cognitive control. More specifically, we predicted different patterns of activation, depending on the mechanism by which ADHD subjects differed from controls in emotional processing. If ADHD participants differed from controls in the processing of positive emotions, we expected to find increased activation in the ADHD participants in regions that are most typically associated with positive affect (e.g. ventral striatum) (Posner et al 2005) during the presentation of positively valenced words. Likewise, if ADHD participants differed from controls in the processing of negative emotions, we expected increased activation in regions associated with negative affect (e.g. insular cortex)(Phan et al 2002) during the presentation of negatively valenced words. Alternatively, if the ADHD participants differed from controls in emotion regulation, we should find altered activation during the presentation of either positively or negatively valenced words because emotion regulation should be engaged to modulate emotional processing regardless of the valence (i.e., positive or negative) of the words presented. Moreover, the task-related activations should be detected in brain regions associated with the regulation of emotion (e.g. medial PFC and hippocampus)(Davidson et al 2000; Posner et al 2009). Lastly, we scanned the ADHD participants while they were on and off stimulant medication. We hypothesized that stimulants would attenuate the atypical emotional processing that we expected to find in the unmedicated ADHD youth.
2. Methods
The Institutional Review Board of the Oregon Health & Science University (OHSU) approved the study procedures. All child participants provided informed assent and a legal guardian provided informed consent.
2.1. Subjects
Participants were 15 adolescents with ADHD and 15 healthy control adolescents. Controls were age- and gender-matched to the ADHD subjects (Table 1). Controls were screened for psychiatric disorders using the Diagnostic Interview Schedule for Children (DISC) - Predictive Scales(Lucas et al 2001) and were excluded if they had any probable, active Axis I disorder. ADHD subjects, and at least one parent, were interviewed by a child psychiatrist and completed the child and parent versions of the DISC(Shaffer et al 1993). ADHD subjects were excluded if they had any active, Axis I disorder in addition to ADHD, other than oppositional defiant disorder (ODD), or conduct disorder (CD). All ADHD subjects were taking stimulant medication as a normal part of their clinical management. ADHD subjects were excluded if they were taking any non-stimulant psychotropic medication (e.g. atomoxetine); control subjects were medication free. Additional exclusion criteria for both groups included: (1) age < 11 or > 16 years; (2) neurological illness or significant head trauma (loss of consciousness > 2 minutes); (3) serious medical problems; (4) pregnancy; (5) IQ < 80; (6) left-handedness; (7) non-native English speakers; and (8) MRI contraindications (e.g. braces).
Table 1.
Demographic and clinical characteristics of the study sample. CDI, Children‘s Depression Inventory; STAI, Spielberger State Anxiety Inventory; FSIQ, Full Scale Intelligence Quotient estimated from the Wechsler Abbreviated Scale of Intelligence. Socioeconomic status was assessed with the Hollingshead Index of Social Position. Pubertal status was assessed with the Puberty Development Scale (PDS).
| ADHD | Healthy Controls | Test Statistic | P value | |
|---|---|---|---|---|
| Age in years | 13.5±1.2 | 13.4±1.2 | t = 0.3 | 0.8 |
| Gender | 13 males; 2 females | 13 males; 2 females | χ2 = 0 | 1.0 |
| Hollingshead Index of Social Position | 32.4±13.9 | 31±11.8 | t = 0.3 | 0.8 |
| FSIQ | 111.4 ± 16 | 114.1±10 | t = 0.5 | 0.6 |
| Pubertal Status | 2.5±0.8 | 2.7±0.7 | Mann–Whitney U = 98 | 0.5 |
| STAI | 45.2±7.3 | 39.5±7.4 | t = 2.1 | 0.04* |
| CDI | 46.4±7.0 | 39.5±0.9 | t = 3.4 | 0.003* |
Indicates a statistically significant difference between the ADHD and control participants. Values in parentheses indicate standard deviations.
Parents completed the Conners’ Parent Rating Scales(Conners et al 1998) and Child Behavior Checklist(Achenbach & Rescorla 2001). All subjects were administered the Edinburgh Handedness Inventory(Oldfield 1971), Hollingshead Index of Social Position(Hollingshead 1975), Children’s Depression Inventory (CDI)(Kovacs 1985), Spielberger State Anxiety Inventory (STAI)(Spielberger & Gorsuch 1970), and Puberty Development Scale (PDS)(Petersen et al 1988). ADHD subjects completed the Wechsler Abbreviated Scale of Intelligence (WASI)(Wechsler 1999); controls completed the short form of the WASI, which consists of the Matrix Reasoning and Vocabulary subtests. Because the ADHD subjects were to complete two MRI scans and because ratings on the CDI and STAI could, in theory, change between the first and second scan, the ADHD subjects completed these measures twice – once at the time of their first MRI scan and again at their second MRI scan. Scores on these measures did not differ significantly at these two-time points (CDI: P=0.36; STAI: P=0.38), and thus we used only the scores obtained with the first MRI scan. ADHD and control subjects did not differ significantly in age, estimated IQ, socioeconomic status, or pubertal stage (Table 1). ADHD subjects had significantly higher levels of depressive symptoms (CDI t-scores for ADHD subjects: 46.4±7.0; Controls: 39.5±0.9; comparison of ADHD subjects and controls: t=3.4; df=28, P=0.003) and anxiety (STAI t-scores for ADHD subjects: 45.2±7.3 Controls: 39.5±7.4; t=2.1; df=28, P=0.04). (The depressive and anxiety levels in both groups were well below levels indicative of clinical significance.) These differences in depressive and anxiety symptoms were controlled for in subsequent analyses. Within the ADHD sample, 13 participants were classified as ADHD – Combined type and two were classified as ADHD – Predominantly Inattentive type.
2.2. fMRI Paradigms
ADHD subjects completed two scanning sessions: one while on their normally prescribed stimulant and another after abstaining from the stimulant for at least 48 hours (i.e., exceeding at least 4 half lives for available stimulant formulations). The order of the scanning sessions was counterbalanced across ADHD subjects to control for medication status. (Seven ADHD participants completed their first scan while unmedicated and eight completed it while medicated.) Control participants completed a single scanning session.
We used two distinct fMRI tasks: a cognitive Stroop and an emotional Stroop(Bush et al 2006; Whalen et al 2006). Each scanning session included one run of the cognitive Stroop and two runs of the emotional Stroop. (The rationale for using one run of the cognitive Stroop and two runs of the emotional Stroop is discussed in the supplemental material.) Task order was counterbalanced across participants. For both tasks, each run consisted of 8 blocks, with 20 trials per block and 15 seconds of fixation at the beginning and end of the run. Trials consisted of 1.5 seconds of stimulus presentation, during which time subjects responded by indicating the number of times the word was presented (Figure 1A & 1B). The total scan time per run was 4 minutes, 30 seconds. The order of presentation consisted of alternating neutral and number word blocks for the cognitive Stroop and alternating neutral and valenced (positive or negative) word blocks for the emotional Stroop (i.e., neutral, negative, neutral, positive, etc.). After the scanning sessions, subjects provided valence ratings (1 = very unpleasant; 3 = neutral; 5 = very pleasant) for the words, to confirm that the words were properly categorized as neutral, negative, and positive. The words selected for the emotional Stroop were matched by word length and frequency of use. To match the number words used in the cognitive Stroop, the neutral words were selected from a single linguistic category - articles of clothing. The words used during the tasks and their valence ratings are presented in the supplemental material.
The fMRI paradigms were programmed in E-Prime (v. 1.0; Psychology Software Tools, Pittsburgh, PA) running on a Dell desktop computer (Dell Computer Corp., Austin, TX). We back-projected the stimuli onto a screen that subjects viewed via a mirror attached to the MRI head coil. Reaction times and responses were collected in the scanner using an MRI compatible button box along with E-prime software.
2.3. Image Acquisition
Images were acquired using a 3.0 Tesla Siemens Magnetom Tim Trio scanner with a 12-channel head-coil at the OHSU Advanced Imaging Research Center. The scanning protocol started with a high-resolution, whole-brain structural image series collected in the sagittal plane using a T1-weighted Magnetization Prepared Rapid Gradient Echo sequence (TI = 900ms, flip angle = 10°, TE = 3.58ms, TR = 2300ms, bandwidth = 180Hz/Px, 256×240 matrix, slice thickness = 1mm). BOLD-weighted functional images were collected in an oblique plane (parallel to the AC-PC line) using T2*- weighted echo-planar imaging (TR=2000ms, TE=30ms, flip angle=90°, FOV=240mm, in-plane resolution=3.8 × 3.8mm, 33 slices covering the whole brain, slice thickness=3.8mm).
2.4. Behavioral Data Analyses
We calculated mean reaction times and error rates for the positive, negative, and neutral words in the emotional Stroop and for the number and neutral words in the cognitive Stroop. If no response was provided for a trial, the trial was not included in the reaction time average, but was included as an incorrect response when calculating error rates. Difference scores between distracter and neutral trials provide a more accurate reflection of attentional biases than do raw scores (van Mourik et al 2005) and thus we calculated distracter effects as follows – reaction time distraction was calculated as the difference in mean reaction times (MRT) between the distracter trials and neutral trials:
Negatively valenced distraction = MRT negative word trials – MRT neutral word trials
Positively valenced distraction = MRT positive word trials – MRT neutral word trials
Cognitive distraction = MRT number word trials – MRT neutral word trials
A similar approach was used to calculate the distraction effects on the basis of error rates:
Negatively valenced distraction = percent error negative word trials – percent error neutral word trials
Positively valenced distraction = percent error positive word trials – percent error neutral word trials
Cognitive distraction = percent error number word trials – percent error neutral word trials
Within-group analyses were conducted using single-sample t-tests. Between group analyses compared the magnitude of the distraction (a) in patients when they were medicated versus unmedicated (paired t-tests), (b) between unmedicated patients and controls (independent-sample t-tests), and (c) between medicated patients and controls (independent-sample t-tests).
2.5. fMRI Data Analyses
Images were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience). Images were slice-time and motion corrected, coregistered with each subject’s T1-weighted scan, normalized into the Montreal Neurological Institute (MNI) space, resampled at 2mm3, and smoothed with a Gaussian kernel of 6mm3 FWHM (Friston et al 1995). A 128-second temporal high-pass filter removed low-frequency noise. For each subject, linear models were constructed using regressors indexing the duration of each block type (neutral and number word blocks for the cognitive Stroop; neutral, negative, and positive word blocks for the emotional Stroop) convolved with the canonical hemodynamic response function. For the emotional Stroop, we generated two contrasts: a) positive blocks vs. neutral blocks (henceforth referred to as neural: positively valenced distraction), and b) negative blocks vs. neutral blocks (henceforth referred to as neural: negatively valenced distraction). For the cognitive Stroop, we generated a single contrast: number blocks vs. neutral blocks (henceforth referred to as neural: cognitive distraction). Within-group analyses were conducted on a) neural: negatively valenced, b) neural: positively valenced, and, c) neural: cognitive distraction by entering the respective contrast images into single-sample t-tests.
2.6. Hypothesis Testing
Group level differences were tested by entering the contrast images for each distracter effect (i.e., neural: positively valenced distraction, neural: negatively valenced distraction, and neural: cognitive distraction) into two-way factorial models that incorporated random effects and covaried for task performance, levels of anxiety and depression, and the presence of comorbid oppositional defiant disorder/conduct disorder (ODD/CD). We used a within-subject factor, distraction, with three levels (positively valenced, negatively valenced, and cognitive distraction). We treated the second factor, group, as a between-subject factor when comparing ADHD patients with controls, and as a within-subjects factor when comparing medicated and unmedicated ADHD participants. To identify brain regions that differed in their response to positively valenced distracters between groups, we isolated group × neural: positively valenced distraction interactions within this these larger models. Group × neural: negatively valenced distraction interactions similarly identified brain regions that differed in their response to negatively valenced distracters between groups. Finally, in order to disentangle the effects of emotional processing from more general cognitive control, we included the neural: cognitive distraction contrast as a covariate. The results from the interaction analyses thus indicate brain regions that differ across the groups in their response to negatively or positively valenced distracters, while controlling for variance associated with the effects of cognitive distracters.
To safeguard against identifying false-positive activations (i.e., Type I errors), we conducted 10,000 Monte-Carlo simulations and determined that a minimum volume of 1600 ml with a connectivity radius of 3.0mm was needed to ensure that a voxel-wise alpha 0.01 would result in a corrected cluster-wise activation probability of < 0.0001. The Monte Carlo simulations were conducted using a version of AlphaSim(Cox 1996) adapted to run on a Matlab platform (http://restfmri.net/forum). Regarding head motion during scanning, we calculated the root mean square (RMS) values of the adjustments needed to realign each subject’s head position into the position that it was in at the beginning of each scanning run. Only runs with less than 2mm RMS of motion were used in these analyses. There were no statistically significant differences in motion across the three groups (data are provided with the supplemental material), but there was a non-significant trend (p = 0.06) for greater motion in unmedicated ADHD subjects than controls. We therefore incorporated into each subject’s linear model six nuisance regressors reflecting motion parameters in three translational directions and rotations.
We localized the regions of activation by first obtaining in SPM the MNI coordinates of the voxel with the peak signal intensity within each area of activation. We translated the MNI coordinates into Talairach coordinates using mni2tal (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). Lastly, we used the Talairach deamon (http://www.talairach.org/daemon.html) to localize the coordinates and obtain an anatomical label. The anatomical labels were confirmed by visual inspection.
2.7. Additional Confounds
Practice effects were a potential confound because the ADHD participants completed the scanning procedures twice, whereas the controls completed the procedures only once. To rule out the possibility that practice effects drove our findings, two steps were taken. First, the scan order for the ADHD participants was counterbalanced by medication status (i.e. half of the ADHD participants completed their first MRI scan while on stimulant medication, whereas the other half completed their first MRI scan unmedicated). Second, subgroup analyses were conducted such that a) the seven ADHD subjects who complete their first scan while unmedicated were compared to controls and b) the eight ADHD subjects who complete their first scan while taking stimulant medication were compared to controls. These subgroup analyses did not differ appreciably from the reported findings.
2.8. Behavioral Correlates
To examine the clinical correlates of emotion-related activation, bivariate correlations were calculated between the task-related activation during the emotional Stroop and clinical measures of symptoms derived from the Conners’ ADHD Parent Rating Scale. Task related activation was calculated for each subject as the sum of the absolute values of the contrast estimates obtained from neural: positively valenced distraction and neural: negatively valenced distraction. For convenience, we term this neural reactivity as it reflects both increases and decreases in activation.
3. Results
3.1. Behavioral Results
Mean reaction times and error rates for the neutral, positive, negative, and number words are presented in Table 2A. All three types of distracters (negatively valenced, positively valenced, and cognitive) were significant in each of the three groups (controls, medicated patients, and unmedicated patients) on the basis of reaction times (Table 2B). On the basis of error rates, cognitive distraction was significant in each of the three groups, negatively valenced distraction was significant only in unmedicated ADHD subjects, and positively valenced distraction was not significant in any of the groups (Table 2B). Between-group comparisons based on reaction times detected no significant differences in distraction effects between the groups (Table 2C). On the basis of error rates, however, the unmedicated ADHD subjects had greater negatively valenced and cognitive distraction than control participants (Table 2C).
Table 2.
Distraction effects of the emotional and cognitive Stroops. (A) Mean reaction time (in milliseconds) and error rate for each trial type (i.e., neutral, positive, negative, and number words). (B) Within-group analyses of distraction effects on the basis of mean reaction time and error rate. (C) Between-group analyses of distraction effects on the basis of reaction time and error rate.
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Neutral Words | Positive Words | Negative Words | Number Words | |||||
| Reaction Time (RT)±SD |
% Error Rate (ER)±SD |
RT±SD | ER±SD | RT±SD | ER±SD | RT±SD | ER±SD | |
| Unmedicated ADHD | 790.9±85.1 | 14.1±14.7 | 842.9±93.7 | 16.3±17.1 | 839.5±93.0 | 17.1±16.4 | 877.0±79.3 | 24.4±18.1 |
| Medicated ADHD | 780.4±87.7 | 11.2±8.7 | 800.3±81.3 | 11.2±9.3 | 830.4±97.0 | 12.8±8.8 | 874.8±67.7 | 17.6±13.4 |
| Controls | 772.5±66.8 | 6.7±6.1 | 790.4±81.2 | 5.5±7.7 | 829.2±79.3 | 5.8±6.9 | 859.1±68.5 | 9.4±4.7 |
| B | |||||||
|---|---|---|---|---|---|---|---|
| Group | Interference | Reaction Time Interference Effects ± SD |
t(df) | p | % Error Rate Interference Effects ± SD |
t(df) | p |
| Unmedicated ADHD | Negative | 35.7±56.4 | 2.5(14) | 0.03* | 3.0±3.6 | 3.2(14) | 0.006* |
| Positive | 40.9±44.5 | 3.6(14) | 0.003* | 2.3±5.9 | 1.5(14) | 0.2 | |
| Cognitive | 47.5±53.0 | 3.5(14) | 0.004* | 8.7±6.6 | 5.1(14) | 0.001* | |
| Medicated ADHD | Negative | 52.2±52 | 6.6(14) | 0.001* | 1.7±5.0 | 1.3(14) | 0.2 |
| Positive | 30.2±29.7 | 3.9(14) | 0.001* | 0.002±5.7 | 0.001(14) | 0.9 | |
| Cognitive | 57.0±49.9 | 4.4(14) | 0.001* | 4.7±6.3 | 2.9(14) | 0.01* | |
| Controls | Negative | 57.2±41.2 | 5.3(14) | 0.001* | −0.9±2.6 | 1.4(14) | 0.2 |
| Positive | 17.9±27.4 | 2.5(14) | 0.02* | −1.2±4.3 | 1.1(14) | 0.3 | |
| Cognitive | 70.2±47.5 | 5.7(14) | 0.001* | 4.0±5.0 | 3.1(14) | 0.008* | |
| C | |||||
|---|---|---|---|---|---|
| Comparison | Interference | Reaction Time Interference Effects | % Error Rates Interference Effects | ||
| t(df) | p | t(df) | p | ||
| Unmedicated ADHD vs. Controls | Negative | 1.2(28) | 0.3 | 3.6(28) | 0.002* |
| Positive | 1.7(28) | 0.1 | 1.8(28) | 0.08 | |
| Cognitive | 1.2(28) | 0.2 | 2.2(28) | 0.04* | |
| Medicated ADHD vs. Controls | Negative | 0.4(28) | 0.7 | 1.8(28) | 0.08 |
| Positive | 1.2(28) | 0.3 | 0.7(28) | 0.5 | |
| Cognitive | 0.7(28) | 0.5 | 0.3(28) | 0.8 | |
| Unmedicated ADHD vs. Medicated ADHD | Negative | 1.3(14) | 0.2 | 0.8(14) | 0.5 |
| Positive | 0.7(14) | 0.5 | 1.3(14) | 0.2 | |
| Cognitive | 0.5(14) | 0.6 | 1.7(14) | 0.1 | |
SD = standard deviation.
Indicates a statistically significant effect.
3.2. Imaging Results
In Table 3, we present the task-related activations for each group (i.e., unmedicated ADHD, medicated ADHD, and controls) during the contrasts — neural: positively valenced, neural: negatively valenced, and neural: cognitive distraction. As presented in Table 3, during the cognitive Stroop, the controls and ADHD participants demonstrated in the neural: cognitive distraction contrast (i.e., Number words > Neutral words and Neutral words > Number words) a pattern of activation that is typically associated with cognitive control(Casey et al 2007; Durston et al 2003; Passarotti et al 2010b). This underscores that the cognitive Stroop task engaged the frontostriatal neural systems that are typically associated with cognitive control(Marsh et al 2006) and can thus serve as a control condition for the effects of cognitive control. In the text below, we focus on the group × distraction interactions, as these are the results most pertinent to our primary hypotheses. The interaction analyses for a) group × neural: positively valenced distraction and b) group × neural: negatively valenced distraction covaried for the effects of cognitive distraction.
Table 3.
Group activation during the cognitive and emotional Stroop tasks. The coordinates and t-values are reported at the peak voxels in each cluster. L, left. R, right. NA, no cluster meets statistical significance. One voxel = 2mm3.
| Talairach coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|
| Region of Activation | X | Y | Z | Region | R/L | Brodmann Area |
Cluster Size |
t value |
| Unmedicated ADHD | ||||||||
| Neural: positively valenced distraction (emotional Stroop) | ||||||||
| Positive words > Neutral words | −55 | −1 | −12 | Middle Temporal Gyrus | L | 21 | 371 | 3.6 |
| −10 | 46 | −4 | Medial Prefrontal Cortex | L | 10 | 248 | 3.4 | |
| Neutral words > Positive words | NA | |||||||
| Neural: negatively valenced distraction (emotional Stroop) | ||||||||
| Negative words > Neutral words | NA | |||||||
| Neutral words > Negative words | 14 | 54 | −6 | Medial Prefrontal Cortex | R | 10 | 5957 | 4.5 |
| Neural: cognitive distraction (cognitive Stroop) | ||||||||
| Number words > Neutral words | −46 | 19 | 34 | Middle Frontal Gyrus | L | 8 | 2712 | 4.4 |
| 2 | 29 | 39 | Middle Frontal Gyrus | R | 8 | 349 | 4 | |
| −16 | −73 | 53 | Precuneus | L | 7 | 1774 | 3.9 | |
| 32 | −50 | 41 | Superior Parietal Lobule | R | 7 | 586 | 3.8 | |
| 51 | 26 | 28 | Middle Frontal Gyrus | R | 9 | 544 | 3.6 | |
| Neutral words > Number words | 46 | −13 | 58 | Precentral Gyrus | R | 6 | 3126 | 4.3 |
| −24 | −35 | −7 | Parahippocampal Gyrus | L | 36 | 620 | 4.3 | |
| −10 | 48 | −6 | Medial Frontal Gyrus | L | 10 | 689 | 4.3 | |
| 57 | 1 | −15 | Middle Temporal Gyrus | R | 21 | 646 | 3.8 | |
| 24 | −39 | −3 | Parahippocampal Gyrus | R | 36 | 365 | 3.7 | |
| Medicated ADHD | ||||||||
| Neural: positively valenced distraction (emotional Stroop) | ||||||||
| Positive words > Neutral words | −46 | 39 | −5 | Middle Frontal Gyrus | L | 47 | 362 | 3.9 |
| Neutral words > Positive words | NA | |||||||
| Neural: negatively valenced distraction (emotional Stroop) | ||||||||
| Negative words > Neutral words | NA | |||||||
| Neutral words > Negative words | NA | |||||||
| Neural: cognitive distraction (cognitive Stroop) | ||||||||
| Number words > Neutral words | −46 | 13 | 36 | Middle Frontal Gyrus | L | 8 | 4593 | 5.7 |
| −4 | −65 | 57 | Superior Parietal Lobule | L | 7 | 3049 | 4.8 | |
| 42 | 16 | 23 | Middle Frontal Gyrus | R | 46 | 802 | 4.3 | |
| 8 | 25 | 36 | Cingulate Gyrus | R | 32 | 569 | 3.9 | |
| Neutral words > Number words | −2 | 54 | −6 | Medial Frontal Gyrus | L | 10 | 1361 | 4.3 |
| Healthy Controls | ||||||||
| Neural: positively valenced distraction (emotional Stroop) | ||||||||
| Positive words > Neutral words | −22 | −82 | −8 | Lingual Gyrus | L | 19 | 2952 | 5.3 |
| −26 | −15 | 43 | Precentral Gyrus | L | 4 | 2847 | 5 | |
| 32 | −13 | 47 | Precentral Gyrus | R | 4 | 764 | 4.3 | |
| −12 | −27 | −5 | Cerebullum, Anterior Lobe | L | NA | 493 | 4.3 | |
| −8 | 54 | 25 | Superior Frontal Gyrus | L | 9 | 409 | 3.9 | |
| Neutral words > Positive words | NA | |||||||
| Neural: negatively valenced distraction (emotional Stroop) | ||||||||
| Negative words > Neutral words | −10 | 56 | 36 | Superior Frontal Gyrus | L | 8 | 578 | 4.7 |
| −16 | −100 | 10 | Cuneus | L | 18 | 342 | 4.5 | |
| Neutral words > Negative words | NA | |||||||
| Neural: cognitive distraction (cognitive Stroop) | ||||||||
| Number words > Neutral words | −44 | −56 | 54 | Superior Parietal Lobule | L | 7 | 1410 | 4.9 |
| −48 | 34 | 24 | Middle Frontal Gyrus | L | 46 | 563 | 4.8 | |
| 50 | 36 | 28 | Middle Frontal Gyrus | R | 9 | 415 | 4.1 | |
| 52 | −48 | 47 | Inferior Parietal Lobule | R | 40 | 1473 | 3.7 | |
| −6 | 22 | 43 | Medial Frontal Gyrus | L | 8 | 304 | 3.3 | |
| Neutral words > Number words | −6 | 50 | −8 | Medial Frontal Gyrus | L | 10 | 337 | 3.3 |
3.2.1. Group × neural: positively valenced distraction
In regards to the unmedicated ADHD and control participants, we found a significant group × neural: positively valenced distraction interaction in the left mPFC (t=4.1, P<0.001, Talairach coordinates: x=−10, y=48, z=−4, Brodmann area (BA) 10). This interaction was driven by significantly greater activation in the left mPFC in the unmedicated ADHD subjects as compared with the control subjects (Figures 2A and 4A). No significant interactions were found when comparing the medicated and unmedicated AHDH participants (Figure 2B) or when comparing controls and medicated ADHD participants (Figure 2C). Independent sample t-test confirmed that activation within the left mPFC was comparable for medicated ADHD participants and controls (Figures 2C and 4A).
Figure 2. Group × Neural: Positively Valenced Distraction Interactions.
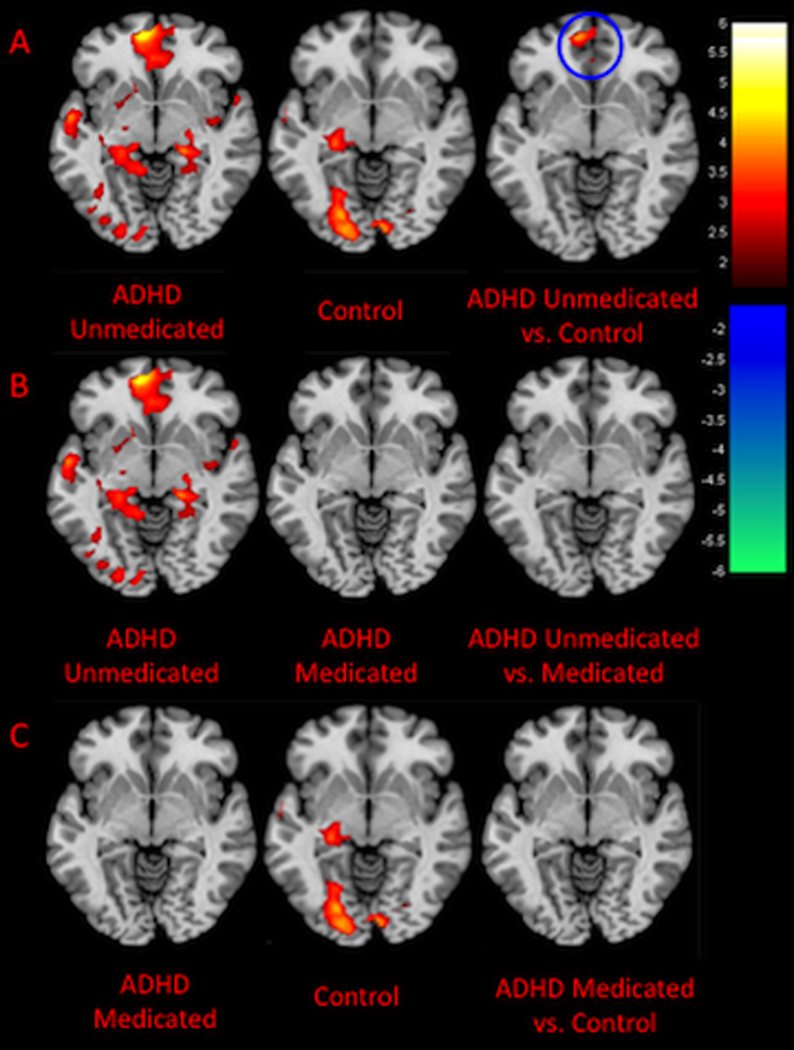
The figures show axial slices through the Talairach z-coordinate −4. Activations are shown in red/orange and deactivations in purple/blue. The color bars indicate the voxel-wise t statistic. Neural: cognitive distraction was included as a covariate. (A) For the unmedicated ADHD sample and controls, a group × neural: positively valenced distraction interaction was detected in the left medial prefrontal cortex (mPFC) as indicated by the blue circle. This interaction was driven by greater activation in the left mPFC in the unmedicated ADHD sample as compared with controls. (B) No group × neural: positively valenced distraction interactions were identified when examining the medicated and unmedicated ADHD samples. (C) No group × neural: positively valenced distraction interactions were identified when examining the medicated ADHD sample and controls.
Figure 4. Medial prefrontal cortex (mPFC) activity.
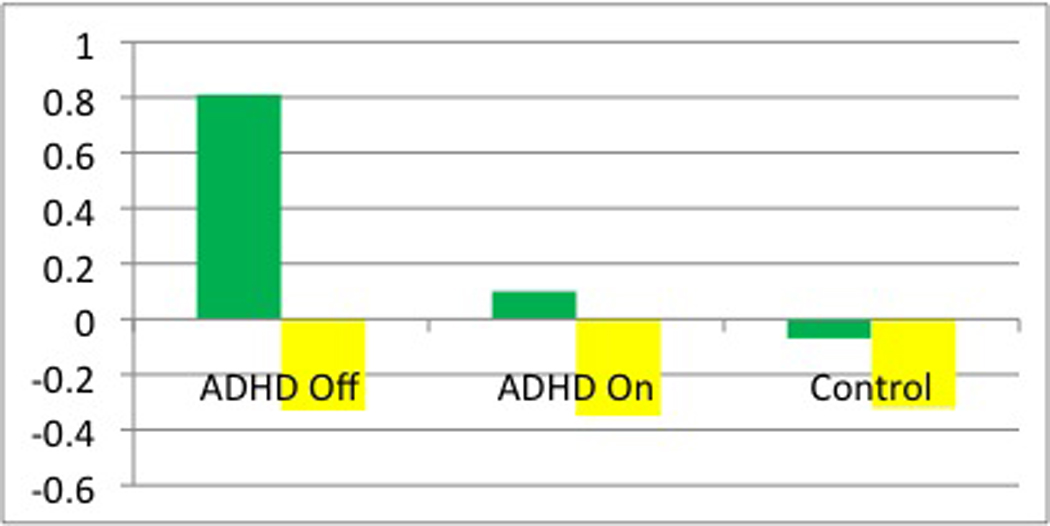
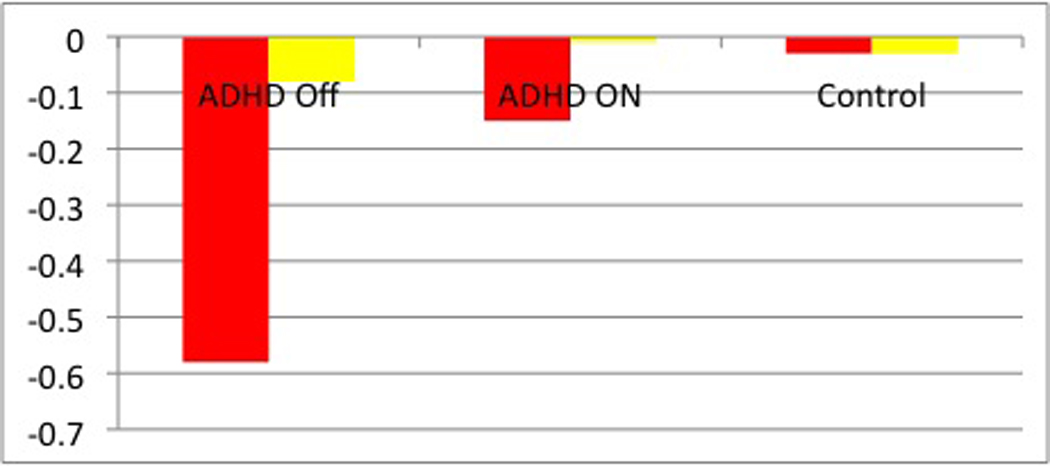
(A) Parameter estimates for the activity in the left mPFC (MNI coordinates: x=−10, y=50, z=−2, Brodmann area (BA) 10). The green bars indicate that during neural: positively valenced distraction, there is greater activation in the left mPFC in the unmedicated ADHD sample as compared with the controls (t=4.1; P<0.001). The yellow bars indicate that during neural: cognitive distraction activation in the left mPFC does not differ across the three groups. (B) Parameter estimates for the activity in the right mPFC (MNI coordinates x=12, y=54, z=−6, BA10). The red bars indicate that during neural: negatively valenced distraction, there is greater deactivation of the right mPFC in the unmedicated ADHD sample as compared with the controls (t=3.4, P=0.001) and in the unmedicated as compared with medicated ADHD sample (t=4.0, P<0.001). The yellow bars indicate that during neural: cognitive distraction, activation in the right mPFC does not differ across the three groups.
3.2.2. Group × neural: negatively valenced distraction
We found a group × neural: negatively valenced distraction interaction in the right mPFC when examining the unmedicated ADHD and control participants (t=3.4, P=0.001, Talairach coordinates: x=12, y=52, z=−8, BA10). This interaction was driven by greater deactivation (i.e., greater activation during the neutral words blocks vs. negative words blocks) in the ADHD participants as compared with controls (Figures 3A and 4B). When examining medicated and unmediated ADHD participants, we found a group × neural: negatively valenced distraction interaction in the mPFC bilaterally (right mPFC: t=4.0, P<0.001, Talairach coordinates: x=16, y=46, z=−12, BA10; left mPFC: t=3.8, P<0.001, Talairach coordinates: x=−22, y=42, z=−11, BA10). These interactions were driven by greater deactivation in unmedicated as compared to medicated ADHD participants in medial prefrontal cortex bilaterally (Figures 3B and 4B). Group × neural: negatively valenced distraction interactions were not detected when examining medicated ADHD participants and controls (Figure 3C). Independent sample t-tests confirmed that activation within the right mPFC was comparable for medicated ADHD participants and controls (Figures 4B).
Figure 3. Group × Neural: Negatively Valenced Distraction Interactions.
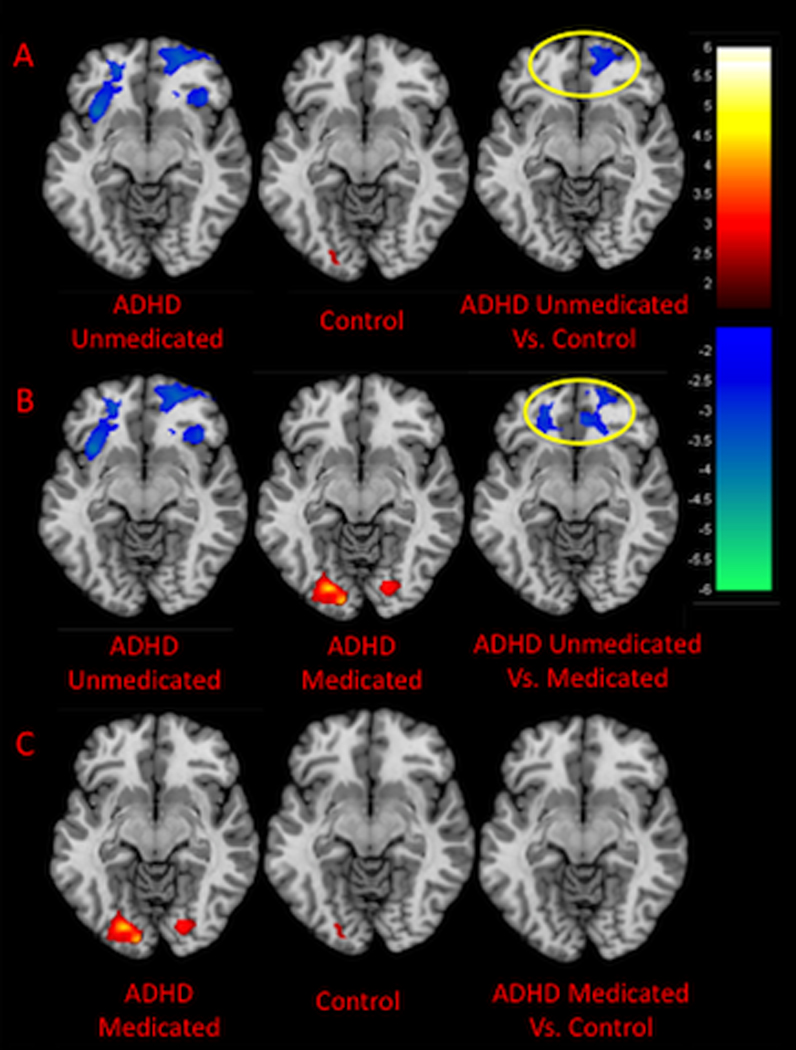
The figures show axial slices through the Talairach z-coordinate −6. Activations are shown in red/orange and deactivations in purple/blue. The color bars indicate the voxel-wise t statistic. Neural: cognitive distraction was included as a covariate. (A) For the unmedicated ADHD sample and controls, a group × neural: negatively valence distraction interaction was detected in the right medial prefrontal cortex (mPFC) as indicated by the yellow circle. This interaction was driven by greater deactivation in the right mPFC in the unmedicated ADHD sample as compared with controls. (B) Group × neural: negatively valence distraction interactions were identified in the mPFC bilaterally when examining the medicated and unmedicated ADHD samples. These interactions were driven by greater deactivation in the mPFC in the unmedicated as compared to unmediated ADHD sample. (C) No group × neural: negatively valence distraction interactions were identified when examining the medicated ADHD sample and controls.
3.3. Clinical Correlates
Task-related activation was calculated for each subject as the sum of the absolute values of the contrast estimates obtained from a) neural: positively valenced distraction and b) neural: negatively valenced distraction. For convenience, we term this neural reactivity as it reflect increases and decreases in activation. Greater reactivity in the mPFC during the emotional Stroop was associated with greater levels of hyperactivity and inattention/cognitive problems, as measured by Conners’ ADHD Parent Rating Scale(Conners 2008) (Figure 5A, Hyperactivity subscale: r=0.47, P=0.02; Figure 5B, Inattention/Cognitive Problems subscale: r=0.41, P=0.04). Reactivity of the mPFC did not correlate with depressive or anxiety symptoms as measure by the CDI and STAI, respectively. The depressive and anxiety symptoms score, however, were well below the clinical range.
Figure 5.
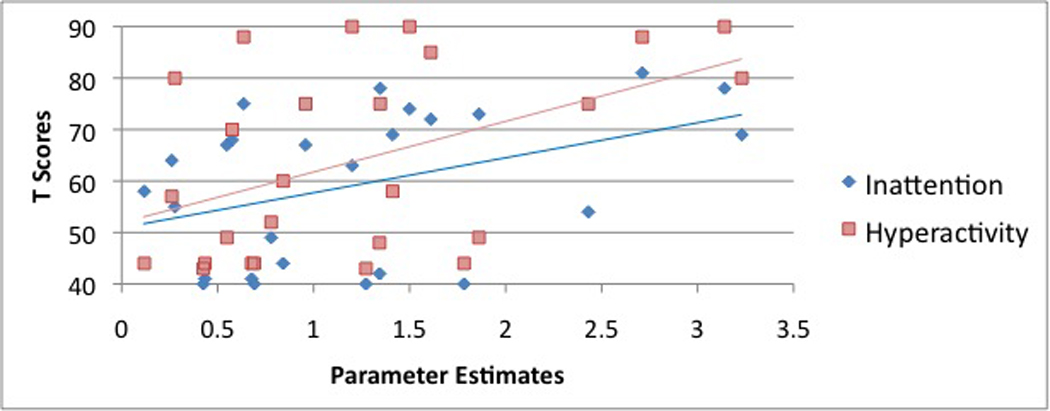
Bivariate correlations were calculated between reactivity of the mPFC during the emotional Stroop and symptoms of a) hyperactivity (red squares) and b) inattention/cognitive problems (blue diamonds), derived from the Conners’ ADHD Parent Rating Scale. Reactivity of the mPFC was calculated for each subject as the sum of the absolute values of the contrast estimates obtained from neural: positively valenced distraction and neural: negatively valenced distraction. Reactivity of the mPFC correlated with the Conners’ ADHD Hyperactivity subscale: r=0.47, P=0.02; and the Inattention/Cognitive Problems subscale: r=0.41, P=0.04.
4. Discussion
This fMRI study employed both an emotional processing task and a cognitive control task in a population of adolescents with ADHD. We used these two tasks to disentangle task-related activations associated with emotional processing from more general deficits in cognitive control. The study had five important findings. First, unmedicated ADHD participants demonstrated increased reactivity in the mPFC relative to healthy controls in the emotional processing task, consistent with their increased EL in clinical presentations(Barkley 2005) and in laboratory tasks(Sonuga-Barke et al 1992). Second, this increased reactivity of the mPFC in the adolescents with ADHD was specific to emotional processing and was detected even after controlling for differences in cognitive control. EL is a well-known clinical feature of ADHD, but argument persists over whether it stems from a reduced ability to inhibit behavioral responses to emotional stimuli(Barkley 2005) or from an actual disturbance in emotional processing(Passarotti et al 2010a; Sonuga-Barke et al 1992). By comparing performance on tasks that engage cognitive control and emotional processing, we were able to demonstrate that dysfunctional emotional processing in ADHD seems to be underpinned by neural alterations independent from those associated with impaired cognitive control. Fourth, stimulants reduced the reactivity of the mPFC toward levels similar to those of healthy controls, consistent with the clinical efficacy of stimulants in reducing EL(Kratochvil et al 2007). Fifth, reactivity of the mPFC correlated with symptoms of both hyperactivity and inattention. This is consistent with recent neuropsychological models of ADHD that stress motivational factors such as an aversion to delay and underscore that ADHD symptoms can be driven by altered emotional processing(Sonuga-Barke 2002).
The function of the mPFC remains an area of debate with some research suggesting that activity within the mPFC indexes changes in emotional valence(Anderson et al 2003; Dolcos et al 2004) and others maintaining that the mPFC is involved in the regulation of emotion(Drevets et al 2008). We found that activity in the mPFC of unmediated ADHD adolescents paralleled the valence of the emotion words presented (i.e., activity increased in the left mPFC for positive words and decreased in the right mPFC for negative words). Consistent with this observation are animal models of emotional processing that demonstrate that mPFC lesions result in apathetic, emotionless behaviors(Fuster 2001; Ongur & Price 2000). Such findings suggest that the mPFC may not regulate emotion, but conversely, may facilitate emotional responses(Damasio et al 1996). Similarly, anatomical studies demonstrate abundant reciprocal connections between the mPFC and limbic structures including the nucleus accumbens and amygdala — structures associated with positive and negative valence(Ongur & Price 2000; Rempel-Clower & Barbas 1998). Together with its connections to the visceromotor cortex, the mPFC is thus anatomically well situated to subserve emotional valence(Ongur & Price 2000). Nevertheless, several neuroimaging studies suggest that activation within the mPFC may also serves to regulate emotion(Drevets et al 2008). One interpretation of our findings is that the normal regulatory function of the mPFC is altered in youth with ADHD. That is, instead of down-regulating affective experiences, the mPFC in ADHD youth may augment, or facilitate, affective responses. Developmental factors are also worth considering as developmental factors may play an important role in shaping the functionality of the mPFC. For example, regional interactions of the mPFC change significantly over development(Fair et al 2008; Fair et al 2009), which may lead to functional modifications of this region with maturation. With regards to ADHD, developmental factors may be particularly salient given the suggestion that ADHD is associated with a delayed neuro-maturational course(Shaw et al 2007). Lastly, the mPFC is important component of the default mode network (DMN) - a neural circuit found to be more active during internally focused states(Raichle & Snyder 2007). Deactivations in the DMN are detected when attention shifts to external stimuli. Abnormal DMN processing has frequently been implicated in ADHD(Fassbender et al 2009; Peterson et al 2009). Our findings may reflect abnormal DMN processing in the unmedicated ADHD participants with the emotional valence of the words indexing the degree of introspection. That is, more negatively valenced words may draw attention to external stimuli to a greater extent than neutral words. For example, a negatively valence word, such as “Kill,” may draw attentional resources toward external threats; this is less likely given a neutral word, such as “Month.” Conversely, a positively valenced word, such as “Happy,” may induce self-reflection to a greater extent than neutral words. Follow-up study is necessary to determine if an interaction exist for ADHD youth between introspection and emotional valence. Nevertheless, Peterson et al.(Peterson et al 2009) recently demonstrated that for ADHD youth, stimulant medication normalizes DMN processing.
Imaging studies investigating stimulants have largely focused on the frontostriatal circuits associated with inhibitory control(Peterson et al 2009; Volkow et al 2005). Our findings, however, suggest that stimulants may have important effects on emotional processing as well. Microdialysis studies suggest that the functionality of the mPFC is highly sensitive to intrasynaptic dopamine levels, consistent with our findings that stimulants (i.e., medications that act on dopamine transmission) markedly altered activation in the mPFC(Karreman & Moghaddam 1996; Moghaddam et al 1990). Clinically, stimulants appear to improve EL for some ADHD youth, while making it worse for others(Smucker & Hedayat 2001). Although our findings demonstrate an attenuation of abnormal emotional processing with stimulants, it should be noted that our study was not a randomized controlled trial. The ADHD participants were taking stimulants with good results at the time of recruitment, so our ADHD sample might have been biased towards youth for whom stimulants have a beneficial effect on emotional processing. Nevertheless, our findings suggest that stimulants may reduce EL by attenuating the atypical response of affective circuits to emotional stimuli.
Several limitations of the study should be considered. To begin, the ADHD subjects were previously exposed to stimulants and therefore the differential activation that we found may have been the product of medication exposure rather than ADHD itself. This is unlikely because instead of causing deviation from the controls, stimulants seemed to attenuate abnormal task-related signal change. Second, significant task performance differences were detected between the unmedicated ADHD and control participants, with the unmedicated ADHD participants generally demonstrating greater distraction effects. This raises the possibility that some of the differences in the imaging results could be attributable to behavioral differences between the groups(Peterson 2003). Our inclusion of reaction times and error rates as covariates in the imaging analyses should attenuate that concern. Lastly, although all of the ADHD participants began the study on a stimulant medication, they were not all taking the same stimulant. It is possible that different stimulants may have different effects on emotional processing; a larger, follow-up study would be necessary to examine this possibility.
In conclusion, by demonstrating that dysfunctional emotional processing in ADHD has neural correlates that are distinct from impaired inhibitory control, our study addressed an important and ongoing debate about the underlying mechanisms that produce emotional reactivity in ADHD youth. Our findings also suggest that in addition to stimulants’ well-known effects on inhibitory control and associated frontostriatal circuitry(Volkow et al 2005), stimulants also affect emotional processing by attenuating abnormal activity within affective circuits. In sum, our findings reinforce the growing literature suggesting that ADHD is a disorder not only of cognition but of emotion as well(Sonuga-Barke et al 1992).
Supplementary Material
Acknowledgements
This work was supported by the American Academy of Child and Adolescent Psychiatry (Posner), the Klingenstein Third Generation Foundation (Posner and Maia), K23 MH091249 (Posner) the United Negro College Foundation (UNCF)/Merck Postdoctoral Science Research Fellowship (Fair), K08 NS52147 (Nagel), P60 AA010760 pilot funds (Nagel). The authors thank Troy Lubianski for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for aseba school-age forms & profiles. Burlington, VA: University of Vermont, Research Center for Children Youth, & Families; 2001. [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Barkley R. ADHD and the nature of self-control. New York: The Guilford Press; 1997a. [Google Scholar]
- Barkley R. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of adhd. Psychological Bulletin. 1997b;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley R. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. New York: The Guilford Press; 2005. [Google Scholar]
- Barkley R, Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:503–513. doi: 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. American Journal of Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Shin LM, Rauch SL. The counting stroop: A cognitive interference task. Nature Protocols. 2006;1:230–233. doi: 10.1038/nprot.2006.35. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, Garrett A, Hinshaw SP, Greenhill LL, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with adhd. American Journal of Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Conners C, Sitarenios G, Parker J, Epstein J. The revised Conners' parent rating scale (cprs-r): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conner's rating scales - 3rd edition. Toronto: Multi-Health Systems; 2008. [Google Scholar]
- Cox R. Afni: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crichton A. An inquiry into the nature and origin of mental derangement. London: Junior, & W. Davies, in the Strand; 1798. Chapter ii: On attention, and its diseases; pp. 254–291. [Google Scholar]
- Damasio A, Everitt B, Bishop D. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions: Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Davidson R, Putnam K, Larson C. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar K, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fmri study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without adhd. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Fair D, Cohen A, Dosenbach N, Church J, Miezin F, Barch D, Raichle M, Petersen S, Schlaggar B. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences. 2008;105:4028. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D, Cohen A, Power J, Dosenbach N, Church J, Miezin F, Schlaggar B, Petersen S. Functional brain networks develop from a local to distributed organization. PLoS Computational Biology. 2009:5. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy W, Cortes C, Mizuiri D, Beckett L, Schweitzer J. A lack of default network suppression is linked to increased distractibility in adhd. Brain Research. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner CD, Frith JB, Poline JB, Heather RS, Frackowiak RS. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Fuster J. The prefrontal cortex - an update time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. Unpublished Manuscript. New Haven, CT: Yale University; 1975. [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: An effect mediated by ventral tegmental area. Journal of Neurochemistry. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The children's depression, inventory (cdi) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kratochvil C, Faries D, Vaughan B, Perwien A, Busner J, Saylor K, Kaplan S, Buermeyer C, Swindle R. Emotional expression during attention-deficit/hyperactivity disorders treatment: Initial assessment of treatment effects. Journal of Child and Adolescent Psychopharmacology. 2007;17:51–62. doi: 10.1089/cap.2006.0018. [DOI] [PubMed] [Google Scholar]
- Lucas C, Zhang H, Fisher P, Shaffer D, Regier D, Narrow W, Bourdon K, Dulcan M, Canino G, Rubio-Stipec M. The disc predictive scales (dps): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the stroop effect: An integrative review. Psychology Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Maedgen J, Carlson C. Social functioning and emotional regulation in the attention deficit hyperactivity disorder subtypes. Journal of Clinical Child and Adolescent Psychology. 2000;29:30–42. doi: 10.1207/S15374424jccp2901_4. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz R, Quackenbush G, Royal J, Skudlarski P, Peterson B. A developmental fmri study of self-regulatory control. Human Brain Mapping. 2006;27:848. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Roth R, Bunney B. Characterization of dopamine release in the rat medial prefrontal cortex as assessed by in vivo microdialysis: Comparison to the striatum. Neuroscience. 1990;36:669. doi: 10.1016/0306-4522(90)90009-s. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Passarotti A, Sweeney J, Pavuluri M. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 2009:1–12. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A, Sweeney J, Pavuluri M. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010a;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A, Sweeney J, Pavuluri M. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Research: Neuroimaging. 2010b;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Peterson B. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Development and Psychopathology. 2003;15:811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
- Peterson B, Potenza M, Wang Z, Zhu H, Martin A, Marsh R, Plessen K, Yu S. An fmri study of the effects of psychostimulants on default-mode processing during stroop task performance in youths with adhd. American Journal of Psychiatry. 2009 doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in pet and fmri. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Development and Psychopathology. 2005;17:715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Russell J, Gerber A, Gorman D, Colibazzi T, Yu S, Wang Z, Kangarlu A, Zhu H, Peterson B. The neurophysiological bases of emotion: An fmri study of the affective circumplex using emotion-denoting words. Human Brain Mapping. 2009;30:883. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M, Snyder A. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower N, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Schwab-Stone M, Fisher P, Cohen P, Placentini J, Davies M, Conners C, Regier D. The diagnostic interview schedule for children-revised version (disc-r): I. Preparation, field testing, interrater reliability, and acceptability. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:643. doi: 10.1097/00004583-199305000-00023. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker W, Hedayat M. Evaluation and treatment of adhd. American Family Physician. 2001;64:817–836. [PubMed] [Google Scholar]
- Sobanski E, Banaschewski T, Asherson P, Buitelaar J, Chen W, Franke B, Holtmann M, Krumm B, Sergeant J, Sonuga-Barke E. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): Clinical correlates and familial prevalence. Journal of Child Psychology and Psychiatry. 2010;51:915–923. doi: 10.1111/j.1469-7610.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--i. The effect of delay on choice. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R. Stai manual for the state-trait anxiety inventory. Consulting Psychologists Press; 1970. [Google Scholar]
- van Mourik R, Oosterlaan J, Sergeant J. The stroop revisited: A meta-analysis of interference control in ad/hd. Journal of Child Psychology and Psychiatry. 2005;46:150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Ding Y. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Whalen PJ, Bush G, Shin LM, Rauch SL. The emotional counting stroop: A task for assessing emotional interference during brain imaging. Natural Protocols. 2006;1:293–296. doi: 10.1038/nprot.2006.45. [DOI] [PubMed] [Google Scholar]
- Williams J, Mathews A, MacLeod C. The emotional stroop task and psychopathology. Psychology Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


