Abstract
Zeta-chain associated protein kinase-70 (Zap70), a Syk family tyrosine kinase, has been reported to be present exclusively in normal T cells, Natural Killer (NK) cells, and B cells, serving as a pivotal regulator of antigen-mediated receptor signaling and development. In this study, we report that Zap70 is expressed in undifferentiated mouse embryonic stem cells (mESCs) and may critically regulate self-renewal and pluripotency in mESCs. We found that Zap70 knocked-down mESCs (Zap70KD) show sustained self-renewal and defective differentiation. In addition, we present evidence that the sustained self-renewal in Zap70KD is associated with enhanced Jak/Stat3 signaling and c-Myc induction. These altered signaling appears to result from up-regulated LIFR and down-regulated SHP-1 phosphatase activity. Based on these results, we propose that, in undifferentiated mESCs, Zap70 plays important roles in modulating the balance between self-renewal capacity and pluripotent differentiation ability as a key regulator of the Jak/Stat3/c-Myc signaling pathway.
Keywords: Zap70, mouse embryonic stem cells, Jak/Stat3, c-Myc, SHP-1, LIFR, self-renewal, pluripotency, stemness
Introduction
Self-renewal and pluripotency, two essential properties of embryonic stem cells (ESCs), are ultimately regulated by combined action of endogenous intrinsic factors and extrinsic signals such as cytokines and growth factors. However, there is a gap in our understanding of the intracellular signaling cascades linking extracellular signals to intrinsic transcriptional cascades. The signals maintaining stemness of murine ESC (mESC) have been extensively studied since leukemia inhibitory factor (LIF) and its cognate signaling pathway through Jak/Stat3 have been identified to be crucial for self-renewal and pluripotency in mESC 1, 2. LIF-mediated pluripotent signaling is initiated by dimerization of gp130 and LIF receptor (LIFR) upon LIF binding, activating Janus-associated tyrosine kinases (JAKs). Activated JAKs phosphorylate tyrosine residues on the intracellular domains of LIFR and gp130, where Stat3 is recruited and then phosphorylated 3. In turn, phosphorylated and subsequently dimerized STAT3 is translocated into the nucleus, where it activates various target genes such as c-Myc, which has a key role(s) in maintaining ESC self-renewal 4. Considering the crucial roles of the Jak/Stat3/c-Myc signaling pathway in regulating self-renewal and pluripotency, this pathway should be finely tuned to maintain the balance between self-renewal and differentiation. However, how Jak/Stat3/c-Myc signaling is finely controlled to maintain the balance between stemness and differentiation is not yet fully understood.
Zeta-chain associated protein kinase-70 (Zap70), a Syk family tyrosine kinase, has only been studied in T cell receptor (TCR) signaling due to its reportedly exclusive expression in T and natural killer (NK) cells 5. The critical roles of Zap70 in TCR-mediated signaling and subsequent immune responses have been well established by numerous studies using Zap70-null mice and Zap70-deficient patients 6, 7. In addition, Zap70 is expressed in pro/pre-B cells and plays a critical role in B cell development 8. Zap70 expression is also found in B cell lymphomas, implying that it plays a role(s) in B cells tumorigenesis 9, 10. In this study, for the first time to our knowledge, we report that Zap70 is expressed in undifferentiated mESCs and plays pivotal roles in controlling stemness. We demonstrate that Zap70 regulates self-renewal and differentiation by modulating the responsiveness of mESCs to LIF. Negative regulation of Jak1/Stat3 signaling by SHP-1 phosphatase activity and up-regulation of LIF receptor expression are two of the mechanisms behind Zap70 expression in mESCs. These results support the view that the function of Zap70 in mESCs is to inhibit excessive Stat3 activity as a means of maintaining stemness of ESCs.
Materials and methods
Reagents and Cell culture
J1 mESCs (cat # SCRC-1010) and DBA/252 mESCs 11 were purchased from ATCC (www.atcc.org). These cells were maintained as described previously 12. Briefly, mES cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 15% fetal calf serum (HyClone), 0.1 mM 2-mercaptoethanol (Sigma), 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM glutamine (Gibco) and 1000 U/ml LIF (Chemicon).
Genetic modification of mESCs
shRNA plasmids targeting mouse ZAP70 were purchased (RMM3981-9590842, Open Biosystems, Huntsville, AL) and transfected into J1 cells with lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. For non-specific control, shRNA targeting for LacZ gene (5’-GCACGTACCTGAATTTCGA-3’) was cloned into pLK0.1 (Open Biosystems) (Brenner and Dolmetsch. 2007). Stable mESCs expressing shRNA for ZAP70 were selected in 1 µg/ml of puromycin-containing culture medium. Knockdown of ZAP70 expression was confirmed with real-time RT-PCR analysis. Zap70-expressing plasmids constructed by cloning PCR-amplified Zap70 cDNA into pcDNA3.1 (Invitrogen). siRNAs targeting to nonspecific gene was purchased from Bioneer (Daejoen, Korea) and siRNAs targeting to Zap70 or SHP-1 were purchased from Dharmacom (Denver, CO).
RNA extraction and real-time RT-PCR
Total RNA was extracted using TRIzol (Invitrogen), and 2~5 µg of total RNA was reverse-transcribed using the SuperScriptII™ First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Real-time RT-PCR was carried out using cDNAs with Quantitect SYBR Green PCR kit (Qiagen, Valencia, CA). Reactions were carried out in triplicates using Exicycler™ 96 realtime quantitative thermal block (Bioneer, Daejeon, Korea). For quantification, target genes were normalized against the glyceraldehyde 3-phosphate dehydrogenase gene (Gapdh). PCR primers used in this study are listed in Table 1.
Immunoblotting
Immunoblotting was performed as previously described method 13. Briefly, cells were washed twice with cold phosphate buffered saline (PBS), lysed with 300 Al of tissue lysis buffer (TLB) (20 mM Tris-base, pH 7.4, 137 mM NaCl, 2 mM EDTA, 1% Triton X-100, 25 mM bglycerophosphate, 2 mM sodium pyrophosphate, 10% glycerol, 1 mM sodium orthovanadate, 1 mM phenylmethysulfonyl fluoride (PMSF), and 1 mM benzamidine), and centrifuged at 20,000g to clarify lysates. Whole-cell extracts were prepared, and 20~50 ug of proteins were resolved on SDS-PAGE using antibodies against ZAP70 (sc-574, Santa Cruz), phospho-ZAP70 (Tyr-493) (#2704, cell signaling), phospho-Stat3 (Tyr-705) (#9131, cell signaling), phospho-JAK (Tyr-1022) (#11149, Signalway antibody), c-Myc (sc-764, Santa Cruz), Oct4 (ab19857, abcam), ERK (sc-154, Santa Cruz), phospho-ERK (Thr 202/Tyr 204, Sigma), actin (sc-1616, Santa Cruz) and α-tubulin (sc-30169, Santa Cruz). Proteins were transferred to PVDF membrane (Perkin Elmer Life Sciences; Boston, MA), blocked for 1–2 h with 5% nonfat dry milk in Tris-buffered saline (TBS) (50 mM Tris-base, pH 7.4, 0.15 M NaCl, and 0.1% Tween-20), and incubated with the primary antibodies in TBS containing 1% BSA solution for 1 to 16 h. Membranes were washed several times in TBS-Tween solution and incubated with HRP conjugated anti-mouse or anti-rabbit antibodies (0.1 Ag/ml). Immunoreactivity was detected by enhanced chemiluminescence (ECL; Amersham, Buckinghamshire, England).
Anexin V analysis
ES cell lines were plated at 500,000 cells/3.5-cm gelatinized plate and cultured for 24 hours in standard ES cell media. The media was changed and cells were cultured for an additional 96 hours at a given concentration of LIF. The cells were collected by trypsinization, stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (BD PharMingen, San Diego, CA), and analyzed by fluorescence-activated cell sorting (FACS) analysis.
Teratoma formation
For teratoma formation assay, cells were trypsinized, and 5 × 105 cells were suspended in a DMEM/Matrigel solution (BD Biosciences Inc) (1:1 ratio (v/v)). The cell/Matrigel suspension was then injected subcutaneously into NOD/SCID mice (Charles River Laboratories, Yokohama, Japan). Six weeks after injection, xenografted masses were harvested, fixed in 10% phosphate-buffered formalin overnight, and subsequently embedded in paraffin was using a Tissue-Tek VIP embedding machine (Miles Scientific, Naperville, IL) and a Thermo Shandon Histocenter2 (Thermo Fisher Scientific, Waltham, MA). Two mm sections were obtained using a Leica RN2065 (Leica, Wetzlar, Germany) and stained with hematoxylin-eosin, Masson’s trichrome, Alcian Blue and analyzed by a trained pathologist. The experiments were reviewed and approved by the Institutional Animal Care and Use Committee of CHA University. All procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published in the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Microarrays
Total RNA was extracted using TRIzol (Invitrogen) and biotinylated cRNA were prepared from 0.55 µg total RNA using the Illumina TotalPrep RNA Amplification Kit (Ambion, Austin, TX) following the manufacturer instructions. Following fragmentation, 1.5 µg of cRNA were hybridized to the Illumina Mouse WG-6 Expression Beadchip according to the manufacturer’s instructions (Illumina, Inc., San Diego, CA). Arrays were scanned with an Illumina Bead Array Reader Confocal Scanner according to the Manufacturer’s instructions. Array data processing and analysis was performed using Illumina BeadStudio v3.1.3 (Gene Expression Module v3.3.8).
Microscopy
Images were captured using an inverted microscopy system (ECLIPSE TE2000; Nikon, Kanagawa, Japan http://www.nikon.com) and analyzed with Nikon ACT-1C for DXM 1200C software (v 1.0.1.5, Nikon Corporation).
Alkaline phosphatase activity
Alkaline phosphatase activity was measured by using AnaSpec’s kit (AnaSpec: # 71230), according to the manufacturer’s instructions.
Statistical analysis
Graphical data are presented as mean ± S.D. Statistical significance among three groups and between groups were determined using one-way or two-way analysis of variance (ANOVA) following Bonferroni multiple comparison post-test and Student t test were applied respectively. Significance was assumed for p < 0.05 (*). Statistical analysis was performed using the SAS statistical package v.9.13 (SAS Inc., Cary, NC http://www.sas.com/).
Results
Expression of ZAP70 in undifferentiated mESCs
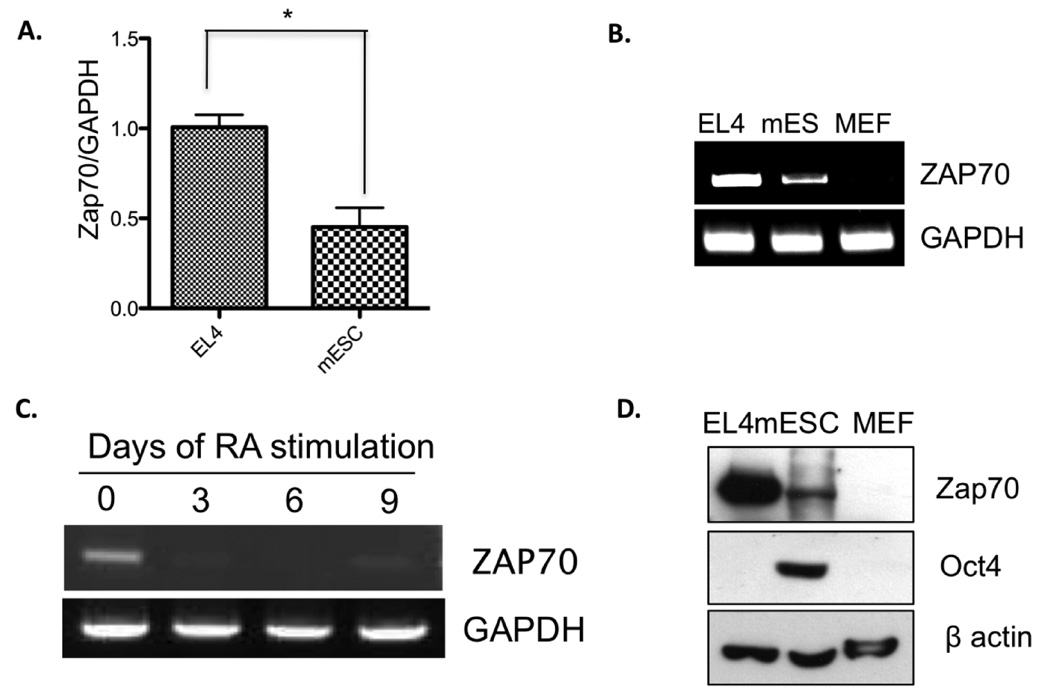
Given that mammalian oocytes and embryonic stem cells (ESCs) are capable of epigenetically reprogramming chromosomes of terminally differentiated cells to the pluripotent state by somatic cell nuclear transfer technique and cell fusion technique, respectively 14–16, we speculated that gene expression comparisons of oocytes and ESCs with those of differentiated cells may reveal important regulators of pluripotency. Toward this goal, we used the immature oocyte-specific transcriptome previously obtained by annealing control primer-polymerase chain reaction technique 17 as the starting platform for the comparison. Interestingly, we found that both immature oocytes and mESCs express Zap70, a protein specifically expressed in only T cells, natural killer cells, and B cells. To confirm this surprising finding, we compared the mRNA expression of Zap70 in the mouse T cell lymphoma line, EL4, and mESCs. As shown in figure 1, Zap70 mRNA expression in mESCs was approximately 50% that of EL4 cells, but it is not detected in mouse embryonic fibroblast cells (Fig. 1A, B). These results suggest the possibility that Zap70 is specifically expressed in undifferentiated mESCs. To test this idea, we further examined Zap70 expression in mESCs during in vitro differentiation induced by retinoic acid (RA) treatment. Indeed, as differentiation proceeds, Zap70 mRNA level dropped (Fig. 1C). In addition, our immunoblotting analysis demonstrated that Zap70 protein expression was evident in T cells and mES cells, but was not detectable in MEFs (Fig. 1D).
Figure 1. Zap70 expression in mESCs.
(A) Zap70 mRNA levels from EL4 and mES were compared by qRT-PCR analyses. *, p < 0.05. (B) Relative levels of Zap70 mRNA from EL4, mES, and MEF cells were compared by RT-PCR analysis of total RNA. GAPDH was used as control of equal mRNA loading. (C) Levels of Zap70 mRNA at the indicated time after retinoic acid–induced differentiation, determined by RT-PCR analysis. GAPDH was used as an equal loading control for RT-PCR. (D) Top panel: Zap70 protein levels from whole-cell extracts of EL4, mES, and MEF cells compared by immunoblotting analysis. Middle panel: Oct4 immunoblotting indicates pluripotency of mESC. Bottom panel: β-actin was used as a loading control.
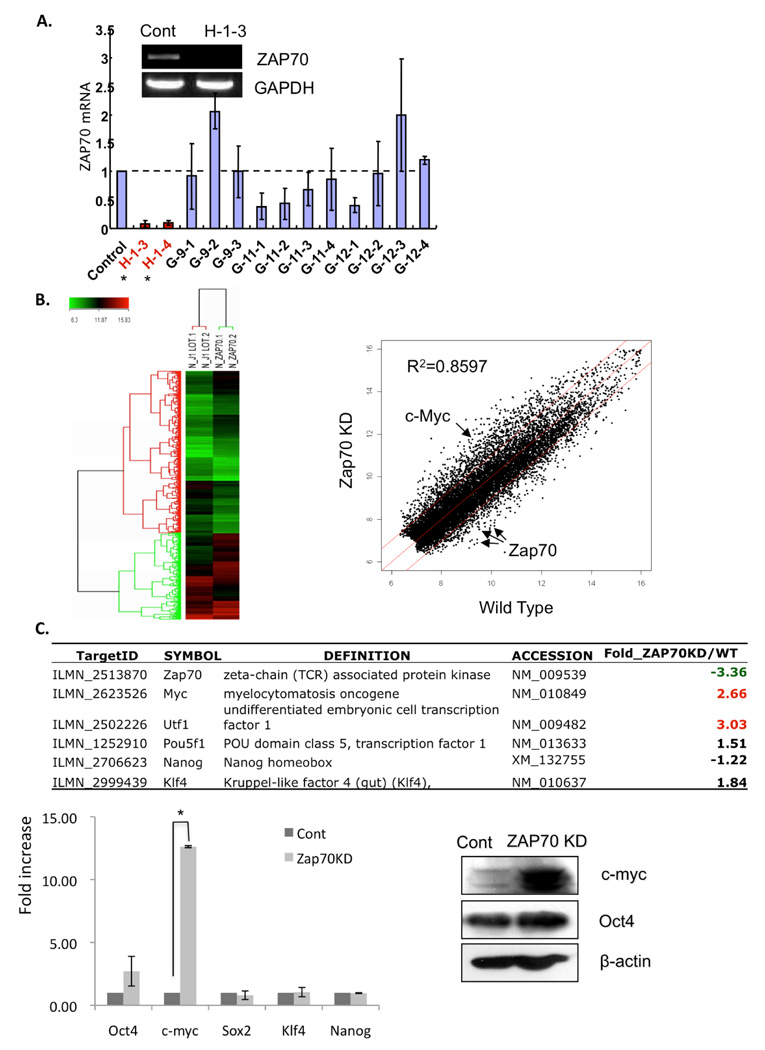
Expression of c-Myc is robustly up-regulated in Zap70 knocked down mESCs via Stat3 activation
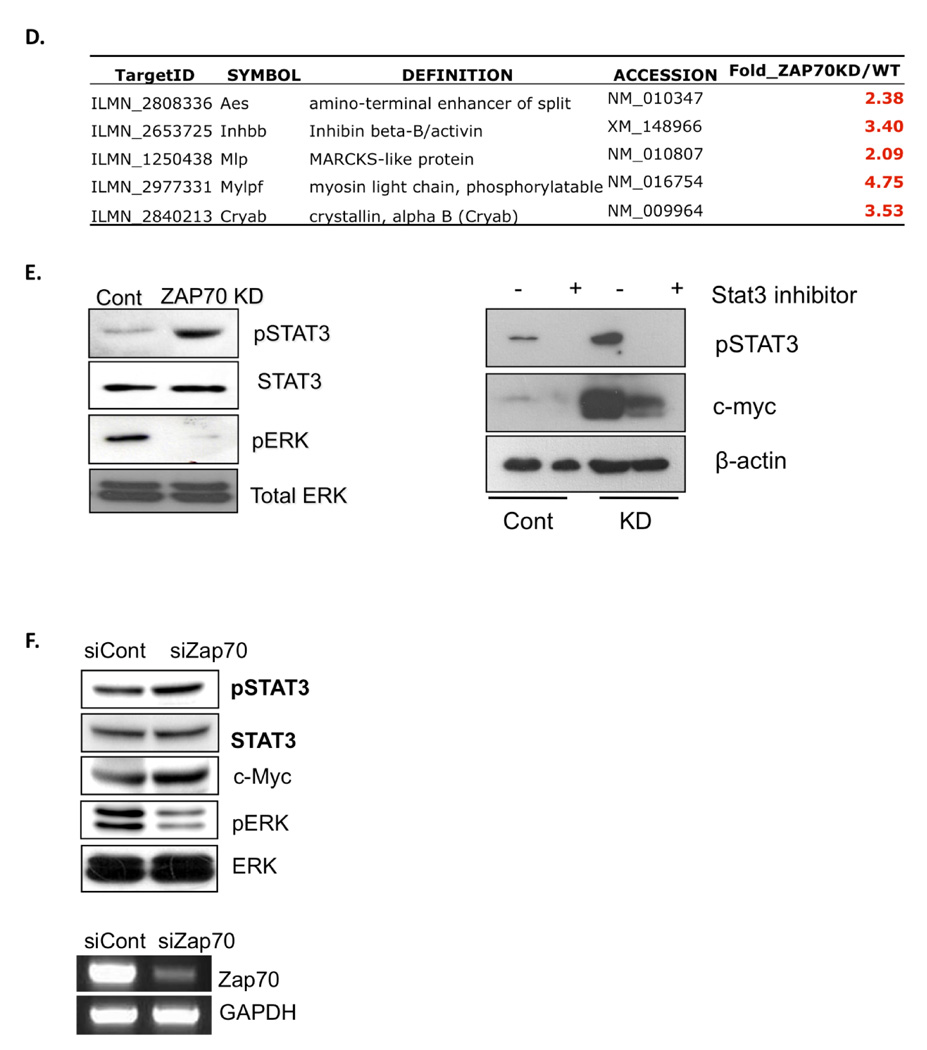
To investigate the potential function of Zap70 in mESCs, we first sought to generate stable mESC lines in which Zap70 expression is knocked down (Zap70 KD). Using a set of Zap70 shRNA plasmids, we successfully established two mESC clones (H-1-3 and H-1-4), in which Zap70 expression was suppressed by approximate 90% compared to control mESCs (Fig. 2A). Under normal mESC culture condition, no distinct morphological alteration was found in Zap70KD compared to the parent mESCs (data not shown). Thus, we performed microarray analysis to compare gene expression profiles of Zap70KD and parental mESCs. Scatter plots of cDNA microarray confirmed that Zap70 mRNA expression is significantly down-regulated in Zap70KD cells and demonstrated significantly altered gene expression profiles (R2 =0.8597) (Fig. 2B); among 12,983 total genes, 1,821 genes (approximately 14%) were determined to be significantly altered in Zap70KD according to a Student’s t-test with a 99% confidence level (up: 1,046 genes, down: 775 genes). Most interestingly, we found that two pluripotency-related genes, i.e., c-Myc 4 and utf1 18 were significantly up-regulated in Zap70KD while other pluripotency marker genes such as Oct4, Sox2, Klf4, and Nanog were not significantly altered (Fig. 2B). The microarray results were confirmed by real-time RT-PCR analysis and up-regulated expression of c-Myc proteins was also confirmed (Fig. 2C). We next attempted to investigate the underlying mechanism of c-Myc up-regulation in Zap70KD mESCs. Since c-Myc expression is dependent on Stat3 transcriptional activity in mESCs or other cell types 19, 20, we hypothesized that high c-Myc expression in Zap70KD may result from up-regulated Stat3 transcriptional activity. In support of this, we found that five out of sixteen Stat3 downstream targets genes 21, were significantly up-regulated in Zap70KD, strongly supporting enhanced Stat3 transcriptional activity (Fig. 2D). Since stat3 transcriptional activity is regulated by phosphorylation at tyrosine 705 and subsequent nuclear translocation 22, we next assessed the level of phosphorylation on Stat3 (Y705) by immunoblotting assay. As shown in figure 2E (left panel), Stat3 phosphorylation was significantly higher in Zap70KD while the total Stat3 was not altered. In contrast, the level of phosphorylated ERK2, which functions in promoting differentiation 12, was significantly reduced. Together, these results strongly suggest that c-Myc gene expression is up-regulated by enhanced Stat3 phosphorylation and subsequent transcriptional activation. To further test the correlation between Stat3 activation and c-Myc induction in Zap70KD, we examined the c-Myc expression level following interference of Stat3 transcriptional activity using Stattic, a pharmacological Stat3 inhibitor 23. As expected, this treatment significantly lowered c-Myc expression, indicating that c-Myc induction in Zap70KD resulted from enhanced Stat3 activity (Fig. 2E, right panel).
Figure 2. Characterization of Zap70 knocked-down (Zap70KD) mESCs.
(A) The Zap70KD cell line was constructed by stable chromosomal integration of a small hairpin RNA (shRNA) plasmid into mouse ES cells. Four independent plasmids (H-1, G-9, G-11, G-12) expressing a shRNA targeting the Zap70 gene were introduced into mESCs for stable cell line construction. qRT-PCR revealed that the H-1-3 and -4 cell lines showed ~90% Zap70 suppression compared to control mESCs. Decreased expression of Zap70 mRNA in the H-1-3 cell line was visualized on an agarose gel after RT-PCR. GAPDH was used as a loading control. (B) Genome-wide expression analysis of Zap70KD (Left panel). Scatter plots comparing the global mRNA expression pattern between wild-type and Zap70KD mESCs (right panel). Red lines indicate two-fold alteration in mRNA expression levels. The positions of selected mRNA are indicated by arrows in the scatter plots (c-Myc as a red and Zap70 as blue dots). (C) Representative pluripotent genes and Zap70 are listed in the table Significant fold changes (two fold above) are indicated by colors (Red: upregulation, Green: downregulation) (top panel). qRT-PCR results for altered pluripotent genes (bottom left panel). *, p < 0.05. Upregulation of c-Myc in Zap70 KD was again validated by immunoblotting (bottom right panel). β-actin was used as a loading control. (D) Upregulation of Stat3 downstream target genes in Zap70KD are listed in the table. Significant fold changes (two-fold above) are indicated by colors, as above. (E) Phosphorylated Stat3, c-Myc and phosphorylated ERK2 were analyzed by immunoblotting analysis from Zap70KD and control cells. Total Stat3 and ERK were used as loading controls (left panel). Stattic, a chemical inhibitor of Stat3, was added to the culture media of Zap70KD and control cells for 24 hrs. Phoshorylation of Stat3 and c-Myc was analyzed by immunoblot. β-actin was used as protein loading control (right panel). (F) Phosphorylated Stat3, c-Myc and phosphorylated ERK1/2 were analyzed by immunoblotting analysis from mESCs transiently transfected with Zap70 specific siRNA for 48 hrs. Knockdown of Zap70 was confirmed by RT-PCR. Abbreviations: Cont, stable mESC cell line constructed by the genomic integration of non-specific shRNA plasmid; Zap70KD, stable Zap70 knockdown mESC cell line; siZap70, siRNA targeting Zap70; siCont, non-specific siRNA
To rule out the possibility that the above results are caused by unexpected genomic alterations and/or any adaptive response accumulated by continuous culture of Zap70KD stable cells, we applied small interfering RNA (siRNA) to achieve transient Zap70 knockdown. As shown in figure 2F, altered responses by Zap70KD such as enhanced Stat3 phosphorylation, up-regulation of c-Myc expression and decreased ERK phosphorylation, were reproduced by this transient suppression of Zap70.
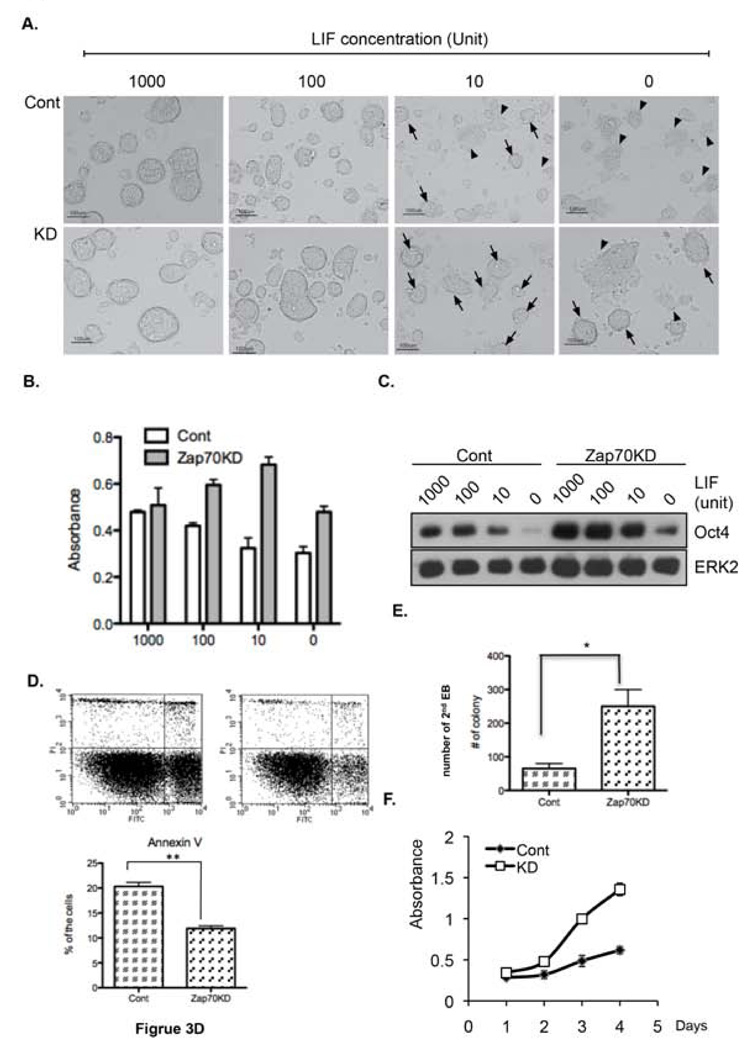
Zap70KD has enhanced self-renewal capacity
Based on the well-characterized role of Stat3 activity to maintain the undifferentiated state of mESCs 2, we speculated that Zap70KD mESCs may exhibit altered self-renewal activity. To address this, we attempted to maintain them in the presence of varying concentrations of LIF (from 0 to 1,000 U). Strikingly, Zap70KD appeared to maintain a typical undifferentiated morphology even under 10 U of LIF (black arrows), which is one hundred times less than in normal ES culture media, whereas control mESCs showed the typical differentiated flat morphology at the same concentration (black arrowheads) (Fig. 3A). For the quantitative analysis of enhanced self-renewal activity in Zap70KD, alkaline phosphatase (AP) activity, which is a typical property of mouse and human embryonic stemness 24, 25, was examined (Fig. 3B). Interestingly, even in the absence of LIF, the AP activity level was almost intact in Zap70KD mESCs (p < 0.5, ns). In contrast, AP activity of wild type mESCs was significantly reduced when LIF concentrations were lower or absent (p < 0.001). In support of enhanced self-renewal capacity of Zap70KD, we also found that Oct4 expression was sustained under lower or no LIF concentration (Fig. 3C). Since LIF stimulation and subsequent Stat3 activation are critical for survival of mESCs 26, 27, we next tested cell survival in Zap70KD and control mESCs under LIF deprivation condition. According to annexin V assay to determine apoptotic cells, apoptotic cell population was much less in Zap70KD following LIF deprivation for four days (Fig. 3D). We next compared the efficiency of secondary EB formation, which reflects the capacity of ESCs to maintain an undifferentiated state and self-renewal capacity 27. In this analysis, primary EBs were collected at approximately day 6, dissociated with trypsin, and replated to form secondary EBs. We found that the efficiency of Zap70KD secondary EB formation was five-fold higher than control cells (Fig. 3E, Fig. S1A). Since self-renewal is defined as the ability to undergo cell divisions without differentiation so as to produce identical pluripotent progeny 28, we next measured the proliferation rate of Zap70KD. Interestingly, we found that Zap70KD mESCs proliferate much rapidly than control (Fig. 3F). ESC markers such as Oct-4, c-Myc, Sox-2, SSEA1 and E-cadherin were all clearly expressed in both Zap70KD and control mESCs, as determined by immunofluorescence staining (Fig. S1B). Taken together, these data demonstrate that Zap70KD mESCs are much less dependent upon LIF for maintaining their undifferentiated state and exhibit significantly enhanced self-renewal capacity.
Figure 3. Enhanced self-renewal activity of Zap70KD.
(A) Zap70KD and control mESCs were maintained for four days with the indicative doses of LIF. Cellular morphologies were compared. Scale bar, 100 um. Black arrows, typical ES colony morphology; black arrowheads, differentiated (flattened) morphology. (B) Alkaline phosphatase activity at the indicated LIF concentrations. Two-way ANOVA (LIF concentration and C1/KD) was applied to determine statistical significance (F3,16 = 20.4, p < 0.001, F3,23 = 20.4, p < 0.001). (C) Oct-4 expression levels were compared by immunoblotting using ERK2 as loading control (D) Apoptotic population under LIF-deprived conditions for four days was measured by annexin V. Left panel: FACS result using PI and annexin V. Right panel: Quantification of annexin-V positive cells (right panel) (E) Primary EBs were dissociated into single cells and re-seeded at a density of 1×106/ml in the same medium as primary EBs for formation of secondary EBs. The number of secondary EBs was counted under a bright microscope. (F) Cell proliferation rate of Zap70KD and control mESCs was quantified by XTT assay. The error bars correspond to three replicates (n=3) and show mean ± s.d. (* p<0.05, ** p<0.01).
Defective differentiation capacity in Zap70KD
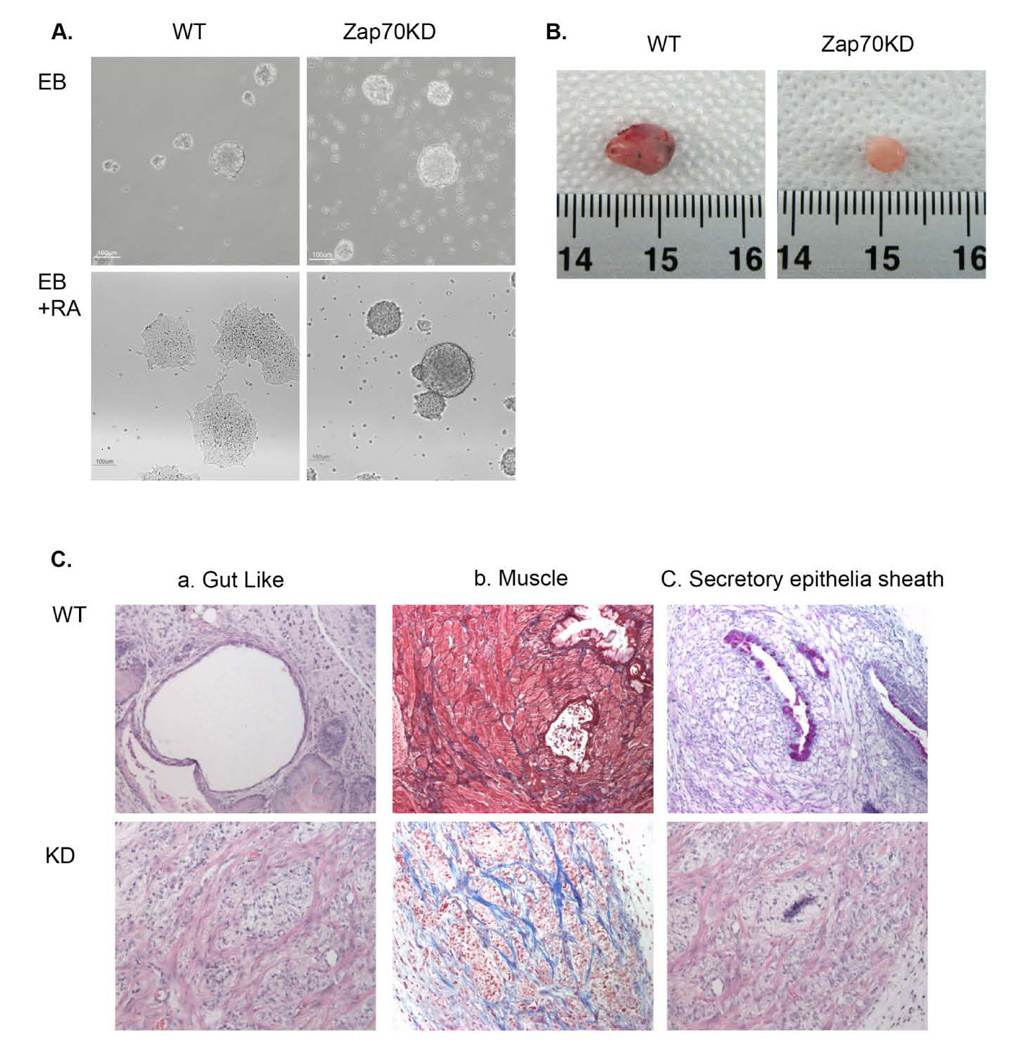
Because the stemness of ESCs is finely tuned by precise balance between maintenance of self-renewal and differentiation capacity, our results prompted us to hypothesize that ZAP70 KD may have altered differentiation potential. To address this, we first investigated the in vitro differentiation following treatment with retinoic acid (RA) for three days. As shown in figure 4A, RA-induced differentiation from Zap70KD EBs was significantly retarded compared to the control. Thus, while control ES cells showed a typical flat morphology of differentiated cells, Zap70KD cells largely retained undifferentiated morphology following RA treatment. This altered differentiation capacity of Zap70KD was further analyzed by comparing teratoma formation. Strikingly, while normal teratomas were well developed from control mESCs within four weeks in all four SCID mice, small size teratoma-like cell mass was formed in only one out of four SCID mice after six weeks of injection of Zap70KD mESCs (Fig. 4B, Table S1). When examined by histological staining, the cell mass obtained from Zap70KD failed to reveal a typical staining for specific lineage cell types and only showed undifferentiated patterns with high collagen accumulation (Fig. 4C; bottom panel). In contrast, teratomas from the wild type mESCs showed typical differentiated tissues consisting of all three germ layers such as gut-like (endoderm), muscle (mesoderm) and secretory epithelia sheath (ectoderm) (Fig. 4C; top panel). We conclude that Zap70KD mESCs show severely impaired differentiation capacity.
Figure 4. Defective differentiation capacity of Zap70KD.
(A) Embryonic bodies (EBs) were formed from either wild type or Zap70KD ES cells after 4 days in culture and differentiation was induced by the addition of retinoic acid. Cell morphology was examined 3 days after differentiation. Scale bar is 100 mm. (B) Morphologies of mESC teratomas obtained from NOD/SCID mice injected with either wild type or Zap70KD are shown. Scale bar, 1cm. (C) Histology staining of mESC induced teratomas stained with H/E, Masson’s trichrome, and Alcian Blue are shown. Gut-lie, muscle, secretory epithelia sheath components were observed in wild type mESC.
Altered LIFR expression and SHP-1 enzymatic activity in Zap70KD mESCs
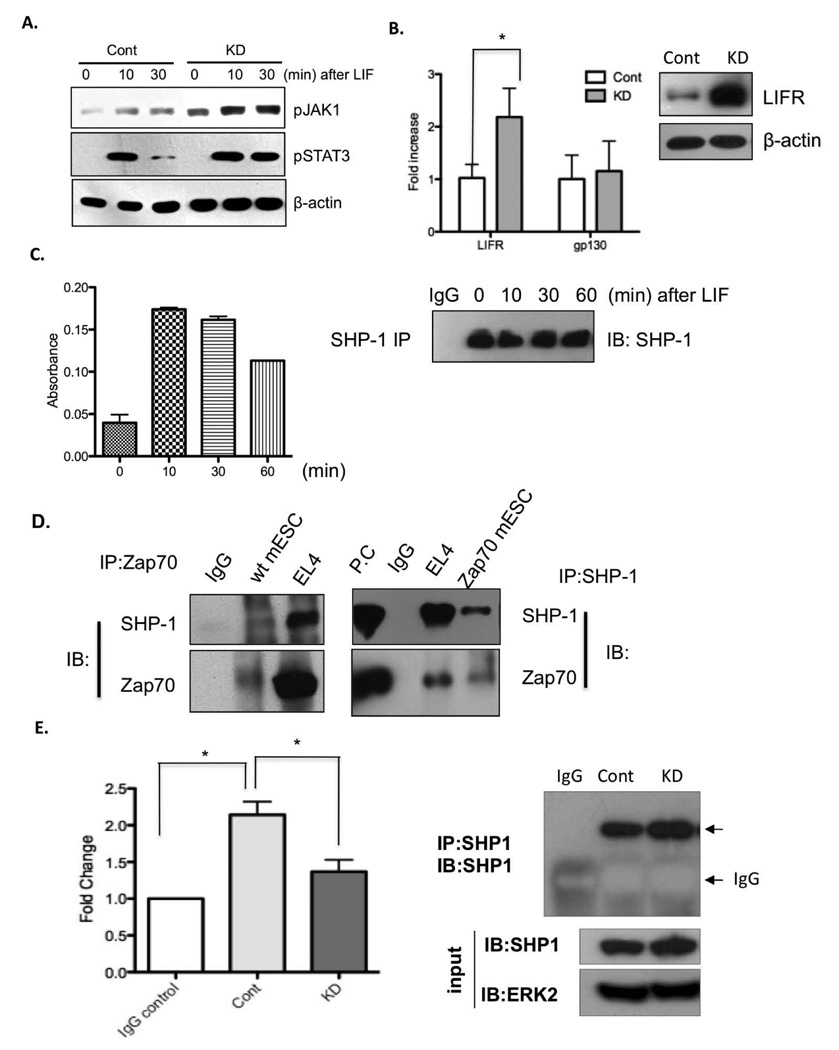
To further investigate the molecular basis of altered balance between self-renewal and pluripotency in Zpa70KD, we next addressed whether Zap70KD influenced Jak1 phosphorylation, which is known to critically regulate Stat3 phosphorylation upon LIF stimulation 29, 30. To this end, Zap70KD and control mESCs were deprived of LIF for 24 hrs (to revert LIF-dependent Jak/Stat3 signaling to basal level) and then stimulated them back with normal LIF concentration for a given time. We found that Jak1 active phosphorylation (Y1022/1023) was more steadily maintained and quickly induced upon LIF stimulation in Zap70KD and, accordingly, induced Stat3 phosphorylation was more steadily maintained (Fig. 5A). Given that Jak1 phosphorylation is initiated by LIF binding to LIFR/gp130 hetero-receptor 3, we next tested if binding of LIF to LIFR/gp130 complex or receptor expression level is altered in Zap70KD. Interestingly, we found that expression of LIFR but not gp130 was significantly up-regulated in Zap70KD (Fig. 5B). These results suggest that up-regulated LIFR and consequent increased LIF binding to the receptor promoted Jak1/Stat3 signaling in Zap70KD, leading to up-regulation of c-Myc. We also noticed that the phosphorylation status of Stat3 remained persistent in Zap70KD compared to control (Fig. 5A), suggesting that negative regulation of Jak/Stat3 is dysfunctional in Zap70KD. Because the tyrosine phosphatase SHP-1, one of the known negative regulators of Jak/Stat3 signaling, is reportedly activated by LIF binding to gp130/LIFR to maintain homeostasis of Stat3 phosphorylation 31, we next measured SHP-1 enzymatic activity following LIF stimulation in control mESCs using in vitro phosphatase assay as previously reported 32. We found that SHP-1 phosphatase activity was robustly activated upon LIF stimulation in control mESCs (Fig.5C). Therefore, dramatic reduction of Stat3 phosphorylation following 30 min LIF stimulation in control mESCs may result from up-regulated SHP-1 enzymatic activity upon LIF stimulation. Since direct association of Zap70 to SHP-1 positively regulates SHP-1 enzymatic activity in T cells 33, we next tested whether Zap70 in mESC interacts with SHP-1. Indeed, we found that Zap70 was associated with SHP-1 in mESCs, as examined by co-immunoprecipitation analysis (Fig. 5D). These results suggest that prolonged Jak1 and Stat3 phosphorylation may result from defective SHP-1 activity caused by its reduced interaction with Zap70 in Zap70KD mESCs. In support of this idea, the enzymatic activity of SHP-1, immunoprecipitated from Zap70KD, was significantly reduced compared to the control (Fig. 5E). Furthermore, transient expression of Zap70 in Zap70KD appeared to restore SHP-1 enzymatic activity, which further supports that Zap70 regulates SHP-1 phosphatase activity in mESCs (Fig. S2).
Figure 5. LIFR expression and SHP-1 phosphatase activity are two mechanisms for Zap70 to regulate Stat3 signals.
(A) mESCs LIF-starved for 24 h were stimulated with LIF for the indicated time and then active phosphorylation of Jak1 (top panel) and Stat3 (middle panel) were determined by immunoblotting. β-actin was used as protein loading control (bottom panel). (B) LIFR and gp130 mRNA level were determined by qRT-PCR and graphically presented. *, p<0.05. LIFR protein expression was shown by immunoblotting analysis. β-actin was used as protein loading control (right panel). (C) mESCs LIF-starved for 24 h were treated with LIF for the indicated time and then harvested. Left panel: SHP-1 enzymatic activity was measured, followed by SHP-1 immunoprecipitation. Right panel: immunoprecipitated SHP-1 levels were compared by immunoblotting (IB) for SHP-1 followed by SHP-1 IP. (D) Co-immunoprecipitation analysis of the interaction of endogenous Zap70 (left panel) or ectopically expressed Zap70 (right panel) with SHP-1. Interaction between Zap70 and SHP-1 in EL4 was used as a positive control. Non-specific IgGs were used as negative control. P.C, positive control from EL4 whole cell lysate. (E) Left panel: SHP-1 enzymatic activity followed by immunoprecipitation. *, p < 0.05. Right panel: Immunoprecipitated (IP) SHP-1 level, determined by immunoblotting (IB) for SHP-1 followed by SHP-1 IP. SHP-1 and ERK2 immunoblotting of whole cell lysate (input) from control mESC (Cont) and Zap70KD (KD) were used as control of equal protein concentration for the IP and phosphatase activity assays.
Transient overexpression of Zap70 in mESCs shows opposite effects of Zap70KD
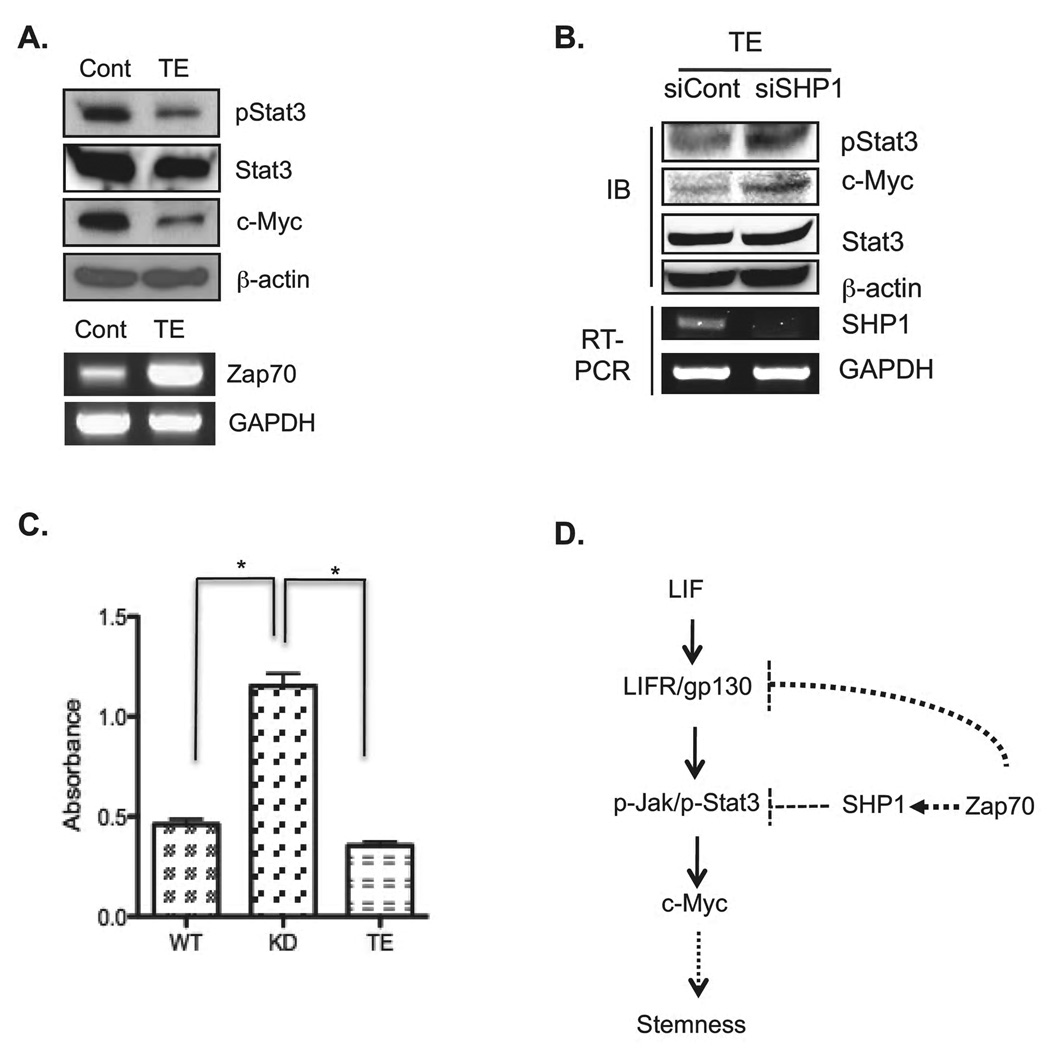
Our loss of function (KD) studies support a novel functional role for Zap70, that of regulating Stat3 signaling activity through modulation of SHP-1 activity and LIFR expression, leading to regulation of c-Myc gene expression. To further substantiate this model, we attempted to generate Zap70-overexpressing mESC clones. In spite of numerous of attempts, no such clone could be established (data not shown). A possible explanation is that Zap70-overexpressing mESCs cannot be stably maintained due to the defective self-renewal. Thus, we over-expressed Zap70 transiently in mESCs (Zap70TE) and analyzed each component of the self-renewal pathway. We found that Zap70 TE mESCs showed reduced levels of Stat3 phosphorylation level and c-Myc expression (Fig. 6A). Interestingly, this transient expression effect of Zap70 toward both Stat3 phosphorylation and c-Myc expression was reverted, at least in part, when SHP-1 expression was suppressed by siRNA (Fig. 6B). Taken together, these results support of our data of Zap70KD and further support that SHP-1 is involved in regulation of Stat3 phosphorylation and c-Myc expression by Zap70. Furthermore, alkaline phosphatase assays showed that transient expression of Zap70 in mESCs significantly lowered the levels of the enzymatic activity (Fig. 6C).
Figure 6. SHP-1 is responsible for the regulation of c-Myc expression.
(A) Phosphorylated Stat3 and c-Myc were analyzed by immunoblotting analysis from mESCs transiently transfected with Zap70 expressing plasmid for 48 hrs. Expression of Zap70 was confirmed by RT-PCR. (B) siSHP-1 or siCont were cotransfected with Zap70 expressing plasmid into mESCs for 48 hrs. Zap70 and SHP-1 expression were analyzed by RT-PCR (bottom panel). Phospho-Stat3 and c-Myc expression in each condition were analyzed by immunoblotting (IB: Top panel). (C) Alkaline phosphatase activity was analyzed from wild type, Zap70KD and mESCs transfected with Zap70 expressing plasmid after LIF-starved for 24 h. *, p < 0.05. (D) Proposed model of Zap70 function in mESCs. Zap70 is a negative regulator of Jak/Stat3 signaling pathway. Interaction of Zap70 with SHP-1 activates SHP-1 phosphatase to suppress Jak/Stat3 signals and subsequent c-Myc expression to maintain the balanced stemness of ESCs. Upregulation of LIFR expression is another mechanism by which Zap70 regulates the balance of self-renewal and pluripotency in mESCs. Abbreviations: Cont, transient expression of GFP; TE, transient expression of Zap70; KD, stable Zap70 knockdown mESC cell line; siSHP1, siRNA targeting SHP-1; siCont, non-specific siRNA
Discussion
Understanding of the pluripotent state of ESCs will facilitate the progress in stem cell research by providing molecular and cellular basis for manipulating their self-renewal versus differentiation properties in vitro. Cell fate decision of stem cells between self-renewal and differentiation is regulated by an intricate regulatory network of extrinsic signals (e.g., cytokines and growth factors) and intrinsic transcriptional factors. In particular, the signal pathway of the LIF mediated Jak/Stat3 signaling and consequent c-Myc induction has been identified to be critical for maintaining the self-renewal and pluripotency in mESCs 1, 2, 4. However, the molecular mechanisms how the LIF/Jak/Stat3 signaling is finely controlled to balance between self-renewal and differentiation have not been fully understood. In this study, we demonstrated that Zap70, whose expression is exclusively reported several immune cells such as T cells, NK cells, pro/pre B cells and B cell leukemia, is prominently expressed in mESCs and plays important roles to regulate the balance between self-renewal and differentiation potential. Our results demonstrate that Zap70 regulates the Jak/Stat3 signaling through consolidating SHP-1 enzymatic activity as well as down-regulation of LIFR expression, leading to modulation of c-Myc gene expression (Fig. 6D). Our Zap70KD analysis shows that SHP1 activity is down-regulated and LIFR and p-STAT3 are up-regulated, resulting in higher expression of c-Myc. It appears that Zap70 regulates mRNA expression of LIFR. However, the level of LIFR may be also regulated by lysosomal degradation, depending on ERK1/2 activity as a negative feedback mechanism 34. In line with this possibility, phospho-ERK1/2 level was reduced in ZAP70KD cells (Fig. 2E and 2F). The precise mechanisms underlying the regulation of LIRF gene expression by Zap70 awaits further investigation.
Another important feature of this study is that promoted Jak/Stat3 signaling in Zap70KD may be caused in part by downregulation of SHP-1 phosphatase activity. Interestingly, SHP-1 enzymatic activity is positively regulated by LIF stimulation to form a negative feedback on Jak/Stat3 signaling 31, as evidenced by the rapid dephosphorylation of Stat3 and increased SHP-1 activity upon LIF stimulation. Since association of SHP-1 to Zap70 is important for full activation of SHP-1 33, lack of Zap70 in Zap70KD may interfere with SHP-1 activation upon LIF stimulation, which is responsible for prolonged Stat3 phosphorylation. It has been widely accepted that SHP-1 functions as a negative regulator of Jak/Stat3 signaling pathway in a number of independent studies 37–39. Therefore, loss of SHP-1 and subsequent up-regulation of Jak/Stat3 activity have been frequently found in many lymphomas 34–36. Because c-Myc is a known target of Stat3 4, 19, 37, it may be responsible for Stat3 mediated tumorigenic growth 38–40 linked to the rapid cell division of mESCs (~12 hours). This interesting possibility was further supported by a recent study demonstrating that c-Myc occupied the promoters of mainly active genes in mESCs and many cell cycle regulatory genes 40. Thus, it is likely that elevated cell proliferation observed in Zap70KD may be caused by upregulated c-Myc expression. SHP-2 is another tyrosine phosphatase that has been reported to negatively affect the self-renewal 41, 42 and regulate differentiation of mESCs through ERK activity 43 or Stat3 phosphorylation 50. Therefore, it will be of great interest to address whether SHP-2 activity/expression is also affected by Zap70 and how this interaction may influence the pluripotent state of mESCs in conjunction with SHP-1. Finally, our results suggest that Zap70 critically regulates pluripotent differentiation potential. Zap70KD exhibits not only severely defective in vitro differentiation following RA treatment but also show defective teratoma formation in vivo. Our data indicate that Zap70 regulates differentiation potential via controlling the level of p-ERK. The molecular mechanisms underlying regulation of the level of p-ERK by Zap70 warrant further investigation.
Conclusion
We found that Zap70 plays important roles to modulate the LIF/Jak/Stat3 signaling pathway as a means of balancing the pluripotent state of mESCs between the maintenance of self-renewal and the differentiation capacity. We propose that two mechanisms underlying regulation of the Jak1/Stat3 signaling by Zap70 in mESCs include: (1) regulation of LIFR mRNA expression and (2) regulation of SHP-1 phosphatase activity. These two mechanisms are expected to function synergistically to modulate excessive Stat3 signaling of mESCs under LIF stimulation to maintain the balanced pluripotent state.
Supplementary Material
Acknowledgements
The authors acknowledge the scientific discussion and statistical analysis provided by Dr. J.S Moon. This work was supported by National Research Foundation of Korea (NRF) funded by the Korea Goverment (KRF-2008-313-C00703), by the Korea Science and Engineering Foundation (KOSEF) of the Korean government (MOST) (20090084075, 313-2008-2-C00703), and by National Institute of Health grant MH087903. This work was also supported by a grant (SC-5130) from the Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea.
Footnotes
Author contribution
Young-Cha, Bo-Hyun Moon, Mi-Ok Lee : Major contributor for collection and assembly of data
Hee-Jin Ahn, Hye-Jin Lee, : Collection and assembly of data
Kyung-Ah Lee, Albert J. Fornace Jr: Conception and design, Data analysis and interpretation
Kwang-Soo Kim, Hyuk-Jin Cha, Kyung-Soon Park : Conception and design, Manuscript writing, Data analysis and interpretation.
References
- 1.Williams R, Hilton D, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda T, Nakamura T, Nakao K, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kristensen D, Kalisz M, Nielsen J. Cytokine signalling in embryonic stem cells. APMIS. 2005;113:756–772. doi: 10.1111/j.1600-0463.2005.apm_391.x. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright P, McLean C, Sheppard A, et al. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Iwashima M, Turck C, et al. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 6.Arpaia E, Shahar M, Dadi H, et al. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 7.Elder M, Lin D, Clever J, et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 8.Schweighoffer E, Vanes L, Mathiot A, et al. Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity. 2003;18:523–533. doi: 10.1016/s1074-7613(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–4614. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 10.Orchard J, Ibbotson R, Davis Z, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004;363:105–111. doi: 10.1016/S0140-6736(03)15260-9. [DOI] [PubMed] [Google Scholar]
- 11.Allen M, Svensson L, Roach M, et al. Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med. 2000;191:859–870. doi: 10.1084/jem.191.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jirmanova L, Afanassieff M, Gobert-Gosse S, et al. Differential contributions of ERK and PI3-kinase to the regulation of cyclin D1 expression and to the control of the G1/S transition in mouse embryonic stem cells. Oncogene. 2002;21:5515–5528. doi: 10.1038/sj.onc.1205728. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Lee M, Moon B, et al. Senescent growth arrest in mesenchymal stem cells is bypassed by Wip1-mediated downregulation of intrinsic stress signaling pathways. Stem Cells. 2009;27:1963–1975. doi: 10.1002/stem.121. [DOI] [PubMed] [Google Scholar]
- 14.Markoulaki S, Meissner A, Jaenisch R. Somatic cell nuclear transfer and derivation of embryonic stem cells in the mouse. Methods. 2008;45:101–114. doi: 10.1016/j.ymeth.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosi D, Rasmussen T. Reprogramming mediated by stem cell fusion. J Cell Mol Med. 2005;9:320–330. doi: 10.1111/j.1582-4934.2005.tb00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tada M, Takahama Y, Abe K, et al. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SJ, Chung HM, Cha KY, et al. Identification of differential gene expression in germinal vesicle vs. metaphase II mouse oocytes by using annealing control primers. Fertil Steril. 2005;83 Suppl 1:1293–1296. doi: 10.1016/j.fertnstert.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Okuda A, Fukushima A, Nishimoto M, et al. UTF1, a novel transcriptional coactivator expressed in pluripotent embryonic stem cells and extra-embryonic cells. EMBO J. 1998;17:2019–2032. doi: 10.1093/emboj/17.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiuchi N, Nakajima K, Ichiba M, et al. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman T, Broome M, Sinibaldi D, et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekkai D, Gruel G, Herry M, et al. Microarray analysis of LIF/Stat3 transcriptional targets in embryonic stem cells. Stem Cells. 2005;23:1634–1642. doi: 10.1634/stemcells.2005-0182. [DOI] [PubMed] [Google Scholar]
- 22.Darnell JJ, Kerr I, Stark G. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 23.Schust J, Sperl B, Hollis A, et al. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 25.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 26.Duval D, Trouillas M, Thibault C, et al. Apoptosis and differentiation commitment: novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ. 2006;13:564–575. doi: 10.1038/sj.cdd.4401789. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Mantel C, Hromas RA, et al. Oct-4 is critical for survival/antiapoptosis of murine embryonic stem cells subjected to stress: effects associated with Stat3/survivin. Stem Cells. 2008;26:30–34. doi: 10.1634/stemcells.2007-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 29.Narazaki M, Witthuhn B, Yoshida K, et al. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Natl Acad Sci U S A. 1994;91:2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishimoto T. Signal transduction through homo- or heterodimers of gp130. Stem Cells. 1994;12 Suppl 1:37–44. discussion 44-35. [PubMed] [Google Scholar]
- 31.Bousquet C, Susini C, Melmed S. Inhibitory roles for SHP-1 and SOCS-3 following pituitary proopiomelanocortin induction by leukemia inhibitory factor. J Clin Invest. 1999;104:1277–1285. doi: 10.1172/JCI7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizuno K, Katagiri T, Maruyama E, et al. SHP-1 is involved in neuronal differentiation of P19 embryonic carcinoma cells. FEBS Lett. 1997;417:6–12. doi: 10.1016/s0014-5793(97)01234-9. [DOI] [PubMed] [Google Scholar]
- 33.Plas D, Johnson R, Pingel J, et al. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 34.Oka T, Ouchida M, Koyama M, et al. Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res. 2002;62:6390–6394. [PubMed] [Google Scholar]
- 35.Delibrias C, Floettmann J, Rowe M, et al. Downregulated expression of SHP-1 in Burkitt lymphomas and germinal center B lymphocytes. J Exp Med. 1997;186:1575–1583. doi: 10.1084/jem.186.9.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q, Wang H, Marzec M, et al. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6948–6953. doi: 10.1073/pnas.0501959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bromberg J, Wrzeszczynska M, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 38.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 39.Ding B, Yu J, Yu R, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeller K, Zhao X, Lee C, et al. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci U S A. 2006;103:17834–17839. doi: 10.1073/pnas.0604129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qu C, Feng G. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 1998;17:433–439. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- 42.Chan R, Johnson S, Li Y, et al. A definitive role of Shp-2 tyrosine phosphatase in mediating embryonic stem cell differentiation and hematopoiesis. Blood. 2003;102:2074–2080. doi: 10.1182/blood-2003-04-1171. [DOI] [PubMed] [Google Scholar]
- 43.Burdon T, Stracey C, Chambers I, et al. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol. 1999;210:30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.