Abstract
The receptor-regulated protein Smad3 is key player in the signaling cascade stimulated by the binding of activin to its cell surface receptor. Upon phosphorylation, Smad3 forms a heterocomplex with Smad2 and Smad4, translocates to the nucleus and acts as a transcriptional co-activator. We have identified a unique isoform of Smad3 that is expressed in mature pituitary gonadotropes. 5' RACE revealed that this truncated Smad3 isoform is transcribed from an ATG site within exon 4 and consists of 7 exons encoding half of the linker region and the MH2 region. In pituitary cells, the truncated Smad3 isoform was phosphorylated upon activin treatment, in a manner that was temporally distinct from the phosphorylation of full-length Smad3. Activin-induced phosphorylation of Smad3 and the truncated Smad3 isoform was blocked by both follistatin and siRNA-mediated knockdown of Smad3. The truncated Smad3 isoform antagonized Smad3-mediated, activin-responsive promoter activity. We propose that the pituitary gonadotrope contains an ultra-short, activin-responsive feedback loop utilizing two different isoforms of Smad3, one which acts as an agonist (Smad3) and another that acts as an intracrine antagonist (truncated Smad3 isoform) to regulate FSHβ production.
Keywords: pituitary, activin, Smad3, FSHβ, transcription
1. Introduction
The members of the transforming growth factor-β (TGFβ) superfamily regulate cell growth, differentiation, apoptosis, morphogenesis, adhesion, and immune response (Roberts et al., 1990). The activins, activin A (ActA) and B (ActB), are members of the TGFβ superfamily; while ActA is more widely expressed and plays an important role in development (Matzuk et al., 1995, 1996), ActB is primarily expressed in human gonadotropes (Uccella et al., 2000), where it acts as a local paracrine factor that stimulates follicle-stimulating hormone β-subunit (FSHβ) expression (Carroll et al., 1989). The activins bind to high-affinity type II receptors (ACVR2 and ACVR2B) and promote the recruitment, trans-phosphorylation, and stabilization of type I receptors into a type II/I receptor complex (Shi and Massague, 2003; Thompson et al., 2003). The phosphorylated type I receptors in turn phosphorylate and activate the intracellular signaling proteins Smad2 and Smad3, which form a complex with a cofactor, Smad4 (Kretzschmar and Massague, 1998). The Smad complex then enters the nucleus, where it acts as a transcriptional regulator by binding target promoters (Massague and Wotton, 2000; Suszko et al., 2005). Previous studies have established that both ActA and ActB stimulate Smad2 and Smad3 phosphorylation and nuclear accumulation in LβT2 cells, leading to a rapid increase in FSHβ primary transcript and mRNA levels (Bernard, 2004; Pernasetti et al., 2001; Suszko et al., 2005). Within the proximal rat and murine FSHβ promoters, Smad complexes bind an 8-bp sequence, GTCTAGAC, called a Smad binding element (SBE) (Lamba et al., 2006). The human, porcine, and ovine FSHβ promoters lack the proximal SBE but are activin responsive, suggesting the presence of additional or alternative SBEs (Lamba et al., 2006).
In female mammals, FSHβ expression occurs in a rapid, exquisitely timed cycle of stimulation and extinction that is regulated through neuroendocrine, endocrine and paracrine/autocrine mechanisms (Ortolano et al., 1988; Schwartz and Levine, 2006). Gonadotropin-releasing hormone (GnRH) is released from the hypothalamus in a pulsatile manner to stimulate the expression and release of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary (Besecke et al., 1996; Levine and Duffy, 1988). The frequency of GnRH pulses varies as a function of hormonal status and reproductive cycle stage. These pulses also regulate the expression of follistatin, which in turn limits the level of free activin (Bauer-Dantoin et al., 1996). Activin can also be blocked by a negative endocrine feedback loop regulated by inhibin from the ovary. During the normal reproductive cycle, the rising and falling of inhibin levels contributes to the regulation of FSHβ expression in the gonadotropes (Cook et al., 2004). In cycling female rats, inhibin A levels are low on the morning of metestrus and increase steadily to a peak on proestrus, then decline to a nadir on the morning of estrous (Woodruff et al., 1996). The loss of inhibin, together with the downregulation of follistatin at the level of the pituitary, permits activin to signal through Smads and cause the short-lived, but imperative, secondary FSH surge (Woodruff et al., 1996). This secondary FSH surge is necessary for the recruitment of the next cohort of follicles into the growing pool (Hoak and Schwartz, 1980).
Within the gonadotrope, the duration of FSHβ expression is regulated by the degradation and turnover of Smad2/3. In general, ubiquitin-dependent protein degradation plays an important role in various biological processes, including transcriptional regulation, signal transduction, immune response and cell cycle regulation (Hershko and Ciechanover, 1998). Ubiquitination of proteins is induced by E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes and E3 ubiquitin ligases (Haas and Siepmann, 1997). E3 ligases recognize target proteins and induce their degradation by 26S proteasomes (Orino et al., 1991). Previous studies have shown that Smads are regulated by proteasomal degradation (Zhu et al., 1999). Activated (phosphorylated) Smad2 is ubiquitinated by E3 ligases after translocation into the nucleus (Lo and Massague, 1999), whereas phospho-Smad3 interacts with the ROC1-SCFFbw1a complex (Cenciarelli et al., 1999; Winston et al., 1999) for proteasomal degradation (Fukuchi et al., 2001). Interestingly, the degradation products of certain proteins, including the transcription factors Cubitus interruptus (Ci) and NF-κB, are biologically active protein fragments produced by the proteasome based on minimal signal sequences within the protein (Tian et al., 2005). Based on the observation of a pituitary-specific Smad protein in our previous studies (personal communication with A. Matouschek), we hypothesized that this protein was a Smad degradation product that could represent a novel, active component in the regulation of TGFβ signaling.
Here, we have characterized this novel Smad protein, which was found to be a truncated isoform of Smad3 that is a product of a pituitary-specific promoter and not ubiquitin-dependent proteolytic processing. The Smad3 isoform is phosphorylated by activin but negatively regulates activin-stimulated FSHβ promoter activity in mature gonadotropes. We believe that this truncated Smad3 isoform functions within an ultra-short cellular feedback loop (or “intracrine” loop) that limits the duration of activin-stimulated FSHβ gene expression in pituitary gonadotropes during the reproductive cycle.
2. Materials and methods
2.1 Animals
CD1 mice (Harlan, Indianapolis, IN) were maintained in accordance with the policies of Northwestern University's Animal Care and Use Committee. Mice were housed and bred at a constant temperature, humidity, and photoperiod (14L:10D), with food and water available ad libitum. Various tissues including pituitary, ovary, brain, liver, muscle, testis, and intestine were collected from 6-day-old mice (n=6) and protein lysates were prepared for immunoblotting. Pituitaries were collected from 8-week-old mice (n=4), minced and plated in serum-free Dulbecco's modified Eagle's medium (DMEM)/F12 (Invitrogen, Carlsbad, CA), and treated with activin A (30 ng/ml) for 1 h, then lysates were prepared for immunoblotting.
2.2 Cell lines
An immortalized mouse pituitary gonadotrope cell line, LβT2 (kindly provided by Dr. P. L. Mellon, University of California, San Diego, CA), was maintained in DMEM/F12 supplemented with 5% (v/v) fetal bovine serum (FBS) and 1% (v/v) antibiotics at 37°C and 5% CO2. KGN human granulosa cells and HEK293FT cells were cultured in DMEM/F12 with 10% (v/v) FBS and 1% (v/v) antibiotics. aT3-1 cells and HaCaT keratinocyte cells were cultured in DMEM with 10% (v/v) FBS and 1% (v/v) antibiotics.
2.3 Recombinant ligands and plasmid constructs
Recombinant human ActA was purified and formulated as reported previously (Pangas and Woodruff, 2002; Antenos et al., 2008). Recombinant ActB (R&D Systems, Minneapolis, MN) was reconstituted in 0.1% (w/v) bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Follistatin was a generous gift of Dr. A.F. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Pituitary Program). Creation of the FSHβ promoter-luciferase (FSHβ-Luc), 3TP-Luc and CAGA-Luc reporter constructs has been reported previously (Burdette and Woodruff, 2007). The Smad3, TGFβRII and Alk5-HA type I receptor expression constructs have been described previously (Suszko and Woodruff, 2006). The Smad3 isoform expression construct was generated by PCR amplification of the 5' RACE product, followed by cloning into the pcDNA 3.1 TOPO-V5 His6 vector. The identity of the plasmids was confirmed by sequence analysis.
2.4 5' rapid amplification of cDNA ends (5' RACE)
RNA from a mouse pituitary gonadotrope cell line, LβT2, was extracted and purified with the RNeasy kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. First-strand cDNA synthesis was performed using the SMARTer™ cDNA synthesis kit per the manufacturer's instructions (Clontech Inc., Mountain View, CA, USA). Prior to the reverse transcription-polymerase chain reaction (RT-PCR), total RNA was treated with calf intestinal phosphatase (CIP) to remove the 5'-phosphate from all RNA species except intact mRNA bearing the the 5' CAP structure. Then, tobacco acid pyrophosphatase (TAP) was used to remove the cap structure from mRNA, leaving the 5'-phosphate exposed and available for subsequent ligation. CIP/TAP pre-treatment increases 5' RACE selectivity by avoiding false PCR amplification products through selection of complete mRNAs from the total RNA population. Next, a synthetic RNA oligonucleotide was added and ligated to the CIP/TAP-treated RNA and the chimeric RNA was reverse transcribed using random primers and SMARTScribe™ Reverse Transcriptase. The resulting cDNA was used as a template for PCR using the reverse Smad3-specific primer with the following sequence: 5'-AAGACCTCCCCTCCGATGTAGTAGA-3'. PCR was performed with Master Mix and UPM (universal primer mix) using an annealing temperature of 68°C for 30 sec.
2.5 Cell treatment and transient transfection
LβT2, αT3-1, KGN and HaCaT cells were plated at 80% confluency in 24-well plates, and then treated with vehicle, ActA (30 ng/ml), ActB (30 ng/ml), TGFβ1 (10 ng/ml) or follistatin (300 ng/ml) at the indicated concentrations and for the indicated times. Cells were then washed in PBS and lysed, and total protein was collected for immunoblotting using the indicated antibodies.
For experiments involving reporter constructs, LβT2 and HEK293FT cells were plated in 24- or 6-well plates at a confluency of 80% one day prior to transfection. LβT2 cells were transfected with 50 ng of pcDNA3.0, full-length Smad3, and Smad3 isoform for luciferase assay, 2 ug of TGFβRII and/or Alk5-HA expression plasmids in 6 well for ligand test, and 500 ng of FSHβ-Luc reporter construct for luciferase assay. Lipofectamine 2000 and Opti-MEM were used for LβT2 cell transfection. HEK293FT was transfected with 500 ng of 3TP-Luc or CAGA-Luc reporter constructs, and with the indicated amounts of full-length Smad3 or Smad3 isoform expression plasmids. pcDNA3.0 empty vector was used to balance the total amount of transfected plasmid. After 18 h, the cells were treated with the indicated ligands at various doses for 6 h in phenol-free, serum-free DMEM/F12 and then harvested for luciferase assay. Emitted luminescence was measured for 30 s using an AutoLumat Luminometer (Berthold Technologies Co., Oak Ridge, TN).
For experiments involving siRNA transfection, LβT2 cells were plated at 60%–70% confluency one day prior to transfection. The cells were transfected with 100 nM of nonspecific control siRNA oligoduplexes (D-001206-09-00; Dharmacon, Inc., Lafayette, CO) or specific siRNA targeted to Smad3 (M-040706-00; Dharmacon, Inc.) for 48 h using Lipofectamine 2000 in Opti-MEM medium (Invitrogen). After 48 h, the cells were treated with for 1 h in control medium or activin A at a concentration of 30 ng/ml.
2.6 Immunoblot analysis
Mouse tissues and cultured cells subjected to tranfection or treatment were lysed, and total protein was isolated for immunoblotting as described previously (Burnette, 1981). Nuclear and cytoplasmic extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific, Waltham, MA) according to the manufacturer's instructions. Extracts were run on NuPAGE® 4–12% Bis-Tris gradient gels (Invitrogen) and transferred onto nitrocellulose membranes using the iBlot™ Dry Blotting system (Invitrogen). Anti-phospho-Smad3 (pSmad3, Ser423/425, C25A9), anti-phospho-Smad2 (pSmad2, 3101), anti-Smad3 (Smad3, C67H9), anti-Smad2 (3122), anti-lamin A/C (2032) and anti-GAPDH (14C10) antibodies were purchased from Cell Signaling Technologies (Beverly, MA). Goat anti-rabbit and mouse horseradish peroxidase-conjugated secondary antibodies were purchased from Invitrogen. Proteins were visualized using ECL Plus detection reagent (Amersham Biosciences, Fairfield, CT) and exposed to x-ray film (Eastman Kodak Co., Elk Grove Village, IL) for varying times.
2.7 Statistical analysis
Values are reported as the mean ± S.E. and were analyzed using Prism Version 4.0a (GraphPad Software, Inc., San Diego, CA). Analysis of variance, followed by the appropriate post-test (Tukey's, Bonferroni or Kruskal-Wallis), was used to evaluate differences between treatments. Statistical significance was reported if P<0.05.
3. Results
3.1 Identification and cloning of the 30 kDa Smad protein
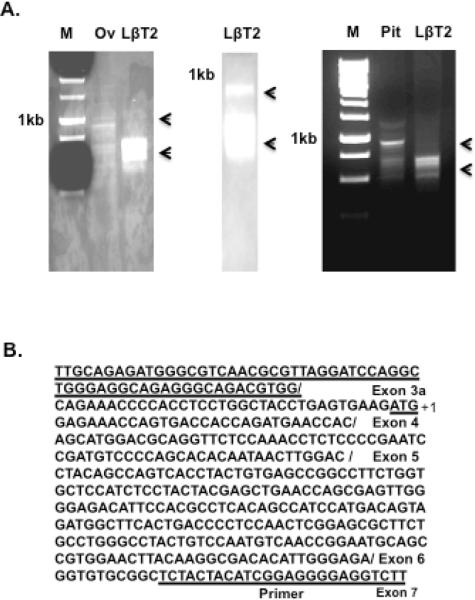
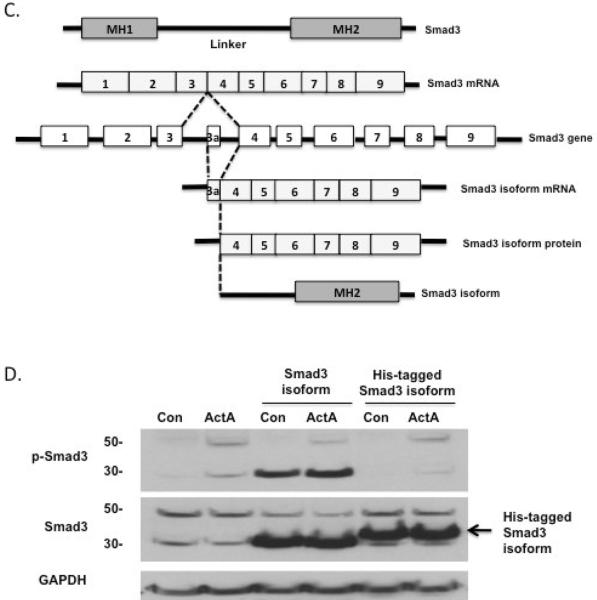
We had identified a 30 kDa phosphoprotein that was pituitary-specific, activin-stimulated, and detected by anti-Smad3 antibodies. To identify this protein product, we performed 5' RACE with a primer directed to mouse Smad3 cDNA isolated from either LβT2 gonadotrope cells or pituitary tissue. 5' RACE produced four bands from LβT2, pituitary cDNA and one major band from mouse ovary tissue cDNA (Fig. 1A). After 35 cycles of PCR, we were able to amplify three small bands and a large band from both LβT2 and pituitary 5' RACE products. We isolated the large PCR product for cloning and sequencing, and confirmed that it was full-length Smad3 cDNA in both cases. We also isolated and cloned the small 5' RACE PCR products, and found by sequence analysis that they all contained exons 4, 5, 6 and 7 of Smad3 but various parts of exon 3 (Fig. 1B and 1C). We identified another ATG site within exon 4 (Fig. 1C), and the expected protein product from this truncated sequence agrees with the 30 kDa protein seen in our immunoblots. Thus, the Smad3 isoform appears to originate from this new transcription site that leads to a truncated mRNA and protein product.
Fig. 1.
Identification and cloning of the Smad3 isoform. (A) 5' RACE PCR was performed to identify full-length Smad3 and a truncated Smad3 isoform (arrows) using 30 cycles (left and right) or 35 cycles (middle). PCR data shows the comparison of 5' RACE PCR products between ovary and LβT2 cells (left), and the comparison of 5' RACE PCR products between pituitary and LβT2 cells (right). The location of the 1 kb marker is shown in all cases. Arrows indicate the 5' RACE PCR products of both full-length and truncated Smad3 isoforms. M, marker; Ov, ovary; LβT2, LβT2 cell line; Pit, pituitary. (B) The sequences show the result of one band identified by 5' RACE. 5' RACE revealed a new exon 3a sequence prior to exon 4 and a new ATG marked +1 within exon 4 (underlined), a possible start site for the truncated Smad3 isoform. The 5' RACE reverse primer in exon 7 is underlined. (C) Schematic of the mouse Smad3 gene structure, with alternative splicing sites and protein products. Full-length Smad3 consists of nine exons and is separated into three functional parts, MH1, linker and MH2. The smaller Smad3 5' RACE product consists of seven exons and two functional parts, half of the linker region and the MH2 region. The smaller Smad3 transcriptional product is referred to as “Smad3 isoform.” (D) The Smad3 isoform identified by 5' RACE, with or without a C-terminal His tag was cloned into an expression vector and overexpressed in LβT2 cells. Untransfected (lanes 1 and 2) and transfected (lanes 3–6) cells were treated with vehicle (Con) or activin A (30 ng/ml) for 1 hour, and the lysates were immunoblotted with anti-phospho-Smad3 antibody (top), anti-Smad3 antibody (middle) or anti-GAPDH as a control (bottom). The Smad3 isoform identified by 5' RACE was identical in size to the small Smad3 band observed in activin-treated pituitary cells and LβT2 cells. Addition of a His tag to the C-terminus of the Smad3 isoform interfered with its phosphorylation.
3.2 Confirmation of the truncated Smad3 isoform 5' RACE PCR product identity
To confirm that 5' RACE product indeed produced a truncated isoform of Smad3, has the same size as the endogenous Smad3 isoform, and has the responsiveness to activin, we cloned the fragment into an expression vector for transfection into LβT2 gonadotrope cells. Transfected cells were treated with vehicle or ActA (30 ng/ml) and their lysates were probed with anti-Smad3 and anti-phospho-Smad3 antibodies. The transfected cells yielded a 30 kDa band that was identical to the band seen in untransfected LβT2 cells (Fig. 1D, lanes 1 and 2 compared with lanes 3 and 4). Modification of full-length Smad3 with a C-terminal 6× His tag interferes with phosphorylation of the p423/425 site, thus making it unrecognizable to anti-phospho-Smad3 antibody. Similar C-terminal modification of the truncated Smad3 isoform also blocks detection by the anti-phospho-Smad3 antibody, suggesting that the 5' RACE product is functional and behaves like full-length Smad3 (Fig. 1D, lanes 5 and 6).
3.3 Tissue expression of Smad3 and Smad2 protein in 6-day-old mice
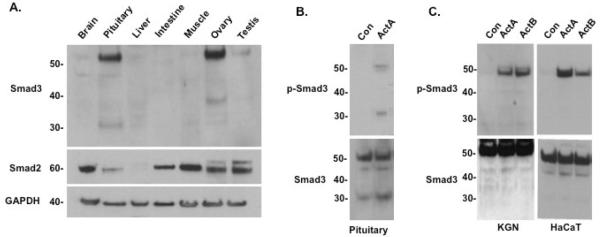
To examine endogenous, tissue-specific expression of the full-length and truncated Smad3 proteins, total protein was isolated from various mouse tissues and probed with anti-Smad3 or anti-Smad2 antibodies (Fig. 2A). The pituitary and ovary showed the highest Smad3 expression (~52 kDa) compared with other tissues. In contrast, brain (not including the pituitary), liver, muscle and testis showed comparably low expression of Smad3. Notably, additional protein bands appeared in the Smad3 immunoblots of both pituitary (30 kDa) and ovary (40 kDa) tissue lysates (Fig. 2A). Smad2 (60 kDa) had a distinct expression pattern, with the highest levels in muscle and brain and the lowest levels in pituitary and liver The Smad2 antibody did not detect bands of lower molecular weight in either the pituitary or ovary lysates, supporting the hypothesis that this truncated protein is specifically derived from Smad3.
Fig. 2.
Tissue-specific expression of a Smad3 isoform in mice and identification of a tissue-specific, activin-induced, phospho-Smad3 isoform protein in mouse pituitary. (A) Immunoblots of mouse tissues with anti-Smad3 antibody (top), anti-Smad2 (middle), or anti-actin (bottom) as a control. Tissue samples were collected from day 6 mice. Pituitary and ovary show a strong fulllength Smad3 band (52 kDa), which is also present to a lesser degree in brain, liver, muscle, and testis. Additional protein bands appeared in the Smad3 immunoblots of pituitary (30 kDa) and ovary (40 kDa). Smad2 expression was high in brain, intestine, muscle, ovary, and testis. (B) Immunoblots of vehicle (Con) or activin A (30 ng/ml)-treated pituitaries from 8-week-old mice, using either anti-phospho-Smad3 antibody (top) or anti-Smad3 antibody (bottom). Unphosphorylated full-length and Smad3 isoform proteins were present in untreated pituitary cells. Activin treatment induced full-length phospho-Smad3 (52 kDa) and the 30 kDa phospho-Smad3 isoform in the pituitary. (C) Immunoblots of KGN (human granulosa, left) and HaCaT (human keratinocyte, right) cells treated with vehicle (Con), activin A or activin B (30 ng/ml) for 1 hour, using either anti-phospho-Smad3 antibody (top) or anti-Smad3 antibody (bottom). Only full-length phospho-Smad3 was detected in these cells.
Since activin is known to induce the phosphorylation of full-length Smad3 (Bernard et at., 2004), we investigated whether the truncated Smad3 isoform would also be phosphorylated in response to activin treatment. Primary cultures of mouse pituitary cells were exposed to ActA 30 ng/ml) for 1 hour then processed for immunoblotting. As expected, ActA induced the phosphorylation of full-length Smad3 (Fig. 2B). Interestingly, the truncated (30 kDa) isoform of Smad3 was also phosphorylated at similar levels upon activin treatment. We also tested the lysates of other cell lines that are known to support Smad3 signaling, including KGN granulosa and HaCaT human keratinocyte cells. Neither anti-Smad3 nor anti-phospho-Smad3 antibodies detected the presence of the 30 kDa band in these cell lines (Fig. 2C). Similarly, only the full-length Smad3 protein was detected in the lysates of T47D, MDA-MB 231 and MCF7 breast cancer cells; JEG3 placental choriocarcinoma cells; CHO-K1 ovary cells; FET colon carcinoma cells; HeLa cervical cancer cells; and HEK293T kidney cells (data not shown). Together, these data suggest that the 30 kDa isoform of Smad3 is specific to pituitary tissue in the mouse.
3.4 Specificity of activin-induced phospho-Smad3 isoform production
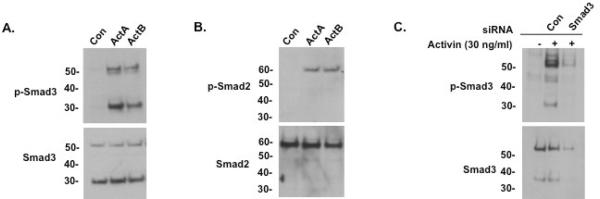
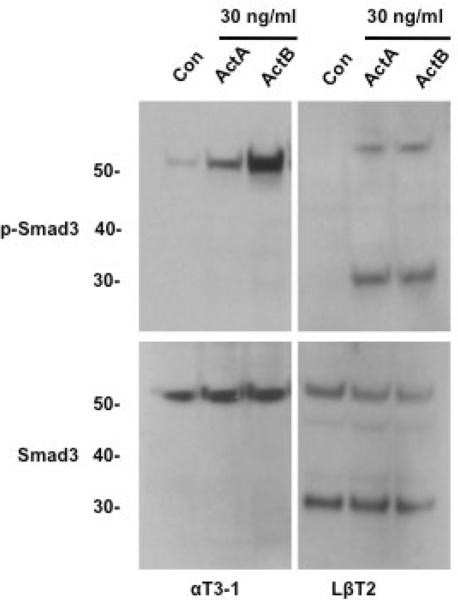
After confirming the pituitary-specific expression of the 30 kDa Smad3 isoform, we further characterized the protein in LβT2 gonadotrope cells. To clarify whether ActA and ActB have differential effects on the induction of the truncated Smad3 isoform, LβT2 cells were treated with each form of activin and probed for phospho-Smad3 levels. Compared to untreated cells, both ActA- and ActB-treated cells displayed increased phosphorylation of full-length (52 kDa) and truncated (30 kDa) phospho-Smad3 protein (Fig. 3A). Total Smad3 levels were similar in both untreated and treated cells. Furthermore, we confirmed that the truncated isoform was specific to Smad3, as anti-Smad2 and anti-phospho-Smad2 antibodies only recognized the 60 kDa full-length Smad2 protein, but not a 30 kDa protein product (Fig. 3B).
Fig. 3.
Characterization of the Smad3 isoform protein in LβT2 cells. (A) Immunoblots of vehicle (Con), activin A (30 ng/ml), or activin B (30 ng/ml)-treated LβT2 cell lysates, using either anti-phospho-Smad3 antibody (top) or anti-Smad3 antibody (bottom). Both of activin A and B treatment induced a full-length phospho-Smad3 (52 kDa) and a phospho-Smad3 isoform protein (about 30 kDa). Unphosphorylated full-length and Smad3 isoform proteins were also present in untreated cells including activin-treated cells. (B) Immunoblots of vehicle (Con), activin A or activin B (30 ng/ml)-treated LβT2 cell lysates, using either anti-phospho-Smad2 antibody (top) or anti-Smad2 antibody (bottom). A single form of phospho-Smad2 (60 kDa) was seen in LβT2 cells upon treatment with activin A or activin B; unphosphorylated Smad2 was detected in untreated cells. (C) Anti-phospho-Smad3 (top) and anti-Smad3 (bottom) immunoblots of LβT2 cells transfected with 100 nM of control (Con) or mouse Smad3-specific siRNA oligoduplexes per well using Lipofectamine 2000, then treated with activin A (30 ng/ml) for 1 hour. Both full-length and small Smad3 and phospho-Smad3 proteins were knocked down by Smad3-specific siRNA.
To further confirm that the truncated protein product detected by anti-Smad3 antibody is truly a Smad3 isoform, we transiently transfected LβT2 cells with Smad3-targeting siRNA oligoduplexes. Levels of both total full-length and truncated Smad3 protein were decreased compared to control siRNA (Fig. 3C, bottom panel). Additionally, we also examined the induction of Smad3 phosphorylation by activin. Cells transfected with control siRNA showed a clear increase in phosphorylated full-length and truncated Smad3 isoforms upon activin treatment (Fig. 3C, top panel). As expected, Smad3-siRNA treated cells detected little to no phosphorylated protein of either size due to the successful knockdown of total protein by the oligoduplexes, supporting the conclusion that the truncated isoform is indeed a derivative of Smad3.
3.5 Response of truncated Smad3 to regulators of activin signaling
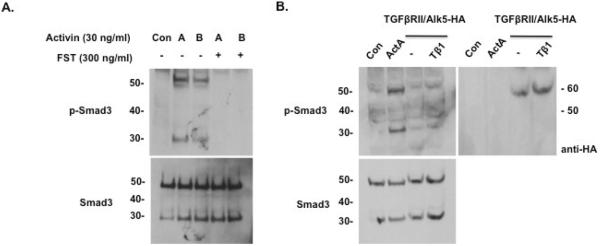
Activin signaling is negatively regulated by follistatin (FST), and thus we determined whether FST would inhibit the phosphorylation of both full-length and truncated Smad3 isoforms in LβT2 cells. Indeed, treatment with FST (300 ng/ml) counteracted the induction of both full-length phospho-Smad3 and phospho-Smad3 isoforms by activin (Fig. 4A). Additionally, we investigated whether the phosphorylation of truncated Smad3 was a specific response to activin or a general product of TGFβ signaling. To this end, we overexpressed TGFβRII and Alk5-HA in LβT2 cells, which do not endogenously express TGFβRII receptor. Upon treatment with TGFβ1 (10 ng/ml), these cells expressed low levels of truncated phospho-Smad3 isoform (Fig. 4B). Transfection efficiency was evaluated by probing the same blot with anti-HA antibody that detected strong expression of Alk5-HA (Fig. 4B, right panel). We note that despite similar levels of total Smad3 in the ActA- and TGFβ1-treated groups, the phosphorylation of both full-length and truncated Smad3 are reduced in response to TGFβ1. We recognize that transient transfection may not permit full expression of TβRII in all cells. Despite this caveat, the data suggest that multiple TGFβ family members regulate Smad3 phosphorylation, but the respons to activin is more potent than TGFβ (Suszko et al., 2006).
Fig. 4.
Response of Smad3 isoform to regulators of activin signaling and to TGFβ1 ligand. (A) Anti-phospho-Smad3 (top) and anti-Smad3 (bottom) immunoblots of LβT2 cells treated with vehicle (Con), activin A or activin B (30 ng/ml) in the absence or presence of follistatin (FST; 300 ng/ml) for 1 hour. Follistatin treatment abolished the induction of both full-length phospho-Smad3 and phospho-Smad3 isoform proteins by activin A and B. (B) Untreated (control) and activin A-treated LβT2 cells were transfected with empty vector. Additionally, LβT2 cells were transfected with expression vectors for TGFbRII and Alk5-HA, and treated with TGFβ1 (Tβ1) for 6 hours. Lysates were blotted with either anti-phospho-Smad3 antibody (left top), anti-Smad3 antibody (left bottom), or anti-HA (right top). Compared with activin A treatment (lane 2), TGFβ showed a much weaker induction of the phospho-Smad3 isoform.
3.6 Characterization of activin A-stimulated phospho-Smad3 isoform accumulation
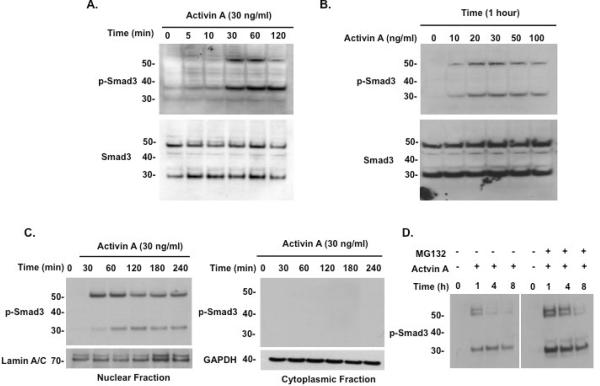
The phosphorylation of full-length Smad3 has a defined time course in response to activin (Fukuchi, et al., 2001). We performed a similar characterization of the truncated Smad3 isoform by treating LβT2 cells with ActA, then harvesting cells at intermittent time points up to 2 hours. Phosphorylation of full-length and truncated Smad3 protein was observed after 30 minutes of ActA treatment (Fig. 5A). The 52 kDa band peaked at 1 hour and decreased by 2 hours, whereas the 30 kDa band persisted through the remainder of the treatment period. There was also a dose-dependent effect of ActA on the levels of both full-length and truncated Smad3 isoforms (Fig. 5B). Levels of both proteins could be induced with 10 ng/ml ActA, and the effect peaked at 30 ng/ml activin A. Interestingly, a higher dose (100 ng/ml) had a less robust inductive effect on the two phospho-Smad3 proteins.
Fig. 5.
Characterization of Smad3 isoform protein in LβT2 cells. (A) Time course of activin A-induced phospho-Smad3 isoform production. LβT2 cells were treated with activin A (30 ng/ml) and harvested at the indicated time points for immunoblotting with anti-phospho-Smad3 antibody (top) and anti-Smad3 (bottom). Full-length phospho-Smad3 (52 kDa) and the phospho-Smad isoform protein (30 kDa) were induced by 30 minutes, while the smaller protein persisted through the 2-hour time point; full-length Smad3 levels peaked at 1 hour and declined thereafter. (B) Dose-dependence of activin A-induced phospho-Smad3 isoform production. LβT2 cells were treated with various concentrations of activin A (10–100 ng/ml) for 1 hour, then harvested for immunoblotting with anti-phospho-Smad3 antibody (top) and anti-Smad3 (bottom). Activin A 10 ng/ml was sufficient to induce both full-length and Smad3 isoform proteins, with the greatest amount of protein achieved at 30 ng/ml activin A. (C) LβT2 cells were treated with activin A (30 ng/ml) and harvested at different time points up to 4 hours, then nuclear and cytoplasmic lysates were prepared for immunoblotting with anti-phospho-Smad3 (top) or anti-Smad3 antibody (not shown). Nuclear fraction lysates were blotted with anti-lamin A/C and cytoplasmic fraction lysates were blotted with anti-GAPDH as loading controls. Both full-length phospho-Smad3 and phospho-Smad3 isoform proteins localized exclusively to the nucleus by 30 minutes of activin treatment. (D) LβT2 cells were treated with vehicle or activin A (30 ng/ml) and the proteasome inhibitor MG132 (50 nM). Cells were harvested at the indicated time points and protein used for immunoblot with anti-phospho-Smad3 antibody. MG132 treatment blocked full-length phospho-Smad3 degradation but had little effect on the phospho-Smad3 isoform.
Upon activin stimulation, phosphorylated Smad3 complexes with Smad4 and translocates into the nucleus, where it acts as a transcriptional regulator (Massague and Wotton, 2000). To test whether truncated phospho-Smad3 also localized to the nucleus upon activin treatment, nuclear and cytoplasmic fractions of ActA-treated LβT2 lysates were prepared. Full-length phospho-Smad3 first appeared in the nuclear fraction by 30 minutes, and its abundance began to decrease 2 hours (Fig. 5C). In contrast, maximum levels of truncated phospho-Smad3 in the nucleus were reached at a later time, between 30 minutes and 1 hour. Truncated phospho-Smad3 levels peaked at 2 hours following ActA treatment and began to decline by 3 to 4 hours (Fig. 5C). In the cytoplasmic fraction, no phospho-Smad3 bands of either size were detected, suggesting that both the full-length and truncated Smad3 isoforms translocate immediately into the nucleus upon phosphorylation by the ligand-bound activin receptor.
3.7 Half-life of the 30-kDa phospho-Smad3 protein
Previous studies have described that Smad3 exists at a steady-state concentration, moving between the nucleus and cytoplasm, and is constitutively degraded (Fukuchi, et al., 2001, Gao, et al., 2009). Upon activin stimulation, phospho-Smad3 translocates into the nucleus and is actively degraded via ubiquitination in the cytoplasm (Zhu et al., 1999). To determine whether the truncated Smad3 isoform is a degradation product of full-length Smad3, LβT2 cells were treated with ActA and either MG132 (50 nM; Fig. 5D) or lactacystin (10 μM; data not shown), which are specific inhibitors of proteasome-dependent proteolysis. MG132 treatment blocked the degradation of full-length phospho-Smad3 and increased its half-life from 1 to 4 hours (Fig. 5D). By contrast, MG132 and lactacystin treatment had little effect on the levels of the truncated Smad3 isoform at any time point following ActA treatment. These results suggest that the truncated Smad3 protein is not a product of proteasomal degradation, and that other mechanisms are likely involved in the production of the Smad3 isoform.
3.8 Functional role of the truncated Smad3 isoform
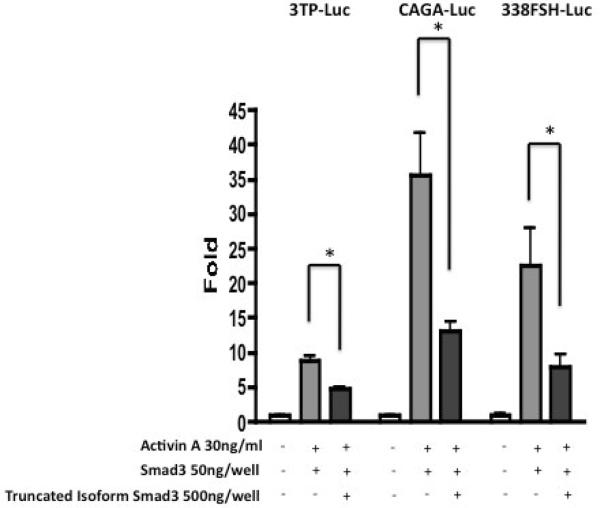
Several groups have shown that Smad2 and Smad3, in complex with Smad4, transduce activin signals that lead to the activation of the FSHβ promoter (Suszko, et al., 2003). To test whether the truncated Smad3 protein has a role in the regulation of FSHβ expression, LβT2 expression, LβT2 cells were transfected with the 338FSHβ-Luc reporter construct and expression vectors for full-length and truncated Smad3. Truncated Smad3 significantly decreased ActA-stimulated 338FSHβ-Luc reporter activity upon co-expression (Fig. 6). This suppressive effect of the truncated Smad3 isoform was also seen on activin-induced 3TP-Luc and CAGA-Luc reporter activation (Fig. 6). These data suggest that the truncated Smad3 isoform inhibits activin-induced activation of the FSHβ promoter, perhaps by competing with the Smad3/4 complex for binding to the SBE.
Fig. 6.
Functional role of the Smad3 isoform protein in activin-regulated promoter activity. HEK293FT cells were transfected with the Smad3 isoform expression plasmid plus the full-length Smad3 expression plasmid and one of two activin-responsive reporter constructs: 3TP-Luc, or CAGA-Luc, and LβT2 cells with FSHβ-Luc; empty vector (pcDNA3.0) was used to adjust total plasmid concentration for each transfection condition. Cells were treated with activin A (30 ng/ml) for 6 hours and then harvested for luciferase assay. For each reporter, promoter activity decreased with increased Smad3 isoform expression. Luciferase activity is expressed as fold induction relative to cells transfected with control vector alone. The data represent the means ± S.E. of three independent experiments performed in quadruplicate.
3.9 Physiological implications of Smad3 isoform-mediated suppression of FSHβ expression
Previous studies have established that FSHβ is only expressed in relatively mature gonadotrope cell lines, such as LβT2, and not in immature gonadotrope lines, such as αT3-1 (Jacobs et al., 2003). Presumably, cell-specific factors are induced during gonadotrope development that direct the temporal expression of FSHβ. Therefore, we determined whether the truncated Smad3 isoform is present in immature gonadotropes, and if its phosphorylation could be induced by activin in these cells. Unlike LβT2 cells, which contained both full-length and truncated phospho-Smad3 upon activin treatment, αT3-1 cells only yielded the full-length protein (Fig. 7). This result strongly supports the idea that the truncated Smad3 functions in the intracrine suppression of FSHβ expression, and that this mechanism is initiated at some point during pituitary gonadotrope development.
Fig. 7.
Differential induction of phospho-Smad3 isoform protein by activin in LbT2 and αT3-1 gonadotrope cells. Immunoblots of vehicle, activin A or activin B-treated αT3-1 or LβT2 cell lysates, using either anti-phospho-Smad3 antibody (top) or anti-Smad3 antibody (bottom). Activin treatment induced full-length phospho-Smad3 (52 kDa) and the phospho-Smad3 isoform (about 30 kDa) in LbT2 cells but not αT3-1 cells. There was also no unphosphorylated form of the Smad3 isoform protein present in αT3-1 cells.
Discussion
Activin is the major positive regulator of FSHβ subunit synthesis, and represents the rate-limiting step for FSH secretion (Attardi and Miklos, 1990). There are several cellular mechanisms that modulate activin-stimulated FSHβ expression in the pituitary gonadotrope, and, together with neuroendocrine and endocrine mechanisms, they contribute to the tight control of FSH production during the female reproductive cycle. Inhibin and follistatin interfere with activin signaling by binding to the activin receptor or to activin itself, respectively. Phosphorylation and Smad4 complex formation are necessary for Smad3 transcriptional activity on the FSHβ promoter. Proteasomal degradation of activin-specific Smads limits the duration of the activin signal. In this report, we describe yet another mechanism that modulates activin-stimulated FSHβ gene expression in the pituitary: an ultra-short intracrine feedback loop involving an activin-induced, Smad3 isoform found only in mature pituitary gonadotrope cell lines and pituitary tissue.
Previous studies have established that a decrease in pituitary follistatin content and increase in free activin A in serum on the afternoon of proestrus leads to a sharp rise in FSHβ mRNA expression that precedes the primary FSH surge (Besecke, et al., 1997). Another peak in FSHβ mRNA levels precedes the secondary serum FSH surge. We propose that the trough between the two FSH surges may be mediated by the suppression of FSHβ mRNA at the intracellular level via a tight negative feedback loop involving the activin-induced phospho-Smad3 isoform.
Overexpression of the Smad3 isoform decreased activin-stimulated FSHβ promoter activity and FSHβ mRNA levels in a dose-dependent manner. The suppressive effect of Smad3 isoform overexpression was not specific to the FSHβ promoter, but was generalized to other activin-responsive promoters (3TP-Luc and CAGA-Luc) that also contain SBEs. The suppressive effect suggests that the Smad3 isoform acts in a competitive manner, but the exact mechanism remains to be determined. The Smad3 isoform may interfere with Smad3/Smad4 complex formation or its association with other cofactors in the nucleus, or it may interfere with binding of Smad3/4 to SBEs on the FSHβ promoter. Alternatively, the Smad3 isoform protein may interact with co-inhibitors or bind to specific suppressor sites in the FSHβ promoter to override the stimulatory effect of Smad3/Smad4 binding. In a similar manner, other pituitary-specific factors such as Foxl2 or Pitx2c regulate the expression of the FSHβ gene through binding to specific promoter sites (Suszko et al., 2003; Lamba et al., 2009).
It is particularly interesting that we were unable to identify the Smad3 isoform protein in αT3-1 cells. The αT3-1 cell line is an immature pituitary gonadotrope line that was developed from gonadotropes originating from e14.5 pituitaries. αT3-1 cells do not express the FSHβ and LHβ subunits, and therefore represent a type of fetal gonadotrope progenitor cell (Brown and McNeilly, 1999; Ellsworth et al., 2006). LβT2 cells are mature gonadotrope pituitary cells that express FSHβ, LHβ and the aGSU subunits, and produce functional FSH and LH dimers (Feng et al., 2008; Brown and McNeilly, 1999). We hypothesize that the capacity to produce the Smad3 isoform may develop as part of gonadotrope differentiation, after the initiation of FSHβ expression. The FSHβ promoter does not respond to activin in either HEK293T or αT3-1 cells, despite the fact that αT3-1 cells are activin-responsive (Fernandez-Vazquez et al., 1996). It is possible that the ultra-short negative feedback loop between activin and the Smad3 isoform protein may involve specific factors not present in immature gonadotropes, but are present in cells that naturally express FSHβ or that are highly differentiated (Pernasetti et al., 2001). The potential interaction between the Smad3 isoform protein and pituitary-specific transcription factors will be pursued in future studies. It is unclear why another Smad3 isoform band was detected in whole mouse ovary lysates; though it lies outside the scope of the current study, further investigation of this phenomenon is underway.
Our findings add to the growing body of evidence that Smad protein variants modulate signaling by TGFβ superfamily members. A splice variant of Smad6, Smad6s, contains a distinct N-terminus and is found only in Homo sapiens and Pan troglodytes, and a novel human variant, termed Smad6B, has been identified in human prostatic and rodent testicular cell lines (Konrad et al., 2008). The function of Smad6B is not yet known. Another Smad3 splice variant (Smad3-Δ3) lacks exon 3, resulting in a Smad protein with a truncated linker region (Kjellman et al., 2004). Again, the function of Smad3-Δ3 is unknown, though it has been shown to have transactivating properties. Yet another Smad protein splice variant, Smad8B, lacks the SSXS site. Smad8B was found to specifically associate with both Smad8 and Smad4, and inhibit BMP signaling (Nishita et al., 1999). Here, we identified a Smad3 isoform protein that is present in both the nucleus and cytoplasm of LβT2 cells as an unphosphorylated protein, suggesting that, like full-length Smad3, the Smad3 isoform protein is present in a steady state that can translocate across the nuclear membrane. We found that the Smad3 isoform protein originates from an alternative promoter upstream of exon 3a. Upon activin treatment and Smad3 phosphorylation, production and translocation of the phospho-Smad3 isoform seems to lag behind the full-length phospho-protein. Degradation of phospho-Smad3 isoform protein is also slower than that of the full-length Smad3. How these properties relate to the proposed feedback mechanism will be investigated.
In conclusion, we have identified a novel, activin-induced, 30 kDa phospho-Smad3 isoform that acts as an intracellular negative regulator of activin-stimulated FSHβ expression. This truncated Smad3 isoform may be an important and specific “intracrine” modulator of FSHβ synthesis and FSH secretion in the mature pituitary gonadotrope. We believe that the proposed ultra-short negative feedback mechanism represents another mechanism by which activin-stimulated FSHβ expression is tightly regulated during the female reproductive cycle.
Highlights.
We identify a dominant negative version of the Smad signaling pathway that controls FSHβ transcription.
The phosphorylation of the full length Smad3 transcription fact permits the rapid rise of pituitary FSH while the activation of the truncated Smad3 extinguishes the signal.
This rapid on-off transcriptional control pathway regulates the gonadotropin surge needed to continue the normal reproductive cycle.
Acknowledgements
The authors gratefully acknowledge the editorial assistance and figure preparation of Alison Kim, Ph.D. and Stacey C. Tobin, Ph.D.
SupportThis work was supported by NIH/NICHD R01 HD037096 and NIH/NICHD R01 HD044464 to T.K. Woodruff.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have nothing to disclose.
References
- Antenos M, Zhu J, Jetly NM, Woodruff TK. An activin/furin regulatory loop modulates the processing and secretion of inhibin alpha- and betaB-subunit dimers in pituitary gonadotrope cells. J. Biol. Chem. 2008;283:33059–33068. doi: 10.1074/jbc.M804190200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi B, Miklos J. Rapid stimulatory effect of activin-A on messenger RNA encoding the follicle-stimulating hormone beta-subunit in rat pituitary cell cultures. Mol. Endocrinol. 1990;4:721–726. doi: 10.1210/mend-4-5-721. [DOI] [PubMed] [Google Scholar]
- Bauer-Dantoin AC, Weiss J, Jameson JL. Gonadotropin-releasing hormone regulation of pituitary follistatin gene expression during the primary follicle-stimulating hormone surge. Endocrinology. 1996;137:1634–1639. doi: 10.1210/endo.137.5.8612495. [DOI] [PubMed] [Google Scholar]
- Bernard DJ. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Mol. Endocrinol. 2004;18:606–623. doi: 10.1210/me.2003-0264. [DOI] [PubMed] [Google Scholar]
- Besecke LM, Guendner MJ, Schneyer AL, Bauer-Dantoin AC, Jameson JL, Weiss J. Gonadotropin-releasing hormone regulates follicle-stimulating hormone-beta gene expression through an activin/follistatin autocrine or paracrine loop. Endocrinology. 1996;137:3667–3673. doi: 10.1210/endo.137.9.8756531. [DOI] [PubMed] [Google Scholar]
- Besecke LM, Guendner MJ, Sluss PA, Polak AG, Woodruff TK, Jameson JL, Bauer-Dantoin AC, Weiss J. Pituitary follistatin regulates activin-mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology. 1997;138:2841–2848. doi: 10.1210/endo.138.7.5279. [DOI] [PubMed] [Google Scholar]
- Brown P, McNeilly AS. Transcriptional regulation of pituitary gonadotrophin subunit genes. Rev. Reprod. 1999;4:117–124. doi: 10.1530/ror.0.0040117. [DOI] [PubMed] [Google Scholar]
- Burdette JE, Woodruff TK. Activin and estrogen crosstalk regulates transcription in human breast cancer cells. Endocr. Relat. Cancer. 2007;14:679–689. doi: 10.1677/ERC-07-0054. [DOI] [PubMed] [Google Scholar]
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carroll RS, Corrigan AZ, Gharib SD, Vale W, Chin WW. Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol. Endocrinol. 1989;3:1969–1976. doi: 10.1210/mend-3-12-1969. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M, Pagano M. Identification of a family of human F-box proteins. Curr. Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- Cook RW, Thompson TB, Jardetzky TS, Woodruff TK. Molecular biology of inhibin action. Semin. Reprod. Med. 2004;22:269–276. doi: 10.1055/s-2004-831902. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol. Endocrinol. 2006;20:2796–2805. doi: 10.1210/me.2005-0303. [DOI] [PubMed] [Google Scholar]
- Feng J, Lawson MA, Melamed P. A proteomic comparison of immature and mature mouse gonadotrophs reveals novel differentially expressed nuclear proteins that regulate gonadotropin gene transcription and RNA splicing. Biol. Reprod. 2008;79:546–561. doi: 10.1095/biolreprod.108.068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vazquez G, Kaiser UB, Albarracin CT, Chin WW. Transcriptional activation of the gonadotropin-releasing hormone receptor gene by activin A. Mol. Endocrinol. 1996;10:356–366. doi: 10.1210/mend.10.4.8721981. [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol. Biol. Cell. 2001;12:1431–1443. doi: 10.1091/mbc.12.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Alarcon C, Sapkota G, Rahman S, Chen P, Goerner N, Macias M, Erdjument-Bromage H, Tempst P, Massague J. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell. 2009;13:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AL, Siepmann TJ. Pathways of ubiquitin conjugation. FASEB J. 1997;11:1257–1268. doi: 10.1096/fasebj.11.14.9409544. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hoak DC, Schwartz NB. Blockade of recruitment of ovarian follicles by suppression of the secondary surge of follicle-stimulating hormone with porcine follicular field. Proc. Natl. Acad. Sci. USA. 1980;77:4953–4956. doi: 10.1073/pnas.77.8.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SB, Coss D, McGillivray SM, Mellon PL. Nuclear factor Y and steroidogenic factor 1 physically and functionally interact to contribute to cell-specific expression of the mouse Follicle-stimulating hormone-beta gene. Mol. Endocrinol. 2003;17:1470–1483. doi: 10.1210/me.2002-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad L, Scheiber JA, Bergmann M, Eickelberg O, Hofmann R. Identification of a new human Smad6 splice variant. Andrologia. 2008;40:358–363. doi: 10.1111/j.1439-0272.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Kjellman C, Honeth G, Jarnum S, Lindvall M, Darabi A, Nilsson I, Edvardsen K, Salford LG, Widegren B. Identification and characterization of a human smad3 splicing variant lacking part of the linker region. Gene. 2004;327:141–152. doi: 10.1016/j.gene.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Massague J. SMADs: mediators and regulators of TGF-beta signaling. Curr. Opin. Genet. Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Mol. Endocrinol. 2009;23:1001–1013. doi: 10.1210/me.2008-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba P, Santos MM, Philips DP, Bernard DJ. Acute regulation of murine follicle-stimulating hormone beta subunit transcription by activin A. J. Mol. Endocrinol. 2006;36:201–220. doi: 10.1677/jme.1.01961. [DOI] [PubMed] [Google Scholar]
- Levine JE, Duffy MT. Simultaneous measurement of luteinizing hormone (LH)-releasing hormone, LH, and follicle-stimulating hormone release in intact and short-term castrate rats. Endocrinology. 1988;122:2211–2221. doi: 10.1210/endo-122-5-2211. [DOI] [PubMed] [Google Scholar]
- Lo RS, Massague J. Ubiquitin-dependent degradation of TGF-beta-activated smad2. Nat. Cell Biol. 1999;1:472–478. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol. Biol. Cell. 2003;14:2809–2817. doi: 10.1091/mbc.E02-07-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- Matzuk M, Kumar T, Shou W, Coerver K, Lau A. Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Recent Prog. Horm. Res. 1996;51:123–154. discussion 155–157. [PubMed] [Google Scholar]
- Nishita M, Ueno N, Shibuya H. Smad8B, a Smad8 splice variant lacking the SSXS site that inhibits Smad8-mediated signalling. Genes Cells. 1999;4:583–591. doi: 10.1046/j.1365-2443.1999.00285.x. [DOI] [PubMed] [Google Scholar]
- Orino E, Tanaka K, Tamura T, Sone S, Ogura T, Ichihara A. ATP-dependent reversible association of proteasomes with multiple protein components to form 26S complexes that degrade ubiquitinated proteins in human HL-60 cells. FEBS Lett. 1991;284:206–210. doi: 10.1016/0014-5793(91)80686-w. [DOI] [PubMed] [Google Scholar]
- Ortolano GA, Haisenleder DJ, Dalkin AC, Iliff-Sizemore SA, Landefeld TD, Maurer RA, Marshall JC. Follicle-stimulating hormone beta subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology. 1988;123:2946–2948. doi: 10.1210/endo-123-6-2946. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Woodruff TK. Production and purification of recombinant human inhibin and activin. J. Endocrinol. 2002;172:199–210. doi: 10.1677/joe.0.1720199. [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. Cell-specific transcriptional regulation of follicle-stimulating hormone-beta by activin and gonadotropin-releasing hormone in the LbetaT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295. doi: 10.1210/endo.142.6.8185. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Flanders KC, Heine UI, Jakowlew S, Kondaiah P, Kim SJ, Sporn MB. Transforming growth factor-beta: multifunctional regulator of differentiation and development. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1990;327:145–154. doi: 10.1098/rstb.1990.0050. [DOI] [PubMed] [Google Scholar]
- Schwartz NB, Levine JE. Ontogeny of gonadotropin-releasing hormone neurons: fishing for clues in medaka. Endocrinology. 2006;147:1074–1075. doi: 10.1210/en.2005-1597. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Suszko MI, Balkin DM, Chen Y, Woodruff TK. Smad3 mediates activin-induced transcription of follicle-stimulating hormone beta-subunit gene. Mol. Endocrinol. 2005;19:1849–1858. doi: 10.1210/me.2004-0475. [DOI] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. Regulation of the rat follicle-stimulating hormone beta-subunit promoter by activin. Mol. Endocrinol. 2003;17:318–332. doi: 10.1210/me.2002-0081. [DOI] [PubMed] [Google Scholar]
- Suszko MI, Woodruff TK. Cell-specificity of transforming growth factor-beta response is dictated by receptor bioavailability. J. Mol. Endocrinol. 2006;36:591–600. doi: 10.1677/jme.1.01936. [DOI] [PubMed] [Google Scholar]
- Thompson TB, Woodruff TK, Jardetzky TS. Structures of an ActRIIB:activin A complex reveal a novel binding mode for TGF-beta ligand:receptor interactions. EMBO J. 2003;22:1555–1566. doi: 10.1093/emboj/cdg156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Holmgren RA, Matouschek A. A conserved processing mechanism regulates the activity of transcription factors Cubitus interruptus and NF-kappaB. Nat. Struct. Mol. Biol. 2005;12:1045–1053. doi: 10.1038/nsmb1018. [DOI] [PubMed] [Google Scholar]
- Uccella S, La Rosa S, Genasetti A, Capella C. Localization of inhibin/activin subunits in normal pituitary and in pituitary adenomas. Pituitary. 2000;3:131–139. doi: 10.1023/a:1011431123208. [DOI] [PubMed] [Google Scholar]
- Winston JT, Koepp DM, Zhu C, Elledge SJ, Harper JW. A family of mammalian F-box proteins. Curr. Biol. 1999;9:1180–1182. doi: 10.1016/S0960-9822(00)80021-4. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137:5463–5467. doi: 10.1210/endo.137.12.8940372. [DOI] [PubMed] [Google Scholar]
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]