Abstract
The ability to retain information in working memory (WM) requires not only the active maintenance of information about specific items, but also the temporal order in which the items appeared. Although many studies have investigated the neural mechanisms of item maintenance, little is known about the neural mechanisms of temporal order maintenance in WM. Here, we used electroencephalography (EEG) to compare neural oscillations during WM tasks that required maintenance of item or temporal order information. Behavioral results revealed that accuracy and reaction times were comparable between the two conditions, suggesting that task difficulty was matched between the item and temporal order WM tasks. EEG analyses indicated that theta (5–7 Hz) oscillations over prefrontal sites were increased during temporal order maintenance, whereas alpha oscillations (9–12 Hz) over posterior parietal and lateral occipital sites were increased during item maintenance. The frontal theta enhancement was primarily evident in high performers on the order WM task, whereas the posterior alpha enhancement was primarily evident in high performers on the item WM task. These results support the idea that frontal theta and posterior alpha oscillations are differentially related to maintenance of item and temporal order information.
Introduction
Working memory (WM) processes support the active maintenance of information so that it can be manipulated or quickly accessed at a later time (Baddeley, 1986, 2003). Numerous studies have reported persistent activity in brain regions such as lateral prefrontal cortex (PFC) (Fuster and Alexander, 1971; Kubota and Niki, 1971; Kojima and Goldman-Rakic, 1982; Miller et al., 1996; Courtney et al., 1998) and parietal cortex (Gnadt and Andersen, 1988; Koch and Fuster, 1989; Snyder et al., 1997; D'Esposito et al., 1999; Curtis et al., 2004; Todd and Marois, 2004) during the maintenance of objects or spatial locations. Little is known, however, about how the temporal order of items in WM is maintained (but see Marshuetz et al., 2000; Amiez and Petrides, 2007). Some models suggest that neural oscillations may facilitate the maintenance of temporal sequence information (Lisman and Idiart, 1995; Jensen, 2006; Lisman and Buzsáki, 2008; Vogel and Fukuda, 2009) although experimental support for these ideas remains to be fully established.
Previous studies have shown that oscillatory power in the theta band (4–8 Hz) over prefrontal regions (Gevins et al., 1997; Jensen and Tesche, 2002; Meltzer et al., 2007, 2008) and in the alpha band (8–12 Hz) over posterior sites (Jensen et al., 2002; Schack and Klimesch, 2002; Tuladhar et al., 2007; Sauseng et al., 2009; Scheeringa et al., 2009) is correlated with WM load. These findings are intriguing because as WM load is increased, the number of temporal order relationships among items in WM also increases, and these relationships might be incidentally maintained (Hasher and Zacks, 1979; Miyashita, 1988; Mangels, 1997). Thus, it is possible that theta and alpha modulations with WM load are related to maintenance of item or temporal order information. No previous study, to our knowledge, has investigated the role of neural oscillations in human temporal order maintenance, and the extent to which item and temporal order maintenance share overlapping or distinct neural signatures remains unclear (Marshuetz and Smith, 2006).
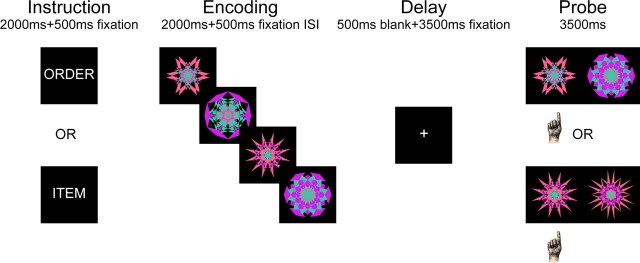
Here, we used electroencephalography (EEG) to directly compare neural oscillations associated with the active maintenance of temporal order information (order trials) with those associated with the maintenance of item information (item trials) (Fig. 1). Both trial types consisted of a memory set of four kaleidoscope images, followed by a 4 s retention delay, and then a probe display consisting of two kaleidoscope images. On item trials, participants decided which of the two kaleidoscope images was presented in the memory set; on order trials, participants decided which of two kaleidoscope images had been presented earlier in the memory set. To ensure that task difficulty on item trials would be comparable to difficulty on order trials, the foil kaleidoscope images on item trials were selected to be highly similar to the corresponding target (Fig. 1). To investigate the role of theta and alpha oscillations in WM maintenance, we examined differences in oscillatory activity during the delay period between item and order trials.
Figure 1.
Schematic diagram of the working memory tasks.
Materials and Methods
Participants.
Thirty-one healthy undergraduate students from the University of California at Davis were recruited in this study. One subject withdrew from the experiment in the middle of the test. Data from four subjects were excluded due to poor memory performance (i.e., <60% accuracy in either the item or order condition) and data from five subjects were discarded due to excessive artifacts in the recorded EEG. Of the remaining 21 subjects, 11 were females. The study was approved by the Institutional Review Board at the University of California at Davis. Written informed consent was obtained from each subject before the experiment.
Stimuli and design.
Stimuli were kaleidoscope images created by overlying three opaque hexagons of different colors, with each hexagon undergoing three rounds of side bisection and random deflection (Voss et al., 2008; Voss and Paller, 2009). Stimuli on each WM trial consisted of four kaleidoscope images with the same three constituent colors but different bisections and deflections in each hexagon. Visually similar foils on the probes of item WM trials were created by using the same three colors and deflecting each color-matched hexagon at similar random angles (<10° difference). By using these special sets of kaleidoscope images, we aimed to match the task difficulty between the two types of WM trials.
Procedure.
Figure 1 illustrates the sequence of events during a WM trial. The experimental paradigm, which was adapted from Amiez and Petrides (2007), consisted of two types of WM trials: item trials and order trials. On each WM trial, participants first saw an instruction word, either “ITEM” or “ORDER”, on the screen for 2000 ms, followed by a fixation cross for 500 ms. After the fixation cross, four kaleidoscope images sequentially appeared on the screen for 2000 ms, each with a 500 ms interstimulus interval. The four kaleidoscope images were then followed by a delay of 4000 ms, after which a test display appeared on the screen for 3500 ms. On order trials, the test display consisted of two kaleidoscope images from the previous sequence, and participants were asked to identify which kaleidoscope image came earlier in the sequence by pressing the appropriate response key. The two kaleidoscope images in the test display were randomly determined by the experimental program on a trial-by-trial basis. On item trials, the test display was comprised of one previously presented kaleidoscope image along with another visually similar foil kaleidoscope image that was not in the sequence. Participants were instructed to identify the old kaleidoscope image on item trials by pressing the appropriate response key. The target kaleidoscope image in the test display of an item trial was randomly chosen from the sequence of kaleidoscope images on a trial-by-trial basis.
Although participants could, in principle, maintain both visual details about each image and temporal order information on both item and order trials, the tasks were sufficiently demanding that it would be very difficult to do so and still perform well on both tasks. Indeed, our pilot testing revealed that some participants tried to do this and, as a result, performed particularly poorly on item trials. We therefore carefully instructed participants to emphasize maintenance of visual details on item trials, as the probes on these trials would require a very detailed memory of each image. Thus, participants were instructed to maintain different aspects of the memory set based on the instruction provided on each WM trial. To ensure that they understood the instructions, participants completed a practice session consisting of four item and four order trials. Following the practice section, participants completed a total of five testing blocks, each comprised of a random sequence of 10 order trials intermixed with 10 item trials. Thus, across the entire experiment, each participant completed 50 order and 50 item trials. Between testing blocks, there was a self-paced short break. Because the kaleidoscope images used in the experiment were meaningless abstract objects, participants were encouraged to focus on the visual details of kaleidoscope images instead of verbalizing them. Post-test debriefing indicated that participants were able to follow the instructions and had not attempted to verbalize the kaleidoscope images.
Electroencephalographic recordings.
The EEG was recorded from 64 silver/silver chloride electrodes mounted in an elastic cap using an ActiveTwo EEG recording system (Biosemi). The positioning of 64 electrodes was in accordance with an extended version of the international 10/20 system (Jasper, 1958). Additional electrodes were placed on the mastoids. The horizontal electrooculogram (EOG) was recorded from electrodes placed to the outer canthi. The vertical EOG was recorded from electrodes above and below the left eye. The EEG and EOG were recorded continuously at 1024 Hz and digitally high-pass filtered at 0.16 Hz and low-pass filtered at 100 Hz.
Data analysis.
We focused our EEG analyses on correct trials only. All data analysis was performed off-line using EEGLAB toolbox (Delorme and Makeig, 2004) and custom MATLAB (MathWorks) scripts. The continuous EEG data were down-sampled into 512 Hz, re-referenced to the average of the right and left mastoids, and high-pass filtered at 0.5 Hz. EEG epochs were extracted for each epoch extending from −1 s preceding to 20 s following the onset of trial instructions (i.e., “ITEM” or “ORDER”) to encompass the entire duration of a WM trial. Each EEG epoch was then baseline-corrected by subtracting the mean voltage before trial onset. To remove eye movement artifacts, the fastICA algorithm (Hyvärinen and Oja, 2000) implemented in EEGLAB toolbox (Delorme and Makeig, 2004) was applied to all EEG epochs. Independent component analysis components associated with horizontal and vertical eye movements were visually identified and removed according to their scalp maps and activity profile (Jung et al., 2000a,b). EEG epochs were then subjected to an artifact rejection procedure in which epochs with amplitude exceeding ±100 μV were discarded.
EEG spectral power was computed by convolving single-trial EEG epochs from each scalp electrode with six cycle complex Morlet wavelets (i.e., mf0σt = 6) (Roach and Mathalon, 2008). Oscillatory power, defined as the square of the modulus of the resulting complex number, was then averaged across trials and log-transformed. To specifically look at neural oscillations during WM maintenance, oscillatory power during the delay was extracted and normalized with respect to oscillatory power during the time window (−150–0 ms) before the onset of trial instruction (i.e., “ORDER” or “ITEM”). We also conducted another set of analyses in which oscillatory power during the delay was not baseline-corrected. The pattern of results was essentially unchanged, so we report the analyses of baseline-corrected data here.
Oscillatory power during the delay was binned into two frequency bands (theta, 5–7 Hz; alpha, 9–12 Hz). The theta and alpha frequency bands defined here were slightly different from the typically defined theta (4–8 Hz) and alpha (8–12 Hz) frequency bands (Gevins et al., 1997; Jensen et al., 2002; Howard et al., 2003; Meltzer et al., 2007). This is because, as a result of frequency smearing after time-frequency decomposition, using the standard, broader frequency bands would reduce our ability to distinguish between oscillatory effects in the theta and alpha frequency bands. We therefore narrowed our theta and alpha frequencies to best characterize neural oscillations at those frequency bands.
Analyses presented here focused on differences in oscillatory power during the delay period of correct item trials compared with the delay period of correct order trials. The time window for these analyses was 600–3400 ms after the onset of the delay to avoid contamination of delay period activity estimates by oscillatory activity during the encoding and probe phases (i.e., due to the inherent temporal imprecision of wavelet analyses). Therefore, only oscillatory effects during the middle part of the maintenance period were subjected to further statistical analyses.
Statistical analyses were made using repeated-measures ANOVA for oscillatory power averaged over latency intervals and electrode clusters. When appropriate, Greenhouse–Geisser correction (Greenhouse and Geisser, 1959) for violation of the sphericity assumption was used to adjust degrees of freedom. Only significant effects involving item and order conditions are reported. Topographic EEG power analyses were conducted by grouping electrodes into nine clusters (frontal vs central vs posterior × left vs middle vs right): middle-frontal cluster (F1, Fz, F2), left-frontal cluster (AF7, F5, F7), right-frontal cluster (AF8, F6, F8), middle-central cluster (Cz, C1, C2), left-central cluster (C3, C5, T7), right-central cluster (C4, C6, T8), middle-posterior cluster (Oz, O1, O2), left-posterior cluster (PO7, P7, P9), and right-posterior cluster (PO8, P8, P10). A significant interaction between conditions of interests (i.e., item and order) and frontal-central-posterior or left-middle-right electrode clusters would suggest that the oscillatory effects are not equally distributed across the scalp. This analysis strategy was used to determine the scalp distributions of theta and alpha oscillations.
Surface Laplacian transformation.
Scalp surface Laplacian estimates were computed using CSD (Current Source Density) Toolbox (Version 1.1) (Kayser and Tenke, 2006a,b). The density of the transcranial current flow was estimated through a surface Laplacian transformation (second spatial derivative) that yields reference-free scalp current density estimates for the direction, location, and intensity of scalp EEG. Single-trial EEG epochs were transformed into reference-free scalp current density estimates using the spherical spline surface Laplacian algorithm (Perrin et al., 1989, 1990) with m-constant = 4 and smoothing constant λ = 10−6. The surface Laplacian-transformed single-trial EEG epochs were then submitted to the time-frequency decomposition procedures mentioned above to estimate oscillatory power over theta and alpha frequency bands. The surface Laplacian transformation minimizes volume-conducted contributions from distant brain regions and, therefore, provides EEG topographies with more sharply localized peaks than those of the scalp potential (Nunez et al., 1994; Tenke and Kayser, 2005; Nunez and Srinivasan, 2006). Therefore, topographic maps of theta and alpha power computed from surface Laplacian transformed EEG were used to complement topographic maps of theta and alpha power calculated from raw EEG to estimate superficial neocortical sources of theta and alpha oscillations.
Results
Behavioral performance
Accuracy was high on both order (74.19% correct; SD, 9.26%) and item (75.62% correct; SD, 7.55%) trials and no significant between-condition differences were observed (t(20) = 0.64, p > 0.50). A 2 × 2 (order–item × correct–incorrect) ANOVA of reaction times revealed that responses were faster on correct (mean, 2355.12 ms; SD, 524.15) than on incorrect (mean, 2638.56 ms; SD, 717.39) trials (F(1,20) = 17.44, p < 0.001). However, there was no significant main effect between order (mean, 2474.53 ms; SD, 619.50) and item (mean, 2513.72 ms; SD, 648.17) conditions (F(1,20) = 0.49, p > 0.49), nor was there a significant condition-by-accuracy interaction (F(1,20) = 1.74, p > 0.20). Overall, the behavioral results suggest that task difficulty was matched between the two conditions. To further examine the relationship between memory performance on item and on order trials, we calculated the correlation between accuracy on the two trial types across subjects. This analysis revealed no significant correlation between them (r(19) = 0.32, p > 0.15), suggesting that, at least to some extent, performance on these tasks was driven by different factors.
EEG analyses
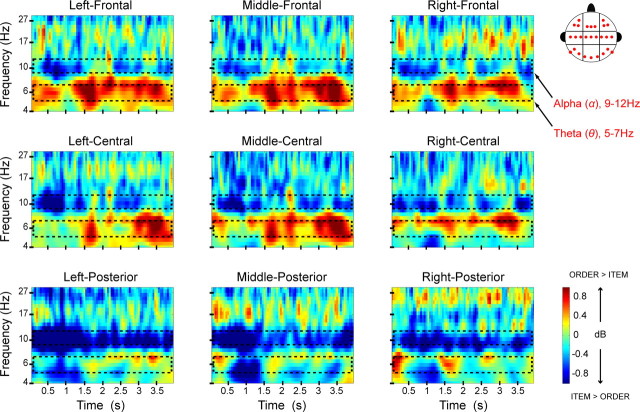
To compare neural activity associated with the maintenance of item and temporal order information, we tested for differences in oscillatory power during the delay period between correct item and correct order trials. We first examined neural oscillations associated with the maintenance of temporal order information by comparing oscillatory power elicited by correct order trials with those elicited by correct item trials in the time window of 600–3400 ms after the onset of the delay. The time window was chosen to minimize the contribution of activity related to stimulus encoding at the beginning and probe-related activity at the end of the delay. As shown in Figure 2, order trials elicited sustained theta (5–7 Hz) power compared with item trials during the maintenance period. The enhancement of theta power during the delay period of order trials was most pronounced over frontal electrode sites (Figs. 2, 3A). This was confirmed statistically in an ANOVA that revealed a significant electrode region-(frontal vs central vs posterior)-by-condition (order vs item) interaction (F(1.2,23.2) = 4.31, p < 0.05) on theta power during the delay.
Figure 2.
Frontal theta is enhanced during order maintenance, whereas posterior alpha is enhanced during item maintenance. Time-frequency spectrograms illustrate the difference in oscillatory power between correct order and correct item trials. The x-axis represents time relative to the onset of the 4 s delay period and the y-axis represents logarithmically spaced frequencies. Effects are separately plotted for each of the nine analyzed electrode clusters. Hotter colors denote relative increases in oscillatory power during order trials; cool colors denote relative increases in oscillatory power during item trials.
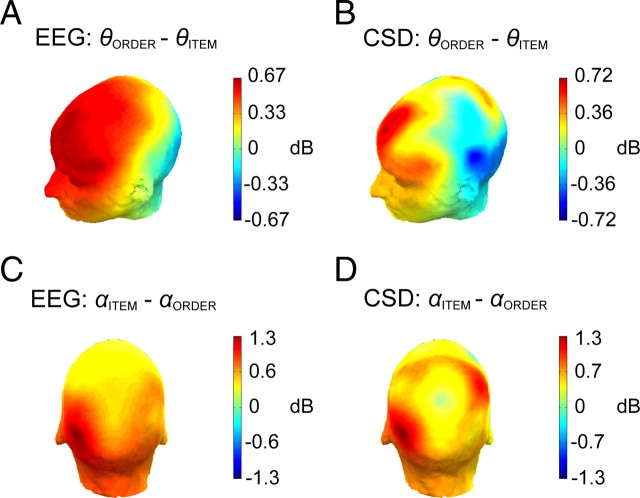
Figure 3.
Topography of frontal theta and posterior alpha effects. A, Topographic map of the difference in oscillatory power between correct order and correct item trials in theta (θ) frequency band (5–7 Hz) in the time window of 600–3400 ms following onset of the delay. B, The same as in A, except that oscillatory power is computed from surface Laplacian-transformed single-trial EEG epochs. C, Topographic map of the difference in oscillatory power between correct item and correct order trials in the alpha (α) frequency band (9–12 Hz) in the time window of 600–3400 ms during the delay. D, The same as in C, except that oscillatory power is computed from surface Laplacian-transformed single-trial EEG epochs. CSD, Current source density.
To identify possible cortical sources of the frontal theta activity, we conducted time-frequency analysis of surface Laplacian-transformed single-trial EEG epochs. The surface Laplacian transformation allows for an estimation of scalp current density (Tenke and Kayser, 2005), which effectively eliminates the contribution of deep and broadly distributed superficial sources (Nunez et al., 1994; Nunez and Srinivasan, 2006), thereby enhancing the relative contributions of sources located in superficial gyral surfaces of the neocortex. Consistent with the raw EEG analysis, the scalp current density analysis revealed an enhancement of frontal theta power effect in the time window of 600–3400 ms during the delay (Fig. 3B). The topography of the theta enhancement is consistent with sources in the medial and lateral PFC.
Figure 2 also shows that item trials elicited stronger alpha (9–12 Hz) power than order trials did during the delay period. An ANOVA on data from the nine electrode clusters confirmed an overall enhancement of alpha power on item trials (F(1,20) = 4.17, p = 0.055). However, this was qualified by a significant electrode region (frontal vs central vs posterior)-by-condition (item vs order) interaction (F(1.8,36.3) = 3.76, p < 0.05), suggesting that the alpha effect was more pronounced over posterior than over frontal electrode sites (Fig. 3C). In addition, a subsidiary ANOVA on data from only the posterior electrode clusters (i.e., P9, P7, PO7; O1, Oz, O2; P8, P10, PO8) revealed a marginal condition-by-hemisphere interaction (F(1.8,36.1) = 2.54, p = 0.098), suggesting that the posterior alpha effect was slightly lateralized to the left hemisphere.
As with the frontal theta effect, we were also interested in identifying the possible superficial cortical sources of the posterior alpha effect. We computed oscillatory power from surface Laplacian-transformed single-trial EEG epochs and found that the alpha power enhancement in delay period on item trials was maximal over posterior and posterior-central electrode sites (Fig. 3D). The topography of these effects is consistent with sources in left posterior parietal and lateral occipital cortex.
Working memory performance and neural oscillations
The analyses described above show that frontal theta oscillations were enhanced during the delay period of order trials, whereas posterior alpha oscillations were enhanced during item trials. We next conducted analyses to determine whether these oscillatory effects were related to the ability to maintain temporal order and item information in WM. In these analyses, we focused on data from middle-frontal (i.e., Fz, F1, F2) and left-posterior (i.e., P7, P9, PO7) electrode clusters, which showed that frontal theta and posterior alpha effects were maximally overlapping across analyses of raw (Fig. 3A,C) and surface Laplacian-transformed (Fig. 3B,D) EEG data during the delay period (600–3400 ms).
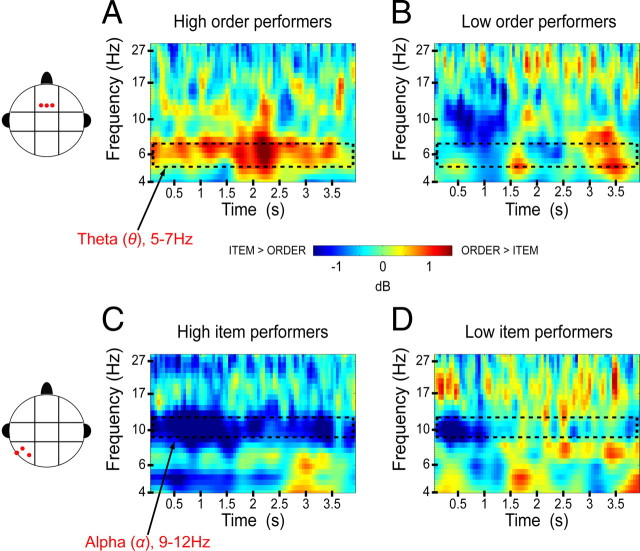
To examine whether the frontal theta and posterior alpha oscillations were related to behavioral performance on order trials, we performed a median-split analysis in which the subjects were divided into two groups based on their accuracy on order trials. We then assessed the extent to which the frontal theta and posterior alpha effects (i.e., theta and alpha power differences between item and order trials) were reliable for both low and high order performers. However, before proceeding to statistical tests, we visually inspected the frontal theta and posterior alpha effects across the 21 subjects and found that there was an outlier whose frontal theta effect was ∼4 SDs above the mean (mean, 0.71 dB; SD, 1.49 dB). Although inclusion or exclusion of the outlier participant did not affect analyses of overall power differences between item and order trials, initial analyses suggested that this subject added noise to the median-split analysis of the frontal theta effect. The outlier was thus excluded from the analysis, which resulted in 10 high order performers and 10 low order performers. Figure 4, A and B, illustrate the frontal theta effect separately for high order and low order performer groups. As is evident in the figure, the frontal theta effect was not significant for participants in the low order performer group (t(9) = 1.53, p > 0.16) but was significant for the high order performer group (t(9) = 2.45, p < 0.05), suggesting that frontal theta oscillations were related to the maintenance of temporal order information. With respect to alpha oscillations in high and low order performers, statistical analyses showed that there was a marginal alpha effect for high order performers (t(9) = 1.97, p > 0.08) but, surprisingly, the alpha effect was significant for low order performers (t(9) = 2.41, p < 0.05), suggesting that frontal theta and posterior alpha effects were in different directions between the high and low order performer groups.
Figure 4.
Theta and alpha effects for high and low performers on the working memory tasks. A, Averaged spectrogram of the order − item power difference for the top 10 order performers at the middle-frontal electrode cluster. B, Averaged spectrogram for the bottom 10 order performers, showing the same contrast and electrode cluster as in A. C, Averaged spectrogram of the order − item power difference for the top 10 item performers at the left-posterior electrode cluster. D, Averaged spectrogram for the bottom 10 item performers, showing the same contrast and electrode cluster as in C.
We next conducted a median-split analysis based on performance on item trials (10 high performers and 10 low performers) to determine the extent to which posterior alpha and frontal theta oscillations were related to maintenance of item information. As shown in Figure 4C, the posterior alpha effect was only reliable for high item performers (t(9) = 3.83, p < 0.01), not low item performers (t(9) = 0.97, p > 0.35) (Fig. 4D), suggesting that alpha oscillations were associated with the maintenance of item information in WM. In contrast, the theta effect was not significant for high item performers (t(9) = 0.14, p > 0.89) and, surprisingly, was significant for low item performers (t(9) = 8.95, p < 0.001).
Overall, the median-split analyses indicated that high order performers showed enhanced frontal theta oscillations on order trials, whereas low order performers showed enhanced posterior alpha oscillations on item trials. Conversely, high item performers showed enhanced posterior alpha oscillations on item trials, whereas low item performers showed enhanced frontal theta oscillations on order trials. These results seem to suggest that there was a negative relationship between frontal theta and posterior alpha oscillations during WM maintenance. To confirm this impression, we ran a correlation analysis in which we quantified the relationship between frontal theta and posterior alpha effects at time windows where these effects were found to be maximal (frontal theta, 1700–3400 ms during the delay; posterior alpha, 600–1700 ms during the delay) (Fig. 2). We found that frontal theta oscillations were negatively correlated with posterior alpha oscillations (r(18) = −0.48, p < 0.05) (Fig. 5). Thus, subjects who showed larger theta enhancements on order trials showed smaller alpha enhancements on item trials, and vice versa. These findings suggest that there may be a tradeoff between the processes associated with frontal theta and posterior alpha oscillations.
Figure 5.
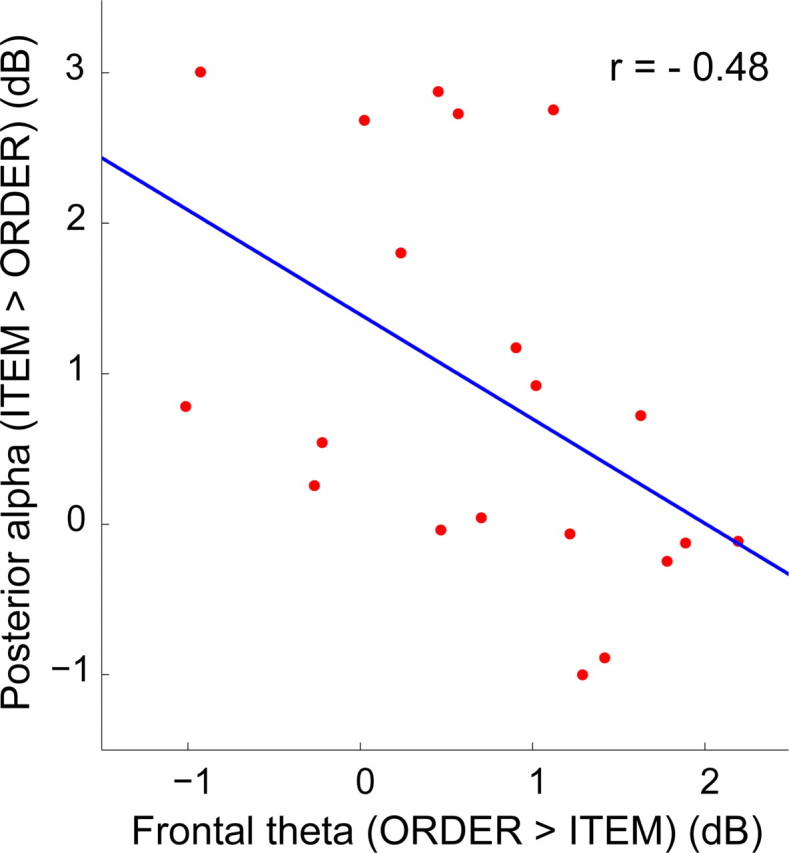
Frontal theta enhancements during order maintenance are negatively correlated with posterior alpha enhancements during item maintenance. The scatter plot depicts individual differences in the alpha power enhancement during item trials at the left-posterior electrode cluster (y-axis) relative to the theta enhancement at the middle-frontal electrode cluster during order trials (x-axis). Pearson product-moment correlation coefficient, r = −0.48. Linear least-squares best fits to the data are superimposed.
Discussion
The current study is, to our knowledge, the first to contrast electrophysiological activity in humans during active maintenance of item and temporal order information in WM. We found evidence for a functional distinction between frontal theta and posterior alpha oscillations with respect to the maintenance of temporal order and item information. Theta power at frontal sites was enhanced during temporal order maintenance, and this effect was robust for high order performers. In contrast, alpha power at posterior sites was enhanced during item maintenance, and this effect was robust for high item performers. Importantly, the comparable behavioral performance between order and item conditions suggests that the observed oscillatory effects cannot be attributed to differences in task difficulty. Moreover, the negative correlation between frontal theta and posterior alpha, as well as the results from the median-split analyses, suggest that theta and alpha oscillations might be associated with the engagement of different kinds of processing that support the maintenance of item versus temporal order information.
Theta oscillations and maintenance of temporal sequences
Consistent with previous reports showing frontal theta enhancement during WM tasks (Gevins et al., 1997; Jensen and Tesche, 2002; Meltzer et al., 2007, 2008), we observed a frontally distributed increase in theta power during the delay period of order trials. Analyses of current density waveforms revealed that the topography of the theta effect was consistent with cortical sources in the medial and lateral PFC. This finding converges with results showing that dorsolateral and medial PFC contribute to the generation of frontal midline theta in monkeys (Tsujimoto et al., 2006) and with results showing that PFC lesions in monkeys (Petrides, 1991), rats (Kesner and Holbrook, 1987) and humans (Petrides and Milner, 1982; Shimamura et al., 1990; McAndrews and Milner, 1991; Milner et al., 1991; Kesner et al., 1994; Mangels, 1997) are associated with impaired temporal order memory. Results from some monkey single-unit recording studies also suggest PFC involvement in maintenance of temporal sequences (Ninokura et al., 2003, 2004; Siegel et al., 2009), including one study that showed phase locking of neuronal spiking to low-frequency oscillations (Siegel et al., 2009). These results suggest that neural oscillations in PFC might play a role in facilitating the maintenance of temporal order information.
Evidence from fMRI studies also suggests a role for the PFC in long-term memory for temporal sequences (Cabeza et al., 1997; Rajah et al., 2008; Jenkins and Ranganath, 2010; Tubridy and Davachi, 2011), but few fMRI studies have investigated WM for temporal order (Marshuetz et al., 2000; Marshuetz and Smith, 2006; Amiez and Petrides, 2007). Notably, in the fMRI study from which the current behavioral paradigm was adapted, Amiez and Petrides (2007) found that medial and dorsolateral PFC activation was enhanced during the encoding and retrieval of temporal WM. The present results extend their findings by demonstrating that the PFC contributes to temporal order maintenance and that theta oscillations play a role in this process.
Although there is evidence for the generation of theta oscillations in PFC (Tsujimoto et al., 2006), the neural mechanism that gives rise to these oscillations is unclear. Electrophysiological studies in rats (Buzsáki, 2002) and humans (Ekstrom et al., 2005, 2007) have shown that theta oscillations are prominent in the hippocampus, and these oscillations may play a role in coordinating interactions between the hippocampus and PFC (Hyman et al., 2005; Jones and Wilson, 2005; Siapas et al., 2005; Benchenane et al., 2010; Sigurdsson et al., 2010). In light of evidence suggesting a role for the hippocampus in sequence representation (Fortin et al., 2002; Kesner et al., 2002), and evidence for relatively unidirectional connections from the hippocampus to PFC (Rosene and Van Hoesen, 1977; Swanson, 1981; Goldman-Rakic et al., 1984; Ferino et al., 1987; Jay et al., 1989; Sesack et al., 1989), it is possible that the hippocampus might play a role in driving prefrontal theta oscillations (Anderson et al., 2010). Further research will be needed to test this hypothesis.
Alpha oscillations and maintenance of item information
Alpha oscillations are often interpreted to reflect cortical idling (Pfurtscheller et al., 1996), but several studies have shown that posterior alpha power increases with the number of items maintained in WM (Jensen et al., 2002; Schack and Klimesch, 2002). Furthermore, a recent study showed that effects of repetitive transcranial magnetic stimulation (rTMS) on alpha oscillations were positively correlated with behavioral performance on an object WM task (Hamidi et al., 2009).
Our data also suggest that alpha oscillations are related to successful maintenance of item information—participants with high performance on item trials showed significant alpha effects, whereas low item performers did not. This finding might indicate that alpha oscillations reflected neural processes that preserved the fidelity of object representations during the memory delay. Notably, the use of similar foils in the probes for item trials required participants to distinctly maintain each individual object and inhibit visual interference during the delay. It is therefore possible that posterior alpha oscillations played a role in the active maintenance of visual details (which would contribute more to item than to order trials), or, alternatively, that alpha enhancement during item trials reflected the increased need to inhibit visual processing (Jensen et al., 2002; van Dijk et al., 2010) during the delay (so as not to disrupt the actively maintained object representations).
A study by Sauseng et al. (2009) provided support for the inhibition account of alpha oscillations. In their study, participants actively maintained visual objects presented in one hemifield while ignoring objects presented in the other hemifield. During WM maintenance, alpha power enhancement was higher over posterior sites ipsilateral to the hemifield corresponding to retained memory items than over contralateral sites, suggesting that posterior alpha oscillations were associated with inhibiting information from the irrelevant hemifield. Moreover, Sauseng et al. (2009) applied 10 Hz repetitive rTMS, thought to entrain alpha oscillations (Klimesch et al., 2003; Thut and Miniussi, 2009), over posterior brain regions during WM delay and found that ipsilateral rTMS increased WM performance, whereas contralateral rTMS elicited the reverse pattern. These findings are consistent with the idea that alpha oscillations are related to inhibiting irrelevant visual stimuli during WM maintenance. It is therefore possible that the alpha enhancement during item trials reflected the need to inhibit visual interference during the delay.
As in numerous previous studies, the current study showed prominent alpha oscillations over posterior sites. Surface Laplacian analyses were consistent with alpha sources in posterior parietal and lateral occipital regions. The locus of the posterior alpha effect is consistent with monkey studies showing that occipital visual areas contribute to the generation of alpha oscillations (Bollimunta et al., 2008; Mo et al., 2011), with analyses of alpha generators during WM maintenance (Tuladhar et al., 2007), and with fMRI studies showing intraparietal and intraoccipital sulci (IPS/IOS) activation during visual WM tasks (Todd and Marois, 2004, 2005). Moreover, Todd and Marois (2005) found a positive correlation between WM capacity (Vogel and Machizawa, 2004) and IPS/IOS activation during the encoding, maintenance, and retrieval phases of a visual WM task. In light of our results showing prominent posterior alpha enhancement in high item performers and a study (Sauseng et al., 2009) demonstrating a correlation between posterior alpha and WM capacity, it is possible that the posterior alpha effect is associated with WM capacity.
Negative relationship between frontal theta and posterior alpha oscillations
Separate analysis of data from high and low performers revealed that frontal theta enhancement was prominent in high order and low item performers, whereas posterior alpha enhancement was associated with high item and low order performers. Furthermore, there was a significant negative correlation between frontal theta and posterior alpha effects. Putting together these results, we speculate that the dynamics between frontal theta and posterior alpha may reflect a tradeoff between different strategies that favor either the maintenance of item or temporal order information. Some participants may adopt strategies emphasizing the maintenance of temporal order information, and they would be expected to perform well on order trials and show a significant frontal theta effect. Other participants, who placed relatively more emphasis on the maintenance of visual details, would be expected to perform well on item trials and exhibit a significant posterior alpha effect. These findings suggest that, although both theta and alpha oscillations are related to WM maintenance (Jensen and Tesche, 2002; Jensen et al., 2002), they are differentially related to processes that support maintenance of order and item information. One potential area for future investigation, motivated by our findings, would be to examine whether artificially induced (e.g., rTMS or transcranial direct current stimulation) oscillations at theta or alpha frequency bands modulate intrinsic neural oscillations and, consequently, influence the maintenance of temporal order and item information, respectively, in WM.
Footnotes
This work was supported by National Institutes of Health Grant R01 MH068721. This paper is dedicated to the memory of Dr. Edward G. Jones. We gratefully acknowledge Dr. Joel Voss for sharing kaleidoscope stimuli with us. We also thank each of the reviewers for their helpful comments and suggestions.
References
- Amiez C, Petrides M. Selective involvement of the mid-dorsolateral prefrontal cortex in the coding of the serial order of visual stimuli in working memory. Proc Natl Acad Sci U S A. 2007;104:13786–13791. doi: 10.1073/pnas.0706220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex. 2010;20:1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford UP: 1986. [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal–prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci. 2008;28:9976–9988. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, Tulving E. Brain regions differentially involved in remembering what and when: a PET study. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Ekstrom A, Viskontas I, Kahana M, Jacobs J, Upchurch K, Bookheimer S, Fried I. Contrasting roles of neural firing rate and local field potentials in human memory. Hippocampus. 2007;17:606–617. doi: 10.1002/hipo.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to the medial prefrontal cortex in the rat. Exp Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive transcranial magnetic stimulation affects behavior by biasing endogenous cortical oscillations. Front Integr Neurosci. 2009;3:14. doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol Gen. 1979;108:356–388. [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Oja E. Independent component analysis: algorithms and applications. Neural Netw. 2000;13:411–430. doi: 10.1016/s0893-6080(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 1989;505:337–340. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O. Maintenance of multiple working memory items by temporal segmentation. Neuroscience. 2006;139:237–249. doi: 10.1016/j.neuroscience.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000a;37:163–178. [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol. 2000b;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns. I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006a;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns. II. Adequacy of low-density estimates. Clin Neurophysiol. 2006b;117:369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Holbrook T. Dissociation of item and order spatial memory in rats following medial prefrontal cortex lesions. Neuropsychologia. 1987;25:653–664. doi: 10.1016/0028-3932(87)90056-x. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO, Fineman B. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32:881–891. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17:1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- Koch KW, Fuster JM. Unit activity in monkey parietal cortex related to haptic perception and temporary memory. Exp Brain Res. 1989;76:292–306. doi: 10.1007/BF00247889. [DOI] [PubMed] [Google Scholar]
- Kojima S, Goldman-Rakic PS. Delay-related activity of prefrontal neurons in rhesus monkeys performing delayed response. Brain Res. 1982;248:43–49. doi: 10.1016/0006-8993(82)91145-3. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34:337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE. Working memory for order information: multiple cognitive and neural mechanisms. Neuroscience. 2006;139:195–200. doi: 10.1016/j.neuroscience.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE, Jonides J, DeGutis J, Chenevert TL. Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. J Cogn Neurosci. 2000;12(Suppl 2):130–144. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- McAndrews MP, Milner B. The frontal cortex and memory for temporal order. Neuropsychologia. 1991;29:849–859. doi: 10.1016/0028-3932(91)90051-9. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Mayes LC, Constable RT. Individual differences in EEG theta and alpha dynamics during working memory correlate with fMRI responses across subjects. Clin Neurophysiol. 2007;118:2419–2436. doi: 10.1016/j.clinph.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, Spencer DD, Constable RT. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex. 2008;18:1843–1855. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgements. Neuropsychologia. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Miyashita Y. Neuronal correlate of visual associative long-term memory in the primate temporal cortex. Nature. 1988;335:817–820. doi: 10.1038/335817a0. [DOI] [PubMed] [Google Scholar]
- Mo J, Schroeder CE, Ding M. Attentional modulation of alpha oscillations in macaque inferotemporal cortex. J Neurosci. 2011;31:878–882. doi: 10.1523/JNEUROSCI.5295-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninokura Y, Mushiake H, Tanji J. Representation of the temporal order of visual objects in the primate lateral prefrontal cortex. J Neurophysiol. 2003;89:2868–2873. doi: 10.1152/jn.00647.2002. [DOI] [PubMed] [Google Scholar]
- Ninokura Y, Mushiake H, Tanji J. Integration of temporal order and object information in the monkey lateral prefrontal cortex. J Neurophysiol. 2004;91:555–560. doi: 10.1152/jn.00694.2003. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electrical fields of the brain. New York: Oxford UP; 2006. [Google Scholar]
- Nunez PL, Silberstein RB, Cadusch PJ, Wijesinghe RS, Westdorp AF, Srinivasan R. A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalogr Clin Neurophysiol. 1994;90:40–57. doi: 10.1016/0013-4694(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Corrigenda EEG 02274. Electroencephalogr Clin Neurophysiol. 1990;76:565. [Google Scholar]
- Petrides M. Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc Biol Sci. 1991;246:299–306. doi: 10.1098/rspb.1991.0158. [DOI] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontaland temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band: an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Ames B, D'Esposito M. Prefrontal contributions to domain-general executive control processes during temporal context retrieval. Neuropsychologia. 2008;46:1088–1103. doi: 10.1016/j.neuropsychologia.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristic of evoked and oscillatory electroencephalographic activity in a human memory scanning task. Neurosci Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MC. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage. 2009;44:1224–1238. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci U S A. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin Neurophysiol. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci. 2009;13:182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Behav Neurosci. 2005;5:144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J Neurophysiol. 2006;95:2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex. 2011;21:272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O. Parietal-occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp. 2007;28:785–792. doi: 10.1002/hbm.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, van der Werf J, Mazaheri A, Medendorp WP, Jensen O. Modulations in oscillatory activity with amplitude asymmetry can produce cognitively relevant event-related responses. Proc Natl Acad Sci U S A. 2010;107:900–905. doi: 10.1073/pnas.0908821107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Fukuda K. In mind and out of phase. Proc Natl Acad Sci U S A. 2009;106:21017–21018. doi: 10.1073/pnas.0912084107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. An electrophysiological signature of unconscious recognition memory. Nat Neurosci. 2009;12:349–355. doi: 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Baym CL, Paller KA. Accurate forced-choice recognition without awareness of memory retrieval. Learn Mem. 2008;15:454–459. doi: 10.1101/lm.971208. [DOI] [PMC free article] [PubMed] [Google Scholar]