Abstract
Matrix metalloproteinases (MMPs) have long been linked to cancer progression owing to their ability to breakdown tissue barriers for metastatic spread. Accordingly, multiple studies have examined the potential value of these enzymes as targets for cancer therapy. Unfortunately, most clinical trials with MMP inhibitors have yielded negative results which has made necessary to re-evaluate the role of these proteases in cancer. Recent works mainly based on the use of mouse models deficient in specific MMPs have revealed that these enzymes play many roles in cancer distinct from matrix destruction, influencing early steps of tumor evolution, and expanding their pro-tumorigenic properties. However, these in vivo studies have also shown that, unexpectedly, some MMP family members like MMP8 may have paradoxical anti-tumor functions. Nevertheless, the final validation of these MMPs as bona fide tumor suppressors requested the identification of the putative genetic or epigenetic changes underlying their inactivation during cancer development. To this purpose, very recent large-scale genomic studies have explored the possibility that MMPs could be genetically altered in a panel of human malignant tumors from different sources. These studies have demonstrated that MMP8 is a frequently mutated gene in human melanoma. Functional analysis of the identified mutations has confirmed that all of them lead to the loss-of-function of MMP8 and enhance the progression of melanoma, thus providing definitive evidence that MMP8 is a tumor-suppressor gene. Parallel studies have extended these findings to other MMP-related metalloproteinases such as ADAMTS15, which has been found to be genetically inactivated in human colorectal cancer. This review describes the identification and validation of some MMPs and related enzymes as anti-tumor proteases and speculates about the molecular mechanisms underlying their protective roles in tumor development. Finally, the review explores the clinical applications derived from the identification of MMPs that favour the host instead of the tumor.
Keywords: matrix metalloproteinases, MMP-8, tumor suppressor gene, mouse model, somatic mutation
The Place of MMPs in the Human Degradome
Proteases play essential roles in a wide variety of biological processes and are also associated with multiple diseases, including cancer.1 This large and growing functional diversity of proteolytic enzymes derives from the occurrence in all organisms of a high number of proteins with different sizes, shapes and catalytic properties but with the common ability to hydrolyze peptide bonds. Analysis of the human genome sequence has shown that our degradome—the complete set of proteases produced by human cells and tissues—is composed of at least 569 proteases or protease-like proteins classified into five catalytic classes and 68 families. Interestingly, the mouse degradome is even more complex and consists of at least 644 proteases and homologues.2 Among this variety of proteolytic enzymes, the matrix metalloproteinases (MMPs)—encoded by 24 distinct genes in human and 23 genes in mouse—have long been considered as important effectors in cancer progression due to their ability to degrade the main protein components of the extracellular matrix and basement membranes, thereby providing an access for tumor cells to the vascular and lymphatic systems and facilitating the generation of metastasis.
Based on this idea, over the last years there has been a sustained interest in the structural and functional characterization of all members of this family of metalloproteinases.3 MMPs can be classified into four groups according to their domain organization. The archetypal MMPs, including collagenases and stromelysins, contain a signal peptide necessary for secretion, a propeptide involved in the maintenance of enzyme latency, a catalytic domain that binds zinc and a hemopexin C-terminal domain important in determining substrate specificity and interactions with tissue inhibitors of metalloproteases. Matrilysins contain the minimal domain organization that is necessary for secretion, latency and catalytic activity but lack the hemopexin C-terminal domain. Gelatinases incorporate three fibronectin II modules within the catalytic domain which improve collagen and gelatin degradation. Finally, convertase-activatable MMPs have a basic insert in the prodomain that is cleaved by furin-like proteases. Among the MMPs that belong to this group there are secreted enzymes or membrane-bound proteins via GPI (glycosylphosphatidylinositol), type I or type II transmembrane segments. Consistent with this structural diversity, MMPs play multiple functions beyond their direct contribution to the degradation of tissue barriers and influence cell behavior, survival and death in many physiological and pathological processes including cancer.
Links Between MMPs and Cancer
The association between MMPs and cancer can be traced back to the 1970s when several studies identified pro-metastatic activities linked to the presence of members of the MMP family such as gelatinases.4 However, further studies over the next three decades have shown that the role of MMPs in cancer progression is much more complex than that derived from their contribution to the late invasive stages of the tumorigenic process. Thus, we now recognize that MMPs may target growth-factor receptors, cytokines, chemokines, cell adhesion molecules, apoptotic ligands and angiogenic factors and contribute to all stages of tumor progression, including proliferation, adhesion, migration, angiogenesis, senescence, apoptosis and evasion of the immune system.5 This wide functional diversity of MMPs in cancer biology together with the establishment of causal relationships between MMP overexpression in tumor or stromal cells and cancer progression prompted the development of clinical trials with a first generation of inhibitors designed to block the proteolytic activity of these enzymes. However, most of these clinical trials using broad-spectrum MMP inhibitors (MMPIs) for the treatment of patients with advanced cancer have yielded disappointing results.6 There are several putative explanations for the poor outcome of these MMPIs, mostly derived from the previous ignorance about the wide structural and functional complexity of this protease family.7 In fact, just three MMPs (MMP1, MMP2 and MMP3) had been described when the first clinical trials with MMPIs were designed. The subsequent discovery of more than 20 distinct MMPs and the identification of the two related families of metalloproteinases called ADAMs (A Disintegrin And Metalloproteinase) and ADAMTSs (ADAMs with ThromboSpondin domains) which can be also inhibited by MMPIs, has made necessary the re-evaluation of the specificity of the inhibitors used in the clinical trials. Additionally, the finding that MMPs are crucial in the early stages of tumor progression suggests that their contribution might be less relevant once metastasis have been generated, thus minimizing the putative beneficial effects of MMPIs administered to cancer patients with advanced disease. Finally, the lack of positive results with MMPI-based trials could also have been derived from deficiencies in the selection of the specific MMP family members which should be targeted in cancer. Thus, the observation that in some patients treated with broad-spectrum MMPIs there was an apparent exacerbation of tumor growth, suggested the possibility that some MMPs might have paradoxical anti-tumor roles. Further studies based on the generation of genetically modified mouse models of loss-of-MMP function have recently provided definitive evidence on the protective role of some MMPs in cancer.
MMPs with Anti-Tumor Properties
After years of considering MMPs as pro-tumorigenic enzymes, studies with mutant mice deficient in MMP8 demonstrated for the first time the in vivo anti-tumor properties of a member of this protease family.8 MMP8 or collagenase-2 is mainly produced by neutrophils and has been implicated in a variety of inflammatory conditions. The unexpected finding that MMP8 has tumor-defying functions derived from the observation that the absence of this protease in MMP8-null mice strongly increased the incidence of skin tumors in these animals. Bone-marrow transplantation experiments in MMP8-deficient mice revealed that neutrophil-derived MMP8 is sufficient to rescue the anti-tumor protection conferred by this metalloproteinase. Molecular and histopathological studies aimed at evaluating the mechanisms underlying the protective function of MMP8 have indicated that the loss of this protease causes important deficiencies in the inflammatory response induced by chemical carcinogens, finally resulting in a sustained inflammation that creates a favorable microenvironment for cancer progression. Further studies have shown that this protective function of MMP8 is likely linked to its ability to participate in the proteolytic processing of inflammatory mediators rather than to its classical role as an efficient collagenase implicated in the degradation of fibrillar collagens.8,9 Consistent with these studies in mice, experimental manipulation of MMP8 expression levels in human breast cancer cells has demonstrated that downregulation of this protease in non-metastatic cells increases its metastatic potential, whereas its overexpression in metastatic cells substantially reduces this potential.10 Recent experiments using MMP8-null mice and syngenic melanoma and lung carcinoma cells overexpressing MMP8 have extended these findings and demonstrated that this metalloproteinase functions as a metastasis suppressor through the modulation of tumor cell adhesion and invasion.11 Finally, analysis of MMP8 expression in human breast carcinomas has revealed that the presence of this enzyme correlates with a lower incidence of metastasis and confers a good prognosis to breast cancer patients. It is also remarkable that similar findings have been reported for patients with oral carcinomas.12 Likewise, and also consistent with the above observations, a high-expression allele of the MMP8 gene is associated with lower susceptibility to metastasis and better survival in breast cancer patients.13
Parallel studies have shown that other MMP family members distinct from MMP8, including MMP12 (macrophage metalloelastase) and MMP26 (matri-lysin-2) can also develop anti-tumor functions in different stages of cancer progression.14–16 Furthermore, other MMPs such as MMP3 (stromelysin-1), MMP9 (gelatinase-B), MMP11 (stromelysin-3) and MMP19, which were originally described as protumorigenic enzymes, appear to play dual roles in cancer and exhibit protective roles in some specific circumstances (reviewed in ref. 17).
Taken collectively, the above findings have provided strong support to the idea that there are several MMPs with the ability to negatively control some aspects of cancer progression, thus favoring the host instead of the tumor. The occurrence of anti-tumor MMPs has also provided some explanations for the lack of success of clinical trials based on the use of a first generation of broad-spectrum MMPIs for cancer therapy. Nevertheless, these observations are still preliminary in many aspects and further studies are necessary to elucidate the molecular mechanisms underlying the cancer-defying functions of the anti-tumor MMPs. Likewise, it is essential to clarify the putative genetic or epigenetic alterations which must undergo these MMPs during tumor progression in order to be validated as bona fide tumor-suppressor genes. Fortunately, very recent studies derived from the availability of the results from large-scale analysis of cancer genomes, have shed light on this important question.
High Throughput Sequencing of Gene Families in Human Cancer
The above discoveries suggested that MMPs might be genetically altered in human cancer. One way to definitely implicate a gene to human cancer is to discover tumor-specific mutations in the gene and to evaluate the functional effects of those mutations. In order to do this, the genes of interest are sequenced from a panel of tumors and the sequence is compared to the reference sequence. DNA from the patient’s constitutional DNA is then analyzed to determine whether the variant in the tumor arose specifically within the tumor, i.e., whether the change is somatic.
In the past, large scale mutational analysis of candidate genes was technically limited. Several important advances have changed this. These include our ability to establish high quality tumor banks, created either through generation of early passage tumor cell lines or xenografts or through selective capture or microdissection of tumor cells. This allows the detection of somatic mutations that are otherwise masked by neighboring normal tissue. In addition, the completion of the human genome sequence coupled with vast improvement in automated methods for large-scale sequence analysis of specific loci have allowed for rapid and robust sequence analysis.18–21 The combination of these advances has now created an opportunity for systematically identifying somatic mutations in human cancers and evaluating the roles of such mutated genes in tumorigenesis.
MMPs are Somatically Mutated in Human Cancer
In the past, it was stated that the expression of MMPs in tumors was secondary to transcriptional changes rather than genetic alterations.5 At that time, there were only two reported genetic alterations in cancers, which were the translocation of the MMP23 genes in neuroblastoma22 and amplification of the MMP24 gene.23 A large systemic analysis of genetic alterations in human breast and colorectal cancers recently uncovered numerous mutations in genes that were originally not associated with cancer. In this study, approximately 22% of the candidate cancer (CAN) genes were involved in cell adhesion, which includes the metalloproteinase family.18 This gave perspective and guidance in how further cancers should be analyzed genetically.
Recently MMPs have been further investigated in melanoma and been shown to have a significant genetic impact on MMPs correlation with cancer.24 As an initial screen to evaluate whether MMPs are genetically altered in melanoma, the coding exons of all MMP members were analyzed in a panel of 32 tumors. Sequences from these regions were PCR amplified and directly sequenced from tumor DNA. Every identified genetic alteration was checked in the matched normal DNA in order to determine whether it was somatic. Every MMP gene that was found to have one somatic mutation or more was evaluated in an additional 48 melanoma samples.
From this analysis a total of 28 somatic mutations in eight MMP genes were identified, involving 23% of the melanoma tumors analyzed. In order to determine whether the alterations are passenger mutations, which are biologically neutral and do not affect the growth of the cancer cell, or driver mutations which are involved in the development of the cancer and have been positively selected over time, it is important to test if the ratio of nonsynonymous:synonymous (NS:S) alterations is significantly higher than the NS:S ratio predicted for non selected passenger mutations. The NS:S alterations detected in the melanoma MMP screen was 28:5, which was significantly higher than the NS:S ratio of 2:1 predicted for nonselected passenger mutations, which led to the conclusion that these are driver mutations. The mutations also had a significant ratio of C:G > T:A transitions, which was noted as melanoma signature.25 MMP8 and MMP27 were the most frequently mutated genes noted in these samples.24 Interestingly, both of these MMPs are on the same chromosome 11 and they both have an identical protein domain structure. Ninety percent of somatic mutations occur in one allele and dominantly develop the cancer. Ten percent of the somatic mutations need both alleles to cause the protein to dysfunction and cause the progression of cancer; these act in a recessive manner and are known as tumor suppressor genes.25 MMP8 was further explored since it was one of the most frequently mutated MMP genes (found in 6.3% of melanoma samples), most of its mutations were accompanied by a loss of heterozygosity and because it has been previously shown to protect against skin tumor development;8 all suggesting that MMP8 is a tumor suppressor gene.
Functional Effects of Wild Type MMP8 and MMP8 Mutations
MMP8 (collagenase-2) belongs to the group of extracellular proteases and has the ability to degrade the extracellular matrix.26 Thus, it has been shown to have excellent capabilities of degrading fibrillar collagens.27 However, MMP8 has many other substrates as well such as other extracellular matrix proteins, proteases, cell adhesion proteins, protease inhibitors, growth factors and chemokines.
Previous in vivo studies characterizing the effects of MMP8 on melanoma metastasis had shown that overexpression of wild type MMP8 in melanoma cells reduces lung metastasis formation. The mechanism for the reduced metastasis was found to be through increased adhesion of cells expressing wild type MMP8 to type I collagen and laminin-1 when compared with control cells. In addition, tumor cells overexpressing wild type MMP8 were shown to have a substantially reduced invasive capability through Matrigel when compared with control cells,11 again suggesting MMP8 to be a tumor suppressor gene.
The nature of the mutations identified in MMP8 described in the previous section also suggested MMP8 to be a tumor suppressor gene. However, in order to confirm this, functional evaluation of the mutations was necessary. To determine the biochemical consequences of the mutations, wild type MMP8 or five of the mutations were overexpressed HEK 293T cells and their matrix metalloproteinase activity was evaluated. Expression of all the MMP8 mutants resulted in significantly less metalloproteinase activity compared to wild type protein. The biological effects of the various MMP8 mutations were then tested. When growth assays on plastic were performed, melanoma cells expressing wild type or mutant MMP8 grew similarly. However, when growth on soft agar was evaluated, wild type MMP8 substantially inhibited the number of colonies that formed. Similarly, when MMP8 was knocked down in a melanoma cell line that expressed wild type MMP8, an increase in anchorage independent growth was seen, thus phenocopying the overexpression results. In vivo analysis of the MMP8 mutations involved subcutaneous injection of melanoma cells overexpressing either wild type or mutant MMP8 into NOD/SCID mice. Mice that were injected with wild type clones had no local tumor ulcerations or lung metastases; however, mice injected with cells overexpressing the various MMP8 mutations developed tumor ulcerations and numerous tumor lung invasions.24 These compelling findings conclude that MMP8 has a protective role, while mutations in MMP8 enhance the progression of melanoma. A summary of the roles that wild type and mutant MMP8 have in tumorigenesis is summarized in Figure 1.
Figure 1.
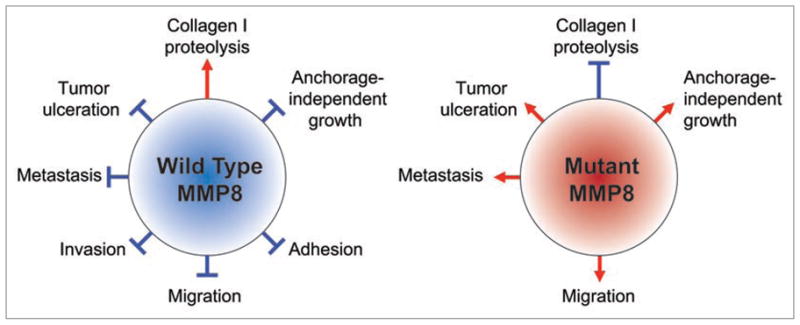
Functional effects of wild type and mutant MMP8. Wild type MMP8 shows tumor suppressive activities by maintaining its proteolytic activity and inhibiting various stages of tumorigenesis, such as migration and metastasis. In contrast, mutant MMP8 loses its proteolytic ability and enhances tumorigenic phenotypes.
Further Genetic Analyses
There has been undeniable evidence showing that the genetic mutations of MMP8 have a significant effect on the functional activity of the protein and that it is a tumor suppressor. It is indeed warranted based on this systematic genetic study that MMPs are genetically evaluated in additional cancer types. In addition, to extend our knowledge of fundamental roles of proteases in cancer progression, a complementary systematic sequence analysis of all ADAMs and the related ADAMTS family would be a worthwhile endeavor. Similar to MMPs, ADAM and ADAMTS belong to a superfamily of zinc-based proteinases, the metzincins.28 Many members of the ADAM and ADAMTS families have been associated with tumorigenesis and tumor progression. The exploration has already begun. Four somatic mutations have been discovered in ADAMTS15 in 50 colorectal cancer samples.29 Functional analysis of these mutations has revealed that knockdown of this protease in colorectal cancer cells promotes in vitro and in vivo tumor growth and invasion, and overexpression of wild type ADAMTS15 reverses these phenotypes. In addition, in vivo analysis has shown that ADAMTS15 knockdown markedly promotes in vivo tumor growth. Thus, functional evaluation of this protease has revealed yet another tumor suppressor gene. It is also remarkable that several ADAMTS genes including ADAMTS1, ADAMTS9, ADAMTS12 and ADAMTS18 have been identified as epigenetically silenced genes in several human carcinomas30–33 further validating the concept that different members of this family of secreted metalloproteinases are inactivated in cancer and may function as anti-tumor proteases.
Clinical Implications
Understanding the molecular changes that arise during cancer development is critical to the development of rationally designed diagnostic and treatment strategies. This is currently the practice with several different types of cancers such as the subclassification of acute myeloid leukemia and the treatment of chronic myeloid leukemia34 and gastrointestinal stromal tumors35 by using drug therapy to inhibit the mutated genes. As mentioned, in the past, MMPs were associated with cancer metastasis5,36 and small molecule inhibitors of MMPs were tested as potential anticancer agents. However, clinical trials using these inhibitors showed no effect and, occasionally, accelerated tumor growth6,17,37 suggesting that an in-depth analysis of the specific role of individual MMPs in particular cancer types is necessary. The studies described above for MMP8 and ADAMTS15 together with previous observations on the anti-tumorigenic properties of different proteases17 have identified anti-targets for diagnostic and therapeutic intervention. Thus, the MMP inhibitors used in clinical trials were possibly inhibiting the antitumor effects of certain MMPs. Recognizing that particular extracellular proteases play a protective role in the development of cancer can change the treatment of malignancies. Perhaps restoring the protective nature of certain MMPs or increasing their proteolytic activity in tumors with a particular MMP mutation or deficiency can hinder the progression of the cancer. This new era in which the sequence of the human genome has been delineated, coupled with advances in high-throughput DNA analyses have created an opportunity to more precisely characterize genetically the human genome and in this case the degradome.38 Genetic, functional and structural evaluation of the roles of mutated proteases in tumorigenesis will allow the discrimination between proteases that promote cancer development and proteases that inhibit it. It is reasonable to expect that such studies can lead to the design of novel inhibitors that are specific to particular proteolytic enzymes leading to individualized medicine and to improved disease management.
Conclusions and Future Work
With the use of high throughput DNA sequencing, over the next couple of years, there can potentially be a revolutionary change in the science of cancer. This has been already accomplished for ADAMTS15 in colorectal cancer and the MMP family in melanoma, and through sequencing and functional analysis, new tumor suppressors have been discovered. This can be advanced and applied to the search for mutations of MMPs and other extracellular proteases in various cancers. The detection of these mutations will initiate further studies in the understanding of the role of these mutations and these particular genes in cancers. Although MMP8 and ADAMT15 are now well established as tumor suppressors, there is much speculation as of their roles in the progression of cancer. Further studies need to be done on these proteins in order to understand the mechanism of their protective roles. There still needs to be additional research done in this area to get a better understanding of these proteases before it can be used in the clinical practice. Nonetheless, the research is heading in the right direction and this will help to answer the questions of the complex nature of cancer, possible roles of various genes in cancer, and new insight into the treatment of malignancies.
Acknowledgments
This work was supported by grants from Ministerio de Ciencia e Innovación-Spain, Fundación “M. Botín”, the European Union FP7-MicroEnviMet (to C.L.-O.) and the Intramural Research Programs of the National Human Genome Research Institute, National Institutes of Health, USA (to Y.S.).
References
- 1.Lopez-Otin C, Bond JS. Proteases: multifunctional enzymes in life and disease. J Biol Chem. 2008;283:30433–7. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quesada V, Ordonez GR, Sanchez LM, Puente XS, Lopez-Otin C. The Degradome database: mammalian proteases and diseases of proteolysis. Nucleic Acids Res. 2009;37:239–43. doi: 10.1093/nar/gkn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochimica et Biophysica Acta. 2009 doi: 10.1016/j.bbamcr.2009.07.004. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–8. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 5.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 7.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–72. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 8.Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–7. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) Faseb J. 2007;21:2580–91. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montel V, Kleeman J, Agarwal D, Spinella D, Kawai K, Tarin D. Altered metastatic behavior of human breast cancer cells after experimental manipulation of matrix metalloproteinase 8 gene expression. Cancer Res. 2004;64:1687–94. doi: 10.1158/0008-5472.can-03-2047. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez-Fernandez A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–63. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- 12.Korpi JT, Kervinen V, Maklin H, Vaananen A, Lahtinen M, Laara E, et al. Collagenase-2 (matrix metalloproteinase-8) plays a protective role in tongue cancer. Br J Cancer. 2008;98:766–75. doi: 10.1038/sj.bjc.6604239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decock J, Long JR, Laxton RC, Shu XO, Hodgkinson C, Hendrickx W, et al. Association of matrix metalloproteinase-8 gene variation with breast cancer prognosis. Cancer Res. 2007;67:10214–21. doi: 10.1158/0008-5472.CAN-07-1683. [DOI] [PubMed] [Google Scholar]
- 14.Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, et al. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–75. doi: 10.1158/0008-5472.CAN-05-4279. [DOI] [PubMed] [Google Scholar]
- 15.Savinov AY, Remacle AG, Golubkov VS, Krajewska M, Kennedy S, Duffy MJ, et al. Matrix metalloproteinase 26 proteolysis of the NH2-terminal domain of the estrogen receptor beta correlates with the survival of breast cancer patients. Cancer Res. 2006;66:2716–24. doi: 10.1158/0008-5472.CAN-05-3592. [DOI] [PubMed] [Google Scholar]
- 16.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, et al. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–55. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumor suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 18.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 19.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, et al. The Genomic Landscapes of Human Breast and Colorectal Cancers. Science. 2007 doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 20.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gururajan R, Lahti JM, Grenet J, Easton J, Gruber I, Ambros PF, et al. Duplication of a genomic region containing the Cdc2L1-2 and MMP21-22 genes on human chromosome 1p36. 3 and their linkage to D1Z2. Genome Res. 1998;8:929–39. doi: 10.1101/gr.8.9.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llano E, Pendas AM, Freije JP, Nakano A, Knauper V, Murphy G, et al. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase a overexpressed in brain tumors. Cancer Res. 1999;59:2570–6. [PubMed] [Google Scholar]
- 24.Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41:518–20. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 27.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–50. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 28.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viloria CG, Obaya AJ, Moncada-Pazos A, Llamazares M, Astudillo A, Capella G, et al. Genetic inactivation of ADAMTS15 metalloprotease in human colorectal cancer. Cancer Res. 2009;69:4926–34. doi: 10.1158/0008-5472.CAN-08-4155. [DOI] [PubMed] [Google Scholar]
- 30.Lind GE, Kleivi K, Meling GI, Teixeira MR, Thiis-Evensen E, Rognum TO, et al. ADAMTS1, CRABP1 and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell Oncol. 2006;28:259–72. doi: 10.1155/2006/949506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo PH, Leung AC, Kwok CY, Cheung WS, Ko JM, Yang LC, et al. Identification of a tumor suppressive critical region mapping to 3p14. 2 in esophageal squamous cell carcinoma and studies of a candidate tumor suppressor gene, ADAMTS9. Oncogene. 2007;26:148–57. doi: 10.1038/sj.onc.1209767. [DOI] [PubMed] [Google Scholar]
- 32.Jin H, Wang X, Ying J, Wong AH, Li H, Lee KY, et al. Epigenetic identification of ADAMTS18 as a novel 16q23. 1 tumor suppressor frequently silenced in esophageal, nasopharyngeal and multiple other carcinomas. Oncogene. 2007;26:7490–8. doi: 10.1038/sj.onc.1210559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moncada-Pazos A, Obaya AJ, Fraga MF, Viloria CG, Capella G, Gausachs M, et al. The ADAMTS12 metalloprotease gene is epigenetically silenced in tumor cells and transcriptionally activated in the stroma during progression of colon cancer. J Cell Sci. 2009;122:2906–13. doi: 10.1242/jcs.050468. [DOI] [PubMed] [Google Scholar]
- 34.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–17. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 35.Sleijfer S, Wiemer E, Verweij J. Drug Insight: gastrointestinal stromal tumors (GIST)—the solid tumor model for cancer-specific treatment. Nat Clin Pract Oncol. 2008;5:102–11. doi: 10.1038/ncponc1037. [DOI] [PubMed] [Google Scholar]
- 36.Freije JM, Balbin M, Pendas AM, Sanchez LM, Puente XS, Lopez-Otin C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003;532:91–107. doi: 10.1007/978-1-4615-0081-0_9. [DOI] [PubMed] [Google Scholar]
- 37.Overall CM, Kleifeld O. Tumor microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Otin C, Overall CM. Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol. 2002;3:509–19. doi: 10.1038/nrm858. [DOI] [PubMed] [Google Scholar]


