Abstract
A neonatal mouse model of intermittent hypoxia (IH) simulating the recurring hypoxia/reoxygenation episodes of apnea of prematurity (AOP) was developed. C57BL/6 P2 pups were culled for exposure to either intermittent hypoxia or intermittent air as control. The IH paradigms consisted of alternation cycles of 20.9% O2 and either 8.0% or 5.7% O2 every 120 or 140 seconds for 6 hours a day during daylight hours from day 2 to day 10 postnatally, i.e., roughly equivalent to human brain development in the perinatal period. IH exposures elicited modest to severe decrease in oxygen saturation along with bradycardia in neonatal mice, which were severity-dependent. Hypomyelination in both central and peripheral nervous systems was observed despite the absence of visible growth retardation. The neonatal mouse model of IH in this study partially fulfills the current diagnostic criteria with features of AOP, and provides opportunities to reproduce in rodents some of the pathophysiological changes associated with this disorder, such as alterations in myelination.
Keywords: mouse model, intermittent hypoxia, infantile apnea, apnea of prematurity, white matter
1. INTRODUCTION
Preterm birth occurs in about 12% of all pregnancies in the United States, when defined as any birth occurring at <37 weeks of postconceptional age. Premature infants are at greater risk for short-term and long-term complications, including disabilities and impediments in growth and mental development. Apnea of prematurity (AOP) correlates with a higher occurrence in preterm infants due to the disturbed control of breathing, leading to apnea, intermittent hypoxia, and bradycardia. The frequency and severity of AOP has been linked to a variety of adverse outcomes including abnormal myelin, synaptic connections, and mental development (Abu-Shaweesh and Martin, 2008, Janvier et al., 2004).
In an effort to further elucidate the role of AOP in brain development it becomes imperative to develop an animal model, the latter then enabling exploration of mechanisms of morbidity, feasibility and validity of therapeutic interventions aiming to ameliorate outcomes in premature babies. The ‘perfect’ mock model is assumed to simulate the pathogenesis of the apneic disorder, manifest the clinical features, and result in similar consequences as those occurring in human disease; another requirement would dictate that the technical process of implementing such model in mammals should be simple and readily feasible. To date however, no single animal model of sleep apnea (SA) has satisfied all of those stipulated requirements. A more realistic and useful alternative approach has consisted in development of multiple partial models, each of which would address a subset of the features characteristically encountered in SA. Among those partial models, rodent models of intermittent hypoxia (IH) during sleep have been widely employed (Table 1), not only because hypoxia/reoxygenation events play a crucial role in producing most of the morbid outcomes in patients, but also for the technical simplicity of implementing IH, and the additional advantage of enabling use of transgenic approaches for functional analyses. Accordingly, an extensive amount of work spanning the last decade has shown that such partial rodent models of sleep apnea-associated reproduce many of the features occurring in SA: IH during sleep, sleep disturbances, and sleep fragmentation. Importantly, most of the known end-organ morbidities observed in patients with SA were also reproduced in this model, including alterations in respiratory control, hypertension, insulin resistance, metabolic syndrome, cardiovascular impairments, atherosclerosis, erectile dysfunction, and neurocognitive and behavioral disturbances. However, animal models to mock those clinical features as seen in AOP, which occur during a critical brain development period, have not well been developed.
Table 1.
Murine models of intermittent hypoxia in pathophysiological study of sleep apnea.
| Stage | Experimental procedure | Feature(s) | Limitation | Developed by |
|---|---|---|---|---|
| adult and aged | intermittent O2 concentration changes | IH feature | only IH feature, hypocapnia, no intrathoracic swing | Gozal D, 2001; Tagaito Y, 2001; Veasey SC, 2004 (most applied) |
| Intermittent ventilatory arrest | IH feature and hypercapnia | sedated /anesthetized, invasive procedure, short-term IH, no intrathoracic swing | Wu Y, 2007 | |
| intermittent O2 concentration changes | IH feature and hypercapnia | only IH feature, no intrathoracic swing | Kanagy NL, 2001 | |
| upper airway collapse/reopening | IH feature, hypercapnia and intrathoracic swing | sedated /anesthetized, invasive procedure, short-term IH | Farré R, 2003 | |
| intermittent airway occlusion | IH feature, hypercapnia and intrathoracic swing | restraint stress | Farré R, 2007 | |
| Intermittent snout obstruction | IH feature, hypercapnia and intrathoracic swing | sedated /anesthetized, short-term IH | Carreras A, 2011 | |
| sleep disturbance and intermittent O2 concentration | IH feature and sleep deprivation/restriction | Uncorrected IH and SD events, no intrathoracic swing | Perry JC, 2007 | |
| neonatal | intermittent O2/CO2 concentration changes | IH feature | only IH feature, no intrathoracic swing maternal effects | Douglas RM, 2010 |
| intermittent O2 concentration changes | IH feature | only in Feature, hypocapnia no intrathoracic swing maternal effects | Fan C, 2005; Pawar A, 2008 | |
| gestational | intermittent O2 concentration changes | IH feature | only IH feature, hypocapnia no intrathoracic swing maternal effects | Gozal D, 2003 |
In this study, we aimed to establish a neonatal mouse model of intermittent hypoxia that would mimic the hypoxia/reoxygenation events in the developing “premature” mammal. The mouse was therefore selected due to the unique immaturity of the brain at birth, equivalent to that of the 30-week gestation newborn human (Hagberg et al., 2002; Marret et al., 1995). Furthermore, specific precautions were implemented to avoid some of the potential confounders of IH. This model of neonatal IH-exposed mouse manifested a modest to severe decrease in oxygen saturation that was accompanied by slow heart rate, and was ultimately associated with hypomyelination in the nervous system.
2. MATERIALS AND METHODS
2.1 Animals
P2 wild-type C57BL/6 pups and nursing dam(s) (Jackson Laboratory, Bar Harbor, ME) were housed in an isolated room equipped with computer-controlled hypoxic chambers. All procedures were supervised by the UofL Research Resources Center - an AALAC approved facility and performed in accordance with the guidelines of the Animal Care and Use Committee of University of Louisville School of Medicine, and the NIH requirements for care and use of laboratory animals.
2.2 Short-term neonatal IH exposures
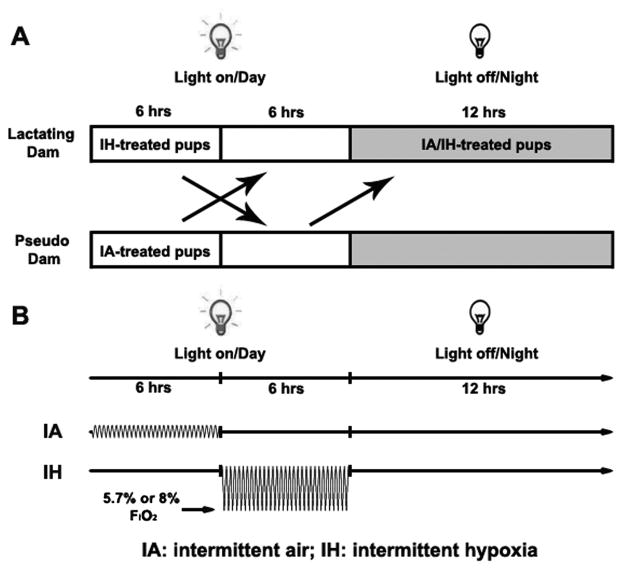
Neonatal C57BL/6 P2 pups with 1.2–1.8g body weight were culled before exposures. Eight pups were divided into two groups–intermittent hypoxia group (IH) and intermittent air control group (IA). Food and water were provided ad libitum within the chamber. The IH paradigm was adopted as alternations of 20.9% O2 and 8.0% or 5.7% O2 leading to 120 s or 140 s cycles (25–30 episodes per hour) for 6 hours a day during daylight hours (Figure 1). The nadir of ambient oxygen level in the chamber was continuously monitored with an oxygen sensor and periodically calibrated via another oxygen analyzer placed at the mouse nose level next to the den area in the cage. The IH exposure window was selected as the period between P2 to P10 neonatal stages, since such period is roughly equivalent to that of human brain development in the perinatal period; just prior to the intense myelination, axonal maturation and synapse formation that occurs immediately thereafter (Hagberg et al., 2002; Marret et al., 1995). The pups under either IA or IH exposure were nursed by a pseudo foster mother in a separate cage with home-cage bedding for 6 hours, and then stayed with their genetic mother for the rest of the day. The non-pregnant pseudo dam had been housed with a lactating dam before the experimentally exposed pups were born and during the 12-hour dark cycle in the days of IA/IH exposures. All animals were assigned to identical custom-designed chambers (15″×16″×16″, Oxycycler model A84XOV, BioSpherix, Lacona, NY) to be operated under 12-hour light-dark cycle (10:00am–10:00pm). Humidity, ambient CO2 (<0.03%) and environmental temperature (26°C) in the chambers were periodically monitored and maintained during exposures. After IH exposures, pups were transferred to room air conditions with the lactating dam until sacrifice for tissue collection.
Figure 1.
Experimental paradigm of a neonatal mouse model of intermittent air (IA) and intermittent hypoxia (IH) exposures. A. Diagram of neonatal groups for IH or IA treatment; B. Schematic profile of IA and IH exposures. FIO2: fraction of inspired oxygen.
2.3 Arterial oxygen saturation, breath and heart rate measurements
Arterial oxygen saturation (SpO2), respiratory rate, and cardiac pulse rate were recorded on unanesthetized P2 to P7 IA- or IH-treated pups using the MouseOx Pulse Oximeter (STARR Life Sciences Corp., Oakmont PA). One sentinel pup from each exposure group was instrumented for two hours and the mouse pup collar clip (XS size for 1–15g body weight) with sensor probe (4 mm) was positioned on the temporal regions of the head. Data were collected before, during and after intermittent hypoxia exposure, and were analyzed with WinDaq Waveform Browser software only during error code-free periods.
2.4 Electron microscopy
Three P10 IH-insulted pups and their IA counterparts were anesthetized by pentobarbital and perfused intracardially with 3% glutaraldehyde in 0.1 M cacodylate buffer (pH7.2) at room temperature. The optic (CNS) and trochlear (PNS) nerves were removed and postfixed in 1% osmium tetroxide in cacodylate buffer. Tissues were rinsed in buffer, dehydrated in graded ethanol and propylene oxide, and embedded in Embed812 epoxy resin (Electron Microscopy Sciences, Fort Washington, PA). Ultrathin sections were prepared from distal ends of the nerves and stained with uranyl acetate/lead citrate. The micrographs were randomly captured from three different fields of the section per animal under a Philips CM10 transmission electron microscope (2–4 optic axons/field, 5–7 trochlear axons/field). The diameters of axons and myelinated fibers were measured via NIH ImageJ software, followed by g-ration (ratio of axonal diameter to fiber diameter) calculation.
2.5 Statistical analyses
All data were shown as mean±SD. The comparisons of three types of exposures (air, 8% O2, 5.7% O2) at different postnatal stages were performed using two-way ANOVA followed by post-hoc tests. Differences were considered as statistically significant for p values <0.05.
3. RESULTS
3.1 Somatic growth of naive, 6-hr daily fast, and 6-hr IA treated newborn pups
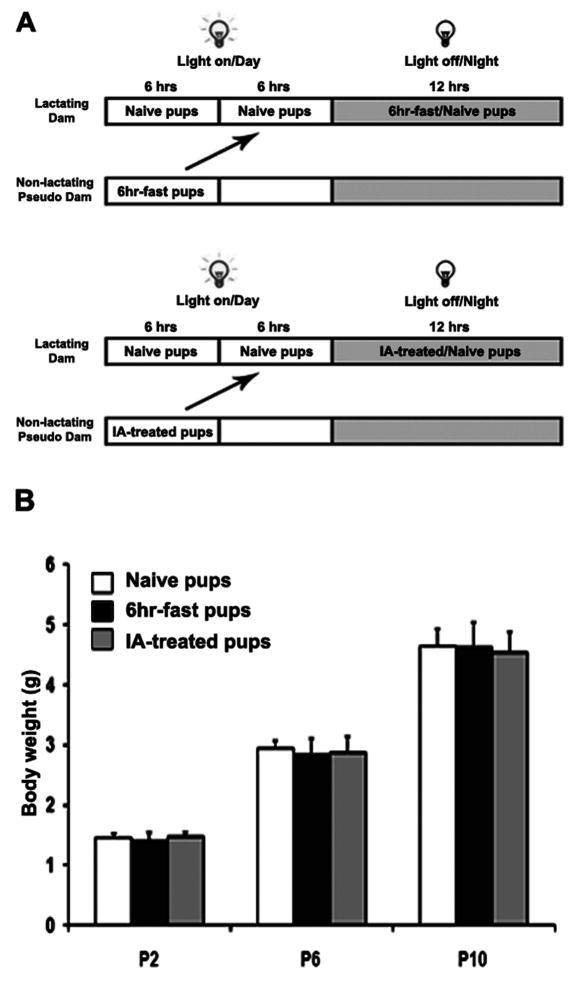
Considering some of the potential confounding factors, including lower lactation capacity and/or nutrients intake due to stress, psychological or airflow noise stress, and fast during the 6-hrs of IA or IH exposures, we compared the body weights of naïve pups with those of pups exposed to 6-hr daily fast, and to those exposed to 6-hr IA exposures (Figure 2A). Significant differences in body weight were observed at the different ages. At the same postnatal stage, there were no significant differences in body weight among the three different treatment groups after 4 days (P6) or 8 days (P10) of treatment (Figure 2B, p>0.05), suggesting that 6-hr daily fast and 6-hr IA exposures for eight days were unlikely to cause malnutrition and growth retardation of pups. Consequently, IA exposures were retained as an adequate control condition for subsequent experiments involving IH exposures.
Figure 2.
Growth in newborn C57BL/6 mice. A. Diagram of experimental procedure for 6-hr daily fast and 6-hr intermittent air exposures; B. Body weights at P2, P6, and P10 in naive (24-hr nursing by lactating dam), 6-hr daily fast, and 6-hr IA treated pups (p>0.05, n=12/each group).
3.2 Effect of intermittent hypoxia on neonatal body and brain weights
Previous studies have shown that gestational intermittent hypoxia leads to restricted fetal and neonatal somatic growth and brain development in rodents (Gozal, et al., 2003; Schwartz et al., 1998). Thus, we examined if neonatal IH would affect somatic and brain weights during development. Compared to IA-treated pups, IH-exposed pups showed slightly less body weight. However, such changes did not reach statistical significance after 8 days of treatment or 20 days of post-exposure, independent of whether 8.0% or 5.7% nadir fraction of inspired oxygen (FIO2) were employed (Figure 3, p>0.05). No differences in brain weight emerged after 8 days exposure to IH cycles and twenty days after cessation of IH exposures (Figure 4, p>0.05). Thus, IH exposures using either 8% or 5.7% FIO2 as the nadir concentration suggest that this exposure paradigm of neonatal IH fails to induce evidence of severe gross retardation in somatic and brain development.
Figure 3.
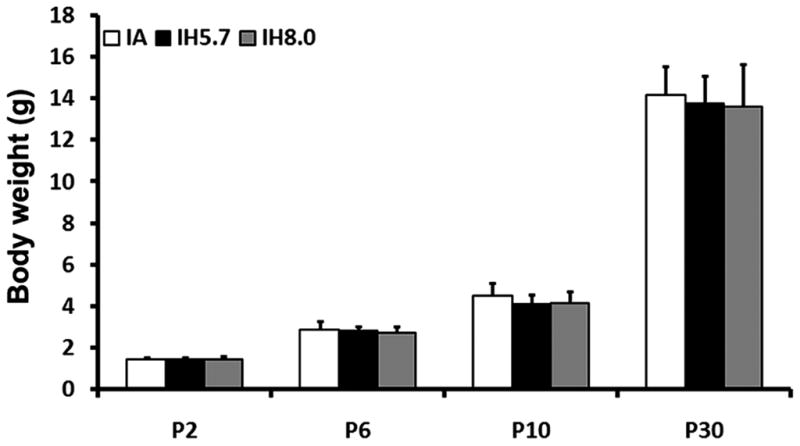
Body weight of neonatal pups after 4-day and 8-day intermittent hypoxia exposures with a nadir of 5.7% FIO2 and 8% FIO2. Body weights were measured at P2, P6, P10, and P30 in 6-hr IA and 6-hr IH treated pups (p>0.05, n=24 for IA groups, n=12 for IH groups).
Figure 4.
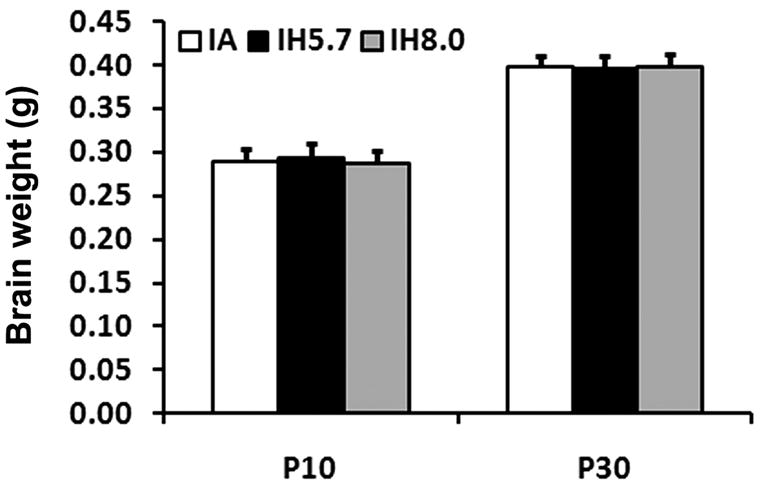
Whole brain weights after 8-day IA or IH (5.7% FIO2 and 8% FIO2) exposures and 20 days later after cessation of exposures (p>0.05, n=12/each group).
3.3 Effect of intermittent hypoxia on neonatal oxygen saturation, heart and breath rates
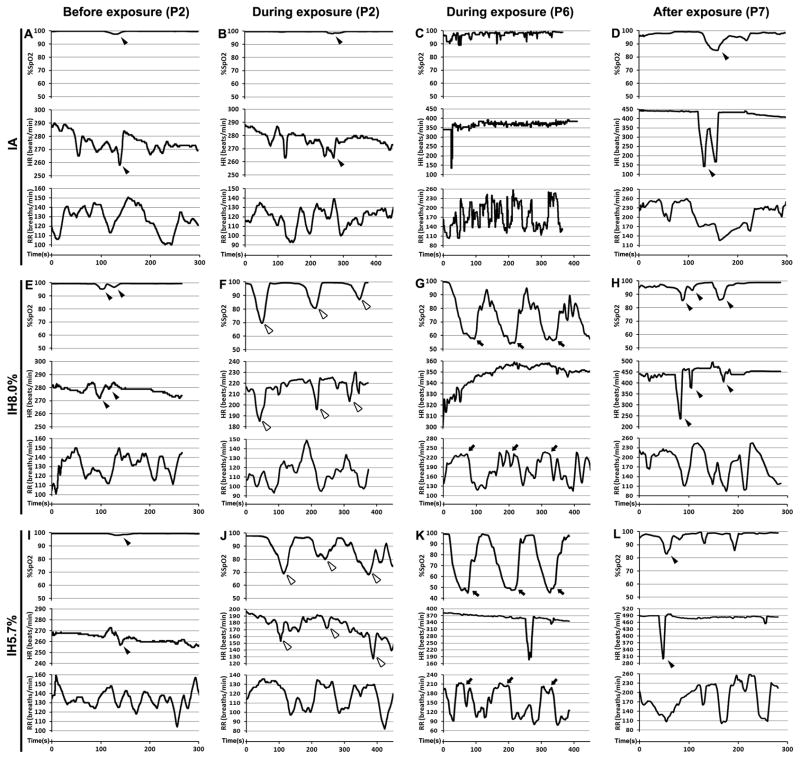
Pulse oximetry, heart and respiratory rates were measured on conscious pups within the chamber before, during, and after IA or IH (5.7% or 8% nadir FIO2) exposures. The resting heart and respiratory rates significantly increased during the development (Figure 5A–D, Table 2). Under the room air or IA exposures, the declined heart beating usually elicited an oxyhemoglobin desaturation on neonatal pups (Figure 5A, B, D, E, H, I, and L; black arrowheads). During the IH exposures, pulse oxyhemoglobin saturation (SpO2) changed in a recurrent manner that paralleled the deoxygenation/reoxygenation cycles (Figure 5F, G, J, and K). Either a modest (8% FIO2) or a more severe (5.7% FIO2) oxygen desaturation returned to normoxic levels similar to IA controls during the 20.9% FIO2 portion of the exposure cycle and after four days exposures (Table 2). Noticeably, heart rate significantly declined during the hypoxic part of the cycle at P2 (Figure 5F and J, white arrowheads) but not at P6 (Figure 5G and K, Table 2). Instead, the breath frequency was increased in response to hypoxia-induced oxyhemoglobin desaturation at P6 (Figure 5G and K, arrows; Table 2). Tachycardia was found in post-hypoxic mice, especially in the IH-exposed group of 5.7% FIO2 (Table 2), while bradycardia episodes occurred sporadically when the respiratory reflex missing (Figure 5D, H and L, black arrowheads).
Figure 5.
Effect of IH on pulse oxygen saturation (SpO2), heart (HR) and respiratory (RR) before, during and after exposures in P2, P6 and P7 neonates. Representative graphs of SpO2, HR, and RR under IA (A–D) and IH (E–L) exposures with a nadir of 8% (E–H) or 5.7% FIO2 (I–L) were shown at lower time scales.
Table 2.
Effect of intermittent hypoxia on neonatal SpO2, HR and RR before, during, and after exposures.
| SpO2, (%) | HR (times/min) | RR (times/min) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before exposure (P2) | During exposure | After exposure (P7) | Before exposure (P2) | During exposure | After exposure (P7) | Before exposure (P2) | During exposure | After exposure (P7) | ||||
| P2 | P6 | P2 | P6 | P2 | P6 | |||||||
| IA | 99.47±0.47 | 99.66±0.40 | 97.30±2.04 | 97.00±2.97 | 275.34±6.49 | 277.16±5.22 | 365.14±23.13 | 402.78±51.68 | 127.77±13.36 | 118.78±10.14 | 132.58±40.00 | 156.94±32.32 |
| IH8.0 | 99.07±0.99 | 79.03±8.85#*,† | 55.87±1.89#* | 95.99±3.71* | 278.42±2.73 | 216.54±8.92*,† | 348.08±10.32 | 442.20±35.03† | 120.59±11.16 | 114.35±12.37 | 185.99±36.42* | 180.29±42.33 |
| IH5.7 | 99.37±0.29 | 71.74±6.89#*,† | 45.83±1.45#* | 96.65±3.56* | 272.14±4.70 | 171.67±16.58*,† | 356.97±32.41 | 478.60±25.22*,† | 122.47±10.00 | 116.28±14.31 | 188.12±42.70* | 181.61±44.70 |
IA: intermittent air; IH: intermittent hypoxia; SpO2: pulse oxygen saturation; HR: heart rate; RR: respiratory rate. Data were collected from 7 mice and shown as mean±SD.
indicates SpO2 values @ the nadirs of F1O2.
represents p<0.05 in the same column comparison.
Represents p<0.05 in the same row comparision.
3.4 Intermittent hypoxia-induced hypomyelination in neonatal mice
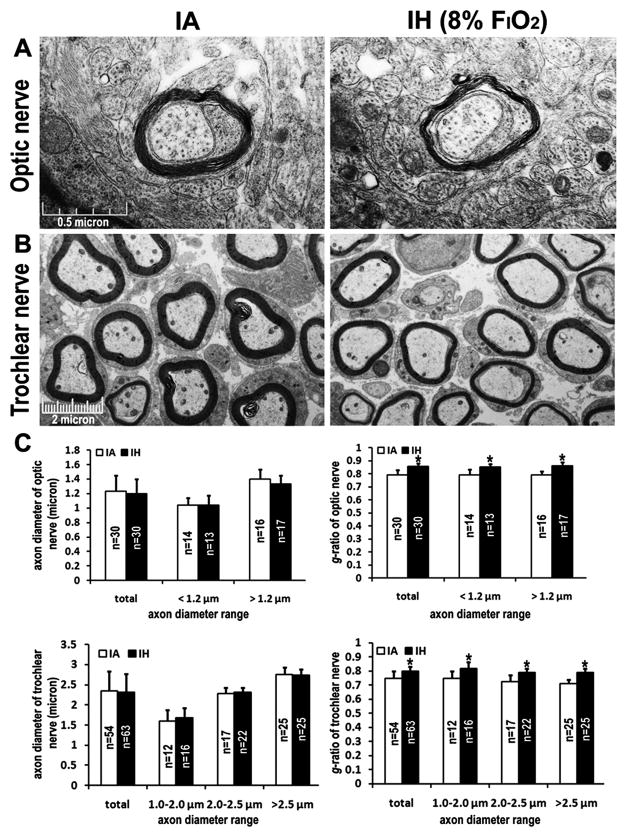
Previous reports of brain imaging studies conducted on adult sleep apnea or congenital central hypoventilation syndrome (CCHS) patients reported extensive axon and myelin alternations in the brain (Macey et al., 2008; Kumar et al., 2008, 2010). Furthermore, peripheral evidence would suggest improved CNS structural development in premature infants who were treated with caffeine for AOP (Doyle et al., 2010). To determine if neonatal IH exposures would cause dysplastic changes in the mouse nervous system, we examined the ultra-structural morphology of the optic nerve and cranial nerves (e.g. trochlear nerve) using electron microscopy (EM) in P10 IH-exposed pups. Such unbiased assessments, which were conducted by an investigator blinded to the nature of the exposure, revealed that IH-exposed axons of optic nerves were myelinated scatteredly and had much thinner myelin lamination than IA-treated fibers (Figure 6A, C, p<0.01). Furthermore, although the size of axons did not differ between IA- and IH-treated groups in the trochlear nerves, the thickness of the myelin sheath relative to the axon diameter was significantly reduced in IH-exposed mice (Figure 6B, C, p<0.01), suggesting that neonatal apnea-associated IH may induce hypomyelination in the developing nervous system.
Figure 6.
Representative electron micrographs of myelin ultra-structure in transverse sections of P10 optic and trochlear nerves under IA or IH (8% FIO2) exposures, respectively. The transverse sections for EM examination were prepared from distal optic nerve (A) and trochlear nerve (B). (C) The average of axon diameters and g-ratios categorized by axon diameter range in IA- and IH-exposed groups. N: the number of total axons randomly captured from three different fields of each section per pup and three pups for each group. Error bar: standard deviation. Scale bar: μm. * represents p<0.01
4. DISCUSSION
In this study, intermittent hypoxia was targeted as the hallmark feature of the model such as to resemble AOP. The severity of the hypoxic stress is dependent on the nadir of FIO2 in the chamber, the duration of FIO2 at the nadir, the number of hypoxic events per hour, the number and timing of hypoxic episodes within a day, and the number of days or weeks of total exposure period. The rodent neonatal models of IH have been reported to adopt 5% (rat) or 11% (mouse) FIO2 with the duration at the nadir from 18 (rat) or 576 (mouse) minutes a day (Fan et al., 2005; Pawar et al., 2008). We set up two hypoxic treatments, 5.7% FIO2 and 8% FIO2, for comparing the different levels of IH impact on gross and neural development. The nadir FIO2 was maintained at 5.7% or 8% for 20 seconds. The paradigm we applied in this study was between 50 to 60 minutes a day, which is much milder than the previous reports. For experimental feasibility and simplicity purposes, we concentrated the hypoxia/reoxygenation episodes into a 6 hours period during daylight. Although there is no consensus regarding the average frequency of apneic events in premature infants, severe events occurring approximately 4–6 times an hour interspersed with more common short apneic episodes (i.e., respiratory pauses > 3 seconds but < 20 seconds without bradycardia and oxygen desaturation) have been observed (Bader et al., 1998; Berterottiere et al., 1990; Henderson-Smart and Steer 2010; Nock et al., 2004). These events may alter carotid body activity and further increase apnea frequency (Al-Matary et al., 2004; Cardot et al., 2007; Nock et al., 2004). In view of these clinical observations and the known higher hypoxic tolerance in mice, we opted for 25~30 hypoxic episodes per hour in our model (Figure 1). Perhaps more importantly, the retained IH profile induced bradycardia and intermittent oxygen desaturation as those found in AOP (Figure 5, Table 2).
The perinatal period is a very susceptible and vulnerable window to environmental perturbations, in which maternal nursing patterns are considered to impose a major adverse impact on long-term development. Exposure of the maternal dam to hypoxic conditions more than two hours a day decreased maternal weight gain and food intake significantly (Schwartz et al., 1998). Repeated episodes of hypoxia and other stressors may also decrease lactation capacity and alter milk composition in the foster dam by activating the hypothalamus-pituitary-adrenal (HPA) axis (Catalani et al., 2010; Ma et al., 2008), where corticosterone is released and then taken by the suckling pups via the milk (Angelucci et al., 1983; Yorty et al., 2004). Additionally, maternal-pup separation and exposure to a new environment can elicit the stress response in both the dam and/or the pups (Moles et al., 2004; Sung et al., 2010). All of the above-mentioned considerations could then result in developmental delay in the offspring, and confound the phenotype elicited by IH. To overcome these limitations, only the original C57BL/6 lactating dam was housed to provide breast milk to the pups under room air conditions (Figure 2). Pup litters were culled to a standard number to ensure adequate nutrition and minimize their somatic growth differences. A female mouse substitute, housing with the lactating dam during the period without IA and IH exposures, acted as a nursing dam to keep mother-offspring interaction in a separate cage containing home-cage bedding when pups were receiving IA or IH treatments, and thus reduce the stress in pups from maternal-pup separation and unfamiliar odor stimuli (pseudo dam and nest). Following these precautionary procedures with our milder IH paradigm, neither body weights (Figure 3), nor brain weights of pups (Figure 4) were significantly reduced in their gross development as a consequence of IH. Nevertheless, the developmental retardation may still occur after a longer period of IH insults due to cumulative effects of hypoxic stress as reported before (Farahani et al., 2008; Soukhova-O’Hare et al., 2006). We should also remark that excessive auditory stimulation may create negative physiologic responses such as apnea, bradycardia, increased blood pressure, changes in oxygen saturation, and alterations in sleep-wake states. However, the rodent auditory system is not mature until P10, since the auditory brainstem response is not yet established or functional (Ehret 1985; Freeman et al., 1999). Therefore, we assumed that the airflow-induced noise in the chambers did not elicit a severe stress response and was not eventually associated with any gross developmental stunt (Figure 3 and 4).
Oxyhemoglobin desaturation and bradycardia are two major signs in AOP. Apena or hypoventilation initially causes a fall in oxygen saturation, which in turn triggers a reflex bradycardia (Adams et al, 1997; Henderson-Smart et al., 1986; Miller and Martin, 1998). The neonatal model of IH in this study demonstrated that both oxygen saturation and heat rate significantly declined in general during IH exposures (Table 2). Recurrent oxygen desaturation was in concert with FIO2 switching (Figure 5F, G, J, and K). At P2, the respiratory center located in the medulla oblongata and respiratory muscles had not fully developed, thus a breathing response to falling oxygen saturation through the peripheral chemoreceptor reflex was still absent or ineffective and induced severe bradycardia events emerging around the nadir of hypoxia/oxygen desaturation episodes (Figure 5F and J, white arrowheads). After four days development, the respiratory frequency was augmented without obvious change in heart rate when oxygen desaturation occurred (Figure 5J and K, arrows), indicating that breathing response is becoming mature and respiratory compensation functions. However, one day later after IH treatment was withdrawn (P7), an accelerated heart beat was observed in IH-treated groups and RR was maintained as the same levels as those in IA controls, suggesting that intermittent hypoxia could elicit long-lasting alterations in cardiac function. Newborn mice show a respiratory pattern of instable breathing similar to that of preterm infants. The number and duration of apneas and periodic breathing episodes are indicators of respiratory instability. Worthy of mention, we selected the C57BL/6 strain in this study for its genetic predisposition of the ventilator instability. Inbred C57BL/6 mice spontaneously exhibit central apneas and post-hypoxic frequency decline and periodic breathing (Han et al., 2002; Stettner et al., 2008), which may compound IH-induced pathophysiologic consequences and lead to a long-term adverse impact on neurodevelopment even after hypoxic insult has been rectified.
Imaging studies have pointed to the occurrence of diffuse injuries in neonatal white matter microstructure in preterm infants (El-Dib et al., 2010; Ment et al., 2009), and multifocal white matter lesions have also been described in CCHS patients (Kumar et al., 2008, 2010). In our mouse model of neonatal IH, hypomyelination was found in both central and peripheral nervous systems of the brain (Figure 6A and B). IH-exposed optic and trochlear nerves had thinner myelin sheaths than the same structures in IA-treated mice without variation of axon size after 8 days exposures (Figure 6C). These findings indicate not only that the selected FIO2 was effective, but also suggested that infantile apnea-associated intermittent hypoxia may adversely involve myelin-forming process in preterm infants. The mechanisms underlying the disrupted myelinogenesis associated with IH insult have not been extensively investigated. It has been reported that chronic sustained hypoxia (SH) during murine perinatal development revealed a diffusive reduction of oligodendrocyte progenitors and disrupted white matter maturation through mechanisms that involve activation of A1 adenosine receptors (Back et al., 2006; Curristin et al., 2002). However, SH and IH induce differential gene activation either in vitro or in vivo (Ryan et al., 2005; Zhou et al., 2008), suggesting that IH may activate different signaling pathways on hypoxia-induced white matter injury. Exploration of white matter impairments in other brain areas and IH-specific pathological mechanisms will definitely require further studies.
5. CONCLUSIONS
We have here reported on a mouse model of intermittent hypoxia aiming to simulate the hypoxia/reoxygenation events occurring in AOP. The model fulfills the current diagnostic criteria for AOP, and reliably reproduced similar pathophysiological alterations, such as intermittent oxyhemoglobin desaturations and bradycardia. Such events over a relatively short period of critical CNS development yielded evidence for abnormal myelination processes, and thus provide a useful experimental tool for future studies on infantile apnea and preterm birth.
Acknowledgments
We thank the analytical microscopy core facility for providing access to EM instruments, and in particular Cathie Caple and Arkadiusz Slusarczyk for technical assistance. We also thank Dr. Robert M. Greene for the substantial support via COBRE program. This work is supported by NIH 2P20RR017702-061A1 (R.M.G., J.C. is COBRE supported junior faculty and co-investigator), Sleep Research Society Foundation/J. Christian Gillin M.D. Research Grant (J.C.), University of Louisville SOM Basic Grant (J.C.), and NIH HL-086662 (D.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatr Pulmonol. 2008;43(10):937–44. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
- Adams JA, Zabaleta IA, Sackner MA. Hypoxemic events in spontaneously breathing premature infants: etiologic basis. Pediatr Res. 1997;42:463–71. doi: 10.1203/00006450-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin Perinatol. 2004;28(4):264–72. doi: 10.1053/j.semperi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Angelucci L, Patacchioli FR, Chierichetti C, Laureti S. Perinatal mother-offspring pituitary-adrenal interrelationship in rats: corticosterone in milk may affect adult life. Endocrinol Exp. 1983;17(3–4):191–205. [PubMed] [Google Scholar]
- Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, Rivkees SA. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60(6):696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- Bader D, Tirosh E, Hodgins H, Abend M, Cohen A. Effect of increased environmental temperature on breathing patterns in preterm and term infants. J Perinatol. 1998;18(1):5–8. [PubMed] [Google Scholar]
- Berterottière D, D’Allest AM, Dehan M, Gaultier C. Effects of increase in body temperature on the breathing pattern in premature infants. J Dev Physiol. 1990;13(6):303–8. [PubMed] [Google Scholar]
- Cardot V, Chardon K, Tourneux P, Micallef S, Stéphan E, Léké A, Bach V, Libert JP, Telliez F. Ventilatory response to a hyperoxic test is related to the frequency of short apneic episodes in late preterm neonates. Pediatr Res. 2007;62(5):591–6. doi: 10.1203/PDR.0b013e318155868e. [DOI] [PubMed] [Google Scholar]
- Carreras A, Wang Y, Gozal D, Montserrat JM, Navajas D, Farré R. Non-invasive system for applying airway obstructions to model obstructive sleep apnea in mice. Respir Physiol Neurobiol. 2011;175(1):164–8. doi: 10.1016/j.resp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Catalani A, Alemà GS, Cinque C, Zuena AR, Casolini P. Maternal corticosterone effects on hypothalamus-pituitary-adrenal axis regulation and behavior of the offspring in rodents. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.10.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Curristin SM, Cao A, Stewart WB, Zhang H, Madri JA, Morrow JS, Ment LR. Disrupted synaptic development in the hypoxic newborn brain. Proc Natl Acad Sci U S A. 2002;99(24):15729–34. doi: 10.1073/pnas.232568799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Ryu J, Kanaan A, Rivero MC, Dugan LL, Haddad GG, Ali SS. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am J Physiol Cell Physiol. 2010;298:C1594–C1602. doi: 10.1152/ajpcell.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, Rees S, Anderson PJ, Inder TE. Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68(5):734–42. doi: 10.1002/ana.22098. [DOI] [PubMed] [Google Scholar]
- Ehret G. Behavioural studies on auditory development in mammals in relation to higher nervous system functioning. Acta Otolaryngol Suppl. 1985;421:31–40. doi: 10.3109/00016488509121754. [DOI] [PubMed] [Google Scholar]
- El-Dib M, Massaro AN, Bulas D, Aly H. Neuroimaging and Neurodevelopmental Outcome of Premature Infants. Am J Perinatol. 2010 doi: 10.1055/s-0030-1254550. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG. Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics. 2005;22:292–307. doi: 10.1152/physiolgenomics.00217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol. 2008;43(1):20–28. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- Farré R, Rotger M, Montserrat JM, Calero G, Navajas D. Collapsible upper airway segment to study the obstructive sleep apnea/hypopnea syndrome in rats. Respir Physiol Neurobiol. 2003;136(2–3):199–209. doi: 10.1016/s1569-9048(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Farré R, Nácher M, Serrano-Mollar A, Gáldiz JB, Alvarez FJ, Navajas D, Montserrat JM. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. Sleep. 2007;30(7):930–3. doi: 10.1093/sleep/30.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S, Plotnik M, Elidan J, Sohmer H. Development of short latency vestibular evoked potentials in the neonatal rat. Hear Res. 1999;137(1–2):51–8. doi: 10.1016/s0378-5955(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21(7):2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167(11):1540–7. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol. 2002;92:1133–1140. doi: 10.1152/japplphysiol.00785.2001. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Butcher-Puech MC, Edwards DA. Incidence and mechanism of bradycardia during apnoea in preterm infants. Arch Dis Childhood. 1986;61:227–232. doi: 10.1136/adc.61.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Steer PA. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst, Rev. 2010;20(1):CD000273. doi: 10.1002/14651858.CD000273.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24(12):763–8. doi: 10.1038/sj.jp.7211182. [DOI] [PubMed] [Google Scholar]
- Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37(2 Part 2):511–5. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res. 2008;64(3):275–80. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Harper RM. Rostral brain axonal injury in congenital central hypoventilation syndrome. J Neurosci Res. 2010;88(10):2146–54. doi: 10.1002/jnr.22385. [DOI] [PubMed] [Google Scholar]
- Ma S, Mifflin SW, Cunningham JT, Morilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coeruleus in response to subsequent immobilization stress. Neuroscience. 2008;154(4):1639–47. doi: 10.1016/j.neuroscience.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–77. [PMC free article] [PubMed] [Google Scholar]
- Marret S, Mukendi R, Gadisseuxc JF, Gressens P, Evard P. Effect of ibotenate on brain development: An excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol. 1995;54:358–370. doi: 10.1097/00005072-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Hüppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8(11):1042–55. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin R, Fox W, editors. Fetal and neonatal physiology. 2. WB Saunders; Philadelphia: 1998. [Google Scholar]
- Moles A, Rizzi R, D’Amato FR. Postnatal stress in mice: does “stressing” the mother have the same effect as “stressing” the pups? Dev Psychobiol. 2004;44(4):230–7. doi: 10.1002/dev.20008. [DOI] [PubMed] [Google Scholar]
- Nock ML, Difiore JM, Arko MK, Martin RJ. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J Pediatr. 2004;144(3):291–5. doi: 10.1016/j.jpeds.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Pawar A, Peng Y, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxcia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JC, D’Almeida V, Souza FG, Schoorlemmer GH, Colombari E, Tufik S. Consequences of subchronic and chronic exposure to intermittent hypoxia and sleep deprivation on cardiovascular risk factors in rats. Respir, Physiol, Neurobiol. 2007;156(3):250–8. doi: 10.1016/j.resp.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Kovach A, Meyer J, McConnell C, Iwamoto HS. Brief, intermittent hypoxia restricts fetal growth in Sprague-Dawley rats. Biol Neonate. 1998;73(5):313–9. doi: 10.1159/000013990. [DOI] [PubMed] [Google Scholar]
- Soukhova-O’Hare GK, Cheng ZJ, Roberts AM, Gozal D. Postnatal intermittent hypoxia alters baroreflex function in adult rats. Am J Physiol Heart Circ Physiol. 2006;290(3):H1157–1164. doi: 10.1152/ajpheart.00767.2005. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Huppke P, Gartner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008;160:21–27. doi: 10.1016/j.resp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Sung YH, Shin MS, Cho S, Baik HH, Jin BK, Chang HK, Lee EK, Kim CJ. Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neurosci, Lett. 2010;470(1):86–90. doi: 10.1016/j.neulet.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O’Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol. 2001;91:2758–2766. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27(2):194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang H, Brautigan DL, Liu Z. Activation of glycogen synthase in myocardium induced by intermittent hypoxia is much lower in fasted than in fed rats. Am J Physiol Endocrinol Metab. 2007;292(2):E469–75. doi: 10.1152/ajpendo.00486.2006. [DOI] [PubMed] [Google Scholar]
- Yorty JL, Schultz SA, Bonneau RH. Postpartum maternal corticosterone decreases maternal and neonatal antibody levels and increases the susceptibility of newborn mice to herpes simplex virus-associated mortality. J Neuroimmunol. 2004;150(1–2):48–58. doi: 10.1016/j.jneuroim.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Zhou D, Wang J, Zapala MA, Xue J, Schork NJ, Haddad GG. Gene expression in mouse brain following chronic hypoxia: role of sarcospan in glial cell death. Physiol Genomics. 2008;32(3):370–9. doi: 10.1152/physiolgenomics.00147.2007. [DOI] [PubMed] [Google Scholar]