Abstract
This study employed quantitative sensory testing (QST) to evaluate pain responses in chronic spinal pain patients at low risk and high risk for opioid misuse, with risk classification based on scores on the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R). Patients were further sub-grouped according to current use of prescription opioids. Of the 276 chronic pain patients tested, approximately 65% were taking opioids; a median split was used to further categorize these patients as being on lower or higher doses of opioids. The highrisk group (n= 161) reported higher levels of clinical pain, had lower pressure and thermal pain thresholds at multiple body sites, had lower heat pain tolerance, and rated repetitive mechanical stimuli as more painful relative to the low-risk group (n= 115; p’s< .01). In contrast, QST measures did not differ across opioid groups. Multiple linear regression analysis suggested that indices of pain-related distress (i.e., anxiety and catastrophizing about pain) were also predictive of hyperalgesia, particularly in patients taking opioids. Collectively, regardless of opioid status, the high-risk group was hyperalgesic relative to the low-risk group; future opioid treatment studies may benefit from the classification of opioid risk, and the examination of pain sensitivity and other factors that differentiate high- and low-risk groups.
Keywords: hyperalgesia, opioid misuse, chronic pain, catastrophizing, anxiety
Introduction
Opioid analgesics remain a treatment of choice for the management of moderate to severe cancer pain, and are increasingly used to treat chronic noncancer pain 24,25,78. However, long-term opioid use is associated with a variety of adverse outcomes, including medication misuse and addiction in some patients 20,53,54,87. Recent substantial increases in the prescription of opioids for chronic noncancer pain, with some studies suggesting a nearly 100% increase over the past decade, have been paralleled by sharp increases in opioid abuse and accidental overdose 5,18,19,90,95.
As a consequence of the growing use of opioids for the treatment of chronic pain, increasing attention has been paid to the misuse and abuse of prescription opioid medication 53,54,87. It has become clear that the prevalence of opioid misuse among chronic pain patients varies widely across settings, with surveys reporting misuse rates from several percent to over 50% of patients 87. Part of this variability is almost certainly related to the complexity of defining medication misuse. For example, recent studies 54 have characterized prescription opioid misuse using a combination of patient-reported compliance checklists (e.g., patients respond to questions about the amount of medication they use daily, whether they have run out of medication early, whether they have sought opioids from other sources, etc.), ratings by the prescribing physician (e.g., did the patient request early refills, etc.), and more “objective” indices such as urine toxicology screens. Such observations have sparked interest in studying individual differences in the propensity to misuse prescription opioids, and recent studies have sought to identify patient-related factors that are associated with a greater or lesser probability of opioid misuse. For example, recent investigations have highlighted the association of factors such as a history of substance abuse or mental health disorders with increased risk for opioid misuse 26,27,70,82,87.
One factor that has not yet received much attention in the opioid misuse literature is individual differences in pain sensitivity (or hyperalgesia). To date, evidence has mounted that opioids can produce a paradoxical amplification of pain sensitivity, a phenomenon termed opioid-induced hyperalgesia (OIH). An increasing body of literature from both clinical and basic science studies has documented this phenomenon, and OIH appears to pose a significant clinical challenge in acute, chronic, and cancer pain settings 14–17,41,49,71,79. While there is some agreement that OIH may reduce opioid efficacy (and perhaps contribute to opioid tolerance), hyperalgesia has not generally been studied as a contributor to opioid misuse, though some reviews have suggested this possibility 14,20,74,79. One recent study in opiate addicts has suggested that individual differences in opioid-induced hyperalgesia were strongly related to important clinical outcomes 74. Relative to controls, abstinent opiate addicts showed reduced pain tolerance, and within the opiate addict group, those who were most pain-sensitive reported the highest levels of clinical pain, the highest levels of distress, and the highest degree of cue-induced drug craving 74. The link between OIH and opioid craving is particularly interesting since self-report of opioid craving was associated with indices of opioid misuse in a 6-month prospective study of a large sample of chronic pain patients maintained on oral opioids 92. Given that hyperalgesia may be associated with opioid craving and other risk factors for opioid misuse, we sought to study links between individual differences in pain responses and opioid risk phenotypes.
In this study, we utilized a well-validated self-report screening measure: the Screener and Opioid Assessment for Patients with Pain (SOAPP-R), a 24-item self-report measure that was developed to improve a clinician’s ability to assess a patient’s risk for opioid abuse. A multi-center longitudinal study showed that this instrument was able to prospectively predict aberrant use of opioid medications among chronic pain patients 1,11,12. The SOAPP-R queries patients about drug craving, substance abuse history, and emotional factors such as distress, anger, and interpersonal conflict. At present, however, despite a confluence of evidence that SOAPP-R scores are associated with risk for opioid misuse 1,11,12,54,92, we know relatively little about the specific mechanisms by which high SOAPP-R scores confer increased risk 53, and no studies have yet investigated hyperalgesia in groups of patients categorized according to opioid misuse risk status on the SOAPP-R.
In this study, chronic spinal pain patients were classified as either low- or high-risk for opioid misuse using a previously-validated SOAPP-R cutoff score. Groups were compared on mechanical and thermal pain responses. We hypothesized that risk for opioid misuse would be associated with hyperalgesia, and we expected this effect to be strongest among patients taking opioids. In addition, we evaluated pain-related negative affect using ratings of anxiety and a measure of pain catastrophizing 13,28. As high levels of general negative affect are associated with elevated opioid craving and opioid withdrawal symptoms 38, and as catastrophizing specifically correlates with individual differences in opioid analgesia 40, we hypothesized that catastrophizing would be associated with a greater degree of hyperalgesia, particularly in the group of patients using opioid medications.
Methods
Study Design and Participants
This was a cross-sectional cohort study performed in a single, large, urban, university-based pain management center. Participants were 276 patients recruited from the Pain Management Center at Brigham & Women’s Hospital. Patients with a diagnosis of spinal pain, with or without radicular symptoms, who were able to speak, read, and write in English, and who had been experiencing pain for at least 6 months, were invited to participate. Patients were excluded if they had a diagnosis of cancer or other malignant disease, or had cognitive limitations that precluded providing self-report data.
Questionnaires
Standard demographic information was collected by self-report. In addition, patients reported what analgesic medications they were currently taking. Patient reports of medications were verified by the research assistant using the electronic medical record system. Published tables were used to convert daily opioid dosages into morphine equivalents, as in other recent studies 56,57. Current 0–10 ratings of back pain severity (0 = no pain to 10 = the worst pain imaginable 69 were also obtained before and after the testing session.
The Screener and Opioid Assessment for Patients with Pain (SOAPP-R) was used to classify patients as either low- or high-risk for opioid misuse. The SOAPP-R is a 24-item, self-administered screening instrument used to help determine risk potential for future aberrant drug-related behavior. Items are rated from 0 = never to 4 = very often (e.g., How often have you felt a craving for medication?), and summed to generate the total SOAPP-R score, which ranges from zero to 96. Additional information about the SOAPP-R, including copies of the questionnaire, is available through the website of a pain education group: http://www.painedu.org/soapp-development.asp. The SOAPP-R has been shown to have good predictive validity, with an area under the curve ratio of 0.88 (95% confidence interval [CI], 0.81–0.95). A cut-off score of 18 showed good sensitivity (0.86) and specificity (0.73) for predicting prescription opioid misuse.
The Pain Catastrophizing Scale (PCS 85) is a well-validated, widely-used, self-report measure of catastrophic thinking associated with pain 28. The PCS has good psychometric properties in pain patients and controls 88. Cronbach’s α for the PCS was above .9, indicating very high levels of internal item consistency 31. The construct of catastrophizing incorporates: magnification of pain-related symptoms, rumination about pain, feelings of helplessness, and pessimism about pain-related outcomes. Individuals rate the extent to which they experience (when they are in pain) the thought or feeling described by each item; scores on this 13-item measure can range from 0–52 (each item is scored 0 = not at all to 4 = all the time).
Session Protocol
Study subjects provided informed consent, and all procedures were approved by the Partners Institutional Review Board at Brigham & Women’s Hospital. Many of these procedures have been described in our previous studies 35,37. Clinical pain ratings (on a 0–10 scale) were obtained before and after the psychophysical testing session, and, as in prior QST studies 36,59, current verbal ratings of anxiety (on a 0–10 scale, with “no anxiety” and “severe anxiety” as the respective anchors) were obtained during the testing session. During the session, subjects were seated comfortably in a reclining chair while they underwent the brief psychophysical testing procedures (the assessment of which lasted approximately 30 minutes) described below:
Quantitative Sensory Testing (QST)
First, participants underwent an assessment of mechanical temporal summation using a set of seven custom-made weighted pinprick stimulators developed by the German research Network on Neuropathic Pain 76,77. These punctuate mechanical probes have a flat contact area of .2 mm in diameter, and exert forces between 8 and 512 mN. Punctate stimuli were delivered to the skin on the dorsum of the middle finger of the right hand. We first determined the lowest force stimulator that produced a sensation of discomfort (128 or 256 mN for most subjects), and then applied a train of 10 stimuli at the rate of 1 per second. Participants rated the painfulness of the first, fifth, and tenth stimulus, and also rated any ongoing pain after-sensations 15 seconds following the final stimulus. All ratings were on a 0–100 verbal pain intensity scale used in previous studies 32,37.
Next, as in previous studies 21,45,51, we bilaterally assessed pressure pain thresholds (PPTh) at several sites. PPTh at the trapezius muscle and the metacarpophalangeal joint of the thumb were determined twice on the right and left sides of the body in a randomized order. At each site, mechanical force was applied using a 0.5-cm2 probe covered with polypropylene pressure-transducing material; pressure was increased at a steady rate of 30 kPA/s until the subject indicated that the pressure was "first perceived as painful".
Finally, contact heat stimuli were delivered using a contact thermode (Medoc Advanced Medical Systems, Ramat Yishai, Israel). Thermal assessment included sampling of warmth and cool thresholds, followed by heat pain thresholds (HPTh) and cold pain thresholds (CPTh), followed by heat pain tolerance (HPTo) all tested on the ventral forearm using a method of limits paradigm with a rate of temperature change of 0.5°C/Sec 33.
Data Analysis
Patients were categorized as a function of their SOAPP-R risk classification (high risk = SOAPP-R total score> 18) and opioid use. Nearly 2/3 of study patients were using opioids; because opioid doses varied so widely across subjects, we performed a median split to divide individuals into those with lower daily opioid doses (i.e., daily morphine equivalents > 0 and ≤ 50 mg) and those with higher daily opioid dosages (i.e., daily morphine equivalents > 50 mg). Group comparisons were performed using a series of factorial analyses of variance (ANOVA) and multivariate analyses of variance (MANOVA) or covariance (MANCOVA). These multivariate statistical approaches are useful in analyzing relationships between predictors and multiple inter-related dependent variables. MANCOVA tests whether groups differ on a combination of outcome variables, and thus provides protection against inflating the false positive rate in testing multiple dependent variables. Group classification according to SOAPP-R score and opioid use were the independent variables. A total of four MANCOVAs were performed; the first evaluated group differences in thermal sensitivity for non-painful stimuli (i.e., warm and cool thresholds), the second examined group differences in pain ratings in response to the punctuate probe stimulation (i.e., ratings of the 1st, 5th, and 10th stimuli, and the “post” ratings of after-sensations), the third tested for group differences in pressure pain thresholds (separately averaged scores for the thumb and trapezius), and the fourth involved thermal pain responses (heat and cold pain thresholds, and heat pain tolerance). Age and sex served as covariates in each of these analyses.
Associations between psychosocial variables (e.g., catastrophizing and anxiety) and pain responses were evaluated using multiple linear regression analyses, with SOAPP-R scores included as a predictor. In order to create a manageable number of dependent variables for these regression analyses, mechanical probe pain ratings were averaged, as were pressure pain thresholds. To create a measure of thermal thresholds, HPTh and CPTh were standard-scored, then cold pain thresholds were reverse-scored before averaging the two (this was done because HPTh and CPTh are “opposite” in their directionality: lower HPTh represents greater pain sensitivity, and higher CPTh represents greater pain sensitivity). Heat and cold pain thresholds are generally highly correlated (r= −.82 in a sample of fibromyalgia patients 80 and r= −.70 in the current sample), and prior studies from our laboratory 35 and others 3,22 have combined multiple pain response measures into unitary indices of pain sensitivity. Regression analyses were performed separately in patients taking opioids and patients not taking opioids. All analyses were performed using SPSS (V.17, Chicago, IL).
Results
ANOVAs revealed no significant main effects (of opioid group or SOAPP-R score) or interaction for age or sex (all p’s > .30), indicating that the groups did not differ on these demographic variables (see Table 1). In all groups, the majority of participants were women, and the average age tended to be in the late forties (see Table 1). We did control for age and sex in the analyses described below, as these demographic variables are often related to pain thresholds and we were interested in their potential association with pain responses in this study. Ratings of clinical pain were higher in the high-risk SOAPP-R groups (p < .01; see Table 1), but there was no effect of opioid group and no interaction. Opioid dose did not vary as a function of SOAPP-R group, and SOAPP-R scores did not vary across opioid categories. For PCS scores and anxiety ratings, there was a strong main effect of risk group (p< .01), but no significant effect of opioid group or interaction (p’s > .2; Table 1).
Table 1.
Characteristics of groups as a function of risk and opioid use (mean ± SD).
| No Opioids | Low Opioids | High Opioids | ||||
|---|---|---|---|---|---|---|
| Low Risk | High Risk | Low Risk | High Risk | Low Risk | High Risk | |
| n= 41 | n= 56 | n= 40 | n= 48 | n= 34 | n= 57 | |
| Age | 49.7 ± 15 | 46.3 ± 14 | 50.3 ± 11 | 49.1 ± 11 | 46.7 ± 11 | 48.3 ± 10 |
| Sex (% Male) | 41% | 31% | 43% | 42% | 38% | 45% |
| Clinical Pain a | 5.7 ± 1.9 | 6.2 ± 2.5 | 5.9 ± 2.0 | 6.2 ± 1.9 | 5.4 ± 2.4 | 6.2 ± 2.7 |
| MEq Units b | 0 ± 0 | 0 ± 0 | 31 ± 14 | 27 ± 14 | 231 ± 205 | 307 ± 279 |
| SOAPP-R a | 11.2 ± 5 | 29.3 ± 8 | 11.8 ± 4 | 31.5 ± 12 | 12.8 ± 4 | 27.5 ± 7 |
| PCS a | 16.0 ± 11 | 32.3 ± 11 | 18.1 ± 9 | 30.7 ± 11 | 18.9 ± 11 | 29.9 ± 11 |
| Anxiety (0–10) a | 0.6 ± 2.0 | 1.1 ± 2.3 | 0.8 ± 2.0 | 0.9 ± 1.9 | 0.4 ± 0.9 | 0.6 ± 1.2 |
MEq Units= Morphine equivalent units (daily dose); PCS= Pain Catastrophizing Scale.
p<0.01 for the main effect of SOAPP-R Risk grouping
p<0.01 for the main effect of opioid classification
Multivariate Analysis of QST Variables
A MANCOVA (controlling for age and sex) examining warm and cool thresholds revealed no effect of opioid group, SOAPP-R score, or interaction (all p’s > .40). This lack of significant effects suggests that the opioid groups (no opioids, lower-dose opioids, high-dose opioids) do not differ in their thermal sensitivity to non-painful stimuli; similarly, the high- and low-risk subjects (as defined by the SOAPP-R) do not differ on these measures of thermal sensitivity.
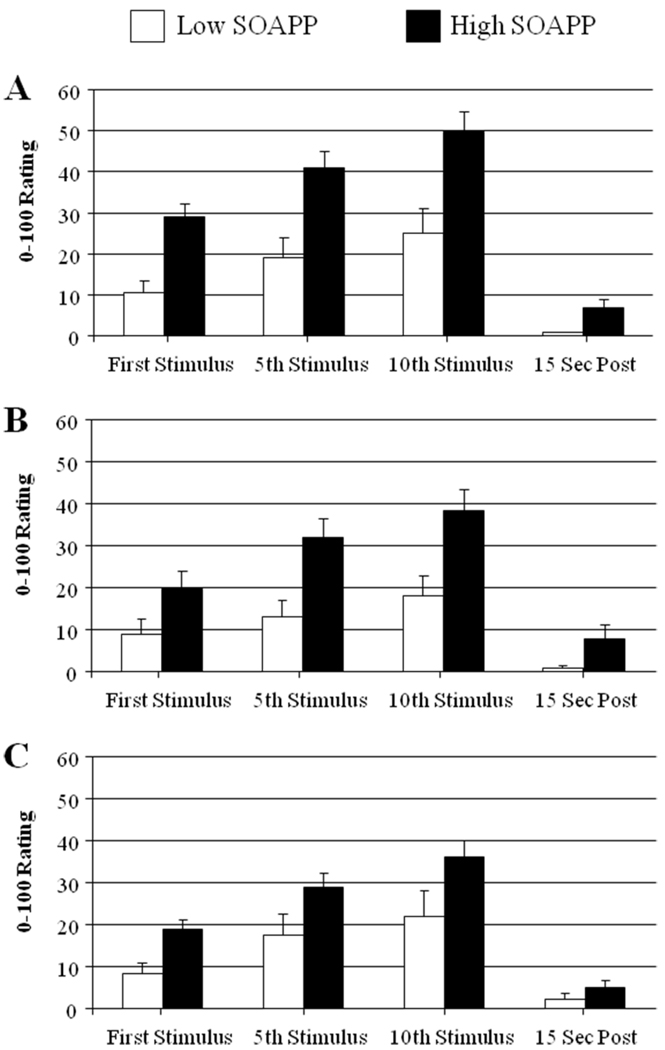
A MANCOVA examining punctate probe pain ratings revealed no effect of opioid group [F(8,532)= 1.3, p= .23], a significant effect of SOAPP-R category [F(4,265)= 9.1, p< .001], and no significant interaction between opioid group and SOAPP-R score (p> .4; Fig. 1). This result indicates that while the opioid groups do not differ in their mechanical pain responses, the high SOAPP-R group did report more intense mechanical pain relative to the low SOAPP-R group.
Figure 1.
Pain ratings (0–100) for punctuate mechanical stimuli as a function of opioid use and risk group (mean ± SEM). Panel A= No Opioids, Panel B= Lower-Dose Opioids, Panel C= Higher-Dose Opioids.
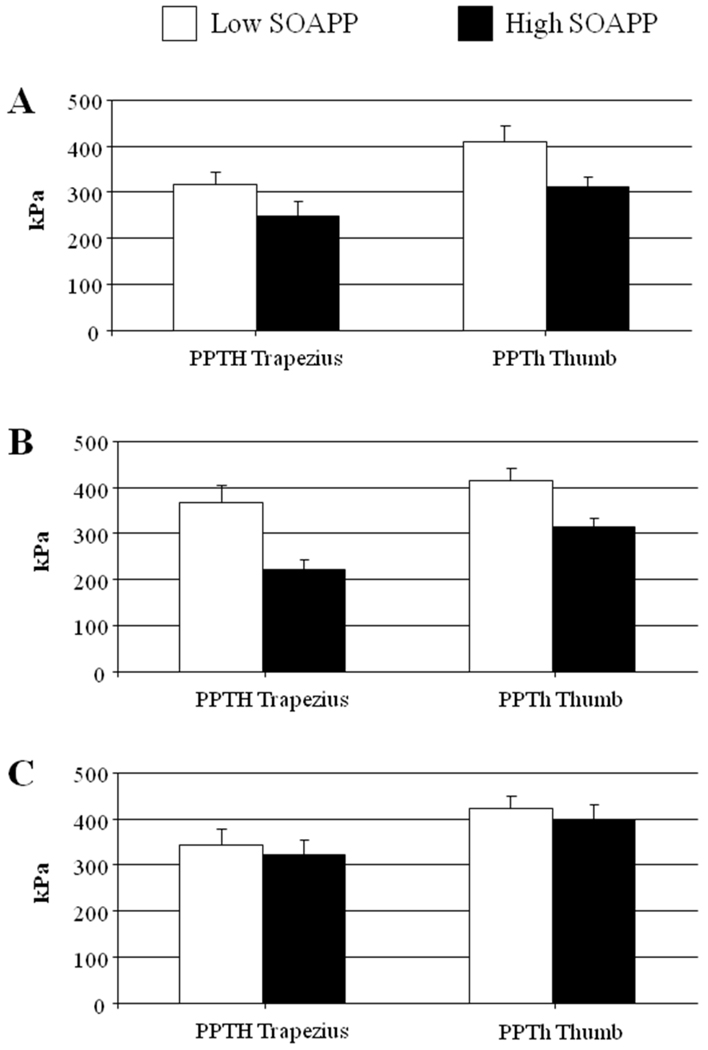
A MANCOVA examining pressure pain thresholds revealed no effect of opioid group [F(4,536)= 1.1, p= .37], a significant effect of SOAPP-R category [F(4,265)= 5.4, p< .01], and no significant interaction between opioid group and SOAPP-R score (p> .2; Fig. 2). Similar to the punctuate probe findings, the opioid groups do not differ in their pressure pain sensitivity, while the high SOAPP-R group does show reduced pressure pain thresholds (i.e., this group is more sensitive to pressure pain) compared to the low SOAPP-R group.
Figure 2.
Pressure pain thresholds (in kPa) as a function of opioid use and risk group (mean ± SEM). Panel A= No Opioids, Panel B= Lower-Dose Opioids, Panel C= Higher-Dose Opioids.
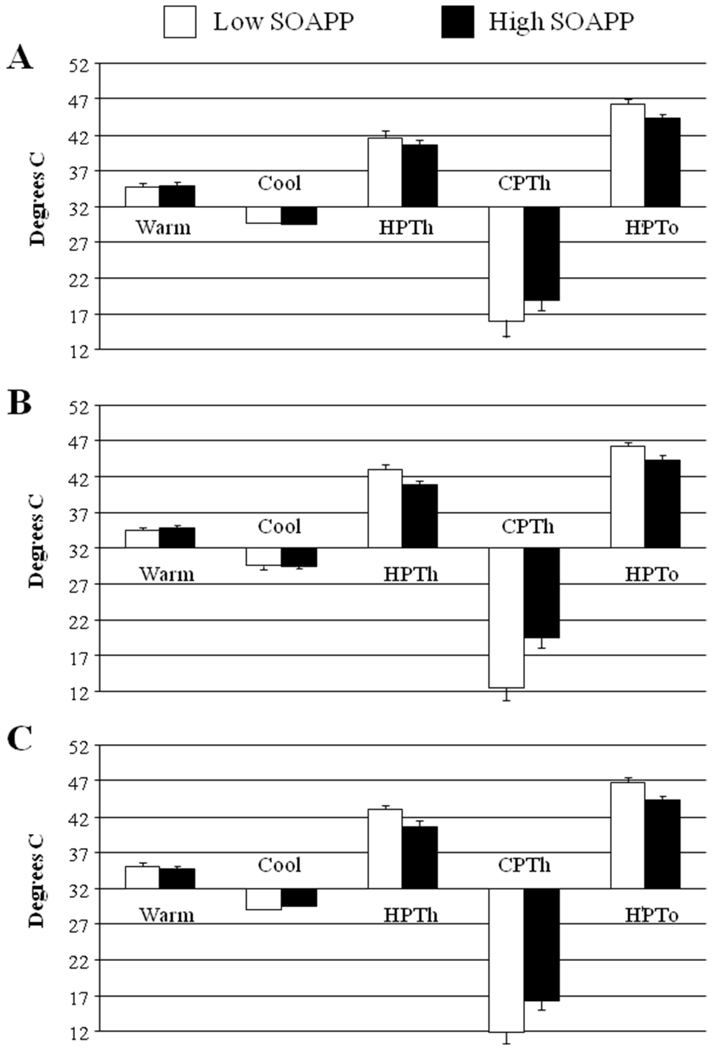
A MANCOVA examining thermal pain responses revealed no effect of opioid group [F(6,530)= 1.7, p=.12], a significant effect of SOAPP-R category [F(3,265)= 7.9, p< .001], and no significant interaction between opioid group and SOAPP-R score (p> .2; Fig. 3). Again, the opioid groups do not differ in their pain sensitivity (in this case, thermal pain sensitivity), while the high SOAPP-R group demonstrates elevated heat and cold pain sensitivity, as well as reduced heat pain tolerance.
Figure 3.
Thermal responses as a function of opioid use and risk group (mean ± SEM). Panel A= No Opioids, Panel B= Lower-Dose Opioids, Panel C= Higher-Dose Opioids.
As some studies have suggested that pain tolerance is a more sensitive measure of opioid-induced hyperalgesia than pain threshold measures 16,23, we performed a separate ANCOVA on heat pain tolerance alone (as this was the only measure of pain tolerance in the present study). Results from this analysis paralleled the multivariate analyses above: there was no effect of opioid group (p= .81), a strong effect of SOAPP-R category ([F(1,268)= 22.5, p< .001], and no significant interaction (p= .57).
Multiple Regressions Predicting QST Variables
Linear regression analysis predicting mean pain ratings of punctuate mechanical stimuli revealed in the first step that women had higher pain ratings than men, both among opioid-using patients and among those not taking opioids (Table 2). Age was not associated with pain ratings within either of the opioid groups. In the second step, psychosocial variables explained 18% of the variance in pain ratings among non-opioid patients and 26% of the variance in pain ratings among patients using opioids. In both groups, higher catastrophizing scores were associated with higher pain ratings while SOAPP-R scores were not significantly related. Anxiety scores were strongly associated with elevated pain ratings only among the group of patients using opioids (Table 2).
Table 2.
Hierarchical linear regression predicting mechanical probe pain ratings.
| No Opioids (n= 97) | Taking Opioids (n= 179) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Step R2 | β | p | Step R2 | β | p | |
| Step 1 | Age | .05 (for step) | −.15 | .17 | .06* | .08 | .34 |
| Sex | −.21 | .05 | −.22 | .01 | |||
| Step 2 | Anxiety | .18** (for step) | .00 | .98 | .26** | .34 | .001 |
| SOAPP-R | .11 | .43 | .13 | .13 | |||
| PCS | .32 | .02 | .24 | .01 | |||
p< .05
p< .01
Linear regression analysis predicting pressure pain thresholds revealed in the first step that men had higher pain thresholds than women, both among opioid-using patients and among those not taking opioids (Table 3). Older age was associated with higher pain thresholds only in the group of patients not on opioids. In the second step, psychosocial variables explained 5% of the variance in pain ratings (not significant) among non-opioid patients and 16% of the variance in pain ratings among patients using opioids. In the group of patients taking opioids, both catastrophizing scores and anxiety ratings were negatively related to pressure pain thresholds (Table 3).
Table 3.
Hierarchical linear regression predicting pain pressure pain threshold.
| No Opioids (n= 97) | Taking Opioids (n= 179) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Step R2 | β | p | Step R2 | β | p | |
| Step 1 | Age | .18** (for step) | .36 | .001 | .07** | .03 | .67 |
| Sex | .29 | .004 | .27 | .001 | |||
| Step 2 | Anxiety | .05 (for step) | −.14 | .16 | .16** | −.31 | .001 |
| SOAPP-R | .19 | .18 | .07 | .45 | |||
| PCS | −.27 | .06 | −.26 | .01 | |||
p< .05
p< .01
Finally, in the linear regression model predicting composite thermal pain thresholds, the first step suggested that older age predicted lower thermal sensitivity (i.e., higher heat pain thresholds) only among the non-opioid group while sex predicted greater thermal sensitivity only within the opioid group (i.e., women were more thermally pain-sensitive than men). In the second step, psychosocial variables explained 4% of the variance in pain ratings among non-opioid patients (not significant) and 17% of the variance in pain ratings among patients using opioids. In the opioid group, higher catastrophizing scores were uniquely related to more thermal pain sensitivity (p< .001) (Table 4).
Table 4.
Hierarchical linear regression predicting pain thermal pain threshold.
| No Opioids (n= 97) | Taking Opioids (n= 179) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Step R2 | β | p | Step R2 | β | p | |
| Step 1 | Age | .25** | .51 | .001 | .04* | .07 | .36 |
| Sex | −.15 | .12 | .20 | .01 | |||
| Step 2 | Anxiety | .04 | .04 | .73 | .17** | −.12 | .13 |
| SOAPP-R | .05 | .71 | .03 | .73 | |||
| PCS | −.24 | .08 | −.40 | .001 | |||
p< .05
p< .01
Discussion
As a consequence of the growing use of opioids for the treatment of chronic pain, increasing attention has been paid to the misuse and abuse of prescription opioid medication 53,54,87. However, many physicians prescribing pain medication have minimal background and training in addiction or drug abuse, and look for ways to assess a given patient’s level of risk for medication misuse. These factors have led to the development of assessment instruments that can be used to aid physicians in identifying which patients are likely to develop problematic patterns of opioid use, and recent studies have sought to evaluate interventions that might reduce such misuse 54. The SOAPP-R was developed to complement current risk assessment practices and to improve a clinician’s ability to assess a patient’s risk for opioid abuse or misuse. Definitions in this area vary widely, and the category of “medication misuse” includes a spectrum of behaviors, from unapproved opioid dosage escalations to running out of medication early, to using illicit drugs in conjunction with prescription opioids, etc. See 11,12,53,54,63,92 for recent examples of studies that operationalize opioid misuse among patients with chronic pain. Prospective studies have confirmed that individuals classified as high-risk on the SOAPP-R do indeed exhibit greater frequencies of aberrant medication-related findings (e.g., escalating medication doses, urine screens that indicate the presence of non-prescribed opioids, etc.) 11,12 in the context of oral opioid treatment of chronic pain, but we know relatively little about the specific mechanisms by which high SOAPP-R scores confer increased risk for opioid misuse 53.
The findings of the present study, which does not constitute a test of the validity of the SOAPP-R, suggest that “high-risk” patients show generalized patterns of hyperalgesia, exhibiting increased sensitivity (including lower pain thresholds, lower pain tolerance, and higher pain ratings) to mechanical and thermal stimuli at multiple body sites. Though the present study is the first to directly examine this question, these results fit well with prior studies in groups of opiate abusers, in which those individuals who were most pain-sensitive and least pain-tolerant during a cold pressor test reported the highest levels of distress and the highest degree of cue-induced drug craving 74, both of which are factors measured by the SOAPP-R. Though we hypothesized that these effects would be most pronounced among patients currently taking opioids, reflecting greater opioid-induced hyperalgesia among high-risk patients 14,20,74,79, those with elevated SOAPP-R scores demonstrated enhanced pain sensitivity even in the non-opioid group. Indeed, we were not able to document the presence of opioid-induced hyperalgesia in this sample; while the high- and low- SOAPP-R groups differed substantially in their pain responses, groups of patients classified on the basis of their opioid use did not differ. Other findings of risk group differences in pain severity and pain catastrophizing were consistent with prior studies, in which those with high SOAPP-R scores showed higher levels of pain intensity and catastrophizing 53.
The lack of apparent OIH in this sample is perhaps not surprising, since a recent review highlighted the mixed findings among cross-sectional studies that evaluate opioid-induced hyperalgesia 41. Other cross-sectional studies that compared opioid-using and opioid-naïve patients have also found no group differences in variables such as cold pain threshold and tolerance 73, ischemic pain tolerance 39, or pressure and thermal pain thresholds 75. In the present set of data, we were unable to detect differences across opioid groups in pain threshold, pain tolerance, or pain ratings. The fact that some other investigations using similar methodologies do detect OIH 15 appears to suggest that its presence or absence may be shaped by sample-specific factors, and highlights the need for additional prospective studies in this area. It is also noteworthy that samples of chronic pain patients often demonstrate a “pre-existing” hyperalgesia that is presumably related to the putative sensitizing effect of chronic pain on the nervous system 58,86. For example, the patients in the current study exhibit lower pressure and heat pain thresholds than demographically similar “control” (i.e., free from chronic pain) samples in previous studies from our laboratory 37,61 and others’ 15. This pre-existing hyperalgesia may add to the difficulty of detecting additional hyperalgesic effects of opioids.
Prior research has consistently revealed broad inter-individual variation in pain sensitivity, evaluated by measuring responses to standardized noxious stimuli under highly controlled conditions 46. Moreover, these individual differences in pain sensitivity (which are associated with SOAPP-R scores in this sample) have demonstrated clinical relevance for shaping long-term pain-related outcomes. A number of surgical studies have examined the relationship between basal pain sensitivity and outcomes such as acute post-operative pain. Among individuals undergoing limb amputation 68, cholecystectomy 4, anterior cruciate ligament repair 96, gynecologic surgery 48, lower abdominal surgery 50, biopsy 81, cesarean section 47,67, and disk surgery 43, pre-surgical QST responses were significantly correlated with acute postoperative pain. In each case, individual differences reflecting greater sensitivity to pain (e.g., lower pain thresholds) were associated with more intense acute post-operative pain. While similar studies of long-term post-operative pain are few, pre-surgical responses to standardized heat stimuli did predict 6-month post-thoracotomy pain outcomes 97, and lower baseline pain thresholds among patients undergoing joint replacement surgery were predictive of more severe joint pain ratings 18 months after surgery 62. Our group has also reported that the most pain-sensitive chronic pain patients obtain the least benefits following multidisciplinary treatment for chronic pain 30, and derive reduced analgesic effects from opioids 34. In the present study, patterns of enhanced pain sensitivity to multiple stimuli, which we observed among the group of patients with elevated SOAPP-R scores (i.e., the “high-risk” patients), may place these individuals at risk for adverse outcomes such as elevated levels of post-procedural pain, or reduced benefit from various types of treatment.
The present findings suggest, in the pattern of results for the regression analyses, that the associations between SOAPP-R scores and hyperalgesia are generally explained by measures of distress, specifically pain-related anxiety and catastrophizing. When included in regression models with these other variables, SOAPP-R scores were not uniquely predictive of mechanical and thermal pain responses, while catastrophizing and anxiety were associated with decreased pain thresholds and increased pain ratings, consistent with a substantial body of prior research indicating that high levels of negative affect are significantly associated with greater pain sensitivity 29,42,44,72,89. Interestingly, these relationships (between measures of distress and hyperalgesia) were strongest among chronic pain patients currently taking opioids, suggesting that processes related to negative affect might interact with processes involved in opioid analgesia. At present, much of the relevant data is cross-sectional, and it is unknown whether opioids contribute to negative affect over time and/or whether persons with the most affective distress tend to be prescribed opioids more frequently or at a higher average daily dose. Recent surveys and healthcare database studies have revealed that individuals with mental health disorders are more likely to be prescribed opioids for pain treatment, and to experience problematic outcomes of opioid therapy, but the causal influences in these studies are generally unclear 5,6,24–27,82–84. The present findings suggest the possibility that some of the driving influences underlying the tendency of high-risk patients to misuse prescription opioids may involve symptoms of anxiety, distress, and catastrophizing. Unfortunately, as we do not have measures of opioid misuse in the present study we are unable to test this hypothesis, which is consistent with current views on the complex, mutifactorial nature of opioid dependence 55.
Much non-human research suggests that affective tone is mediated by endogenous opioids, and processes related to negative mood have been associated with a deactivation of μ-opioid receptors in particular brain regions 2. Catastrophizing, in particular, has been associated with greater post-operative use of opioid analgesics after a painful surgery 52, implying that catastrophizing was correlated with reduced benefit of opioids per unit of medication administered. In addition, measures of negative affect and pain sensitivity have been shown to relate to the magnitude of μ-opioid analgesia in patients with chronic pain; chronic back pain patients who were highest in negative affect showed roughly 50% less morphine analgesia than those with lower levels of negative affect 93, and among neuropathic pain patients, those with the greatest heat pain sensitivity obtained the least benefit from opioids 34. Among healthy adults, similar findings have been observed, as higher levels of catastrophizing were correlated with reduced acute analgesic benefit (measured as a function of changes in pain threshold and tolerance) of IV pentazocine, a kappa-opioid agonist 40. It is important to note that catastrophizing and anxiety form part of a larger construct of negative affect, which includes a variety of cognitive and emotional processes 60,65,91,93,94, and future studies may benefit from “clustering” patients using a variety of indices. Overall, additional prospective research is needed to clarify the manner in which negative affective processes may shape the interactions between opioids (both endogenous and exogenous) and the central nervous system’s processing of pain-related information.
A number of limitations should be considered when interpreting the findings of this study. First, this work is cross-sectional rather than prospective in nature, which does not allow temporal characterization of the potential changes in pain sensitivity in differing groups of patients over the course of opioid therapy. Second, we do not have information on subjects’ current or recent misuse of medications, which might be an important factor shaping individual differences in pain sensitivity. It is possible that the “high-risk” patients in the present study are not fully representative of high-risk patients in general, if, for example, many of the high-risk patients who were abusing their medications were discharged from the practice (and thus were not included in the present study). Third, we did not measure potentially important variables such as the duration of opioid therapy, the recency of medication dosing in relation to the testing, or the use of adjunctive medications. In practice, in a setting such as this one, many patients have been on and off of different opioids for a number of years, making it challenging to quantify with any degree of precision their lifetime opioid exposure. Past studies from this clinic indicate that over 2/3 of patients have been on opioids for at least 2 years, and that the average duration of opioid therapy is approximately 5 years 63,64,66, suggesting that the large majority of opioid-using patients in this sample were likely to have been on these medications long term. In the future, however, follow-up studies will need to account for the nature of the specific opioid medication used (e.g., short-acting vs. long-acting), how recently the last dose was taken, etc. Fourth, we were unable to separately examine specific opioids in this study (because the wide variety of medications would have made for very small group sizes); it is possible that specific opioids are more or less likely to generate a hyperalgesic state in users, and future studies may benefit from examining large groups of patients using a single opioid agent, or from directly comparing different medications. Fifth, we did not directly measure other potentially important psychosocial processes that might be captured by the SOAPP-R. For example, anger and anger expression style have shown consistent relationships to pain responses and pain-relevant physiological processes 7–10. The SOAPP-R contains some items that tap constructs such as anger and hostility (e.g., how often have others told you that you had a bad temper?”), and it is possible that these or other specific factors are partially responsible for the observed associations between SOAPP-R scores and hyperalgesia. In future studies on this topic, we plan to specifically administer measures of the various constructs assessed by the SOAPP-R (e.g., anger, distress, substance use history) to determine which are most highly related to individual differences in pain sensitivity. The present analyses do suggest that pain-related emotional distress (when measured using indices of anxiety and catastrophizing) does statistically account for the associations between SOAPP-R scores and pain sensitivity in this sample.
Despite these limitations, the present study is the first to suggest that patients at elevated risk for prescription opioid misuse (as measured by the SOAPP-R) demonstrate enhanced sensitivity to pain. Whether the presence of hyperalgesia contributes directly to the misuse of opioids (e.g., high-risk patients may escalate their doses of opioids in an attempt to counteract their hyperalgesic state) is not yet known, but may be a fruitful line of research to pursue in future prospective studies. In addition, these findings suggest that measures of negative affect and distress are likely to account for the observed association between high-risk status on the SOAPP-R and enhanced sensitivity to pain, and that negative affective processes may make an especially strong contribution to hyperalgesia in patients taking opioids.
Acknowledgments
This work was supported by NIH Grants K23 AR051315 (RRE), DA024298 (RNJ), and by awards from the American College of Rheumatology (RRE) and Arthritis Foundation (RRE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Perspective: This study demonstrates that chronic spinal pain patients at high risk for misuse of prescription opioids are more pain-sensitive than low-risk patients, whether or not they are currently taking opioids. Indices of pain-related distress were important predictors of pain sensitivity, particularly among those patients taking opioids for pain.
Disclosures: None of the authors have any financial or other conflicts of interest with regard to this study or its findings.
References
- 1.Akbik H, Butler SF, Budman SH, Fernandez K, Katz NP, Jamison RN. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP) J.Pain Symptom.Manage. 2006;32:287–293. doi: 10.1016/j.jpainsymman.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur.J.Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Bhalang K, Sigurdsson A, Slade GD, Maixner W. Associations among four modalities of experimental pain in women. J.Pain. 2005;6:604–611. doi: 10.1016/j.jpain.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90:261–269. doi: 10.1016/S0304-3959(00)00406-1. [DOI] [PubMed] [Google Scholar]
- 5.Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, Campbell CI, Merrill JO, Silverberg MJ, Banta-Green C, Weisner C. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol.Drug Saf. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen.Hosp.Psychiatry. 2009;31:564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruehl S, Burns JW, Chung OY, Chont M. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neurosci.Biobehav.Rev. 2009;33:475–491. doi: 10.1016/j.neubiorev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruehl S, Burns JW, Chung OY, Quartana P. Anger management style and emotional reactivity to noxious stimuli among chronic pain patients and healthy controls: The role of endogenous opioids. Health Psychology. 2008;27:204–214. doi: 10.1037/0278-6133.27.2.204. [DOI] [PubMed] [Google Scholar]
- 9.Burns JW, Bruehl S, Chung OY, Magid E, Chont M, Goodlad JK, Gilliam W, Matsuura J, Somar K. Endogenous opioids may buffer effects of anger arousal on sensitivity to subsequent pain. Pain. 2009;146:276–282. doi: 10.1016/j.pain.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JW, Quartana PJ, Bruehl S. Anger inhibition and pain: conceptualizations, evidence and new directions. J.Behav.Med. 2008;31:259–279. doi: 10.1007/s10865-008-9154-7. [DOI] [PubMed] [Google Scholar]
- 11.Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-Validation of a Screener to Predict Opioid Misuse in Chronic Pain Patients (SOAPP-R) J.Addict.Med. 2009;3:66–73. doi: 10.1097/ADM.0b013e31818e41da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J.Pain. 2008;9:360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell CM, Edwards RR. Mind-body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Transl.Res. 2009;153:97–101. doi: 10.1016/j.trsl.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang G, Chen L, Mao J. Opioid tolerance and hyperalgesia. Med.Clin.North Am. 2007;91:199–211. doi: 10.1016/j.mcna.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Malarick C, Seefeld L, Wang S, Houghton M, Mao J. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143:65–70. doi: 10.1016/j.pain.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin.J.Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 17.Colvin LA, Fallon MT. Opioid-induced hyperalgesia: a clinical challenge. Br.J.Anaesth. 2010;104:125–127. doi: 10.1093/bja/aep392. [DOI] [PubMed] [Google Scholar]
- 18.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006a;83 Suppl 1:S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006b;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat.Rev.Rheumatol. 2010;6:191–197. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- 21.Dhondt W, Willaeys T, Verbruggen LA, Oostendorp RA, Duquet W. Pain threshold in patients with rheumatoid arthritis and effect of manual oscillations. Scand.J.Rheumatol. 1999;28:88–93. doi: 10.1080/030097499442540. [DOI] [PubMed] [Google Scholar]
- 22.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum.Mol.Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 23.Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–96. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 24.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann.Intern.Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin.J.Pain. 2010a;26:1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edlund MJ, Martin BC, Fan MY, Braden JB, Devries A, Sullivan MD. An Analysis of Heavy Utilizers of Opioids for Chronic Noncancer Pain in the TROUP Study. J.Pain Symptom.Manage. 2010b;40:279–289. doi: 10.1016/j.jpainsymman.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: Results from the TROUP Study. Drug Alcohol Depend. 2010c;112:90–98. doi: 10.1016/j.drugalcdep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards RR, Bingham CO, III, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006a;55:325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 29.Edwards RR, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat.Rev.Rheumatol. 2011 doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 30.Edwards RR, Doleys DM, Lowery D, Fillingim RB. Pain tolerance as a predictor of outcome following multidisciplinary treatment for chronic pain: differential effects as a function of sex. Pain. 2003;106:419–426. doi: 10.1016/j.pain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Edwards RR, Giles J, Bingham CO, III, Campbell C, Haythornthwaite JA, Bathon J. Moderators of the Negative Effects of Catastrophizing in Arthritis. Pain Med. 2010;11:591–599. doi: 10.1111/j.1526-4637.2010.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur.J.Pain. 2009a;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratoryinduced pain. Pain. 2004;111:335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Edwards RR, Haythornthwaite JA, Tella P, Max MB, Raja S. Basal heat pain thresholds predict opioid analgesia in patients with postherpetic neuralgia. Anesthesiology. 2006b;104:1243–1248. doi: 10.1097/00000542-200606000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Edwards RR, kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin.J.Pain. 2006c;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RR, Wasan AD, Bingham CO, III, Bathon J, Haythornthwaite JA, Smith MT, Page GG. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res.Ther. 2009b;11:R61. doi: 10.1186/ar2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elkader AK, Brands B, Callaghan R, Sproule BA. Exploring the relationship between perceived inter-dose opioid withdrawal and patient characteristics in methadone maintenance treatment. Drug Alcohol Depend. 2009;105:209–214. doi: 10.1016/j.drugalcdep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Fillingim RB, Doleys DM, Edwards RR, Lowery D. Clinical characteristics of chronic back pain as a function of gender and oral opioid use. Spine. 2003;28:143–150. doi: 10.1097/00007632-200301150-00010. [DOI] [PubMed] [Google Scholar]
- 40.Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sexrelated psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol.Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Fishbain DA, Cole B, Lewis JE, Gao J, Rosomoff RS. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med. 2009;10:829–839. doi: 10.1111/j.1526-4637.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 42.France CR, Keefe FJ, Emery CF, Affleck G, France JL, Waters S, Caldwell DS, Stainbrook D, Hackshaw KV, Edwards C. Laboratory pain perception and clinical pain in post-menopausal women and age-matched men with osteoarthritis: relationship to pain coping and hormonal status. Pain. 2004;112:274–281. doi: 10.1016/j.pain.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Geiss A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114:104–117. doi: 10.1016/j.pain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243–250. doi: 10.1016/S0304-3959(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 45.Gerecz-Simon EM, Tunks ER, Heale JA, Kean WF, Buchanan WW. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin.Rheumatol. 1989;8:467–474. doi: 10.1007/BF02032098. [DOI] [PubMed] [Google Scholar]
- 46.Gracely R. Studies of pain in human subjects. In: Wall P, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1999. pp. 385–407. [Google Scholar]
- 47.Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer EZ. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–1426. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 48.Granot M, Zimmer EZ, Friedman M, Lowenstein L, Yarnitsky D. Association between quantitative sensory testing, treatment choice, and subsequent pain reduction in vulvar vestibulitis syndrome. J.Pain. 2004;5:226–232. doi: 10.1016/j.jpain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ, Rounsefell B. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J.Pain. 2009;10:316–322. doi: 10.1016/j.jpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Hsu YW, Somma J, Hung YC, Tsai PS, Yang CH, Chen CC. Predicting Postoperative Pain by Preoperative Pressure Pain Assessment. Anesthesiology. 2005;103:613–618. doi: 10.1097/00000542-200509000-00026. [DOI] [PubMed] [Google Scholar]
- 51.Incel NA, Erdem HR, Ozgocmen S, Catal SA, Yorgancioglu ZR. Pain pressure threshold values in ankylosing spondylitis. Rheumatol.Int. 2002;22:148–150. doi: 10.1007/s00296-002-0211-1. [DOI] [PubMed] [Google Scholar]
- 52.Jacobsen PB, Butler RW. Relation of cognitive coping and catastrophizing to acute pain and analgesic use following breast cancer surgery. J.Behav.Med. 1996;19:17–29. doi: 10.1007/BF01858172. [DOI] [PubMed] [Google Scholar]
- 53.Jamison RN, Link CL, Marceau LD. Do pain patients at high risk for substance misuse experience more pain? A longitudinal outcomes study. Pain Med. 2009;10:1084–1094. doi: 10.1111/j.1526-4637.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 54.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. Pain. 2010;150:390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jan SA. Introduction: landscape of opioid dependence. J.Manag.Care Pharm. 2010;16:S4–S8. doi: 10.18553/jmcp.2010.16.S1-B.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kidner CL, Gatchel RJ, Mayer TG. MMPI disability profile is associated with degree of opioid use in chronic work-related musculoskeletal disorders. Clin.J.Pain. 2010;26:9–15. doi: 10.1097/AJP.0b013e3181af13ed. [DOI] [PubMed] [Google Scholar]
- 57.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J.Bone Joint Surg.Am. 2009;91:919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuner R. Central mechanisms of pathological pain. Nat.Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 59.Kuzminskyte R, Kupers R, Videbech P, Gjedde A, Fink P. Increased sensitivity to supra-threshold painful stimuli in patients with multiple functional somatic symptoms (MFS) Brain Res.Bull. 2010;82:135–140. doi: 10.1016/j.brainresbull.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Lee JE, Watson D, Frey Law LA. Lower-order pain-related constructs are more predictive of cold pressor pain ratings than higher-order personality traits. J.Pain. 2010a;11:681–691. doi: 10.1016/j.jpain.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, Edwards RR. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res. 2011 doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. J.Bone Joint Surg.Br. 2008;90:166–171. doi: 10.1302/0301-620X.90B2.19640. [DOI] [PubMed] [Google Scholar]
- 63.Michna E, Jamison RN, Pham LD, Ross EL, Janfaza D, Nedeljkovic SS, Narang S, Palombi D, Wasan AD. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin.J.Pain. 2007;23:173–179. doi: 10.1097/AJP.0b013e31802b4f95. [DOI] [PubMed] [Google Scholar]
- 64.Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Palombi D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J.Pain Symptom.Manage. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Mounce C, Keogh E, Eccleston C. A principal components analysis of negative affect-related constructs relevant to pain: evidence for a three component structure. J.Pain. 2010;11:710–717. doi: 10.1016/j.jpain.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, Jamison RN. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J.Pain. 2008;9:254–264. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Nielsen PR, Norgaard L, Rasmussen LS, Kehlet H. Prediction of post-operative pain by an electrical pain stimulus. Acta Anaesthesiol.Scand. 2007;51:582–586. doi: 10.1111/j.1399-6576.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- 68.Nikolajsen L, Ilkjaer S, Jensen TS. Relationship between mechanical sensitivity and postamputation pain: a prospective study. Eur.J.Pain. 2000;4:327–334. doi: 10.1053/eujp.2000.0194. [DOI] [PubMed] [Google Scholar]
- 69.Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33:90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 70.Park J, Lavin R. Risk Factors Associated With Opioid Medication Misuse in Community-dwelling Older Adults With Chronic Pain. Clin.J.Pain. 2010;26:647–655. doi: 10.1097/AJP.0b013e3181e94240. [DOI] [PubMed] [Google Scholar]
- 71.Prommer EE. Opioid-induced pain. J.Clin.Oncol. 2008;26:3464–3465. doi: 10.1200/JCO.2008.17.3633. [DOI] [PubMed] [Google Scholar]
- 72.Pukall CF, Binik YM, Khalife S, Amsel R, Abbott FV. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002;96:163–175. doi: 10.1016/s0304-3959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 73.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - New perspective of opioid-induced hyperalgesia. Pain. 2008;15:431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Ren ZY, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204:423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reznikov I, Pud D, Eisenberg E. Oral opioid administration and hyperalgesia in patients with cancer or chronic nonmalignant pain. Br.J.Clin.Pharmacol. 2005;60:311–318. doi: 10.1111/j.1365-2125.2005.02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006a;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 77.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur.J.Pain. 2006b;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 78.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp.Clin.Psychopharmacol. 2008;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679–684. [PubMed] [Google Scholar]
- 80.Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain. 2008;140:420–428. doi: 10.1016/j.pain.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Soyupek S, Bozlu M, Armagan A, Ozorak A, Perk H. Does experimental pain assessment before biopsy predict for pain during transrectal ultrasound-guided prostate biopsy? Urology. 2007;70:681–684. doi: 10.1016/j.urology.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 82.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan BJ, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150:332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch.Intern.Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 86.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J.Pain. 2009;10:1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin.J.Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 88.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319–324. doi: 10.1016/S0304-3959(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 89.Verbunt JA, Seelen HA, Vlaeyen JW, Bousema EJ, van der Heijden GJ, Heuts PH, Knottnerus JA. Pain-related factors contributing to muscle inhibition in patients with chronic low back pain: an experimental investigation based on superimposed electrical stimulation. Clin.J.Pain. 2005;21:232–240. doi: 10.1097/00002508-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999–2006. NCHS Data Brief. 2009:1–8. [PubMed] [Google Scholar]
- 91.Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin.J.Pain. 2007;23:307–315. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- 92.Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield SF, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin.J.Pain. 2009;25:193–198. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 94.Wasan AD, Kaptchuk TJ, Davar G, Jamison RN. The association between psychopathology and placebo analgesia in patients with discogenic low back pain. Pain Med. 2006;7:217–228. doi: 10.1111/j.1526-4637.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 95.Weisner CM, Campbell CI, Ray GT, Saunders K, Merrill JO, Banta-Green C, Sullivan MD, Silverberg MJ, Mertens JR, Boudreau D, Von Korff M. Trends in prescribed opioid therapy for non-cancer pain for individuals with prior substance use disorders. Pain. 2009;145:287–293. doi: 10.1016/j.pain.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100:115–119. doi: 10.1097/00000542-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 97.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]