Abstract
Background
A continuing controversy exists about whether, asbestos exposure is associated with significant lung function impairments when major radiological abnormalities are lacking. We conducted a systematic review and meta-analysis in order to assess whether asbestos exposure is related to impairment of lung function parameters independently of the radiological findings.
Methods
MEDLINE was searched from its inception up to April 2010. We included studies that assessed lung function parameters in asbestos exposed workers and stratified subjects according to radiological findings. Estimates of VC, FEV1 and FEV1/VC with their dispersion measures were extracted and pooled.
Results
Our meta-analysis with data from 9,921 workers exposed to asbestos demonstrates a statistically significant reduction in VC, FEV1 and FEV1/VC, even in those workers without radiological changes. Less severe lung function impairments are detected if the diagnoses are based on (high resolution) computed tomography rather than the less sensitive X-ray images. The degree of lung function impairment was partly related to the proportion of smokers included in the studies.
Conclusions
Asbestos exposure is related to restrictive and obstructive lung function impairment. Even in the absence of radiological evidence of parenchymal or pleural diseases there is a trend for functional impairment.
Keywords: Asbestos, lung function, chest X-ray, computed tomography, meta-analysis
Introduction
Asbestos fibres are one of the most pervasive environmental hazards because of their worldwide use in the last 100 years as a cheap and effective thermal, sound and electrical insulation material, especially in the construction, shipping and textile industries. The general public is also exposed to asbestos, mainly from deterioration and reconstruction or destruction of asbestos contaminated buildings, worn vehicle brake linings and from the deterioration of asbestos-containing products. In spite of outright bans or restrictions in nearly all industrialised countries nowadays, approximately 125 million workers are occupationally exposed to asbestos worldwide [1] and it is estimated that at least 100,000 die annually from complications of asbestos exposure [2]. In addition to mesothelioma, lung and laryngeal cancer, asbestos has long been known to cause non-malignant pleural fibrosis, (i.e. circumscript pleural plaques (PP), or diffuse pleural thickening (DPT)), pleural effusions, rounded atelectasis and lung fibrosis (asbestosis). Since inhalation of high doses of asbestos fibres may lead to a variety of functional impairments, the monitoring of workers who have been exposed to asbestos, particularly of their lung function, has gained in importance over the years. The identification of functional abnormalities is also relevant for compensation issues. While compromised lung function in pronounced disease is widely accepted, controversies still remain about a possible relationship between earlier or milder non-malignant asbestos-induced pleural or parenchymal fibrosis and reduced lung function measurements [3-11]. The American Thoracic Society and the American College of Chest Physicians [12,13], in particular, have lamented the lack of definitive knowledge in the prevalence and clinical relevance of asbestos-induced obstructive airway diseases and have determined to make this a priority for investigation and elucidation.
We have conducted a systematic review and a meta-analysis of the literature with the aim of identifying and quantifying alterations of lung function parameters in subjects occupationally exposed to asbestos. The leading question was whether occupational exposure to asbestos leads to impairments of lung function independently from the non-malignant radiological findings (i.e. normal chest radiograph (X-ray) or (high resolution) computed tomography (HR)CT, pleural plaques and diffuse pleural thickening or asbestosis).
Materials and methods
Selection criteria
We included publications that assessed lung function parameters and radiological imaging (chest X-Ray or (HR)CT) in persons with occupational exposure to asbestos. Only studies that applied an internationally accepted quality standard for lung function testing (i.e. ATS standard, ERS standard) and that provided information about the corresponding reference values or used reference group were considered. We included only studies reporting lung function parameters expressed as percent-predicted with a corresponding dispersion measure (i.e. standard deviation, standard error or confidence interval) and assigned them to one of the following radiological categories:
A. "Normal imaging", i.e. absence of pleural or lung parenchymal abnormalities.
B. "Pleural fibrosis", i.e. presence of pleural plaques and/or diffuse pleural thickening.
C. "Asbestosis", i.e. parenchymal fibrosis with or without pleural fibrosis.
To be included, studies had to provide data on the proportion of smokers among participants or on the dose (pack-years).
In a few potentially relevant studies the authors failed to report all information listed above (e.g. reference values, quality standards, dispersion measures), thus we tried to contact the authors in order to collect the missing data. Only three authors sent additional information that enabled us to include their publication in the meta-analysis.
Search strategy
MEDLINE was searched from its inception to April 2010 via PubMed with the following search strategy:
("Asbestosis"[Mesh] OR ("Pleural Diseases"[Mesh] AND "Asbestos"[Mesh]) OR ("occupational exposure"[Mesh] AND "Asbestos"[Mesh]) OR ("Lung diseases"[Mesh] AND "Asbestos"[Mesh])) AND "Respiratory Function Tests"[Mesh] AND ("occupational diseases"[Mesh] OR "occupational health"[Mesh] OR "occupational exposure"[Mesh])
We applied the following PubMed limits in order to increase the specificity of our search:
("humans"[MeSH Terms] AND (English[lang] OR German[lang]) AND "adult"[MeSH Terms]) NOT ("Bronchoalveolar Lavage"[MeSH] OR "Neoplasms"[Mesh] OR "Case Reports "[Publication Type]).
Additionally, we scanned congress proceedings, reference lists of relevant articles and searched our own archive for further potentially relevant publications not identified through the electronic search.
Data extraction
We extracted information on sample size, exposure to asbestos, proportion of non-smokers, radiological imaging method and lung function reference values together with the estimates for vital capacity (VC), forced expiratory volume in the first second (FEV1) and FEV1/VC with their corresponding SD, SE or 95% CI. Most of the studies reported forced vital capacity (FVC), but in some papers it was not clear whether FVC or slow (relaxed) vital capacity (SVC) was measured. Data were extracted by at least two of the authors independently from each other and discrepancies were solved by consensus after discussion. (HR)CT-based diagnoses were favoured over those based on X-rays when both were available.
Data synthesis and statistical methods
We performed a meta-analysis to produce pooled estimates of VC, FEV1 and FEV1/VC for each of our designated radiological categories (A, B or C). Within each radiological category, we conducted subgroup analysis according to the type of imaging method used for the diagnosis (X-ray or (HR)CT).
Some studies reported results for different degrees of radiological impairments within the same category (e.g. different ILO scores for asbestosis). In these cases, we pooled the subgroup estimates from the same study with a fixed effects model to obtain a single estimate for each study within each radiological category (A-C).
A random effects model was used to calculate overall estimates for each radiological category.
We calculated I2 as an indicator for the degree of heterogeneity across studies. Values of I2 under 25% indicate low, up to 60% medium and over 75% considerable heterogeneity, making it advisable to perform the analysis using the random effects model [14]. In order to assess whether any observed between-study heterogeneity could be explained through study characteristics other than radiological imaging procedure, we also performed subgroup analysis for the proportion of never-smokers. For this purpose, we divided the study pool into two categories: studies with <25% of participants reporting to have never-smoked and studies with >= 25% of participants reporting to have never-smoked.
A second subgroup analysis was done for mean duration of asbestos exposure, dividing the study pool into two categories: studies reporting mean exposure duration longer than the median duration of the whole sample vs. studies with mean exposure duration shorter than median duration. In addition, we performed meta-regression analysis with the proportion of never-smokers and with the years of asbestos-exposed occupation.
All calculations were performed with the software Comprehensive Meta-Analysis 2.0. (Biostat™, Englewood, USA). Forest plot graphics were produced with Meta-Analyst Software [15]
Results
A total of 542 papers were identified by the electronic literature database search and a further 46 papers through manual searching in congress reports, reference scanning and from our own archive (Figure 1). After scanning titles and abstracts, 289 articles were selected for a detailed assessment of the full publication. From these 289 articles, 30 met the inclusion criteria for the meta-analysis. The most frequent reasons for exclusion were lack of information about lung function parameters and/or about radiological diagnoses and lack of reporting statistical dispersion measures.
Figure 1.
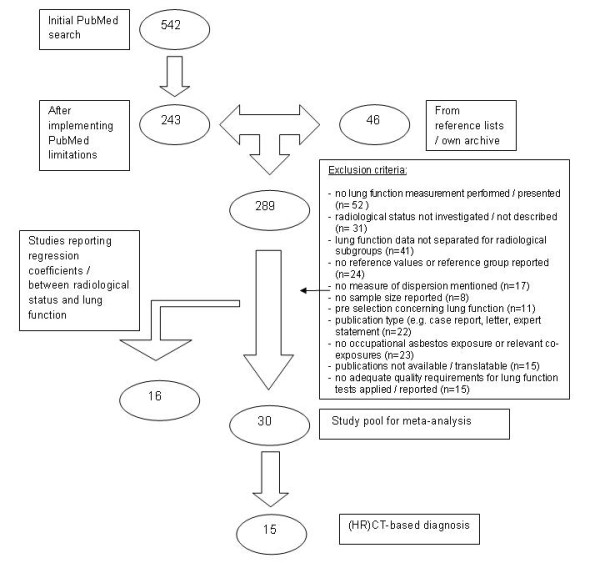
Flow chart - Study selection process.
We included 27 cross-sectional studies, one case-control and two follow-up studies, comprising a total of 15,097 subjects of which the data for 9,921 were reported appropriately for inclusion in our meta-analysis. The characteristics of the included studies are shown in Table 1. Sample size ranged from 19 to 3,383. Some studies focussed on a specific occupation (e.g. asbestos manufacturing, insulation and cladding work, shipyard, asbestos industries, asbestos cement factory, ceiling tiles and wallboards, railway, ironworker, sheet metal, construction carpenters and millwrights) while others included subjects from different occupational fields. The mean duration of occupational exposure to asbestos was reported in 22 studies (i.e. 73% of the study sample) and ranged from 8.4 ± 6.1 to 32.7 ± 6.7 years (mean ± SD). The latency time (i.e. the time since first exposure) was reported in only 9 studies (i.e. 30%) and ranged from 24.5 ± 5.7 to 43.3 ± 6.7 years (mean ± SD). Estimations of asbestos fibre concentration (i.e. fibre-years) were reported only rarely [16,17].
Table 1.
Characteristics of included studies
| Reference | Study type | Study size | N (in meta-analysis) | Asbestos exposure | Smoking habits | Radiological chest imaging | Lung function | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occupation | Duration (yr) | Latency (yr) | non smokers (%) | Pack-years | Quality requirements | Reference values | ||||||||
| mean | SD | Mean | SD | mean | SD | |||||||||
| Ameille et al. 2004 [70] | CS | 287 | 228 | asbestos industry | 25.8 | 9.4 | 33.2 | 9.4 | 38.1 | nr | nr | HRCT | ATS 1987 | ATS 1987 |
| Begin et al. 1993 [71] | CS | 61 | 46 | asbestos industry | 22.0 | 15.6§ | nr | nr | 21.3 | 28.0 | 23.4§ | X-ray/HRCT | Bates 1971 | Bates 1971 |
| Begin et al. 1995 [72] | CS | 207 | 96 | diverse | 26.0 | 13.7§ | nr | nr | 13.5 | 29.4 | 20.6§ | X-ray/HRCT | Bates 1971 | Bates 1971 |
| Van Cleemput et al. 2001 [16] | CS | 94 | 73 | asbestos industry | 25.0 | 1.4 | nr | nr | 15.0 | 10.9 | 20.6 | HRCT | ECSC/ERS | Quanjer 1993 |
| Delpierre et al. 2002 [55] | CS | 97 | 38 | asbestos industry | 19.0 | 2.0 | nr | nr | 37.0 | nr | nr | X-ray | Quanjer 1983 | Quanjer 1993 |
| Garcia-Closas and Christiani 1995 [60] | CS | 631 | 541 | construction/millwright | 20.0 | 10.2 | nr | nr | 33.1 | 24.1 | 21.3 | X-ray | ATS 1987 | Crapo 1981 |
| Hall and Cissik 1982 [24] | CS | 135 | 113 | diverse | #18.0 | 11.2 | nr | nr | 40.7 | #21.2 | 19.5 | X-ray | (ATS) OSHA 1978 | Knudson 1983 |
| Harkin et al. 1996 [73] | CS | 107 | 37 | diverse | nr | nr | 32.5 | 9.5§ | 21.6 | 29.2 | 23.3§ | X-Ray/HRCT | ATS 1986 | Knudson 1983 |
| Jarad et al. 1992 [74] | CS | 60 | 60 | diverse | 10m | 1-35r | 34m | 21-60r | 13.3 | 21m | 0-76r | X-Ray/HRCT | ATS 1979 (Cotes) | Cotes 1979 |
| Kee et al. 1996 [75] | CC | 1150 | 93 | shipyard/construction | 25.5 | 12.1 | 41 | 11.3 | nr | 23.9 | 25.7 | HRCT | ATS 1987 | Crapo 1981; ATS 1987 |
| Kouris et al. 1991 [76] | CS | 996 | 913 | ceiling and wall | 8.4 | 6.1 | 26.8 | 5.1 | nr | 17.6 | 19.1 | X-ray | ATS 1979 | Crapo 1981 |
| Lilis et al. 1991 [59]* | CS | 2790 | 1536 | asbestos insulation | nr | nr | 35.1 | 7.2§ | 46.6 | nr | nr | X-ray | ATS 1987 | ATS 1987 |
| Nakadate et al. 1995 [77] | FU | 242 | 27 | asbestos industry | nr | nr | nr | nr | 26.9 | nr | nr | X-ray | ATS 1978 | Pneumoconiosis law of Japan 1978 |
| Neri et al. 1996 [25] | CS | 119 | 38 | diverse | 10.9 | 6.1 | 24.5 | 5.7 | 26.3 | 14.0 | 11.9 | X-Ray/HRCT | ATS 1987 | Paoletti 1985 |
| Niebecker at al. 1995 [9] | CS | 382 | 194 | diverse | nr | nr | nr | nr | 28.9 | nr | nr | X-ray | according to ERS/ATS | EGKS 1971 |
| Ohar et al. 2004 [4] | CS | 3383 | 3240 | diverse | nr | nr | 41.1 | 10.3 | 21.8 | 38.9 | 29.4 | X-ray | ATS 1987 | ATS 1987 |
| Oldenburg et al. 2001 [26] | CS | 43 | 43 | diverse | 30.7 | nr | nr | nr | 27.9 | nr | nr | X-ray and CT | ATS 1987 | Brändli 1996 |
| Oliver et al. 1988 [56] | CS | 383 | 359 | railway | 29.2 | 13.4 | 35.6 | 15.0 | 26.2 | 23.4 | 25.1 | X-ray | ATS 1979,1987 | Crapo 1981 |
| Paris et al. 2004 [17] | CS | 706 | 51 | asbestos industry | 24.9 | 9.1 | nr | nr | #31.4 | nr | nr | X-ray/HRCT | ATS 1986 | Quanjer 1993 |
| Petrovic et al. 2004 [18] | CS | 120 | 120 | asbestos cement fabric | 20.0 | 9.8 | nr | nr | 100 | - | - | X-ray | CECA 1972 | Quanjer 1993 |
| Piirilä et al. 2005 [78] | CS | 590 | 367 | diverse | #25.7 | 9.4 | nr | nr | 3.0 | #21.0 | 13.7 | HRCT | ERS (Quanjer 1992) | Viljanen 1982 |
| Prince et al. 2008 [79] | CS | 19 | 19 | diverse | nr | nr | nr | nr | 15.8 | 23.5 | 14.5 | X-ray/CT | ATS 2005 | Knudson 1983 |
| Robins and Green 1988 [57] | CS | 182 | 73 | asbestos industry | 30.2 | nr | nr | nr | 18.8 | 22.9 | 16.3 | X-ray | Crapo 1981 | Crapo 1981 |
| Rösler and Woitowitz 1990 [19] | CS | 144 | 20 | diverse | 15.6 | 6.0 | nr | nr | 100 | - | - | X-ray | according to ERS/ATS | Quanjer 1983 |
| Rui et al. 2004 [61] | FU | 103 | 103 | diverse | 25.0 | 7.0 | nr | nr | 36.0 | nr | nr | HRCT | CECA 1971 | Quanjer 1983 |
| Schwartz et al. 1990 [58] | CS | 1211 | 1209 | sheet metal | 32.7 | 6.7 | nr | nr | 20.3 | 26.9 | 29.4 | X-ray | ATS 1972 | Knudson 1983 |
| Schwartz et al. 1993 [33] | CS | 60 | 60 | sheet metal | >= 1 | nr | >= 20 | nr | 22.0 | 28.2 | 23.0 | X-ray | ATS 1979 | Moris 1971; Goldman 1959 |
| Sette et al. 2004 [80] | CS | 87 | 82 | cement/chrysotile miner | #13.4 | 11.7 | nr | nr | nr | #30.7 | 21.9 | CT | ATS 1995 | Pereira 1992 |
| Vierikko et al. 2010 [81] | CS | 627 | 86 | diverse | #18.2 | 11.7 | #43.3 | 6,7 | #16,9 | #15.5 | 16,9 | HRCT | according to ERS/ATS | Viljanen 1982 |
| Zejda 1989 [82] | CS | 81 | 56 | asbestos cement industry | 17.4 | 6.9 | nr | nr | 16.1 | nr | nr | X-ray | CECA 1965 | Quanjer 1993 |
Main characteristics of the Studies included in the meta-analysis. SD: standard deviation, CI: confidence interval CC: Case-control, CS: Cross-sectional; FU: follow-up; nr: not reported; m: median; r: range; X-Ray: chest X-ray; HRCT: high resolution computer tomography; CT: computer tomography; #:for the included subjects; §: calculated from SE. *Additional information obtained from [83]
Except for two studies [18,19], all included current and/or former smokers. The proportion of participants reporting to be never-smokers ranged across the studies from only 3% to 100% (median 26.2%), with three studies not reporting the proportion of never-smokers. Smoking severity was reported in 18 of the studies that included smokers and ranged from 14.0 ± 11.9 to 38.9 ± 29.4 pack-years (mean ± SD).
Radiological imaging was done relying exclusively on chest X-ray in 15 studies and relying exclusively on CT or HRCT in 7 studies. Eight studies considered both chest X-ray and CT/HRCT. Mainly VC, FEV1 or FEV1/VC, or combinations of these parameters, were reported. Some studies provided additional parameters, but due to their scarcity and heterogeneity in assessment methods we did not include them in the meta-analysis. In all studies, lung function test results were acquired according to a quality standard, with the majority (67%) following the American Thoracic Society (ATS) standard procedure available at the time. There was considerable heterogeneity regarding the reference values used to calculate "percent of predicted", with a total of 12 different reference values used across the included studies. The most frequently used reference values were those proposed by Quanjer 1983/1993 [20,21] (n = 5 studies), followed by those of the ATS [22] and Knudson 1983 [23] (both in 4 studies each).
Quantitative data synthesis
Figures 2, 3 and 4 provide an overview of the pooled estimates of lung function parameters according to radiological findings.
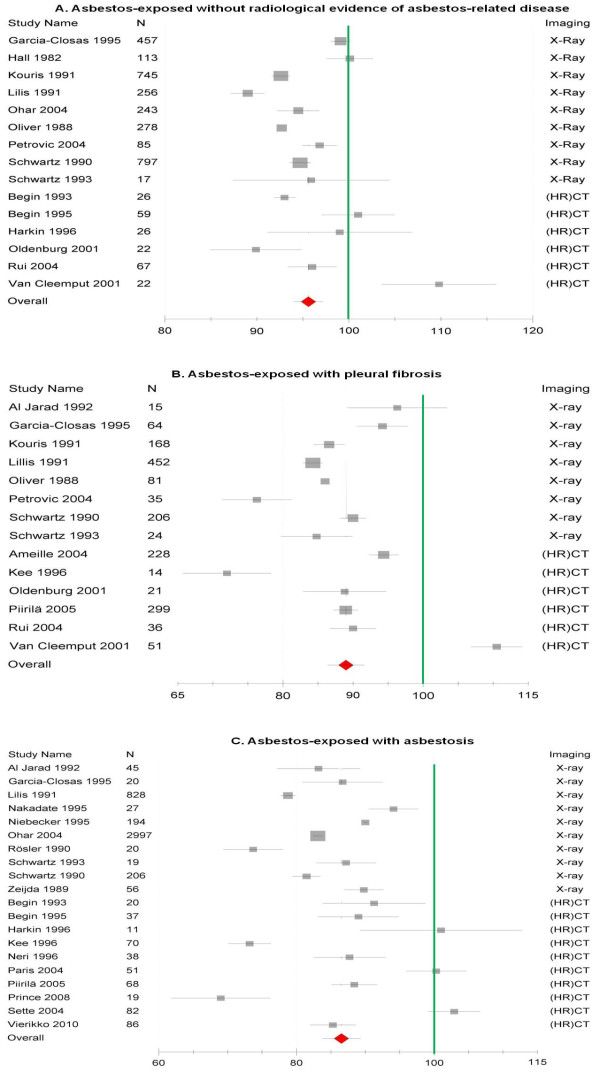
Figure 2.
Forest plot of FVC (expressed as percent predicted with 95%CI) in asbestos-exposed collectives grouped according to the radiological status. 2A shows the subgroups without asbestos-related diseases, 2B shows the subgroups with pleural fibrosis and 2C shows the subgroups with asbestosis.
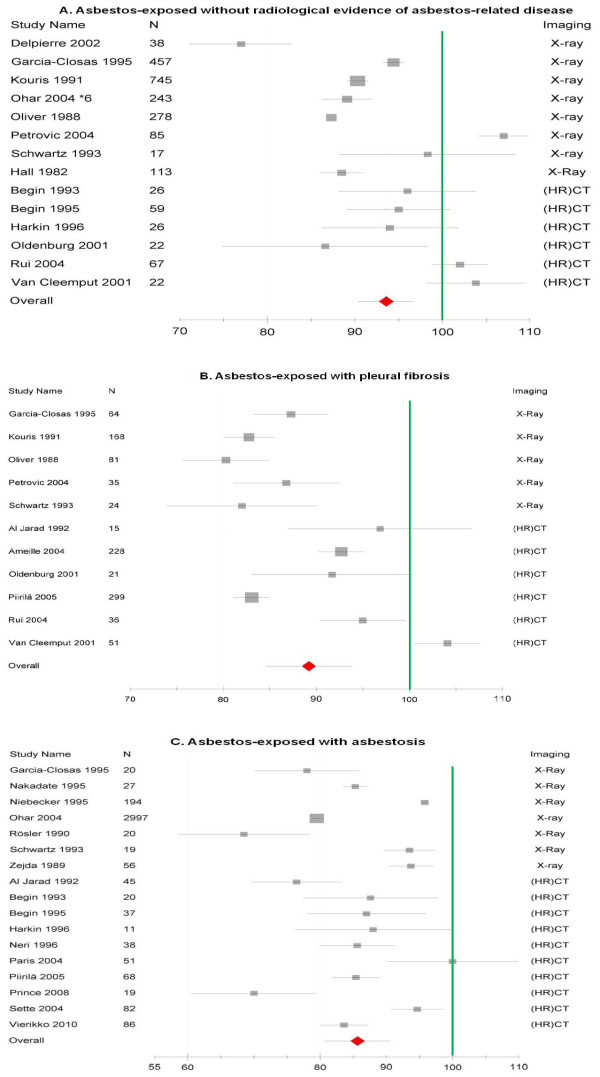
Figure 3.
Forest plot of FEV1 (expressed as percent predicted with 95%CI) in asbestos-exposed collectives grouped according to the radiological status. 3A shows the subgroups without asbestos-related diseases, 3B shows the subgroups with pleural fibrosis and 3C shows the subgroups with asbestosis.
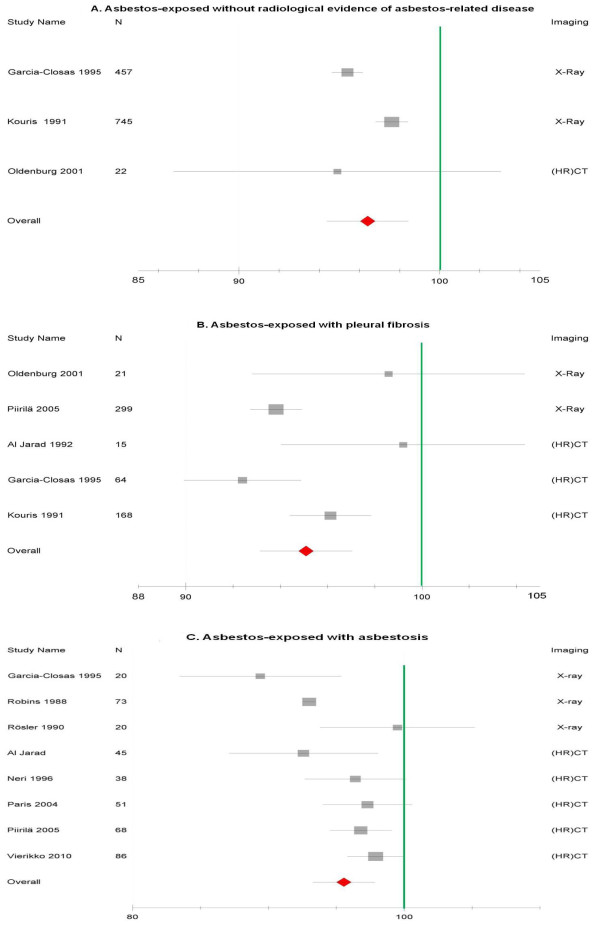
Figure 4.
Forest plot of FEV1/FVC (expressed as percent predicted with 95%CI) in asbestos-exposed collectives grouped according to the radiological status. 4A shows the subgroups without asbestos-related diseases, 4B shows the subgroups with pleural fibrosis and 4C shows the subgroups with asbestosis.
Vital capacity
Vital capacity (VC, FVC) was the parameter most commonly reported in an adequate manner for inclusion in our meta-analysis. Overall, asbestos-exposed workers showed an impairment of vital capacity when compared with reference values (Figure 2). This impairment of vital capacity was already manifest in workers without radiological evidence of asbestos-related pleural or parenchymal diseases (95.7%-predicted; 95%-CI 93.9, 97.3). The loss of vital capacity was most accentuated in subjects with radiological findings of asbestosis (86.5%-predicted; 95%-CI 83.7, 89.4). The subgroup analysis based on the radiological procedure showed lower estimates of vital capacity in all three radiological categories among studies using conventional chest X-ray compared with those using (HR)CT (Table 2).
Table 2.
Estimates of lung function according to radiological findings.
| Overall | Studies with X-ray | Studies with (HR)CT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Estimate | 95% CI | I2 (%) | n | Estimate | 95% CI | I2 (%) | n | Estimate | 95% CI | I2 (%) | |
| FVC (% predicted) | ||||||||||||
| Normal imaging | 15 | 95.7 | 93.9-97.3 | 94.8 | 9 | 94.9 | 92.9-96.9 | 96.2 | 6 | 97.1 | 94.2-100.1 | 89.1 |
| Pleural fibrosis | 14 | 89.0 | 86.5-91.5 | 96.1 | 8 | 87.1 | 83.9-90.4 | 89.5 | 6 | 91.6 | 87.8-95.4 | 96.8 |
| Asbestosis | 20 | 86.5 | 83.7-89.4 | 98.2 | 10 | 84.8 | 80.8-88.8 | 98.9 | 10 | 88.5 | 84.3-92.7 | 95.8 |
| FEV1 (% predicted) | ||||||||||||
| Normal imaging | 14 | 93.6 | 90.6-96.5 | 97.3 | 8 | 91.4 | 87.7-95.1 | 98.0 | 6 | 97.4 | 92.5-102.2 | 64.7 |
| Pleural fibrosis | 11 | 89.2 | 84.7-93.7 | 93.7 | 5 | 83.9 | 77.2-90.5 | 42.0 | 6 | 93.7 | 87.6-99.9 | 95.8 |
| Asbestosis | 17 | 85.7 | 80.6-90.7 | 98.8 | 7 | 85.5 | 77.8-93.1 | 99.5 | 10 | 85.8 | 79.2-92.5 | 80.8 |
| FEV1/FVC (% predicted) | ||||||||||||
| Normal imaging | 3 | 96.4 | 94.3-98.5 | 86.9 | 2 | 97.4 | 92.5-102.2 | 64.7 | 1 | 94.9 | 86.8-103.0 | - |
| Pleural fibrosis | 5 | 95.4 | 92.7-98.1 | 68.7 | 2 | 93.7 | 87.6-99.9 | 95.8 | 3 | 96.3 | 92.6-100.1 | 68.1 |
| Asbestosis | 8 | 95.5 | 94.1-96.9 | 83.8 | 3 | 85.8 | 79.2-92.5 | 80.8 | 5 | 97.0 | 95.7-98.3 | 0.0 |
Comparison of imaging procedure.
Estimates for forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and the ratio of both parameters (FEV1/FVC) for each radiological subgroup. Results are shown for all included studies as well as separated according to the radiological method used for the diagnosis (conventional chest X-ray or (high resolution) computed tomography. Estimates are expressed as percent predicted together with confidence interval (CI) and I2 as a measure of heterogeneity, n = number of studies included in each subgroup.
Heterogeneity was very high in all three radiological subgroups (I2 >90%) and remained after subgroup analysis according to radiological procedure.
FEV1
As for vital capacity, asbestos-exposed workers showed an impairment of FEV1 which was already present in workers with no radiological evidence of asbestos-related disease and was considerably more pronounced in subjects with radiological signs of asbestos-related pleural and/or parenchymal diseases (Figure 3). Again, the subgroup analysis showed differences between studies using chest X-ray and studies using (HR)CT (Table 2). The differences between both imaging procedures were particularly pronounced for subjects identified as having asbestos-related pleural disease. For this group of patients, the estimate of FEV1 obtained from the subgroup of studies using conventional X-ray was about 10 percent lower than estimate obtained from HR(CT) studies (83.9%-predicted; 95% CI 77.2, 90.5 vs. 93.7%-predicted; 95% CI 87.6, 99.9) (Table 2).
Heterogeneity was also very high for these analysis (I2 >90%), but decreased to some extent when grouping studies according to radiological technique.
FEV1/VC
FEV1/VC was less commonly reported in an adequate manner for inclusion in our analysis. Slight FEV1/VC reductions were already seen in workers even without radiological signs of disease, and were similar to those seen for workers with evidence of pleural disease and for those with signs of lung fibrosis related to asbestos (Figure 4). As for the other lung function parameters, there were differences between studies according to the radiological method used, with a tendency to lower FEV1/VC among the studies using chest X-ray.
Heterogeneity was considerable (I2 >60%) but not as pronounced as for the other lung function parameters.
Subgroup analysis and meta-regression
Smoking
Few studies reported estimates stratified by smoking status and radiological category. The proportion of never-smokers was reported in 27 studies. The lung function estimates derived from the subgroup analysis showed greater impairment among studies with more than 25% of participants reporting to be never-smokers for subjects without radiological evidence of asbestos-related disease and in those with pleural fibrosis (Table 3). In the group of workers showing radiological evidence of asbestosis lung function impairments were strongest and a bit more pronounced in the subgroup of studies with a lower proportion of never-smokers.
Table 3.
Estimates of lung function according to radiological findings.
| Overall | Studies with <25% non-smokers | Studies with >25% non-smokers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Estimate | 95% CI | I2 (%) | n | Estimate | 95% CI | I2 (%) | n | Estimate | 95% CI | I2 (%) | |
| FVC (% predicted) | ||||||||||||
| Normal imaging | 14 | 96.1 | 93.9-98.2 | 95.1 | 6 | 98.1 | 94.6-101.6 | 88.0 | 8 | 94.9 | 92.3-97.5 | 96.6 |
| Pleural fibrosis | 12 | 90.3 | 87.4-93.3 | 96.5 | 6 | 93.2 | 88.9-97.5 | 95.9 | 6 | 87.7 | 83.7-91.8 | 95.4 |
| Asbestosis | 18 | 86.4 | 83.2-89.6 | 98.1 | 12 | 85.9 | 81.9-89.8 | 83.7 | 6 | 87.4 | 81.9-92.7 | 98.9 |
| FEV1 (% predicted) | ||||||||||||
| Normal imaging | 13 | 93.9 | 90.0-97.8 | 97.4 | 5 | 97.5 | 90.9-104.1 | 35.4 | 8 | 92.0 | 87.2-96.8 | 98.3 |
| Pleural fibrosis | 10 | 89.9 | 84.1-95.7 | 93.6 | 5 | 91.5 | 83.2-99.9 | 96.3 | 5 | 88.5 | 80.4-96.5 | 86.2 |
| Asbestosis | 16 | 85.2 | 81.4-89.1 | 98.9 | 11 | 84.2 | 79.5-88.8 | 92.2 | 5 | 87.6 | 80.7-94.4 | 97.5 |
| FEV1/FVC (% predicted) | ||||||||||||
| Normal imaging | 2 | 95.4 | 94.6-96.2 | 0.0 | 2 | 95.4 | 94.6-96.2 | 0.0 | - | - | - | |
| Pleural fibrosis | 4 | 95.4 | 91.5-99.3 | 62.5 | 2 | 95.9 | 90.6-101.3 | 74.9 | 2 | 94.9 | 89.2-110.5 | 73.2 |
| Asbestosis | 8 | 95.6 | 93.2-97.9 | 83.8 | 4 | 96.3 | 94.2-98.4 | 55.3 | 4 | 95.3 | 92.2-98.3 | 89.8 |
Subgroup analysis according to % of never-smokers.
Estimates for forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and the ratio of both parameters (FEV1/FVC) for each radiological subgroup. Results are shown for all included studies as well as separated according to the proportion of non-smokers included in each subgroup (less ore more than 25%). Estimates are expressed as percent predicted together with confidence interval (CI) and I2 as a measure of heterogeneity, n = number of studies included in each subgroup.
In the regression analysis of the effect of the proportion of non-smokers on estimates of FEV1, those studies with a higher proportion of never-smokers tended to show less impairment of this parameter (not statistically significant) for all three radiological categories.
Table 4 shows the results of three studies [24-26] reporting estimates for non-smokers and smokers without radiological evidence of parenchymal disease. These papers suggest mainly a synergistic effect of smoking and asbestos exposure.
Table 4.
Asbestos-exposed workers without radiological evidence of parenchymal disease stratified by smoking status.
| Non-smokers | Smokers | ||||||
|---|---|---|---|---|---|---|---|
| Studie | n | % predicted | SD | n | % predicted | SD | |
| Hall 1982 | FEV1 | 46 | 101.0 | 13.6 | 67 | 92.5 | 14.9 |
| FVC | 102.2 | 11.6 | 99.2 | 13.4 | |||
| Neri 1996 | FEV1 | 34 | 90.9 | 15.6 | 47 | 92.0 | 14.0 |
| FVC | 89.7 | 14.9 | 90.9 | 14.3 | |||
| FEV1/FVC | 100.3 | 10.9 | 100.2 | 6.8 | |||
| Oldenburg 2001 | FEV1 | 12 | 105.7 | 13.6 | 31 | 83.6 | 25.1 |
| FVC | 96.1 | 10.9 | 86.7 | 12.6 | |||
| FEV1/FVC | 102.3 | 4.39 | 94.5 | 18.6 | |||
Differences in forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and the ratio of both parameters (FEV1/FVC) between asbestos exposed non-smokers and smokers without radiological evidence of asbestosis. Estimates expressed as percent predicted together with standard deviation (SD) and I2 as a measure of heterogeneity, n = number of subjects included in each subgroup.
Duration of asbestos exposure
Mean exposure duration was reported in 23 studies. The data was heterogeneous (Table 5). FEV1 was consistently better across all radiological categories in the subgroup of studies with a mean exposure length of more than 22 years. In contrast, FEV1/VC was consistently better across all radiological subgroups for the studies with shorter mean exposure duration. The results for FVC were inconsistent. The regression analysis, however, indicated that lower FVC and FEV1 could be expected with increasing mean exposure duration.
Table 5.
Estimates of lung function according to radiological findings.
| Overall | Studies <22 yr. mean exposure | Studies >22 yr. mean exposure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Estimate | 95% CI | I2 (%) | n | Estimate | 95% CI | I2 (%) | n | Estimate | 95% CI | I2 (%) | |
| FVC (% predicted) | ||||||||||||
| Normal imaging | 11 | 96.2 | 94.4-98.0 | 95.9 | 4 | 97.0 | 94.2-99.8 | 96.5 | 7 | 95.7 | 93.4-98.0 | 90.8 |
| Pleural fibrosis | 11 | 89.2 | 85.6-92.8 | 96.9 | 2 | 81.8 | 73.2-90.3 | 92.8 | 9 | 90.8 | 86.8-94.8 | 98.0 |
| Asbestosis | 12 | 87.4 | 82.2-92.6 | 95.5 | 5 | 87.9 | 79.9-95.9 | 96.1 | 7 | 87.0 | 80.2-93.9 | 95.0 |
| FEV1 (% predicted) | ||||||||||||
| Normal imaging | 11 | 93.7 | 89.3-98.1 | 97.9 | 5 | 91.8 | 85.5-98.1 | 97.4 | 6 | 95.5 | 89.3-101.7 | 96.1 |
| Pleural fibrosis | 9 | 89.2 | 83.9-94.5 | 94.8 | 2 | 84.7 | 73.5-95.8 | 35.5 | 7 | 90.6 | 84.6-96.5 | 95.5 |
| Asbestosis | 10 | 86.8 | 82.3-91.2 | 84.2 | 5 | 86.4 | 80.3-92.5 | 90.4 | 5 | 87.1 | 80.6-93.6 | 66.7 |
| FEV1/FVC (% predicted) | ||||||||||||
| Normal imaging | 3 | 96.4 | 94.3-98.5 | 86.9 | 2 | 96.5 | 94.3-98.7 | 93.4 | 1 | 94.9 | 86.2-103.6 | - |
| Pleural fibrosis | 4 | 95.5 | 92.9-96.2 | 68.2 | 1 | 96.2 | 94.4-97.8 | - | 3 | 93.8 | 91.9-95.8 | 48.1 |
| Asbestosis | 7 | 95.8 | 93.8-97.9 | 86.1 | 3 | 97.7 | 95.9-99.5 | 0.0 | 4 | 94.6 | 92.0-97.2 | 83.2 |
Subgroup analysis by mean exposure duration.
Differences in forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and the ratio of both parameters (FEV1/FVC) between subgroups with a mean exposure duration of less (<22 yr.) and more for than 22 years (>22 yr.). Results are shown for each radiological subgroup. Estimates are expressed as percent predicted together with confidence interval (CI) and I2 as a measure of heterogeneity, n = number of studies included in each subgroup.
Discussion
Several population-based studies provide evidence of asbestos exposure contributing significantly to the burden of airway diseases, but a detailed assessment of exposure was generally neither presented nor performed in such studies [27-29]. The pleural plaque incidence in the general population is in the range of 0.02 to 12.8% [30] and is 80-90% attributable to asbestos exposure [31]. The initial concern about the potential adverse effects of asbestos on lung function was vindicated in clinical as well as epidemiologic studies over many years [12,13]. The present meta-analysis has considered the major lung function parameters VC, FEV1, FEV1/VC, for asbestos-exposed workers grouped, according to their radiological diagnosis, into three groups: "absence of pleural and lung parenchymal fibrosis", diagnosed with "pleural fibrosis" (PP and/or DPT) or "asbestosis with or without pleural fibrosis". Overall, our analysis shows a statistically significant reduction of VC, FEV1 and FEV1/VC among workers exposed to asbestos compared to the general population (i.e. reference values).
The severity of the observed impairments is related to the degree of radiological abnormalities indicative of pleural fibrosis and asbestosis. Overall, VC and FEV1 scores were lowest for those workers showing radiological findings of asbestosis, followed by those with signs of pleural fibrosis. Workers exposed to asbestos with normal radiological findings (either X-ray or (HR)CT) exhibited significantly better VC and FEV1 scores than those with radiological abnormalities, but their decreased values indicate some degree of lung function impairment. FEV1/VC was slightly reduced in all groups. This reduction was more evident in the subgroups with radiological abnormalities. These differences between groups persisted mostly when the studies were analysed separately, according to the radiological methods used (either X-ray or (HR)CT), although less pronounced for the (HR)CT-based studies of the three subgroups of patients. In general, studies with (HR)CT based diagnosis report milder lung function impairments than those using conventional X-ray due to the higher sensitivity of the (HR)CT for mild grades of pleural disorders and asbestosis.
A positive relationship between the severity of functional impairment and the radiologically defined degree (score) of asbestos-related pleural and/or pulmonary fibrosis was already reported in a few studies [32-34]. As shown the absence of characteristic radiological findings does not exclude lung function abnormalities. Our meta-analysis revealed statistically significant deterioration in the lung function parameters for asbestos workers without any evidence of radiological abnormalities. These findings extend the meta-analysis by Filippelli, Martines et al [35] who found statistically significant reductions in all investigated lung function parameters in subjects exposed to asbestos, although the authors did not account for different radiological findings. Regression models reported in some of the included studies indicate that the radiological findings can only explain a small part of the variability in these parameters. Other authors have also reported a medium to low explanatory power of radiological findings for other lung function parameters [33,32].
There is evidence from clinical studies that discrepancies between lung function and radiological findings can be due to asbestos-induced pulmonary alterations not radiologically detectable. These studies describe multiple cellular lesions, apoptosis, inflammatory and profibrogenic responses, using histopathology and electron microscopy, as well as the synthesis of associated mediators and oxygen radicals [36-40]. It has been estimated that exposure to an asbestos fibre dose [41] of 25 fibre-years represents the inhalation of about 55 billion asbestos fibres [42], of which a significant proportion is deposited in the lung.
Our findings indicate not only the presence of restrictive but also of obstructive ventilation patterns in workers exposed to asbestos, either with or without asbestos-related radiological abnormalities: an issue of controversial discussion.
Recently, Dement et al. [43] found an overall COPD prevalence of 18.9% in asbestos workers/insulators. In their collective of older construction and trade workers, at the US Department of Energy with mixed exposure at nuclear sites, the prevalence of COPD was of 23% among those only with pleural changes and 32.3% among those with both pleural and parenchymal changes [43]. Conversely, Ameille et al. [44] reported a lack of association between occupational exposure to asbestos and airway obstruction. They determined that FEV1/FVC and FEV25-75 did not differ through the cumulative exposure classes and there was no significant correlation between cumulative exposure to asbestos and pulmonary function parameters nor with the proportion of abnormal pulmonary function tests [44]. However, these authors did not include a non-exposed control group and report generally elevated values for FVC, FEV1, FEV1/FVC and residual volume (RV), which can be explained by the selected study population (volunteers for a screening programme without previous severe respiratory disease).
Bias and limitations
The degree of lung function impairment may have been underestimated due to bias in the included studies. Two main sources of not negligible underestimation of adverse health effects in actual occupational cohort studies are the dilution effect and the comparison bias [45]. The dilution effect results from the inclusion of not or very low exposed workers in the study cohort. The comparison bias results from a healthy hire effects at the beginning of exposure history. The lung function of blue collar workers - like the ones included in our study - is typically better than the references taken from the general population (i.e. over 100% predicted) [46,47]. In those workers lung function values studied at a single time point may be still within the norm despite an underlying considerable absolute decrease since the start of exposure (e.g. a FEV1 fall from 115% to 95%). Comparison bias results also from the healthy worker effect in the course of the working life. Subjects with relevant health impairments may change their occupation or have a shortened work life and thus may not be available for recruiting to later lung function assessment based on occupation or worksite. For example Fell et al. [48] hypothesized in their investigation on respiratory symptoms and ventilatory function of workers exposed to cement dust that individuals susceptible to adverse respiratory effects from cement dust may have quitted work and therefore dropped out of the exposed groups. The authors found a high prevalence (55%) of respiratory symptoms and COPD in the group of former cement workers visited at home, underlying the importance of included former workers. These biases are probably present in the studies included in our systematic review, since most of them had a cross-sectional design not accounting for changes in lung function over time and in general did not consider former workers.
In our meta-analysis, there is a high degree of heterogeneity (high I2) across the studies, which we acknowledged by using a random effects model. Heterogeneity is caused by variations in the individual study populations as well as differences in study methods.
With respect to the study design, a major source of heterogeneity is the quality of lung function tests and the variety of references values used in the studies. We included predicted values, as given by the various authors with their considerable variation. For example, the reference values of Quanjer et al. [20,21] have been shown to be at least 10% too low for current normal populations [49-53], thus leading to an underestimation of the effects of asbestos exposure. The same is true for some other reference values based on inadequate reference populations.
The issue of the study population as a source of heterogeneity includes the following aspects: First, studies differed considerably in the duration of occupational exposure to asbestos, ranging from less than 1 year to over 30 years. The subgroup analysis indicated that the results for FEV1 and for FEV1/VC were negatively related to the duration of exposure. The meta-regression analysis indicated an inverse relationship between exposure duration and FVC and FEV1 (i.e. lower estimates with increasing mean exposure duration). However, this can only explain a small amount of heterogeneity. There are also major differences between studies regarding the intensity of exposure because of the wide variety of tasks and occupations studied. Since only two studies [41,54] reported an estimation of exposure intensity (i.e. fibre-years), we could not explore this source of heterogeneity in subgroup or regression analysis. Similarly, mean latency times were only reported in nine of the included studies, thus subgroup analysis or meta-regression to explore heterogeneity could not be performed.
An additional source of heterogeneity may be the differences in the distribution of confounders, such as smoking or co-exposure to other occupational noxae. Regarding co-exposures most of the studies provided little information and we could not explore this potential source of heterogeneity in detail.
An important question concerns the interaction between smoking and asbestos exposure. Only a few studies accounted for smoking in their analysis appropriately. In one of the two studies that included only never-smokers [18], reduced VC was reported for both asbestos-exposed workers without and with pleural fibrosis, and an impairment of FEV1 was seen in those with pleural fibrosis. The other study considering only never-smokers examined patients with asbestosis. Here all lung function parameters were correspondingly impaired [19].
Niebecker and colleagues showed for patients with asbestosis that the degree of impairment was greater among smokers [9]. Some of the included studies [16,33,55-61] reported multivariate linear regression models including smoking as an explanatory variable (among others). The results of these analyses suggest an association of lung function impairments with pleural abnormalities independent of smoking, i.e. when pleural fibrosis is present then impairments in lung function can be observed in both smokers and non-smokers.
At the study level, the results of subgroup analysis according to the proportion of never-smokers were inconsistent and partly counterintuitive, since for some parameters, the higher the proportion of non-smokers in a study, the lower were the estimates. An additional analysis using the mean pack-years - as an indication of the dose - was not performed, because one third of the included studies did not report the information.
Therefore our approach does not allow a clear differentiation of smoking effects from those of asbestos, mainly due to the shortcomings or the failure to report findings of the included studies but provides evidence that the observed impairment in lung function in the absence of radiological signs of asbestos-related parenchymal disease cannot be attributed solely to smoking and that asbestos exposure plays a causal role.
A recent meta-analysis [35], which did not consider radiological findings, demonstrated independent significant effects of smoking as well as of asbestos exposure (i.e. a synergistic effect), both for forced expiratory flow (FEF25-75, FEF50) as well as thoracic gas volume (TGV) and RV/TGV. In this analysis, the influence of asbestos exposure was stronger than that of smoking for FEV1/VC and airway resistance, whereas smoking had a stronger effect on FEF25-75. Evidence for a synergistic detrimental effect of smoking and asbestos exposure on airflow limitation has also been reported in several additional studies (FEV1 [62,41,61,64], FEV1/VC [65,66,9,10,4,25,61,26], FEF25-75 [66,3,25,10,43] and FEF75-85 [66,3]).
It has to be acknowledged that our study does not allow answering the question whether the observed statistically significant lung function impairments at the population level are also of clinical relevance at the individual level. Indeed, in clinical practice the diagnosis of an obstructive defect requires a FEV1/FVC of less than 70% and a FEV1 over 80% from predicted is considered to represent mild impairment in an individual [67]. Our pooled estimates are within the normal limits applied to individuals (even when considering the lower limits of the confidence interval). Small decreases in group mean values however do not preclude clinically important disease. For example a group of workers exposed to asbestos with moderate dyspnoea had mean FVC of 96%, mean FEV1 of 94% and mean FEV1/FVC of 95% of predicted [68], which are similar to our pooled estimates. In one study, lung function impairments, particularly airflow obstruction, have been associated with increased mortality in asbestos exposed workers [69].
Conclusions
We conclude that asbestos exposure causes restrictive as well as obstructive lung function impairment. Asbestos-exposed workers may present lung function impairments even in the absence of radiological evidence of asbestos-related pleural fibrosis or asbestosis.
Our systematic review demonstrates that despite the large number of studies about the health hazards from occupational exposure to asbestos, there is a need for further research, especially on the role of smoking, occupational co-exposure (e.g. other mineral dusts, welding fumes) and possible synergistic effects on the development of functional impairment, particularly chronic airway obstruction, in asbestos-exposed workers. Such studies should include measurement of CO diffusion capacity, airway resistance and flow volume curves in a consistent approach. Furthermore, our study underlines the necessity for an international agreement on lung function reference values within the individual ethnic groups, to facilitate comparison between different studies.
Abbreviations
CI: confidence interval; DL, CO: CO diffusion capacity; DPT: diffuse pleural thickening; FEF: forced expiratory flow; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; HRCT: high resolution computed tomography; PP: pleural plaques; RV: residual volume; SD: standard deviation; SE: standard error; SVC: slow (relaxed) vital capacity; TGV: thoracic gas volume; TLC: total lung capacity; VC: vital capacity; X-ray: chest radiograph
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors had full access to all data. XB had the original idea for the paper and vouches for the integrity of the analysis. DW, UM and MVG extracted and analysed the data. All authors collaborated in interpreting the data and writing the manuscript and read and approved the final manuscript.
Contributor Information
Dennis Wilken, Email: dennis.wilken@hamburg.de.
Marcial Velasco Garrido, Email: mvelasco@uke.uni-hamburg.de.
Ulf Manuwald, Email: ulf.manuwald@bgv.hamburg.de.
Xaver Baur, Email: baur@uke.uni-hamburg.de.
Acknowledgements and Funding
We thank Kevan Wiley for proof-reading our manuscript. There has been no external financial support or funding of the study, any person who contributed to this study or the preparation of the manuscript.
References
- LaDou J. The asbestos cancer epidemic. Environ Health Perspect. 2004;112:285–290. doi: 10.1289/ehp.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILO resolution concerning asbestos. http://www.ilo.org/wcmsp5/groups/public/---ed_norm/---relconf/documents/meetingdocument/wcms_gb_297_3_1_en.pdf
- Kilburn KH, Warshaw RH. Airways obstruction from asbestos exposure. Effects of asbestosis and smoking. Chest. 1994;106:1061–1070. doi: 10.1378/chest.106.4.1061. [DOI] [PubMed] [Google Scholar]
- Ohar J, Sterling DA, Bleecker E, Donohue J. Changing patterns in asbestos-induced lung disease. Chest. 2004;125:744–753. doi: 10.1378/chest.125.2.744. [DOI] [PubMed] [Google Scholar]
- Enright P. Comment on spirometry. J Occup Med. 1987;29:842. [PubMed] [Google Scholar]
- Edelman P. Asbestos and air flow limitation. J Occup Med. 1987;29:264–265. [PubMed] [Google Scholar]
- Jones RN, Glindmeyer HW, Weill H. Review of the Kilburn and Warshaw Chest article--airways obstruction from asbestos exposure. Chest. 1995;107:1727–1729. doi: 10.1378/chest.107.6.1727. [DOI] [PubMed] [Google Scholar]
- Smith DD. Failure to prove asbestos exposure produces obstructive lung disease. Chest. 2004;126:1000. doi: 10.1378/chest.126.3.1000. [DOI] [PubMed] [Google Scholar]
- Niebecker M, Smidt U, Gasthaus L, Worth G. [The incidence of airway obstruction in asbestosis] Pneumologie. 1995;49:20–26. [PubMed] [Google Scholar]
- Sue DY, Oren A, Hansen JE, Wasserman K. Lung function and exercise performance in smoking and nonsmoking asbestos-exposed workers. The American review of respiratory disease. 1985;132:612–618. doi: 10.1164/arrd.1985.132.3.612. [DOI] [PubMed] [Google Scholar]
- Antonescu-Turcu AL, Schapira RM. Parenchymal and airway diseases caused by asbestos. Curr Opin Pulm Med. 2010;16:155–161. doi: 10.1097/MCP.0b013e328335de61. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170:691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- Banks DE, Shi R, McLarty J, Cowl CT, Smith D, Tarlo SM, Daroowalla F, Balmes J, Baumann M. American College of Chest Physicians consensus statement on the respiratory health effects of asbestos. Results of a Delphi study. Chest. 2009;135:1619–1627. doi: 10.1378/chest.08-1345. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleemput J, De Raeve H, Verschakelen JA, Rombouts J, Lacquet LM, Nemery B. Surface of localized pleural plaques quantitated by computed tomography scanning: no relation with cumulative asbestos exposure and no effect on lung function. Am J Respir Crit Care Med. 2001;163:705–710. doi: 10.1164/ajrccm.163.3.2006017. [DOI] [PubMed] [Google Scholar]
- Paris C, Benichou J, Raffaelli C, Genevois A, Fournier L, Menard G, Broessel N, Ameille J, Brochard P, Gillon JC, Gislard A, Letourneux M. Factors associated with early-stage pulmonary fibrosis as determined by high-resolution computed tomography among persons occupationally exposed to asbestos. Scand J Work Environ Health. 2004;30:206–214. doi: 10.5271/sjweh.781. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ostojic L, Peric I, Mise K, Ostojic Z, Bradaric A, Bota B, Jankovic S, Tocilj J. Lung function changes in pleural asbestosis. Coll Antropol. 2004;28:711–715. [PubMed] [Google Scholar]
- Rösler JA Woitowitz HJ Schuckmann F, Schopper-Jochum J. Lungenfunktionsveränderungen bei Nichtrauchern mit Asbeststaublungenerkrankungen Bericht über die 30 Jahrestagung der Deutschen Gesellschaft für Arbeitsmedizin 1990Stuttgart: Gentner; 113–118.17272937 [Google Scholar]
- Quanjer PH. Standardized lung function testing. Bull Eur Physiopathol Respir. 1983;19:1–96. [PubMed] [Google Scholar]
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- American Thoracic Society. ATS/ERS Task Force: Standardisation of lung function testing. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. The American review of respiratory disease. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- Hall SK, Cissik JH. Effects of cigarette smoking on pulmonary function in asymptomatic asbestos workers with normal chest radiograms. Am Ind Hyg Assoc J. 1982;43:381–386. doi: 10.1080/15298668291409901. [DOI] [PubMed] [Google Scholar]
- Neri S, Boraschi P, Antonelli A, Falaschi F, Baschieri L. Pulmonary function, smoking habits, and high resolution computed tomography (HRCT) early abnormalities of lung and pleural fibrosis in shipyard workers exposed to asbestos. Am J Ind Med. 1996;30:588–595. doi: 10.1002/(SICI)1097-0274(199611)30:5<588::AID-AJIM6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Oldenburg M, Degens P, Baur X. Asbest-bedingte Lungenfunktionseinschränkungen mit und ohne Pleuraplaques. Atemwegs- und Lungenkrankheiten. 2001;27:422–423. [Google Scholar]
- Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, Milton D, Schwartz D, Toren K, Viegi G. American Thoracic Society Statement: Occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167:787–797. doi: 10.1164/rccm.167.5.787. [DOI] [PubMed] [Google Scholar]
- Lebowitz MD. Occupational exposures in relation to symptomatology and lung function in a community population. Environ Res. 1977;14:59–67. doi: 10.1016/0013-9351(77)90066-4. [DOI] [PubMed] [Google Scholar]
- Viegi G, Prediletto R, Paoletti P, Carrozzi L, di Pede F, Vellutini M, di Pede C, Giuntini C, Lebowitz MD. Respiratory effects of occupational exposure in a general population sample in north Italy. The American review of respiratory disease. 1991;143:510–515. doi: 10.1164/ajrccm/143.3.510. [DOI] [PubMed] [Google Scholar]
- Clarke CC, Mowat FS, Kelsh MA, Roberts MA. Pleural plaques: a review of diagnostic issues and possible nonasbestos factors. Arch Environ Occup Health. 2006;61:183–192. doi: 10.3200/AEOH.61.4.183-192. [DOI] [PubMed] [Google Scholar]
- Henderson D, Rantanen J, Barnhart S, Dement JM, De Vuyst P, Hillerdal G, Huuskonen MS, Kivisaari L, Kusaka Y, Lahdensuo A, Langard S, Mowe G, Okubo T, Parker JE, Roggli VL, Rödelsperger K, Rösler J, Woitowitz HJ, Tossavainen A. Asbestos, asbestosis and cancer: the Helsinki criteria for diagnosis and attribution. Scand J Work Environ Health. 1997;23:311–316. [PubMed] [Google Scholar]
- Copley SJ, Lee YC, Hansell DM, Sivakumaran P, Rubens MB, Newman Taylor AJ, Rudd RM, Musk AW, Wells AU. Asbestos-induced and smoking-related disease: apportioning pulmonary function deficit by using thin-section CT. Radiology. 2007;242:258–266. doi: 10.1148/radiol.2421051167. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Galvin JR, Yagla SJ, Speakman SB, Merchant JA, Hunninghake GW. Restrictive lung function and asbestos-induced pleural fibrosis. A quantitative approach. J Clin Invest. 1993;91:2685–2692. doi: 10.1172/JCI116507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedova J, Dlouha B, Rychla L, Neuwirth J, Brabec M, Pelclova D, Fenclova Z. Lung function impairment in relation to asbestos-induced pleural lesions with reference to the extent of the lesions and the initial parenchymal fibrosis. Scand J Work Environ Health. 2003;29:388–395. doi: 10.5271/sjweh.745. [DOI] [PubMed] [Google Scholar]
- Filippelli C, Martines V, Palitti T, Tomei F, Mascia E, Ferrante E, Tomei G, Ciarrocca M, Fioravanti M. [Meta-analysis of respiratory function of workers exposed to asbestos] G Ital Med Lav Ergon. 2008;30:142–154. [PubMed] [Google Scholar]
- Shukla A, Lounsbury KM, Barrett TF, Gell J, Rincon M, Butnor KJ, Taatjes DJ, Davis GS, Vacek P, Nakayama KI, Nakayama K, Steele C, Mossman BT. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-delta knockout mice. Am J Pathol. 2007;170:140–151. doi: 10.2353/ajpath.2007.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Nishimura Y, Maeda M, Kumagai N, Hayashi H, Chen Y, Kusaka M, Kishimoto T, Otsuki T. Cytokine alteration and speculated immunological pathophysiology in silicosis and asbestos-related diseases. Environ Health Prev Med. 2009;14:216–222. doi: 10.1007/s12199-008-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay D, Kamp DW. Asbestos-induced pulmonary toxicity: role of DNA damage and apoptosis. Exp Biol Med (Maywood) 2003;228:650–659. doi: 10.1177/153537020322800602. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Sancini G, Mantegazza F, Chiappino G. Translocation pathways for inhaled asbestos fibers. Environ Health. 2008;7:4. doi: 10.1186/1476-069X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal BD. Apoptosis in lung fibrosis and repair. Chest. 2002;122:293S–298S. doi: 10.1378/chest.122.6_suppl.293S. [DOI] [PubMed] [Google Scholar]
- Ohlson CG, Bodin L, Rydman T, Hogstedt C. Ventilatory decrements in former asbestos cement workers: a four year follow up. Br J Ind Med. 1985;42:612–616. doi: 10.1136/oem.42.9.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitowitz HJ. Die Situation asbestverursachender Berufskrankheiten. Asbestos European Conference. 2003. http://www.hvbg.de/e/asbest/konfrep/konfrep/repbeitr/woitowitz_enpdf
- Dement JM, Welch L, Ringen K, Bingham E, Quinn P. Airways obstruction among older construction and trade workers at Department of Energy nuclear sites. Am J Ind Med. 2010;53:224–240. doi: 10.1002/ajim.20792. [DOI] [PubMed] [Google Scholar]
- Ameille J, Letourneux M, Paris C, Brochard P, Stoufflet A, Schorle E, Gislard A, Laurent F, Conso F, Pairon JC. Does Asbestos Exposure Cause Airway Obstruction, in the Absence of Confirmed Asbestosis? Am J Respir Crit Care Med. 2010;182:526–530. doi: 10.1164/rccm.200812-1815OC. [DOI] [PubMed] [Google Scholar]
- Parodi S, Gennaro V, Ceppi M, Cocco P. Comparison bias and dilution effect in occupational cohort studies. Int J Occup Environ Health. 2007;13:143–152. doi: 10.1179/oeh.2007.13.2.143. [DOI] [PubMed] [Google Scholar]
- Hernberg S. "Negative" results in cohort studies--how to recognize fallacies. Scand J Work Environ Health. 1981;7(Suppl 4):121–126. [PubMed] [Google Scholar]
- Baillargeon J. Characteristics of the healthy worker effect. Occup Med. 2001;16:359–366. [PubMed] [Google Scholar]
- Fell AK, Thomassen TR, Kristensen P, Egeland T, Kongerud J. Respiratory symptoms and ventilatory function in workers exposed to portland cement dust. J Occup Environ Med. 2003;45:1008–1014. doi: 10.1097/01.jom.0000083036.56116.9d. [DOI] [PubMed] [Google Scholar]
- Baur X, Isringhausen-Bley S, Degens P. Comparison of lung-function reference values. Int Arch Occup Environ Health. 1999;72:69–83. doi: 10.1007/s004200050341. [DOI] [PubMed] [Google Scholar]
- Koch B, Schaper C, Ittermann T, Volzke H, Felix SB, Ewert R, Glaser S. [Reference values for lung function testing in adults--results from the study of health in Pomerania" (SHIP)] Dtsch Med Wochenschr. 2009;134:2327–2332. doi: 10.1055/s-0029-1242688. [DOI] [PubMed] [Google Scholar]
- Roberts CM, MacRae KD, Winning AJ, Adams L, Seed WA. Reference values and prediction equations for normal lung function in a non-smoking white urban population. Thorax. 1991;46:643–650. doi: 10.1136/thx.46.9.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Forced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord-Trondelag Study. Eur Respir J. 2001;18:770–779. doi: 10.1183/09031936.01.00255301. [DOI] [PubMed] [Google Scholar]
- Peric I, Arar D, Barisic I, Goic-Barisic I, Pavlov N, Tocilj J. Dynamics of the lung function in asbestos pleural disease. Arh Hig Rada Toksikol. 2007;58:407–412. doi: 10.2478/v10004-007-0033-3. [DOI] [PubMed] [Google Scholar]
- Delpierre S, Delvolgo-Gori MJ, Faucher M, Jammes Y. High prevalence of reversible airway obstruction in asbestos-exposed workers. Archives of environmental health. 2002;57:441–445. doi: 10.1080/00039890209601435. [DOI] [PubMed] [Google Scholar]
- Oliver LC, Eisen EA, Greene R, Sprince NL. Asbestos-related pleural plaques and lung function. Am J Ind Med. 1988;14:649–656. doi: 10.1002/ajim.4700140604. [DOI] [PubMed] [Google Scholar]
- Robins TG, Green MA. Respiratory morbidity in workers exposed to asbestos in the primary manufacture of building materials. Am J Ind Med. 1988;14:433–448. doi: 10.1002/ajim.4700140407. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Fuortes LJ, Galvin JR, Burmeister LF, Schmidt LE, Leistikow BN, LaMarte FP, Merchant JA. Asbestos-induced pleural fibrosis and impaired lung function. The American review of respiratory disease. 1990;141:321–326. doi: 10.1164/ajrccm/141.2.321. [DOI] [PubMed] [Google Scholar]
- Lilis R, Miller A, Godbold J, Chan E, Selikoff IJ. Pulmonary function and pleural fibrosis: quantitative relationships with an integrative index of pleural abnormalities. Am J Ind Med. 1991;20:145–161. doi: 10.1002/ajim.4700200203. [DOI] [PubMed] [Google Scholar]
- Garcia-Closas M, Christiani DC. Asbestos-related diseases in construction carpenters. Am J Ind Med. 1995;27:115–125. doi: 10.1002/ajim.4700270111. [DOI] [PubMed] [Google Scholar]
- Rui F, De Zotti R, Negro C, Bovenzi M. [A follow-up study of lung function among ex-asbestos workers with and without pleural plaques] Med Lav. 2004;95:171–179. [PubMed] [Google Scholar]
- Chien JW, Au DH, Barnett MJ, Goodman GE. Spirometry, rapid FEV1 decline, and lung cancer among asbestos exposed heavy smokers. Copd. 2007;4:339–346. doi: 10.1080/15412550701601340. [DOI] [PubMed] [Google Scholar]
- Zitting A, Huuskonen MS, Alanko K, Mattsson T. Radiographic and physiological findings in patients with asbestosis. Scand J Work Environ Health. 1978;4:275–283. doi: 10.5271/sjweh.2698. [DOI] [PubMed] [Google Scholar]
- Yates DH, Browne K, Stidolph PN, Neville E. Asbestos-related bilateral diffuse pleural thickening: natural history of radiographic and lung function abnormalities. Am J Respir Crit Care Med. 1996;153:301–306. doi: 10.1164/ajrccm.153.1.8542134. [DOI] [PubMed] [Google Scholar]
- Bagatin E, Neder JA, Nery LE, Terra-Filho M, Kavakama J, Castelo A, Capelozzi V, Sette A, Kitamura S, Favero M, Moreira-Filho DC, Tavares R, Peres C, Becklake MR. Non-malignant consequences of decreasing asbestos exposure in the Brazil chrysotile mines and mills. Occup Environ Med. 2005;62:381–389. doi: 10.1136/oem.2004.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH, Einstein K, Bernstein J. Airway disease in non-smoking asbestos workers. Archives of environmental health. 1985;40:293–295. doi: 10.1080/00039896.1985.10545935. [DOI] [PubMed] [Google Scholar]
- Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (updated 2010) http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf
- Demers RY, Neale AV, Robins T, Herman SC. Asbestos-related pulmonary disease in boilermakers. American journal of industrial medicine. 1990;17:327–339. doi: 10.1002/ajim.4700170305. [DOI] [PubMed] [Google Scholar]
- Moshammer H, Neuberger M. Lung function predicts survival in a cohort of asbestos cement workers. Int Arch Occup Environ Health. 2009;82:199–207. doi: 10.1007/s00420-008-0322-4. [DOI] [PubMed] [Google Scholar]
- Ameille J, Matrat M, Paris C, Joly N, Raffaelli C, Brochard P, Iwatsubo Y, Pairon JC, Letourneux M. Asbestos-related pleural diseases: dimensional criteria are not appropriate to differentiate diffuse pleural thickening from pleural plaques. Am J Ind Med. 2004;45:289–296. doi: 10.1002/ajim.10341. [DOI] [PubMed] [Google Scholar]
- Begin R, Ostiguy G, Filion R, Colman N, Bertrand P. Computed tomography in the early detection of asbestosis. Br J Ind Med. 1993;50:689–698. doi: 10.1136/oem.50.8.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begin R, Filion R, Ostiguy G. Emphysema in silica- and asbestos-exposed workers seeking compensation. A CT scan study. Chest. 1995;108:647–655. doi: 10.1378/chest.108.3.647. [DOI] [PubMed] [Google Scholar]
- Harkin TJ, McGuinness G, Goldring R, Cohen H, Parker JE, Crane M, Naidich DP, Rom WN. Differentiation of the ILO boundary chest roentgenograph (0/1 to 1/0) in asbestosis by high-resolution computed tomography scan, alveolitis, and respiratory impairment. J Occup Environ Med. 1996;38:46–52. doi: 10.1097/00043764-199601000-00016. [DOI] [PubMed] [Google Scholar]
- Jarad NA, Strickland B, Pearson MC, Rubens MB, Rudd RM. High resolution computed tomographic assessment of asbestosis and cryptogenic fibrosing alveolitis: a comparative study. Thorax. 1992;47:645–650. doi: 10.1136/thx.47.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee ST, Gamsu G, Blanc P. Causes of pulmonary impairment in asbestos-exposed individuals with diffuse pleural thickening. Am J Respir Crit Care Med. 1996;154:789–793. doi: 10.1164/ajrccm.154.3.8810620. [DOI] [PubMed] [Google Scholar]
- Kouris SP, Parker DL, Bender AP, Williams AN. Effects of asbestos-related pleural disease on pulmonary function. Scand J Work Environ Health. 1991;17:179–183. doi: 10.5271/sjweh.1713. [DOI] [PubMed] [Google Scholar]
- Nakadate T. Decline in annual lung function in workers exposed to asbestos with and without pre-existing fibrotic changes on chest radiography. Occup Environ Med. 1995;52:368–373. doi: 10.1136/oem.52.6.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piirila P, Lindqvist M, Huuskonen O, Kaleva S, Koskinen H, Lehtola H, Vehmas T, Kivisaari L, Sovijarvi AR. Impairment of lung function in asbestos-exposed workers in relation to high-resolution computed tomography. Scand J Work Environ Health. 2005;31:44–51. doi: 10.5271/sjweh.847. [DOI] [PubMed] [Google Scholar]
- Prince P, Boulay ME, Page N, Desmeules M, Boulet LP. Induced sputum markers of fibrosis and decline in pulmonary function in asbestosis and silicosis: a pilot study. Int J Tuberc Lung Dis. 2008;12:813–819. [PubMed] [Google Scholar]
- Sette A, Neder JA, Nery LE, Kavakama J, Rodrigues RT, Terra-Filho M, Guimaraes S, Bagatin E, Muller N. Thin-section CT abnormalities and pulmonary gas exchange impairment in workers exposed to asbestos. Radiology. 2004;232:66–74. doi: 10.1148/radiol.2321030392. [DOI] [PubMed] [Google Scholar]
- Vierikko T, Jarvenpaa R, Toivio P, Uitti J, Oksa P, Lindholm T, Vehmas T. Clinical and HRCT screening of heavily asbestos-exposed workers. Int Arch Occup Environ Health. 2010;83:47–54. doi: 10.1007/s00420-009-0462-1. [DOI] [PubMed] [Google Scholar]
- Zejda J. Diagnostic value of exercise testing in asbestosis. Am J Ind Med. 1989;16:305–319. doi: 10.1002/ajim.4700160309. [DOI] [PubMed] [Google Scholar]
- Lilis R, Miller A, Godbold J, Chan E, Selikoff IJ. Radiographic abnormalities in asbestos insulators: effects of duration from onset of exposure and smoking. Relationships of dyspnea with parenchymal and pleural fibrosis. Am J Ind Med. 1991;20:1–15. doi: 10.1002/ajim.4700200102. [DOI] [PubMed] [Google Scholar]