Abstract
ATP-Binding Cassette (ABC) transporters are efflux pumps frequently associated with multidrug resistance in many biological systems, including malaria. Antimalarial drug-resistance involves an ABC transporter, PfMDR1, a homologue of P-glycoprotein in humans. Twenty years of research have shown that several single nucleotide polymorphisms in pfmdr1 modulate in vivo and/or in vitro drug susceptibility. The underlying physiological mechanism of the effect of these mutations remains unclear. Here we develop structural models for PfMDR1 in different predicted conformations, enabling the study of transporter motion. Such analysis of functional polymorphisms allows determination of their potential role in transport and resistance. The bacterial MsbA ABC pump is a PfMDR1 homologue. MsbA crystals in different conformations were used to create PfMDR1 models with Modeller software. Sequences were aligned with ClustalW and analysed by Ali2D revealing a high level of secondary structure conservation. To validate a potential drug binding pocket we performed antimalarial docking simulations. Using aminoquinoline as probe drugs in PfMDR1 mutated parasites we evaluated the physiology underlying the mechanisms of resistance mediated by PfMDR1 polymorphisms. We focused on the analysis of well known functional polymorphisms in PfMDR1 amino acid residues 86, 184, 1034, 1042 and 1246. Our structural analysis suggested the existence of two different biophysical mechanisms of PfMDR1 drug resistance modulation. Polymorphisms in residues 86/184/1246 act by internal allosteric modulation and residues 1034 and 1042 interact directly in a drug pocket. Parasites containing mutated PfMDR1 variants had a significant altered aminoquinoline susceptibility that appears to be dependent on the aminoquinoline lipophobicity characteristics as well as vacuolar efflux by PfCRT. We previously described the in vivo selection of PfMDR1 polymorphisms under antimalarial drug pressure. Now, together with recent PfMDR1 functional reports, we contribute to the understanding of the specific structural role of these polymorphisms in parasite antimalarial drug response.
Introduction
Efforts to control Plasmodium falciparum malaria are currently reliant on vector control and chemotherapy. Unfortunately, the parasite has demonstrated a persistent ability to circumvent antimalarial drug efficacy through resistance-conferring mutations, as most dramatically illustrated by the collapse of chloroquine as worldwide mainstay chemotherapy [1]. Lack of an effective alternative chemotherapy led to a documented rise in the public health impact of malaria and a significant increase in the disease's related mortality [2], [3].
A cornerstone event in malaria chemotherapy occurred in Thailand, during the 1990s: the recovery of the efficacy of mefloquine (MQ) through its combination with artesunate [4], [5]. Following this successful implementation, conceptually similar artemisinin derivative combination therapies (ACT) were progressively adopted worldwide. Consequently, ACT is presently recognised as an absolute central factor in P. falciparum malaria control [6]. It has been proposed that P. falciparum resistance to ACT could evolve [7], [8]. Indeed, recent reports have provided the first indications that resistance to ACTs may be emerging in natural parasite populations [9], [10]. If such resistance spreads widely, our drug-based efforts to control malaria will be severely held back.
Drug treatments and policies, purposely engineered to avoid the development of Multi-Drug Resistance (MDR) mechanisms are urgently required. This challenge demands a fundamental understanding of the details of the resistance mechanisms utilised by P. falciparum.
In higher mammals, the ATP-binding cassette (ABC) superfamily subclass B1, typified by the mammalian P-glycoprotein (Pgp) [11], have been consistently linked to drug-resistance phenotypes in a large range of organisms [12]. P. falciparum contains in its proteome a Pgp-homologue (PfMDR1) [13], [14]. Single nucleotide polymorphisms (SNPs) in its coding gene (pfmdr1) have been shown to be associated with differential in vivo and in vitro parasite responses to a significant range of central ACT antimalarial partner drugs, including amodiaquine [15], [16], mefloquine [7], [17]–[19], lumefantrine [8], [20] and, importantly, artemisinin [18], [19]. PfMDR1 is hence considered an important potential candidate for mediating ACT resistance [21], [22]. However the biophysics and mechanistic role of these polymorphisms remains poorly understood.
In the present study we demonstrate 3D structural models for PfMDR1 and merge available, but never fully integrated, molecular/functional data on this transporter and the functional effects of its main polymorphisms, including in vitro antimalarial susceptibility.
Recent observations of the effects of the clinical use of different antimalarial upon allele selection are discussed in terms of their contribution to drug resistance and mechanism of action contributing to evidence based view of antimalarial implementation.
Results
PfMDR1 structure
PfMDR1 is a member of the ABC protein superfamily. It is a homologue of Human Pgp-1 composed of two symmetric parts (defined as domain 1 and 2, from N-terminal to C-terminal). Each domain has a transmembrane domain (TMD), composed of three external loops (EL) and two internal helixes (IH) that link six transmembrane regions (TM) followed by a nucleotide binding domain (NBD) (Figure S1).
As a reference for our model, we considered the bacterial ABC lipid flippase (MsbA). This transporter is structurally and functionally related to eukaryotic MDR-type proteins [23]. The documented determination of MsbA structures captured in different conformations allows the basis for structural and motion extrapolations for other ABC full transporters [24].
Comparison of the PfMDR1 primary and secondary structure with those of MsbA, revealed the existence of structure/function conservation between the two proteins, reflected by a ∼22% identity in total homology of PfMDR1 aminoacid residue sequence. In addition, the alignment showed a righteous match in the aminoacid type homology and consequently in secondary structure, pointing towards a structural and functional conservation (Figure S1).
The main sequence divergence in homology occurs in a part of the PfMDR1 NBD's. In NBD1 there is an aminoacid frame insertion after the Q-loop and in NBD2 the insertion occurs after the P-loop. Another observed insertion is located in the TM5 and in its equivalent region of the second domain, designated TM10. TM5 and TM10 in PfMDR1 are expected to be longer than those observed to the MsbA structures. The consistence of this divergence in the homologous halves supports the existence of symmetry between the two domains of ABC transporters (Figure S1).
Structural changes in PfMDR1
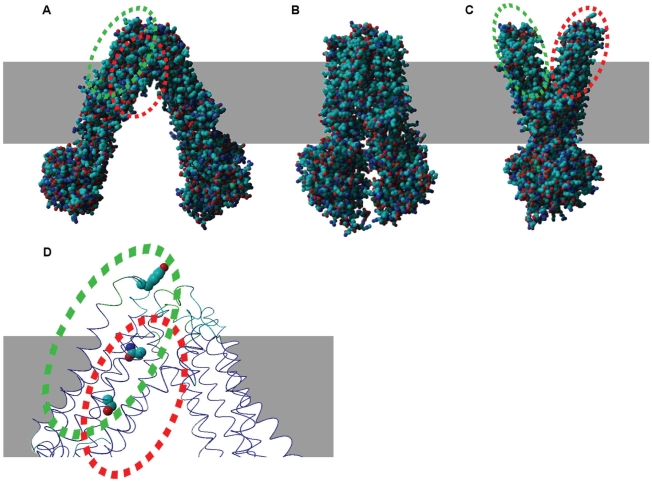
It has been previously demonstrated that a large range of motion is required for the MsbA transporter to function [24]. For PfMDR1, two hinges whose movement allow for different conformations correspond to EL2 (residue 189–194) and EL3 (residue 311–315) and the equivalent hinge EL5 (residue 929–933) and EL6 (residue 1055–1059) (Figure 1 A–C).
Figure 1. PfMDR1 structure models.
PfMDR1 structures are shown in different conformations: inverted V shape (open-apo) based in bacterial MsbA 3B5w structure (A), close-apo based in 3B5x (B) and V shape based in 3B60 structure (C). Panel (D) shows localization of residues 86 (EL1), 1034 and 1042 (TM11) in the open state. Dashed green circle represents residue 86 location and red dashed circle 1034 and 1042.
The TMDs communicate with NBDs through IH contact. A particular characteristic is the intertwined interface between the two halves of the transporter, interlocking TM4/TM5/IH2 with NBD2 and TM10/TM11/IH4 with NBD1. Recently, the importance of this structural feature for ABC transporter substrate binding, signalling, and transport was shown. The bonding of the nucleotide transmits a structural change to the TMs via IH's, resulting in an outward-facing conformation activating transport [25], [26].
Pfmdr1 single nucleotide polymorphisms: implications for PfMDR1 substrate transport capacity
In Africa, two major PfMDR1 variants are found that differ at 3 aminoacid residues: N86Y, F184Y and D1246Y. The haplotype NFD is associated with decreased parasite sensitivity to arylaminoalcool quinoline drugs (e.g. mefloquine, lumefantrine). Accordingly, selection of this haplotype has been consistently observed during artemether-lumefantrine, (Coartem®, Novatis AG, Basel) treatment [8], [20], [27], whereas the alternative haplotype YYY, is linked to decreased sensitivity to 4-aminoquinoline drugs such as chloroquine and amodiaquine [15], [28]–[31].
EL1 is known to be N-glycosylated in the human multidrug transporter Pgp-1 [32]. Asparagines at position 84 and 86 in the EL1 of PfMDR1 were predicted to be glycosylated with a potential of 0.56 and 0.73 respectively (Figure S2). When we replaced in the sequence 86N residue for the tyrosine variant (86Y), the potential glycosylation site was abolished. However, because of the low N-glycosylation capability in P. falciparum, its physiological impact remains to be investigated. [33].
For the MsbA transporter, the EL1, which connects TM1 and TM2, is also proposed to be important in open-apo conformation stability through contact with EL6 which links TM11 and TM12. In order to establish a protein nucleotide-bound conformation, the PfMDR1 EL1 and EL6 split and lose interaction transforming the transporter in a V-shape (Figure 1A–C). Indeed, in MsbA, the a.a. residue 56 localized in the EL1 region was shown to interact covalently through spontaneous disulphide bounding with the opposing monomer by cysteine cross-linking experiments in the ATP unbound form [34] demonstrating the proximity between the EL1 and EL6 loops. A similar structural arrangement has been reported to occur in human Pgp, highlighting the importance of TM1 and TM11 terminals at EL's closeness, in ABC transporters function [35].
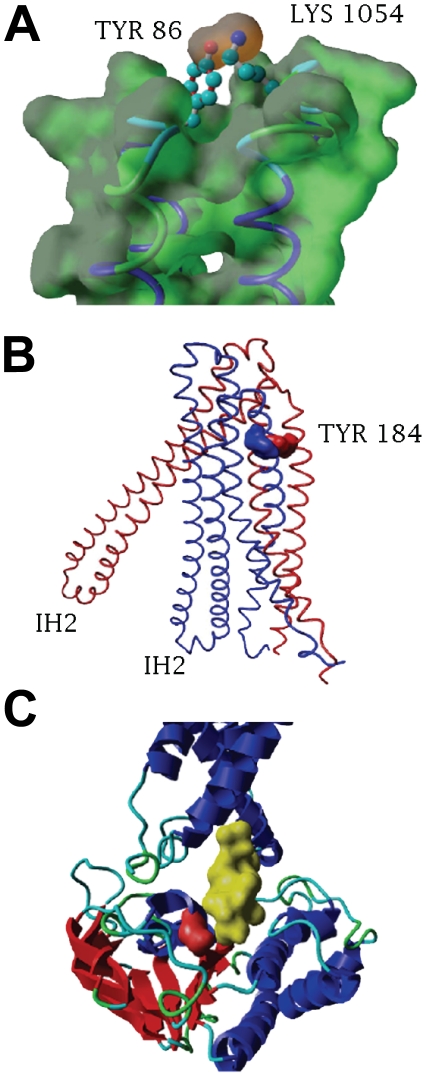
Analyzing the contact residue in the PfMDR1 EL6 we found that the side-chain of 86Y in EL1 localizes in parallel with residue 1054K (Figure 2A). TM1 and TM11 interactions have been shown in human Pgp to be fundamental for the correct positioning of TMs, while modulating the transporter affinity in a drug specific manner [25], [36]. Accordingly, a significant change in drug affinity caused by the TM1 located N86Y mutation was observed [37] suggesting an analogous biophysical role when residue 86 is mutated to a tyrosine.
Figure 2. Structural localization of PfMDR1 polymorphisms.
A) Interaction of EL1-EL6 aromatic side-chain of residue 86Y approximation to the cationic side-chain 1054K in the resting state. Interaction is shown in the Van der Waals surface (dark orange) between the O (red atom) of the 86Y side-chain with N (blue atom) in 1054K side-chain. B) Location of residue 184 in TM3 shown in two different conformation structures - open (red) and close (blue). C) Location of residue 1246 in NBD2 - Residue 1246 surface is shown in red localized in the cleft interacting with IH2 (yellow aminoacids surface) in the opposed domain.
PfMDR1 a.a. residue 184 is embedded in TM3, facing the transporter out surface. Analogous to human P-glycoprotein, a mutation in TM3 was shown to alter transport kinetics, independent from drug binding capacity [38]. TM3 is located in the middle of TMD1 surrounded by TM1–TM2 on one side and TM4–TM6 in the other. TM4 and TM5 are closely associated with TM3 in the nucleotide bound conformation (Figure 2B). These major changes, between close and open conformation, are due to the flexibility of EL3 and EL4, which constitute an important hinge in the transporter structure from an “inverted V” shape to a “V” shape and enabling membrane transport interference [24]. In such mobile structures small changes, such as tyrosine to phenylalanine (being both aromatic aminoacids), may cause disturbance in protein dynamics.
The Y184F SNP appears to alter PfMDR1 kinetics but not drug specificity [37], [39] as showed in the pfmdr1 allelic exchange experiments investigating antimalarial resistance [18]. When compared with the other SNPs in PfMDR1, polymorphism at position 184 exhibit a weaker antimalarial resistance association in vivo [7], [20], [40]–[45].
Polymorphisms located in the NBD of ABC transporters were reported to alter protein transport kinetics through ATP hydrolysis capacity alteration [46] or by altering the communication between the NBD and the transmembrane domains [47]. Residue 1246 is located in the NBD2 near the Q-loop and is part of the cleft in NBD2 that interacts with IH2 at the N-terminal (Figure 2C), and is essential for ABC transporter function [48]. PfMDR1 ATPase basal activity was shown not to be blocked by the D1246Y mutation alone. Reduction of PfMDR1 ATPase activity was also reported to occur only when 1246Y is associated with 1034S or 1042D, but not alone [39].
Our model suggests that the functional impact of the D1246Y mutation occurs through interference with the NBD/TM communication, which is required for ABC transporter function. Furthermore, this effect is substrate specific, since no significant change was observed in halofantrine or vinblastine transport by this mutation, suggesting that it is important for substrates which bind to the TM11 binding pocket (described below), as demonstrated for QN transport [37].
PfMDR1 drug-binding pocket
Transmembrane regions in ABC transporters are involved in ligand docking. Among the five naturally occurring functional PfMDR1 polymorphisms involved in antimalarial resistance, two are located in TM11, residues 1034 and 1042 (Figure 1D). Polymorphisms localised in this region of the human homologous transporter Pgp interfere with drug transport, suggesting the existence of a drug-binding pocket in this region [49].
In our model, the 1034/1042 and 86 positions co-localize in three dimension at a close region in the open state conformation of the protein (V shaped), whereas TM1 and TM11 come closer (Figure 1A and 1D). Spatial analysis shows that residue 86 in EL1 is in contact with the digestive vacuole (DV) lumen (Figure 1A–C), while the residues 1034 and 1042 alternate between facing the cytosol in the open state (V shaped, Figure 1A) and DV lumen in the closed state (Figure 1C).
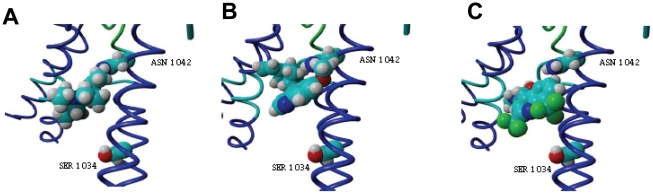
Several studies propose residues 1034 and 1042 as part of an antimalarial binding pocket [19], [39]. Using a refined model from the open state conformation we performed docking analysis in TM11 for several antimalarial drugs in order to characterize 1034 and 1042 residues as a drug binding site. Mefloquine (MQ), quinine (QN), and chloroquine (CQ) docked in the proposed binding site, preferentially interacting with residue 1042. The energies of docking for the best pose were estimated to be: −6.89 Kcal/mol for CQ (Figure 3A), −7.86 for QN (Figure 3B) and −5.69 for MQ (Figure 3C).
Figure 3. Docking of antimalarial in TM11.
Docking of CQ (A), QN (B) and MQ (C) at residues 1042 and 1034 (serine and aspartic acid respectively) in TM11 is shown. The energies of docking for best pose were estimated to be −6.89 Kcal/mol for CQ, −7.86 Kcal/mol for QN and −5.69 Kcal/mol for MQ.
When residues 1042 and 1034 were mutated, the estimated docking capacity of binding was altered, strengthening the hypothesis that these residues are actively involved in the drug binding site. Introduction of a single mutation 1034C abolished the docking of MQ and QN but not CQ. CQ binds to the mutated TM11 (1034C) with an estimated energy of −7.55 Kcal/mol.
The single mutation of N1042D and the double mutation 1034C/1042D abolished the docking of all tested antimalarial.
Cellular physiology of antimalarial transport by PfMDR1 and resistance
Together with PfMDR1, multidrug resistance in P. falciparum was shown to be highly associated with another gene, the chloroquine resistance transporter gene (pfcrt). Both transporters localize in the DV, with opposed directional flux characteristics. PfMDR1 is proposed to be a DV importer [50] whilst PfCRT was shown to be an antimalarial DV exporter [51]. To study the physiology of PfMDR1/PfCRT interference towards antimalarial resistance, we evaluate the contribution for antimalarial resistance of isogenic PfMDR1 mutant's clones with a CQ resistant (7G8) or sensitive (D10) genetic background (Table S1).
The target for antimalarial aminoquinoline is well known to be the parasite's DV. For this reason, chloroquine (CQ), amodiaquine (AQ) and desethylamodiaquine (DEAQ) were used as probe drugs. In order to study in exclusive the effect of PfMDR1 mutations, verapamil was used to block the PfCRT 76T variant.
To test the contribution of PfMDR1 for aminoquinoline we compared the index of resistance between parasites harbouring a wild type (SND) or resistant (CDY) haplotype at residues 1034, 1042 and 1246. These experiments were conducted in two different pfcrt genetic backgrounds carrying a 76T (7G8) or 76K allele (D10).
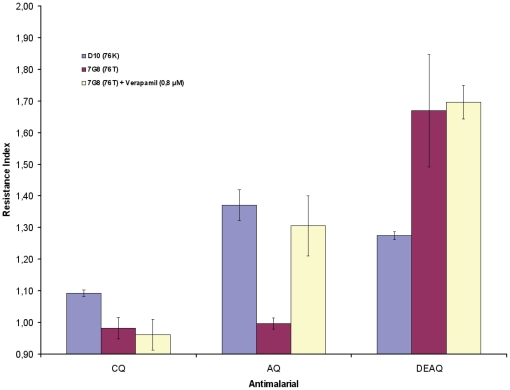
Our results show that PfMDR1 contributed to resistance for all tested antimalarial. The differential effect of PfMDR1 for different aminoquinoline susceptibility was as follow: chloroquine<amodiaquine<desethylamodiaquine (Fig. 4). This association is related with the lipophobicity characteristics of the tested drugs and the PfCRT background. The corresponding Log D (pH 7.2) values for these drug are CQ:0.045<(DEAQ:1.183<AQ:2.60) (taken from [52]). In general, the involvement of PfMDR1 was stronger for AQ and DEAQ than the effect observed for CQ.
Figure 4. Contribution of PfMDR1 for antimalarial resistance.
PfMDR1 Resistance Index (RI) was calculated, for each given antimalarial, as follow: for D10 - RI = D10CDY EC50/D10SND EC50 and equally for 7G8 - RI = 7G8CDY EC50/7G8SND EC50. Verapamil was used to block PfCRT.
In the PfCRT 76K background (D10), the contribution of PfMDR1 was observed even for CQ while in 7G8 clone (76T) is only detected for DEAQ. Although, when PfCRT is blocked with verapamil in 7G8 clone, a significant increase is observed also for AQ to levels comparable with D10 index for AQ (Fig. 4).
These observations support the hypothesis that PfMDR1 is a vacuolar importer. In the presence of the mutant PfMDR1 (blocking antimalarial DV import), sensitivity to aminoquinoline is driven by its capacity to passively enter the DV membrane, demonstrated here through the correlation between the PfMDR1 resistance index and aminoquinoline lipophobicity characteristics.
Interestingly, taken together with the inverse association of aminoquinoline lipophobicity pattern for PfCRT-based resistance, an explanation may present itself for the in vivo co-selection of opposed functional haplotypes (highly effective PfCRT efflux and deficient PfMDR1 influx transporters) to reduce the dynamics of aminoquinoline accumulation in the DV, especially for hydrophobic compounds.
Discussion
In the present work we propose a model for PfMDR1 structure and motion during transport. The existence of extensive literature describing the functional and molecular epidemiologic impact of polymorphisms in this transporter together with knowledge of the structure of ABC transporters, made it possible to predict the functional role of mutant residues in PfMDR1.
Our structural analyses, in the light of previous allelic exchange and transport kinetics studies [18], [19], [37], [39], demonstrate the existence of an internal allosteric modulation of protein transporting capacity by the three key polymorphisms herein studied. Accordingly, PfMDR1 variant 86Y/184Y/1246Y relates to a PfMDR1 low-performance quinoline antimalarial transporter. The inverse variant (86N/184F/1246D) relates to a higher-performance PfMDR1 transporter, being coherently associated with gene copy number amplification and its effects in terms of enhanced resistance to MQ and QN [7], [53].
The two most common PfMDR1 selected variants observed in African parasite populations harbour 86N/184F/1246D or 86Y/184Y/1246Y [8], [15], [20], [28]. Hence, the suggested altered allosteric control of transporter activity proposed by the model seems to be the main molecular phenotype associated with the influence of PfMDR1 in African parasites.
We describe that residues in TM11 (1034 and 1042) are related to phenotype modulation by altering a drug pocket in PfMDR1. The contact of residue 86 with the end of TM11 may explain an observed change in drug specificity through the alteration of PfMDR1 drug pocket conformation [37]. Polymorphisms in TM11 are geographically fixed in Asia and South America. This observation suggests that different genetic background/environment defines the fixation of a particular mechanism for PfMDR1 functional modulation.
From a mechanistic perspective, the haplotype encoding 86N, 1034S, 1042N and 1246D residues is related to a functional PfMDR1 importer into the DV [50]. Again, field observations show this haplotype to be related to mefloquine and lumefantrine resistance [7], [8], [20], [53]. This importer variant is oppositely related (promotes sensitivity) to CQ and amodiaquine (AQ) which acts within the DV [15], [28]–[31].
From a dynamic perspective, PfCRT antimalarial vacuolar efflux may affect the contribution of PfMDR1 to resistance. Supporting the hypothesis of a vacuolar antimalarial accumulation dynamic through PfCRT/PfMDR1 interaction, is the natural selection of functionally inversed polymorphisms of PfCRT and PfMDR1. PfCRT 76T promotes vacuolar efflux [54], [55] and is associated with PfMDR1 86Y (abrogates quinolines transport) [37]. Both forms are selectively associated with, as for example, CQ and AQ malaria chemotherapy [15], [28]–[31]. Oppositely, PfCRT 76K with PfMDR1 86N are associated with selection by aminoalcohol antimalarial drugs, such lumefantrine [8], [56].
Based on different genetic backgrounds, PfCRT/PfMDR1 dynamics can be either pro- or con- tolerance as has been previously reported to occur in a compensatory manner depending on the mechanism of action of the antimalarial [57].
We previously reported the importance of natural selection of different variants of PfMDR1 with different antimalarial chemotherapies [8], [15], [20], [28]. Now we describe how residues in PfMDR1 may be involved in drug resistance using a structural model.
In conclusion, we describe a model for structural changes associated with transport motion and we suggest the presence of a drug binding pocket in PfMDR1. Our results support recent findings suggesting this transporter as being a vacuolar antimalarial importer and propose a structural basis for the importance of residues 86, 184, 1034, 1042 and 1246 for drug specificity and how mutations at these residues may interfere with the composition of a drug pocket in TM11. The proposed models are expected to contribute to the prediction of the effects of other less studied PfMDR1 SNPs, while being a potential tool for the design of antimalarial drugs targeting this essential P. falciparum transporter.
Materials and Methods
Parasites and drug susceptibility assays
Four P. falciparum clones in which the pfmdr1 1034, 1042 and/or 1246 loci have been modified through allelic exchange [18] were selected for this study. They were obtained from the Malaria Research and Reference Reagent Resource Center (MR4©). Two clones were derived from the CQ sensitive D10 clone from Papua New Guinea i.e MRA-563: D10 pfmdr1 SND (autologous transfectant) (D10SND) and MRA-565: D10 pfmdr1 CDY (D10CDY). Two clones were derived from the CQ resistant clone 7G8 from Brazil i.e MRA-566: 7G8 pfmdr1 SND (7G8SND) and MRA-567: 7G8 pfmdr1 CDY (autologous transfectant). The clones were defrosted, adapted to continuous culture in supplemented RPMI-1640 and 5% haematocrite and then synchronized with sorbitol, according to established protocols.
The influence of the pfmdr1 1034C, 1042D and/or 1246Y SNPs on parasite response to CQ, AQ and DEAQ was determined with an HRP2-ELISA based assay in vitro as previously described [58]. In vitro cultured parasites were diluted to an initial parasitemia of 0.05% and aliquoted into microculture 96-well plates pre-dosed with ascending concentrations of 0–404 nM CQ, 0–67 nM AQ or 0–156 nM DEAQ. We also added VP 0.8 µM to a parallel setup.
After incubation at 37°C for 72 h, the samples were freeze-thawed, transferred and processed in pre-coated ELISA plates (Cellabs, Australia) for spectrophotometric analysis (Multiskan EX, Thermo Labsystems®, Helsingfors, Finland) of HRP2 produced during parasite growth. The IC50 and values were determined using HN-NonLin V1.05 Beta © H. Noedl 2001 (http://malaria.farch.net).
Modelling
Protein sequences and structures from bacterial MsbA 3B5w (open-apo with inverted V shape), 3B5x (closed-apo) and 3B60 (open-apo with V shape) upon nucleotide-bind were downloaded from PDB database (www.pdb.org). PfMDR1 sequence is deposited in NCBI Protein database with accession number XP_001351787.
Sequences were aligned with blosum62 matrix in MultiAlin server [59] with manual refinement. Since MsbA is formed by a homodimer, PfMDR1 sequence was analyzed dividing sequence protein in the two symmetric halves and terminals trimmed to the homologous TMD and NBD. Residues 37–642, corresponding to TMD1 and NBD1 and residues 763–1400 corresponding to TMD2 and NBD2, were used in the alignment.
Alignment was then analyzed with Ali2D software (http://toolkit.tuebingen.mpg.de/ali2d), developed by the Department of Protein Evolution, Max-Planck-Institute for Developmental Biology (Germany) for sequence identity and secondary structure similarities. Secondary structure was determined with Psipred algorithm [60] and aminoacids group coloured with Mview [61].
Models were generated using Modeller software [62]. For structures modelling and analysis, divergent residues 479–486 and 496–531 in NBD1, IH4 987–998 and residues 1181–1227 were absent in the alignment. MaxSprout software at European Bioinformatics Institute server was used to generate protein backbone and side chain co-ordinates from the C-(alpha) trace [63]. The halves of the transporter were assembled by superimposition in the MsbA structures using the Mustang algorithm and adjusted manually [64]. Yasara [65] and WinCoot [66] software were used for visualization and refinement.
N-Glycosylation sites prediction
Prediction of N-Glycosylation sites at the EL1 was performed using NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/). The protein sequence including the predicted EL1 plus four boundary aminoacids comprising residues 79–91 was evaluated. A threshold of 0.5 was applied.
Drug pocket modeling and docking
The model based in the 3B5w structure was further refined in WinCoot for docking studies. Docking was performed with Arguslab software [67]. Residues 1034 and 1042 in TM11 were defined as drug binding pockets and docking boxes defined as minimum volume for binding of 12.5×13×12.5 Å for CQ and QN and 13×13×13 Å for MQ were used. Best pose for the lowest energy of binding was considered. The CQ, QN and MQ structures were design and refined at PRODRG2 server [68].
Supporting Information
Protein sequences alignment of PfMDR1 and different MsbA structures. The alignment describes the primary percentage of overall matching between PfMDR1 protein as well as secondary and tertiary structures features. Aminoacids letters are coloured by group identity default palette defined by Mview software. Background colour shows secondary structure, grey - coiled; brown- helix and yellow- beta-sheet. On top of the alignment is annotated the ABC conserved motifs as well as main structural characteristics: TM-transmembranes; EL- external loop; IH- internal helix. Stars identify functional residues in PfMDR1 transport: N86Y, Y184F, S1034C, N1042D and D1246Y.
(PDF)
N-glycosylation sites prediction. External loop 1 sequence (residues 79–91) was screened for putative N-glycosylation sites. Two asparagines localize in this loop at positions 84 and 86. Predictive potential sites obtained with NetNglyc 1.0 software.
(PDF)
PfCRT and PfMDR1 coding genotypes for clones D10SND, D10CDY, 7G8SND and 7G8CDY.
(PDF)
Acknowledgments
We would like to acknowledge Dr. Richard Culleton for his valuable comments and suggestions to improve this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: PEF and MIV are recipients of PhD grants from Fundação para a Ciência e Tecnologia (FCT)/Ministerio da Ciência e Ensino Superior, Portugal - MCES (ref. SFRH/BD/28368/2006 and ref. SFRH/BD/28393/2006, respectively). This study was supported by the Swedish Research Council (2005-6682), Åke Wiberg's Foundation (PU), Fredrik and Ingrid Thuring's Foundation (PU), EDCTP project IP.2007.31060.002 (JPG) and Styrelsen for Internationellt Utvecklingssamarbete (SIDA) – (SWE-2009-165) (JPG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 2.Marsh K. Malaria disaster in Africa. Lancet. 1998;352:924. doi: 10.1016/S0140-6736(05)61510-3. [DOI] [PubMed] [Google Scholar]
- 3.Trape JF, Pison G, Preziosi MP, Enel C, Desgrees du Lou A, et al. Impact of chloroquine resistance on malaria mortality. C R Acad Sci III. 1998;321:689–697. doi: 10.1016/s0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 4.White NJ. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia. 1999;41:301–308. [PubMed] [Google Scholar]
- 5.WHO. Antimalarial Drug Combination Therapy. 2001. Report of a WHO Technical Consultation. WHO/CDS/RBM/200135 Geneva, Switzerland World Health Organization.
- 6.Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisowath C, Stromberg J, Martensson A, Msellem M, Obondo C, et al. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J Infect Dis. 2005;191:1014–1017. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- 9.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 10.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen DW, Fojo A, Chin JE, Roninson IB, Richert N, et al. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986;232:643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- 12.Holland IB, Cole SPC, Kuchler K, Higgins CF. 2003. ABC proteins: from bacteria to Man Academic press.
- 13.Foote SJ, Thompson JK, Cowman AF, Kemp DJ. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989;57:921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- 14.Wilson CM, Serrano AE, Wasley A, Bogenschutz MP, Shankar AH, et al. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989;244:1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren G, Gil JP, Ferreira PM, Veiga MI, Obonyo CO, et al. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect Genet Evol. 2006;6:309–314. doi: 10.1016/j.meegid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Sa JM, Twu O, Hayton K, Reyes S, Fay MP, et al. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc Natl Acad Sci U S A. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes D, Rungsihirunrat K, Nogueira F, Seugorn A, Gil JP, et al. Molecular characterisation of drug-resistant Plasmodium falciparum from Thailand. Malar J. 2002;1:12. doi: 10.1186/1475-2875-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 19.Sidhu AB, Valderramos SG, Fidock DA. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol Microbiol. 2005;57:913–926. doi: 10.1111/j.1365-2958.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- 20.Sisowath C, Ferreira PE, Bustamante LY, Dahlstrom S, Martensson A, et al. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Trop Med Int Health. 2007;12:736–742. doi: 10.1111/j.1365-3156.2007.01843.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen N, Chavchich M, Peters JM, Kyle DE, Gatton ML, et al. De-amplification of pfmdr1-containing amplicon on chromosome 5 in Plasmodium falciparum is associated with reduced resistance to artelinic acid in vitro. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.01421-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavchich M, Gerena L, Peters J, Chen N, Cheng Q, et al. Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:2455–2464. doi: 10.1128/AAC.00947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siarheyeva A, Sharom FJ. The ABC transporter MsbA interacts with lipid A and amphipathic drugs at different sites. Biochem J. 2009;419:317–328. doi: 10.1042/BJ20081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci U S A. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loo TW, Bartlett MC, Clarke DM. Processing mutations disrupt interactions between the nucleotide binding and transmembrane domains of P-glycoprotein and the cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem. 2008;283:28190–28197. doi: 10.1074/jbc.M805834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oancea G, O'Mara ML, Bennett WF, Tieleman DP, Abele R, et al. Structural arrangement of the transmission interface in the antigen ABC transport complex TAP. Proc Natl Acad Sci U S A. 2009;106:5551–5556. doi: 10.1073/pnas.0811260106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dokomajilar C, Nsobya SL, Greenhouse B, Rosenthal PJ, Dorsey G. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob Agents Chemother. 2006;50:1893–1895. doi: 10.1128/AAC.50.5.1893-1895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmgren G, Hamrin J, Svard J, Martensson A, Gil JP, et al. Selection of pfmdr1 mutations after amodiaquine monotherapy and amodiaquine plus artemisinin combination therapy in East Africa. Infect Genet Evol. 2007;7:562–569. doi: 10.1016/j.meegid.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 30.Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob Agents Chemother. 2007;51:3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutherland CJ, Alloueche A, Curtis J, Drakeley CJ, Ord R, et al. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg. 2002;67:578–585. doi: 10.4269/ajtmh.2002.67.578. [DOI] [PubMed] [Google Scholar]
- 32.Schinkel AH, Kemp S, Dolle M, Rudenko G, Wagenaar E. N-glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J Biol Chem. 1993;268:7474–7481. [PubMed] [Google Scholar]
- 33.Gowda DC, Davidson EA. Protein glycosylation in the malaria parasite. Parasitol Today. 1999;15:147–152. doi: 10.1016/s0169-4758(99)01412-x. [DOI] [PubMed] [Google Scholar]
- 34.Buchaklian AH, Funk AL, Klug CS. Resting state conformation of the MsbA homodimer as studied by site-directed spin labeling. Biochemistry. 2004;43:8600–8606. doi: 10.1021/bi0497751. [DOI] [PubMed] [Google Scholar]
- 35.Loo TW, Bartlett MC, Clarke DM. ATP hydrolysis promotes interactions between the extracellular ends of transmembrane segments 1 and 11 of human multidrug resistance P-glycoprotein. Biochemistry. 2005;44:10250–10258. doi: 10.1021/bi050705j. [DOI] [PubMed] [Google Scholar]
- 36.Taguchi Y, Kino K, Morishima M, Komano T, Kane SE, et al. Alteration of substrate specificity by mutations at the His61 position in predicted transmembrane domain 1 of human MDR1/P-glycoprotein. Biochemistry. 1997;36:8883–8889. doi: 10.1021/bi970553v. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez CP, Rotmann A, Stein WD, Lanzer M. Polymorphisms within PfMDR1 alter the substrate specificity for anti-malarial drugs in Plasmodium falciparum. Mol Microbiol. 2008;70:786–798. doi: 10.1111/j.1365-2958.2008.06413.x. [DOI] [PubMed] [Google Scholar]
- 38.Safa AR, Stern RK, Choi K, Agresti M, Tamai I, et al. Molecular basis of preferential resistance to colchicine in multidrug-resistant human cells conferred by Gly-185----Val-185 substitution in P-glycoprotein. Proc Natl Acad Sci U S A. 1990;87:7225–7229. doi: 10.1073/pnas.87.18.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lekostaj JK, Amoah LE, Roepe PD. A single S1034C mutation confers altered drug sensitivity to PfMDR1 ATPase activity that is characteristic of the 7G8 isoform. Mol Biochem Parasitol. 2008;157:107–111. doi: 10.1016/j.molbiopara.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basco LK, Ringwald P. Molecular epidemiology of malaria in Cameroon. X. Evaluation of PFMDR1 mutations as genetic markers for resistance to amino alcohols and artemisinin derivatives. Am J Trop Med Hyg. 2002;66:667–671. doi: 10.4269/ajtmh.2002.66.667. [DOI] [PubMed] [Google Scholar]
- 41.Chaiyaroj SC, Buranakiti A, Angkasekwinai P, Looressuwan S, Cowman AF. Analysis of mefloquine resistance and amplification of pfmdr1 in multidrug-resistant Plasmodium falciparum isolates from Thailand. Am J Trop Med Hyg. 1999;61:780–783. doi: 10.4269/ajtmh.1999.61.780. [DOI] [PubMed] [Google Scholar]
- 42.Chen N, Russell B, Fowler E, Peters J, Cheng Q. Levels of chloroquine resistance in Plasmodium falciparum are determined by loci other than pfcrt and pfmdr1. J Infect Dis. 2002;185:405–407. doi: 10.1086/338470. [DOI] [PubMed] [Google Scholar]
- 43.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, et al. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol. 2000;108:13–23. doi: 10.1016/s0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 44.Price RN, Cassar C, Brockman A, Duraisingh M, van Vugt M, et al. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother. 1999;43:2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zalis MG, Pang L, Silveira MS, Milhous WK, Wirth DF. Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Am J Trop Med Hyg. 1998;58:630–637. doi: 10.4269/ajtmh.1998.58.630. [DOI] [PubMed] [Google Scholar]
- 46.Carrier I, Gros P. Investigating the role of the invariant carboxylate residues E552 and E1197 in the catalytic activity of Abcb1a (mouse Mdr3). FEBS J. 2008;275:3312–3324. doi: 10.1111/j.1742-4658.2008.06479.x. [DOI] [PubMed] [Google Scholar]
- 47.Loo TW, Bartlett MC, Clarke DM. Processing mutations located throughout the human multidrug resistance P-glycoprotein disrupt interactions between the nucleotide binding domains. J Biol Chem. 2004;279:38395–38401. doi: 10.1074/jbc.M405623200. [DOI] [PubMed] [Google Scholar]
- 48.Dalmas O, Orelle C, Foucher AE, Geourjon C, Crouzy S, et al. The Q-loop disengages from the first intracellular loop during the catalytic cycle of the multidrug ABC transporter BmrA. J Biol Chem. 2005;280:36857–36864. doi: 10.1074/jbc.M503266200. [DOI] [PubMed] [Google Scholar]
- 49.Kajiji S, Talbot F, Grizzuti K, Van Dyke-Phillips V, Agresti M, et al. Functional analysis of P-glycoprotein mutants identifies predicted transmembrane domain 11 as a putative drug binding site. Biochemistry. 1993;32:4185–4194. doi: 10.1021/bi00067a005. [DOI] [PubMed] [Google Scholar]
- 50.Rohrbach P, Sanchez CP, Hayton K, Friedrich O, Patel J, et al. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. EMBO J. 2006;25:3000–3011. doi: 10.1038/sj.emboj.7601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Broer S, et al. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science. 2009;325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 52.Bray PG, Hawley SR, Mungthin M, Ward SA. Physicochemical properties correlated with drug resistance and the reversal of drug resistance in Plasmodium falciparum. Mol Pharmacol. 1996;50:1559–1566. [PubMed] [Google Scholar]
- 53.Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez CP, McLean JE, Rohrbach P, Fidock DA, Stein WD, et al. Evidence for a pfcrt-associated chloroquine efflux system in the human malarial parasite Plasmodium falciparum. Biochemistry. 2005;44:9862–9870. doi: 10.1021/bi050061f. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez CP, McLean JE, Stein W, Lanzer M. Evidence for a substrate specific and inhibitable drug efflux system in chloroquine resistant Plasmodium falciparum strains. Biochemistry. 2004;43:16365–16373. doi: 10.1021/bi048241x. [DOI] [PubMed] [Google Scholar]
- 56.Sisowath C, Petersen I, Veiga MI, Martensson A, Premji Z, et al. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis. 2009;199:750–757. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang H, Patel JJ, Yi M, Mu J, Ding J, et al. Genome-wide compensatory changes accompany drug- selected mutations in the Plasmodium falciparum crt gene. PLoS One. 2008;3:e2484. doi: 10.1371/journal.pone.0002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, et al. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother. 2005;49:3575–3577. doi: 10.1128/AAC.49.8.3575-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 61.Brown NP, Leroy C, Sander C. MView: a web-compatible database search or multiple alignment viewer. Bioinformatics. 1998;14:380–381. doi: 10.1093/bioinformatics/14.4.380. [DOI] [PubMed] [Google Scholar]
- 62.Sali A. Modeling mutations and homologous proteins. Curr Opin Biotechnol. 1995;6:437–451. doi: 10.1016/0958-1669(95)80074-3. [DOI] [PubMed] [Google Scholar]
- 63.Holm L, Sander C. Database algorithm for generating protein backbone and side-chain co-ordinates from a C alpha trace application to model building and detection of co-ordinate errors. J Mol Biol. 1991;218:183–194. doi: 10.1016/0022-2836(91)90883-8. [DOI] [PubMed] [Google Scholar]
- 64.Konagurthu AS, Whisstock JC, Stuckey PJ, Lesk AM. MUSTANG: a multiple structural alignment algorithm. Proteins. 2006;64:559–574. doi: 10.1002/prot.20921. [DOI] [PubMed] [Google Scholar]
- 65.Krieger E, Koraimann G, Vriend G. Increasing the precision of comparative models with YASARA NOVA–a self-parameterizing force field. Proteins. 2002;47:393–402. doi: 10.1002/prot.10104. [DOI] [PubMed] [Google Scholar]
- 66.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 67.Thompson MA. ( http://www.arguslab.com) ArgusLab. In: 4.0.1, editor. Planaria Software LLC. Seattle, WA.
- 68.Schuttelkopf AW, van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein sequences alignment of PfMDR1 and different MsbA structures. The alignment describes the primary percentage of overall matching between PfMDR1 protein as well as secondary and tertiary structures features. Aminoacids letters are coloured by group identity default palette defined by Mview software. Background colour shows secondary structure, grey - coiled; brown- helix and yellow- beta-sheet. On top of the alignment is annotated the ABC conserved motifs as well as main structural characteristics: TM-transmembranes; EL- external loop; IH- internal helix. Stars identify functional residues in PfMDR1 transport: N86Y, Y184F, S1034C, N1042D and D1246Y.
(PDF)
N-glycosylation sites prediction. External loop 1 sequence (residues 79–91) was screened for putative N-glycosylation sites. Two asparagines localize in this loop at positions 84 and 86. Predictive potential sites obtained with NetNglyc 1.0 software.
(PDF)
PfCRT and PfMDR1 coding genotypes for clones D10SND, D10CDY, 7G8SND and 7G8CDY.
(PDF)