Abstract
Vascular factors have been shown to affect the rate of AD progression. However, the effect of the APOE ε4 allele on rate of progression has been ambiguous. Little research to date has examined an interaction between vascular factors and the APOE ε4 allele in predicting decline among AD patients. 216 participants with incident AD from a population of elderly persons in Cache County, Utah, were followed for a mean of 3.3 years and 4.2 follow-up visits. A history of vascular risk factors and conditions and anti-hypertensive use was assessed at the diagnostic visit. Linear mixed effects models tested interactions between the vascular factors, APOE ε4, and time as predictors of clinical progression on the Mini-Mental State Exam (MMSE) and Clinical Dementia Rating-Sum of Boxes (CDR-SB). Multiple comparisons were corrected using the Holm-Bonferroni method. There was a 3-way interaction between stroke, APOE ε4 and time in predicting MMSE decline (LR χ2 = 10.32, 2 df, p = 0.006). For the CDR-SB, there were 3-way interactions between the APOE ε4, time and either myocardial infarction (LR χ2 = 17.83, 2 df, p = 0.0001) or stroke (LR χ2 = 11.48, 2 df, p = 0.003. Results suggest a complex relationship between the APOE ε4 and vascular factors in predicting cognitive and functional progression. Among individuals with a history of stroke or MI at baseline, progression of AD is influenced by APOE ε4 carrier status and varies by time after AD diagnosis.
Keywords: Alzheimer’s disease, population-based, disease progression, APOE, vascular factors, stroke, myocardial infarction
Introduction
The ε4 allele of the Apolipoprotein E (APOE) gene is the strongest known genetic risk factor for late-onset Alzheimer’s dementia (AD) [1, 2]. Compared to non-carriers, heterozygous carriers of one ε4 allele are 3–4 times more likely to develop AD, while the risk for homozygous carriers is even higher [2, 3]. Carriers of an ε4 allele are also at an increased risk of vascular disease including myocardial infarction [4], carotid atherosclerosis [5], and hypertension [6]. While these vascular factors are also known risk factors for AD, the combination of the ε4 allele and vascular factors is especially potent in predicting AD risk [7, 8].
While the majority of studies have focused on APOE as a risk factor for AD, some have examined the association between ε4 allele carrier status and rate of AD progression, with conflicting results. The presence of an ε4 allele has been associated with faster progression after a diagnosis of AD [9–11] while others suggest the ε4 allele is associated with slower progression [12–14] or that there is not an association [15–18]. In our sample of incident cases of AD from the Cache County, Utah population, we reported that the ε4 allele was associated with a lower MMSE score at the time of AD diagnosis but did not affect rate of progression on either the MMSE or CDR- Sum of Boxes (CDR-SB) [19].
We and others have previously shown that vascular factors affect the rate of dementia progression after a diagnosis of AD [20, 21] [9]. Given that the APOE ε4 allele is also associated with risk of vascular disease, it is important to determine whether the interaction between vascular factors and ε4 contribute to rate of progression. One previous small study reported that a history of heart disease or stroke was associated with faster cognitive decline only among APOE ε4 carriers [9]. The aim of the present study was to determine whether the APOE ε4 allele interacted with vascular factors in predicting rate of cognitive and functional decline among the sample of incident AD cases in the Cache County Dementia Progression Study followed up to 11 visits over 9 years.
Materials and Methods
Participants and Dementia Diagnosis
The design and sampling methods of the study have previously been described in detail [19, 22]. Briefly, DPS originated from the longitudinal, population-based Cache County Study on Memory in Aging (CCSMA), which has examined the prevalence, incidence, and risk factors of dementia in a U.S. county recognized for its residents’ longevity. In its first wave, CCSMA enrolled 90% of the 5677 county residents aged 65 years or older. Three triennial incidence waves were subsequently completed. Individuals with incident dementia have been followed prospectively in the Cache County Dementia Progression study (DPS).
All DPS participants were identified from the multi-stage procedures of the CCSMA, which have been reported elsewhere [19, 22]. Briefly, using diagnostic assessments involving cognitive screening and in-home evaluation by a trained team, a study geropsychiatrist and neuropsychologist reviewed data from each participant at each CCSMA wave and assigned preliminary diagnoses of dementia according to DSM-III-R criteria [23]. Neuroimaging and laboratory studies were used as part of the diagnostic work-up to further clarify dementia type. The age of onset was assigned as the age when the participant unambiguously met DSM-III-R criteria for dementia. Dementia severity was rated on the Clinical Dementia Rating (CDR; [24]) and health status according to the General Medical Health Rating [25]. A panel of experts consisting of neurologists, geropsychiatrists, neuropsychologists, and a cognitive neuroscientist reviewed all available clinical and neuropathological data and possible and probable AD were diagnosed according to NINCDS-ADRDA criteria. [26] Vascular dementia was diagnosed following National Institute of Neurological Diseases and Stroke and Association International pour la Recherche et l’Enseignement en Neurosciences (NINDS/AIREN) criteria [27]. All study procedures were approved by the institutional review boards of Utah State, Duke, and Johns Hopkins Universities.
Measures of Dementia Progression
Outcomes reflecting AD dementia were the MMSE [28] and the CDR sum of Boxes (CDR-SB; [24]). The MMSE is a global measure of cognition that is widely used in clinical trials assessing potential treatments on AD progression [29]. Similar to methods previously employed in DPS [19, 21] a sensory/motor MMSE adjusted score was calculated by discarding items missed due to sensory/motor impairment (e.g., severe vision or hearing loss, motor weakness, tremor, etc.), calculating the percent correct, and rescaling the final score on a 30-point scale.
The CDR [24] examines functioning in six domains: memory, orientation, judgment/problem solving, community affairs, home/hobbies, and personal care. The CDR is assessed with a semi-structured interview and has excellent reliability and validity [30]. Scores include a composite score (CDR-composite) and Sum of Boxes (CDR-SB), which is the sum of ratings in each of six domains with a range of 0 (no impairment) to 30 (maximum impairment in all domains). CDR-SB was chosen as the principal outcome here, instead of the composite, because of its greater range and demonstrated sensitivity to change in MCI and AD (e.g. [31]).
Assessment of Vascular Factors
Information on all vascular-related variables was obtained at the baseline visit (i.e. visit at which dementia was diagnosed). We previously examined both the utility of a vascular index and individual vascular risk factors in predicting AD progression [21]. The vascular index was adapted from the stroke risk profile developed in the Copenhagen City Heart Study (CCHS) [32]. This index was similar to the Framingham Stroke Risk Profile [33] but incorporated more self-reported data and was therefore closer to methods of the present study. However, the results of the vascular index were null because some vascular factors were found to be associated with a more rapid rate of decline (Atrial fibrillation (AF), systolic hypertension, and angina) while others (history of coronary artery bypass graft surgery (CABG), diabetes, and anti-hypertensive medications) were associated with a slower rate of decline. Thus, we have also focused on individual vascular factors for the present analyses. The following were considered and included because they were originally part of the vascular index: systolic blood pressure (SBP); history of atrial fibrillation (AF), diabetes mellitus (DM), myocardial infarction (MI), smoking, angina or coronary artery bypass surgery (CABG); and current anti-hypertensive medication use. Systolic blood pressure was the average of two measurements obtained by a nurse at the baseline visit, 5 minutes apart. Information on AF, DM, MI, angina, and CABG was obtained via self- and proxy-report. Ascertainment of medication use in this study has been previously described [34] and relied on visual inspection of all available medication vials at each visit. If participants were institutionalized, this information was obtained from nursing home records. We classified participants as current antihypertensive medication users if they were regularly (≥4 times per week) taking a medication from the following drug classes at baseline: angiotensin converting enzyme inhibitors, beta-blocking anti-adrenergics, calcium ion channel blockers, and diuretics.
Statistical Analyses
The present analyses assessing dementia progression focused only on those individuals with at least one follow-up visit. Differences in baseline demographic and health-related characteristics between those with only a baseline visit and those with one or more follow-up visits were examined using Fischer’s Exact test for categorical variables and Student’s t-test for continuous variables. Differences in the prevalence of vascular disease by APOE ε4 genotype (any ε4 allele vs. none) were assessed using Fisher’s exact test.
To model non-linear effects of the interaction between vascular factors and APOE genotype on dementia progression, we examined average change in MMSE and CDR-SB from the visit at which dementia was first diagnosed, using mixed effects models and treating subject-specific intercepts and linear change with time as random effects. This approach, used previously in DPS [19] allowed us to assess the effects of key fixed factors, such as age, on average rate of change, while accounting for the dependence between within-subject repeated measures and for non-linear change with respect to time. Because our analysis revealed significant non-linear time effects for both the MMSE and CDR-SB, we included a time-squared term and appropriate time-squared terms in all examined interactions. The quadratic term allows a better assessment of the cognitive trajectories at later states of the disease. Furthermore, the quadratic terms allowed us to investigate whether the relationship between APOE and vascular factors affected the rate of cognitive decline over the whole post-onset period or whether it was more evident at earlier stages of the disease. Interaction terms were retained in the models if the comparison between likelihood ratio (LR) test statistics between models with and without the interaction terms was significant (p<0.05).
The following variables have previously been found to be associated with progression in MMSE and CDR-SB in this population of AD participants [19]. They were, therefore, included as covariates in the present analyses: baseline age, gender, education, and dementia duration – the age differences from dementia onset to when a diagnosis was made. Education, gender and APOE genotype were determined at Wave 1 of the CCSMA. APOE genotype was determined from buccal DNA using a standard protocol [22]. The a priori p-value was set at p<0.05. Corrections for multiple comparisons were examined within each outcome using the Holm-Bonferroni method [35]. All analyses were conducted using STATA Version 11.1 (StataCorp, College Station, TX).
Results
There were 327 cases of incident AD with baseline MMSE or CDR-SB scores. The current analyses included 216 participants (66.1%) diagnosed with incident AD who had at least one follow-up visit and an available MMSE or CDR-SB score at baseline. The majority were female (65.8%), Caucasian (99.1%) and had mild dementia (mean composite CDR = 1.1, SD = 0.6) at baseline. One hundred eleven individuals (33.9%) lacked any follow-up, the majority (n = 88, 79.3%) due to death. As previously reported [19], these individuals were older and had a lower MMSE at diagnosis compared to those with follow-up data. The prevalence of vascular factors at baseline by follow-up status is shown in Table 1. While there were no significant differences at the p<0.05 level, individuals without a follow-up were more likely to have had a MI (19.1% vs. 11.6%, p=0.091) and to be taking anti-hypertensive medication (54.6% vs. 43.5%, p=0.062) at baseline.
Table 1.
Prevalence of baseline vascular factors among those with and without a follow-up
| Vascular Factor | n=327 (all) | no follow-up (n=111) | Any follow-up (n=216) | p-value |
|---|---|---|---|---|
| N/(%) | N/(%) | N/(%) | ||
| SBP (cont) | 128.8 (17.7) | 129.9 (18.0) | 127.4 (17.6) | 0.267 |
| SBP >=140 | 73/293 (24.9%) | 20/103 (29.1%) | 43/190 (22.6%) | 0.258 |
| Atrial Fibrillation | 45/327 (13.8%) | 17/111 (15.3%) | 28/216 (13.0%) | 0.612 |
| Angina | 46/325 (14.2%) | 17/110 (15.5%) | 29/215 (13.5%) | 0.618 |
| MI | 46/326 (14.1%) | 21/110 (19.1%) | 25/216 (11.6%) | 0.091 |
| CABG | 19/327 (5.8%) | 9/110 (8.1%) | 10/216 (4.65%) | 0.218 |
| Diabetes | 54/327 (16.5%) | 19/111 (17.1%) | 35/216 (16.2%) | 0.474 |
| Current Anti-htn med | 154/326 (47.2%) | 60/110 (54.6%) | 94/216 (43.5%) | 0.062 |
| Stroke | 16/327 (4.89%) | 8/111 (7.2%) | 8/216 (3.7%) | 0.182 |
Of the 216 participants with follow-up data, average time in the study was 3.3 years (SD = 2.2; max = 9.9 years) with a mean of 4.2 study visits (SD = 2.4; max = 11 visits). Ninety-seven (44.9%) of those with a follow-up had at least one APOE ε4 allele. The prevalence of baseline vascular factors did not differ by APOE ε4 allele carrier status (Table 2).
Table 2.
Prevalence of vascular factors by Apoe E4 genotype (any vs. none) among participants with at least one follow-up (n=216)
| Vascular Factor | No E4 (n=119) | Any E4 (1 or 2) (n=97) | p-value | |
|---|---|---|---|---|
| N/(%) | N/(%) | |||
| SBP >=140: | Present | 24 (22.2%) | 19 (23.2%) | 1.000 |
| Absent | 84 (77.8%) | 63 (76.8%) | ||
| Atrial Fibrillation: | Present | 19 (16.0%) | 9 (9.3%) | 0.160 |
| Absent | 100 (84.0%) | 88 (90.7%) | ||
| Angina: | Present | 16 (13.6%) | 13 (13.4%) | 1.000 |
| Absent | 102 (86.4%) | 84 (86.6%) | ||
| MI: | Present | 14 (11.8%)) | 11 (11.3%) | 1.000 |
| Absent | 105 (88.2%) | 86 (88.7%) | ||
| CABG: | Present | 7 (5.8%) | 3 (3.1%) | 0.517 |
| Absent | 112 (94.1%) | 94 (96.9%) | ||
| Diabetes: | Present | 22 (18.5%) | 13 (13.4%) | 0.357 |
| Absent | 97 (81.5%) | 84 (86.6%) | ||
| Current Anti-htn Med: | Present | 48 (40.3%) | 45 (47.4%) | 0.335 |
| Absent | 71 (59.7%) | 51 (52.6%) | ||
| Stroke: | Present | 4 (3.4%) | 4 (4.1%) | 1.000 |
| Absent | 115 (96.6%) | 93 (95.9%) |
p-value based on Fischer’s Exact test
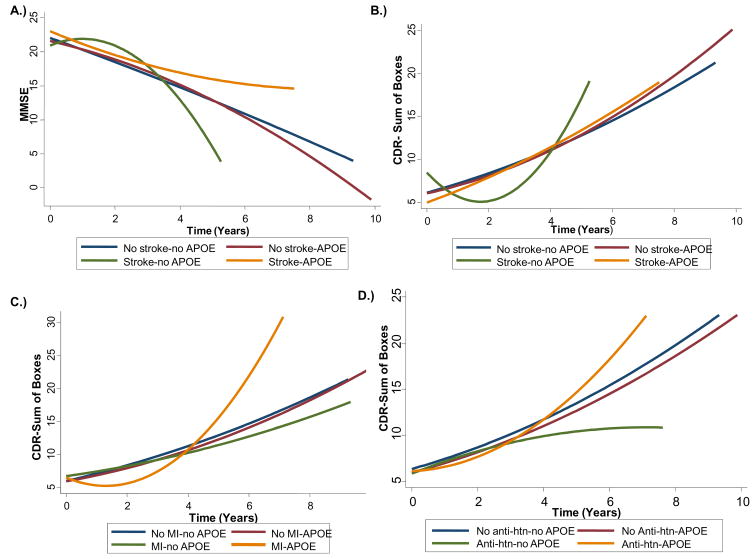
Using multivariate mixed effects models (Table 3), presence of an APOE ε4 allele, regardless of vascular risk factor status, did not predict progression on either the MMSE or CDR-SB. However, there was a significant 3-way interaction (Table 3 and Figure 1a) between stroke, APOE ε4 allele and time (LR χ2 = 10.32, 2 df, p = 0.006), suggesting differential rate of progression for those with vs. without a history of stroke and those with vs. without an ε4 allele. Because higher scores on MMSE and lower scores on CDR-SB denote higher cognitive and functional status (respectively), “faster decline” is denoted by a negative coefficient for MMSE but a positive coefficient for CDR-sb. Figure 1a displays this model relationship such that individuals with a history of stroke and APOE ε4 allele initially declined faster on the MMSE compared to individuals without a history of stroke and no APOE ε4 allele, but their rate of decline decreased over time and they experienced a slower rate of decline relative to the other groups.
Table 3.
Examination of an interaction between vascular factors and APOE as a predictor of cognitive and functional progression on the MMSE and CDR-Sum.
| Vascular factor | MMSE*,† | CDR-Sum*,† | ||
|---|---|---|---|---|
| b (95% CI) | LR test | b (95% CI) | LR test | |
| SBP | ||||
| SBP * APOE * time | 0.01 (−0.03, 0.05) | χ2 = 0.60, 2 df, p = 0.741 | −0.01 (−0.04, 0.03) | χ2 = 0.28, 2 df, p = 0.867 |
| SBP * APOE * time2 | 0.001 (−0.01-0.01) | 0.001 (−0.01-0.01) | ||
| Atrial Fibrillation | ||||
| AF * APOE * time | 1.13 (−1.31, 3.58) | χ2 = 3.31, 2 df, p = 0.192 | −0.92 (−2.92, 1.07) | χ2 = 3.98, 2 df, p = 0.137 |
| AF * APOE * time2 | −0.31 (−0.64, 0.03) | 0.28 (−0.01, 0.57) | ||
| Angina | ||||
| Angina * APOE * time | 1.04 (−1.64, 3.71) | χ2 = 2.77, 2 df, p = 0.251 | −0.32 (−2.48, 1.83) | χ2 = 0.20, 2 df, p = 0.906 |
| Angina * APOE * time2 | 0.08 (−0.47, 0.63) | 0.01 (−0.42, 0.43) | ||
| Myocardial Infarction (MI) | ||||
| MI * APOE * time | 0.03 (−2.41, 2.47) | χ2 = 1.99, 2 df, p = 0.370 | −1.85 (−3.92, 0.21) | χ2 = 17.83, 2 df, p = 0.0001‡ |
| MI * APOE * time2 | −0.18 (−0.54, 0.18) | 0.61 (0.29, 0.93) | ||
| CABG | ||||
| CABG * APOE * time | −1.21 (−5.98, 3.55) | χ2 = 0.40, 2 df, p = 0.818 | −1.88 (−6.01, 2.26) | χ2 = 3.10, 2 df, p = 0.212 |
| CABG * APOE * time2 | 0.32 (−0.66, 1.30) | 0.72 (−0.18, 1.62) | ||
| Diabetes | ||||
| Diabetes * APOE * time | −1.06 (−2.91, 0.80) | χ2 = 1.29, 2 df, p = 0.525 | 0.91 (−0.65, 2.48) | χ2 = 1.41, 2 df, p = 0.494 |
| Diabetes * APOE * time2 | 0.09 (−0.12, 0.29) | −0.09 (−0.27, 0.09) | ||
| Current Anti-htn Med | ||||
| Med * APOE * time | 1.19 (−0.44, 2.83) | χ2 = 2.03, 2 df, p = 0.362 | −0.96 (−2.36, 0.44) | χ2 = 7.20, 2 df, p = 0.027 |
| Med * APOE * time2 | −0.14 (−0.43, 0.15) | 0.34 (0.08, 0.60) | ||
| Stroke | ||||
| Stroke * APOE * time | −4.56 (−9.50, 0.38) | χ2 = 10.32, 2 df, p = 0.006‡ | 6.02 (1.95, 10.10) | χ2 = 11.48, 2 df, p = 0.003‡ |
| Stroke * APOE * time2 | 1.36 (0.48, 2.23) | −1.32 (−2.08, −0.56) | ||
Using Mixed Effects Regression, all models adjusted for time, time2, baseline age, sex, education, dementia duration at baseline, any APOE E4 allele, and all baseline vascular factors.
A positive coefficient for MMSE represents better performance whereas a negative coefficient for CDR-Sum represents a better performance.
Bolded values remain significant after Holm-Bonferroni correction for multiple comparison.
Figure 1.
Interactions between APOE ε4 carrier status, and either a history of stroke, myocardial infarction (MI), or baseline anti-hypertensive (anti-htn) use on rate of progression on the MMSE and CDR-Sum. A) Interaction between APOE ε4 carrier status and stroke on MMSE progression; B) Interaction between APOE ε4 carrier status and stroke on CDR-Sum progression; C) Interaction between APOE ε4 carrier status and MI on CDR-Sum progression; D) Interaction between APOE ε4 carrier status and baseline anti-htn use on CDR-Sum progression.
For progression on the CDR-SB (Table 3), there were significant three-way interactions between the APOE ε4 allele, time and either MI (LR χ2 = 17.83, 2 df, p = 0.0001) or stroke (LR χ2 = 11.48, 2 df, p = 0.003). There was a trend for anti-hypertensive medication use, but this did not hold up after corrections for multiple comparisons (LR χ2 = 7.20, 2 df, p = 0.027). Figure 1b–d show these associations in greater detail. Similar to the results of the MMSE, individuals with a history of stroke and APOE ε4 allele initially had a faster rate of decline on CDR-SB (b = 6.02), compared to individuals without a history of stroke and no APOE ε4 allele, but their rate of decline decreased over time and they experienced a slower rate of decline relative to non-carriers. In the three-way interaction between MI, APOE, and time, individuals with a history of MI and who were APOE ε4 carriers initially had a slower CDR-SB rate of decline compared to those without a history of MI and who were not APOE ε4 allele carriers, but their rate of decline accelerated over time such that they had a faster rate of decline relative to the other groups.
Discussion
In this population-based study of incident AD cases, we previously reported that vascular factors affected rate of decline on the MMSE and CDR-Sum. In this paper, we have described interactions between vascular factors and APOE ε4 carrier status. Results suggest strong three-way interactions between the presence of an APOE ε4 allele, time and stroke on the rate of MMSE decline. They also suggest interactions between the presence of an APOE ε4 allele, time, and either stroke or MI on rate of CDR-SB decline.
Vascular factors have consistently been shown to affect the rate of AD progression [9, 20, 21]. However, the effect of the APOE ε4 allele on rate of progression has been more ambiguous [9–18]. Little research to date has examined an interaction between vascular factors and the presence of an APOE ε4 allele in predicting decline among AD patients. Autopsy studies have reported that the APOE ε4 allele is associated with greater Alzheimer pathology burden, including neuritic senile plaques (e.g. [36]) and neurofibrillary tangles (e.g. [37], and also with greater vascular burden, including small vessel arteriolosclerosis, amyloid angiopathy, and other microvascular changes (e.g. [38]). Vascular factors are presumably related to more vascular pathology but a higher prevalence of senile plaques have also been reported at autopsy of middle-aged individuals with coronary artery disease [39]. Thus, the combination of having an APOE ε4 allele likely leads to more vascular pathology, but also may lead to more Alzheimer pathology, and may therefore be especially potent in predicting incident AD and/or rate of progression after a diagnosis.
To date, just one small study has reported interactions between vascular factors and the APOE ε4 allele in predicting cognitive decline among AD patients [9]. Among ε4 carriers, higher total cholesterol, LDL-cholesterol, stroke, and heart disease were associated with faster cognitive decline. In the present study, we also reported strong three-way interactions between the presence of an APOE ε4 allele, time, and stroke in predicting decline on MMSE and between APOE ε4 allele, time, and either stroke or MI in predicting decline on the CDR-sum. Interestingly, we found that AD patients with a history of stroke and an APOE ε4 allele had less cognitive and functional progression over time compared to those with a history of a stroke and no APOE ε4 allele. While this finding is in contrast to the previous study examining this relationship among AD patients [9], it is congruent with studies that have reported no effect of stroke on incident dementia in APOE e4 allele carriers but did find increased risk in those without an APOE ε4 allele. Presumably, a similar relationship could exist after the clinical onset of AD. Importantly, our findings suggest that this relationship is complex and varies by time from AD diagnosis. Thus, individuals with a history of stroke or MI, or who use anti-hypertensives at baseline, do not progress linearly over time after a diagnosis of AD. Rather, their progression is influenced by APOE ε4 carrier status and varies by time from AD diagnosis.
There are alternative explanations for the time-varying relationship between these vascular factors, APOE ε4 carrier status, and cognitive and functional decline after a diagnosis of AD. First, there’s the possibility of differential survival such that those who have a stroke and an APOE ε4 allele are more likely to die, and those who did not die are more robust to the effects of this interaction on cognitive and functional progression after AD. However, the literature supporting this reasoning is equivocal at best and several studies have not found the APOE ε4 allele to be a risk factor for mortality or poor outcome after stroke [40–42]. Second, the timing of stroke prior to onset of dementia could be important. After a stroke, several people have an initial cognitive decline and then some recovery. While this could explain the initial time-varying effects reported in the present study, stroke as a significant contributor for dementia was included as exclusionary criteria for a diagnosis of AD in this study and, therefore, likely did not occur immediately prior to memory symptoms and AD diagnosis. Lastly, vascular factors were examined at the visit at which incident AD was diagnosed in this study. It is likely that multiple individuals had additional vascular events, including stroke, after a diagnosis of AD. Future analyses will examine the time-varying effects of vascular factors on rate and recovery of cognitive and functional decline in AD patients.
There are several strengths to this study. This was a longitudinal population-based study with incident AD cases, thereby attenuating selection bias found in clinical studies of Alzheimer progression. Second, the participants have been well-characterized over many years of observation (from the Cache County Memory Study and the DPS). Third, participants with primary or co-morbid Vascular Dementia were excluded so as to prevent circularity with regards to vascular risk factors, APOE, and diagnosis of AD. Despite these strengths several limitations warrant consideration. Information on vascular factors was primarily based on self- and proxy-report. As underreporting is more likely than over-reporting, these findings are conservative and, therefore, an interaction between vascular factors and APOE could play a larger role in AD progression. Second, despite having a larger sample size than previous papers, a sample size of 216 with at least one follow-up is small and leads to reduced power at longer durations of follow-up. Thus, the study warrants replication in a population-based study of incident cases with larger sample sizes, particularly when examining the effects of stroke. Third, dates of onset for vascular factors were not available and it is possible that the time at which the vascular factor occurs prior to AD-dementia onset could impact decline. Finally, the Cache County population is primarily Caucasian and of northern European descent. Thus, the results obtained here may not generalize to populations with different ethnic representation.
Acknowledgments
This research was supported by the following NIH grants: R01AG21136, R01AG11380, R01AG18712, K24-AG027841, R01-HG02213, R01-HG005092.
Footnotes
Disclosure Statement: The authors had access to the data at all times and retain the data. Funding was obtained from NIH grants. All participants provided informed consent and the study was approved by the Johns Hopkins University, Utah State University, and Duke University Institutional Review Boards.
References
- 1.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 4.Anand SS, Xie C, Pare G, Montpetit A, Rangarajan S, McQueen MJ, Cordell HJ, Keavney B, Yusuf S, Hudson TJ, Engert JC. Genetic variants associated with myocardial infarction risk factors in over 8000 individuals from five ethnic groups: The INTERHEART Genetics Study. Circ Cardiovasc Genet. 2009;2:16–25. doi: 10.1161/CIRCGENETICS.108.813709. [DOI] [PubMed] [Google Scholar]
- 5.Elosua R, Ordovas JM, Cupples LA, Fox CS, Polak JF, Wolf PA, D’Agostino RA, Sr, O’Donnell CJ. Association of APOE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J Lipid Res. 2004;45:1868–1875. doi: 10.1194/jlr.M400114-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Niu W, Qi Y, Qian Y, Gao P, Zhu D. The relationship between apolipoprotein E epsilon2/epsilon3/epsilon4 polymorphisms and hypertension: a meta-analysis of six studies comprising 1812 cases and 1762 controls. Hypertens Res. 2009;32:1060–1066. doi: 10.1038/hr.2009.164. [DOI] [PubMed] [Google Scholar]
- 7.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 8.Beeri MS, Rapp M, Silverman JM, Schmeidler J, Grossman HT, Fallon JT, Purohit DP, Perl DP, Siddiqui A, Lesser G, Rosendorff C, Haroutunian V. Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology. 2006;66:1399–1404. doi: 10.1212/01.wnl.0000210447.19748.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65:1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- 11.Craft S, Teri L, Edland SD, Kukull WA, Schellenberg G, McCormick WC, Bowen JD, Larson EB. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology. 1998;51:149–153. doi: 10.1212/wnl.51.1.149. [DOI] [PubMed] [Google Scholar]
- 12.Frisoni GB, Govoni S, Geroldi C, Bianchetti A, Calabresi L, Franceschini G, Trabucchi M. Gene dose of the epsilon 4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer’s disease. Ann Neurol. 1995;37:596–604. doi: 10.1002/ana.410370509. [DOI] [PubMed] [Google Scholar]
- 13.Stern Y, Brandt J, Albert M, Jacobs DM, Liu X, Bell K, Marder K, Sano M, Albert S, Del-Castillo Castenada C, Bylsma F, Tycko B, Mayeux R. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer’s disease. Ann Neurol. 1997;41:615–620. doi: 10.1002/ana.410410510. [DOI] [PubMed] [Google Scholar]
- 14.Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS. Individual growth curve analysis of APOE epsilon 4-associated cognitive decline in Alzheimer disease. Arch Neurol. 2005;62:454–459. doi: 10.1001/archneur.62.3.454. [DOI] [PubMed] [Google Scholar]
- 15.Dal Forno G, Rasmusson DX, Brandt J, Carson KA, Brookmeyer R, Troncoso J, Kawas CH. Apolipoprotein E genotype and rate of decline in probable Alzheimer’s disease. Arch Neurol. 1996;53:345–350. doi: 10.1001/archneur.1996.00550040085017. [DOI] [PubMed] [Google Scholar]
- 16.Kleiman T, Zdanys K, Black B, Rightmer T, Grey M, Garman K, Macavoy M, Gelernter J, van Dyck C. Apolipoprotein E epsilon4 allele is unrelated to cognitive or functional decline in Alzheimer’s disease: retrospective and prospective analysis. Dement Geriatr Cogn Disord. 2006;22:73–82. doi: 10.1159/000093316. [DOI] [PubMed] [Google Scholar]
- 17.Bracco L, Piccini C, Baccini M, Bessi V, Biancucci F, Nacmias B, Bagnoli S, Sorbi S. Pattern and progression of cognitive decline in Alzheimer’s disease: role of premorbid intelligence and ApoE genotype. Dement Geriatr Cogn Disord. 2007;24:483–491. doi: 10.1159/000111081. [DOI] [PubMed] [Google Scholar]
- 18.Growdon JH, Locascio JJ, Corkin S, Gomez-Isla T, Hyman BT. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer’s disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- 19.Tschanz JT, et al. Progression of Cognitive, Functional and Neuropsychiatric Symptom Domains in a Population Cohort with Alzheimer’s Dementia: The Cache County Dementia Progression Study. Am J Geriatric Psychiatry. doi: 10.1097/JGP.0b013e3181faec23. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regan C, Katona C, Walker Z, Hooper J, Donovan J, Livingston G. Relationship of vascular risk to the progression of Alzheimer disease. Neurology. 2006;67:1357–1362. doi: 10.1212/01.wnl.0000240129.46080.53. [DOI] [PubMed] [Google Scholar]
- 21.Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitner JC, Munger R, Lyketsos CG. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- 22.Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 23.APA. Diagnostic and statistical manual of mental disorders. American Psychatric Association; 1987. [Google Scholar]
- 24.Hughes C, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. The British Journal of Dementia. 1982:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 25.Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren A, Sheppard JM, Baker A, Brandt J. The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Mohs RC, Schmeidler J, Aryan M. Longitudinal studies of cognitive, functional and behavioural change in patients with Alzheimer’s disease. Stat Med. 2000;19:1401–1409. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1401::aid-sim432>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177-178. [DOI] [PubMed] [Google Scholar]
- 31.Pavlik VN, Doody RS, Massman PJ, Chan W. Influence of premorbid IQ and education on progression of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:367–377. doi: 10.1159/000095640. [DOI] [PubMed] [Google Scholar]
- 32.Truelsen T, Lindenstrom E, Boysen G. Comparison of probability of stroke between the Copenhagen City Heart Study and the Framingham Study. Stroke. 1994;25:802–807. doi: 10.1161/01.str.25.4.802. [DOI] [PubMed] [Google Scholar]
- 33.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 34.Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- 35.Holm S. A simple sequentially rejective multiple test procedure. Scandanavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 36.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 38.Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- 39.Sparks DL, Hunsaker JC, 3rd, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11:601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- 40.Gromadzka G, Baranska-Gieruszczak M, Sarzynska-Dlugosz I, Ciesielska A, Czlonkowska A. The APOE polymorphism and 1-year outcome in ischemic stroke: genotype-gender interaction. Acta Neurol Scand. 2007;116:392–398. doi: 10.1111/j.1600-0404.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 41.Sarzynska-Dlugosz I, Gromadzka G, Baranska-Gieruszczak M, Ciesielska A, Czlonkowska A. APOE does not predict poor outcome 1 year after ischemic stroke. Neurol Res. 2007;29:64–69. doi: 10.1179/174313206X152528. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Gonzalez NA, Sudlow CL. Effects of apolipoprotein E genotype on outcome after ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77:1329–1335. doi: 10.1136/jnnp.2006.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]