Abstract
Background
Recently, oral tobacco products have been marketed specifically towards cigarette smokers. These products come in different nicotine doses and formulations (snus vs. lozenge). To date, little research has been conducted to determine how smokers respond to these products. The goal of this study was to examine if smokers prefer certain oral tobacco products based on their specific characteristics.
Methods
Smokers interested in quitting underwent a sampling phase and a treatment phase. The sampling phase consisted of testing five different products varying in nicotine dose (high vs. moderate vs. low) and formulation (snus vs. lozenge): General Snus, Camel Snus, Marlboro Snus, Stonewall and Ariva. Each product was sampled in the natural environment on separate days. At the end of the sampling period, subjects chose which product they would use during the 2-week cigarette abstinence phase.
Results
General Snus (high nicotine) was not preferred by any smoker. No significant differences in preferences were observed across the other tobacco products. During the smoking cessation phase, Camel Snus was generally associated with greater craving relief and satisfaction, reduced use of cigarettes, and greater abstinence during follow-up compared to other products.
Conclusion
There were no differences in preferences for four of the five oral tobacco products but higher nicotine oral tobacco products were associated with better cessation outcomes among smokers who chose these products.
Keywords: Oral tobacco products, tobacco product testing, cessation
1. Introduction
In the past several years, there has been an increase in oral tobacco products that have been specifically manufactured and marketed to smokers (e.g., Camel or Marlboro Snus). These products are often promoted as a substitute for smoking, particularly in situations where smokers cannot smoke. The impact these tobacco products are likely to have on public health is unknown. Important issues that need to be addressed include the toxicity of the products, how they will be used, and their potential to reduce or induce harm in an individual and on a population level. The major concerns that have been raised by the public health community include: 1) potential “gateway” effect (e.g., initiating with smokeless and switching to cigarettes) (Severson et al., 2007; Tomar, 2003); 2) dual use of smokeless tobacco and cigarettes, which might lead to increased exposure to toxicants and disease risk (Accortt et al., 2002; Teo et al., 2006); and 3) increased or sustained prevalence of tobacco use through recruitment of new users, relapse of ex-smokers, or maintenance of tobacco use in current smokers who might have otherwise quit (see Hatsukami et al., 2004 for review). Some scientists have pointed to the Swedish experience to demonstrate there is a reduced risk of becoming a smoker among male primary snus users compared to non-primary snus users (Ramstrom and Foulds, 2006). In addition, use of snus by smokers has been associated with decreased cigarette smoking and increased chances of becoming abstinent (Bates et al., 2003; Fagerstrom and Schildt, 2003; Gartner and Hall, 2010; Gartner et al., 2007; Ramstrom and Foulds, 2006; Stegmayr et al., 2005; Stenbeck et al., 2009). Other studies do not support some of these findings (Tomar, 2002; Tomar, 2007), particularly in specific populations (Stenbeck et al., 2009).
Even with the influx of these products on the market, very little research has been conducted on whether oral tobacco products are appealing to smokers and the characteristics that would make them appealing. That is, oral tobacco products vary not only in formulation (tobacco in a pouch vs. tobacco lozenge) and flavor, but also vary in the amount of nicotine that they contain (Stepanov et al., 2008).
The primary aims of this exploratory study were to assess the preference by smokers of five oral tobacco products that differed in formulation and dose of nicotine and the effects of the selected products during a 2-week cessation trial.
2. Methods
This multisite study (University of Minnesota and Oregon Research Institute) recruited cigarette smokers through mailings and various media advertisements that stated that cigarette smokers were needed for a study of new oral tobacco products that may help them quit smoking. Individuals interested in the study telephoned the research clinics, and were then informed about the study and initially screened in order to determine whether they met the following inclusion criteria: 1) smoking at least 10 cigarettes daily for the past year and with no current use of other nicotine-containing products; 2) motivation to quit of 7 or higher on a 0 to 10 scale (0=not at all interested in quitting, 10=very much interested in quitting); 3) in good physical health; 4) no contraindications for oral tobacco use, such as mouth sores or significant dental problems; and 5) stable, good mental health with no recent history of substance abuse. Women who were pregnant or nursing were excluded
Smokers who met the inclusion criteria attended an orientation meeting where they were introduced to the oral tobacco products and informed of the study procedures. Subjects then signed a consent form and completed questionnaires including tobacco use history, demographics, Fagerstrom Test for Nicotine Dependence (FTND, Heatherton et al., 1991) and medical history form.
2.1. Baseline
Subjects underwent one week of baseline data collection while smoking at their normal rate. Smoking diaries were kept in real time on a 3 × 5 card. Withdrawal symptoms assessed by the Minnesota Nicotine Withdrawal Scale (MNWS, Hughes and Hatsukami, 1998; Hughes and Hatsukami, 1986) were recorded on a written diary completed at the end of each day.
2.2. Taste Testing
After baseline data collection and prior to the cessation phase, subjects were shown the oral tobacco products, but were blind to the product brand and packaging. They were asked to sample several flavors of three different oral products to be used during the first week of sampling (see Table 1 for flavor and nicotine levels for each tobacco product). They were provided one pouch/lozenge of each of the flavors and asked to place it in their mouths for a sufficient time to determine their flavor preference. Products were sampled in random order. They were given a mouth rinse of water between each flavor sampling. Subjects then selected the flavor they found most appealing for each of the three different oral tobacco products. This taste testing procedure was repeated during the second week with the two remaining oral tobacco products.
Table 1.
Study products for sampling phase
| Study Product | Flavors | Range in free nicotine mg/portion1 | ||
|---|---|---|---|---|
| Stonewall | Wintergreen | Java | Natural | 0.28–0.57 |
| Camel Snus | Frost | Mellow | 1.74–1.97 | |
| Ariva | Wintergreen | Java | 0.24–0.25 | |
| Marlboro Snus | Spearmint | Peppermint | Rich | 0.14–0.38 |
| General Snus | Portion | 3.37 |
Analyzed by Irina Stepanov (personal communication)
2.3. Sampling Weeks
Subjects continued to be blinded to the brands of snus (but not oral tobacco lozenges) during the Sampling Weeks to minimize a brand preference (e.g., Camel smokers partial to Camel packaging). Subjects were instructed on how to use the different types of oral tobacco products (e.g., place the product between the gum and cheek or upper lip, keep it there for at least 30 minutes and avoid any acidic beverages and foods during this time to maximize nicotine absorption).
Subjects were given instructions on which product to use on a specified day. Ten pouches/lozenges of each product of the subject’s preferred flavor were dispensed. Subjects were instructed to be abstinent from cigarettes after midnight and were told that they were to use only the assigned product on that day until 1 pm (or for at least the first five waking hours). Subjects were informed that they must use at least three pouches/lozenges of the assigned product prior to 1 pm. After 1 pm (or at least a five hour period since awakening), they could smoke their usual cigarettes and were allowed to use the remainder of the day’s assigned product ad libitum if desired. On the next day, the subject only smoked their cigarettes. Subjects alternated between sampling a product and resuming smoking the next day. After sampling the third product during the first week, the subject returned to the clinic to return unused products and to obtain products to be used for the next week. Sampling Week 2 followed the same procedures as Week 1 but involved sampling only two products.
Subjects recorded their product sampling behaviors on a daily diary in addition to any cigarettes smoked during the required abstinent period and similarly after 1 pm for the rest of the day. On smoking days, subjects were asked to record number of cigarettes smoked.
At the end of Sample Week 2, subjects completed a Perception of Products questionnaire, which assessed products on relief from withdrawal, relief from craving, ease of use, and general satisfaction or likeability. Subjects then selected the product that they would use for the next two weeks during smoking cessation.
2.4. Treatment Weeks
Subjects quit smoking on Monday following the end of Sample Week 2 and attended clinic visits on Days 3, 7 and 14 of cigarette abstinence. Daily diaries of product and cigarette use and withdrawal symptoms were kept through the cessation period.
At every clinic visit, vital signs, weight, tobacco use amount, Product Evaluation Scale, MNWS, an adverse events form, and use of concomitant medications were evaluated. The Product Evaluation Scale is a 7-point Likert-type scale (1=not at all, 7=extremely) modified from the Cigarette Evaluation Scale (Westman et al., 1992) where subjects rate the oral products on a number of dimensions. Scale scores address three factors reflecting product satisfaction, psychological reward, and aversiveness. In addition, items that have been used to evaluate various medicinal nicotine products were also incorporated in the Product Evaluation Scale (Schneider et al., 2004). Subjects returned all unused products to confirm that the data entered in their diary entries matched the number of pouches/lozenges that had been used. Brief standardized behavioral counseling for cessation was provided starting at the end of Sample Week 2 visit to facilitate cessation. During these sessions, techniques to alleviate withdrawal were not discussed to avoid contaminating the withdrawal assessments. Topics covered included previous quitting experience, garnering social support, and relapse prevention skills.
2.5. Follow-Up
Follow-up clinic visit occurred 1-week post treatment after termination of all tobacco products to assess smoking status. A follow-up phone call at Week 9 (4 weeks post-tobacco use) was conducted to assess any concerns associated with being a subject in the study. Participants were paid for participation based on the number of visits (up to $300).
2.6. Biochemical measures
Carbon monoxide level was obtained at all visits. A first morning urine sample was obtained at the baseline visit, after 2 weeks on product of choice and at 1 week off of all tobacco products. Urines were analyzed for total cotinine.
2.7. Statistics
All categorical variables for description of subject disposition and characteristics were analyzed using Chi-square test or Fisher’s exact test, and all continuous variables were analyzed using two-sample t test. Amount of tobacco products and cigarettes used during sampling period were analyzed using one-way ANOVA to evaluate overall and study site-specific effects of sample products. Use of chosen product and cigarettes, Product Evaluation Scale (3 factor scores and 9 individual questions) and MNWS (total withdrawal and craving) measured during the treatment period were analyzed using general linear mixed models which included site, product chosen, day, their two-way and three-way interaction terms as fixed effects, and random intercept and slope as random effects. For total withdrawal and craving from MNWS, baseline values were also included as a covariate in the mixed models. Time to relapse (smoking even one puff) was measured in days from the start of 2-week treatment period to the end of first 7 days of follow-up period. Survival probabilities were estimated using Kaplan-Meier’s method. The effects of site and product chosen on surviving from relapse were determined using Cox’s proportional hazard models. Point prevalence abstinence for the last 7 days of treatment period and first 7 days of follow-up verified by CO and proportion of continued product use at 1 and 4-weeks after treatment were calculated and compared between sites and among chosen products using Chi-square test or Fisher’s exact test. All the statistical analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC, USA) and level of significance was considered <0.05. The p values reported for multiple comparisons were unadjusted because of the exploratory nature of this study.
3. Results
3.1. Subjects
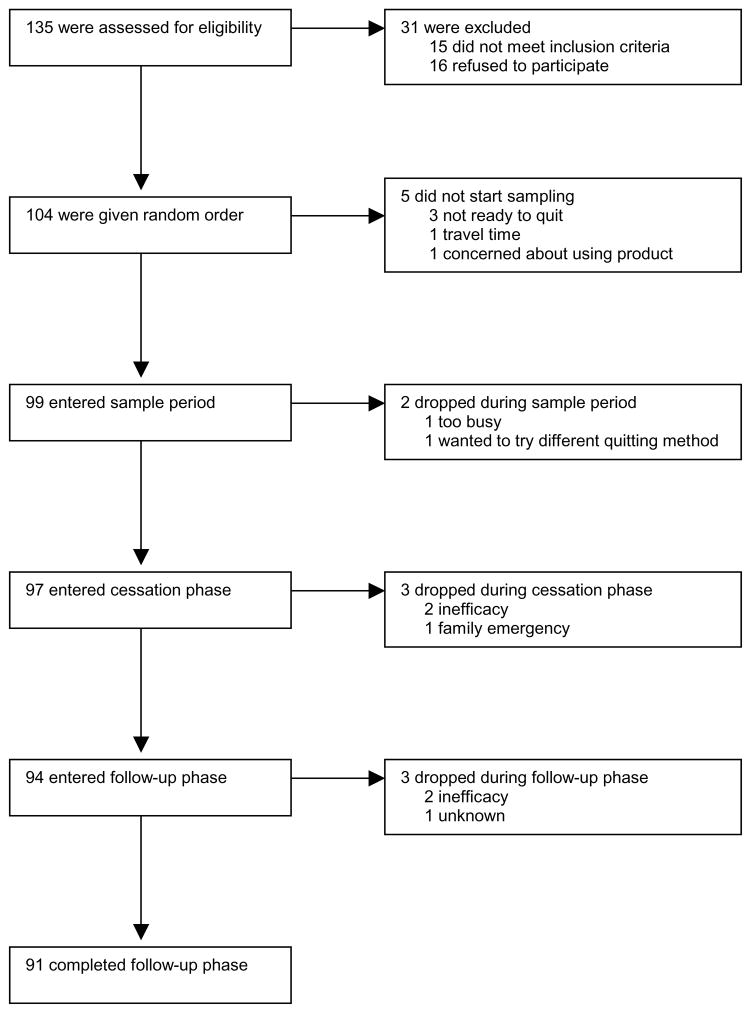
A total of 135 subjects completed questionnaires at the orientation meeting (N=71 at Minnesota and N=64 at Oregon); 104 were assigned to a random order for sampling five oral tobacco products (N=57 at Minnesota and N=47 at Oregon); 99 subjects entered the sampling phase (N=55 at Minnesota and N=44 at Oregon). The number of subjects and reasons for dropping out of the study at various time-points are illustrated in Figure 1.
Figure 1.
Disposition of the subjects
No significant differences were observed in drop-out rates between two sites during any of study periods including sampling, treatment and follow-up (p>0.05 for all). Among those who entered the sampling period (N=99), the mean age was 40.10 (SD=13.22), 64 subjects were male, 64 had greater than a high school education, 91 subjects were employed and 54 subjects were making less than $30,000 per year. The only significant differences between the sites was income with people being more likely to make less than $30,000 at Oregon site compared to Minnesota (p=0.015; 68% vs. 44%). With regards to tobacco use history, the mean cigarettes smoked per day was 19.8 ± 8.1, duration of smoking was 15.7 ± 12.4, FTND score was 5.1 ± 2.1 and the mean motivation to quit was 9.14 ± 1.01 (8.93 ± 1.06 for Minnesota, 9.41 ± 0.87 for Oregon, p=0.02) on a 10 point scale. Subjects who dropped out prior to the sampling phase (n=36) compared to those who entered this phase (n=99) tended to be older (47.0 ± 12.9 vs. 40.1 ± 13.2, p = 0.009), but no other significant differences were observed. The data from both study sites were combined when results showed no differences between sites.
3.2. Product use during sampling
Table 2 shows the amount of each product and cigarettes that were used during the sampling period. No significant differences were observed in the amount of products sampled across the different products. In addition, no significant differences were observed in the number of cigarettes smoked during the sampling of each product.
Table 2.
Amount of each product used during the sampling period
| Variable | Study Product | N | Median | Mean | SD | P value (ANOVA) |
|---|---|---|---|---|---|---|
| Use of Product | ||||||
| Ariva | 98 | 3 | 3.69 | 1.54 | 0.102 | |
| Camel Snus | 98 | 3 | 3.82 | 1.92 | ||
| General Snus | 98 | 3 | 3.22 | 1.55 | ||
| Marlboro Snus | 98 | 3 | 3.63 | 1.49 | ||
| Stonewall | 99 | 3 | 3.71 | 1.57 | ||
| Use of Cigarettes | ||||||
| Ariva | 98 | 10 | 12.18 | 6.64 | 0.950 | |
| Camel Snus | 95 | 11 | 11.82 | 6.87 | ||
| General Snus | 97 | 11 | 12.67 | 6.90 | ||
| Marlboro Snus | 94 | 10.5 | 12.16 | 7.65 | ||
| Stonewall | 96 | 11 | 12.24 | 7.16 | ||
3.3. Response to the product after sampling all products
Subjective response to each item of the Perception of Product questionnaire (see Table 3) was analyzed assuming a uniform distribution (20% for each product) under a null hypothesis (no preferences across products). There were significant differences compared to a uniform distribution of responses across products for the items “relief from withdrawal” (p=0.006), “easier to use” (p<0.0001), “more satisfying” (p=0.005) and “product liked best” (p<0.001). For “relief of urges,” there was no significant difference relative to uniform distribution across the products (p=0.147). For all items, fewer people reported positive responses to General Snus.
Table 3.
Subjective responses on effects of oral tobacco product (percent who endorsed one of the study products for each variable) at the end of sampling phase
| Variable | Study Product
|
||||
|---|---|---|---|---|---|
| Camel Snus | Marlboro Snus | General Snus | Stonewall | Ariva | |
| Relief from withdrawal | 28.87% | 17.53% | 7.22% | 26.80% | 19.59% |
| Relief from urges | 28.57% | 19.39% | 12.24% | 21.43% | 18.37% |
| Easier to use | 20.41% | 20.41% | 1.02% | 23.47% | 34.69% |
| More satisfying | 26.53% | 25.51% | 5.10% | 20.41% | 22.45% |
| Product liked best | 27.55% | 23.47% | 1.02% | 23.47% | 24.49% |
At the end of the sampling period, no one chose General Snus to be used during the 2-week abstinence period. Among the other study products, there were no statistically significant differences in percent of subjects who chose each of the products (Marlboro Snus 23.5%, Camel Snus 27.6%, Ariva 24.5%, Stonewall 24.5%); p = 0.947 assuming a uniform distribution of 25% for each of 4 choices under null hypothesis.
3.4. Product use during treatment
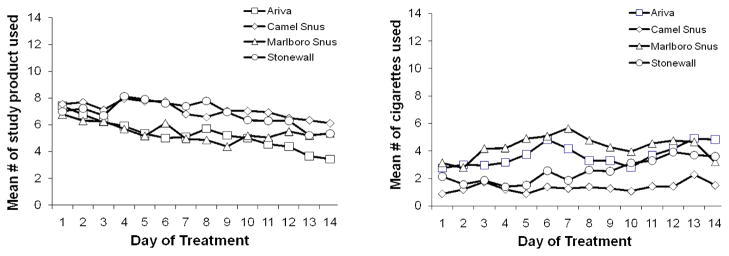
The average amount of chosen product and regular cigarettes used during the treatment period is also illustrated Figure 2. There were no significant differences in the amount of product used during the treatment period across the four chosen products (p=0.592). Amount of product use decreased significantly over the 14-day treatment period (p<0.0001); this decreasing time trend did not vary significantly by products chosen (p=0.230). At the end of the treatment period, the amount of product use varied by product chosen; subjects who chose Ariva used significantly less amount than those who chose Stonewall (p=0.045) and Camel Snus (p=0.018).
Figure 2.
The average amount of study product and cigarettes used during treatment period
Subjects at the Minnesota site smoked significantly less cigarettes per day than those in Oregon (p=0.003). The mean cigarettes per day also varied by product chosen; subjects who chose Marlboro Snus reported smoking more cigarettes than those who chose Stonewall (p=0.012) and Camel Snus (p=0.007). Cigarettes per day increased significantly over the 14-day treatment period (p<0.001); this increasing time trend of cigarettes per day did not vary significantly by products chosen (p=0.875).
3.5. Product response during treatment
Table 4 shows the significant results from the Product Evaluation Scale across the different products. Significant differences across products were observed for two factors, “satisfaction” and “psychological reward,” but not for “aversion.” For “satisfaction,” site effects were observed with Minnesota subjects endorsing higher least square mean satisfaction scores than Oregon (Mean=4.6, SE=0.2 vs. Mean=3.9, SE=0.2, respectively, p = 0.002). For other items on the Product Evaluation Scale, significant product differences were observed for “craving relief,” “withdrawal relief” and “enough nicotine.” In general, products with higher levels nicotine were rated more highly (e.g., more satisfaction, more craving relief, enough nicotine) than products with lower levels of nicotine. No significant differences were observed for “enjoy sensation,” “too much nicotine,” “easy to use,” “side effects,” and “comfortable using the product.” There was a significant site by product interaction (p=0.032) for “concerned about dependence.” Subjects in Minnesota rated more concern about dependence with Camel Snus than in Oregon (Mean=3.4, SE=0.4 vs. Mean=1.8, SE=0.4, respectively, p=0.009). Subjects in Minnesota site reported a higher score for “concerned about dependence” with the Camel Snus versus the other products.
Table 4.
Effects of oral tobacco product on subjective responses during treatment phase
| Variable | Study Products
|
||||
|---|---|---|---|---|---|
| Least Square Mean (SE) | |||||
| Camel Snus | Marlboro Snus | Stonewall | Ariva | p-value | |
| Satisfaction1 | 4.7 (0.2) | 4.2 (0.3) | 4.3 (0.2) | 3.8 (0.2) | 0.035 |
| Psychological Reward2 | 2.9 (0.2) | 2.7 (0.2) | 3.2 (0.2) | 2.4 (0.2) | 0.037 |
| Craving relief3 | 3.9 (0.2) | 3.2 (0.3) | 4.0 (0.3) | 3.1 (0.2) | 0.031 |
| Withdrawal relief4 | 4.3 (0.2) | 3.8 (0.3) a | 4.5 (0.2) | 3.5 (0.2) | 0.021 |
| Enough nicotine5 | 4.3 (0.2) | 4.0 (0.3) | 4.8 (0.3) | 3.6 (0.2) | 0.021 |
| Concerned about dependence6 | 3.4 (0.4) | 2.0 (0.4) | 1.8 (0.5) | 2.0 (0.4) | Site × product 0.032 |
| Craving (MNWS)7 | 1.6 (0.2) | 2.4 (0.2) | 2.4 (0.2) | 2.2 (0.2) | 0.010 |
Likert Scale, 1=not at all, 7=extremely;
Camel Snus vs. Ariva, p=0.004;
Stonewall vs. Ariva, p=0.005, Camel Snus vs. Ariva, p=0.045;
Camel Snus vs. Ariva, p=0.028, Camel Snus vs. Marlboro Snus, p=0.051, Stonewall vs. Marlboro Snus, p=0.042, Stonewall vs. Ariva, p=0.023;
Camel Snus vs. Ariva, p=0.008, Stonewall vs. Ariva, p=0.002, Stonewall vs. Marlboro Snus, p=0.054;
Stonewall vs. Ariva, p=0.002;
Signifcance only for Minnesota site, Camel Snus vs. Ariva, p=0.013, Camel Snus vs. Marlboro Snus, p=0.022, Camel Snus vs. Stonewall, p=0.013;
0=none, 4=severe; Camel Snus vs. Ariva, p=0.025, Camel Snus vs. Marlboro Snus (p=0.007), Camel Snus vs. Stonewall, p=0.003.
Site, visit and product chosen for treatment had significant impact on craving score on the MNWS after controlling for craving score at baseline. Mean craving score was significantly lower at the Minnesota site compared to Oregon site (Mean=2.0, SE=0.1 vs. Mean=2.4, SE=0.1, respectively, p=0.035). Craving scores decreased over visits (p=0.001) and were different across products (p=0.010). Mean craving scores for Camel Snus was significantly lower than the other products. Total withdrawal scores significantly decreased over time (p<0.0001) but did not differ across site or products after controlling for the baseline value.
3.6. Abstinence at the end of treatment and at follow-up
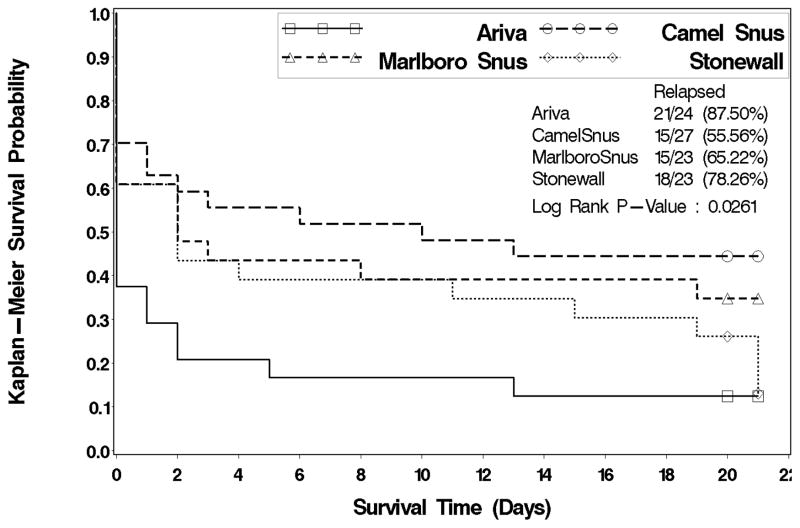
Survival probabilities varied significantly by site (log-rank test, p=0.049). Subjects in Minnesota were about half as likely to relapse as subjects in Oregon (p=0.023, hazard ratio and 95% CI: 0.562, 0.343–0.923). Survival probabilities differed significantly by product (log-rank test, p=0.026; See Figure 3). Compared to subjects who chose Ariva, those who chose Camel Snus were significantly less likely to relapse (p=0.002, hazard ratio and 95% CI: 0.349, 0.178–0.685). The CO-verified point prevalence abstinence for the last 7 days of treatment period was significantly greater (p=0.022) in Minnesota (23/54, 42.6%) than in Oregon (10/44, 22.7%), but abstinence was not significantly associated with the product chosen for treatment (p=0.344). The CO-verified point prevalence abstinence for the first 7 days of follow-up period was also significantly greater (p=0.008) in the Minnesota site (26/54, 48.2%) than that in Oregon (10/44, 22.7%). With this outcome variable, abstinence was significantly associated with the chosen product (p=0.044). Subjects who chose Camel Snus had the highest point prevalence abstinence (16/27, 59.3%) followed by those who chose Marlboro Snus (7/23, 30.4%), Stonewall (7/24, 29.2%) and Ariva (6/24, 25.0%). Camel Snus was associated with significantly higher rates of abstinence than Ariva (p=0.014), Stonewall (p=0.031) and near significant higher rates with Marlboro Snus (p=0.056). No significant site and product interaction effects were observed for either point prevalence abstinence (p > 0.05).
Figure 3.
Survival curve for time to relapse by chosen product
3.7. Continued tobacco product use at follow-up
There were 44 subjects who continued to use tobacco products 1 week after the treatment period (9/24, 37.5%, Ariva; 11/24, 45.8%, Stonewall, 18/27, 66.7%, Camel Snus, 6/23, 26.1%, Marlboro Snus). People who chose Camel Snus for treatment were significantly more likely to continue using it during the first week of follow-up than those who chose Ariva (p=0.037) and Marlboro Snus (p=0.004), but there were no significant differences between Camel Snus versus Stonewall on continued product use (p=0.134). At 4 weeks after the treatment period, several participants continued to use their products (6/24, 25.0%, Ariva, 5/24, 20.8%, Stonewall, 13/27, 48.2%, Camel Snus, 4/23, 17.4%, Marlboro Snus). Two of the four Marlboro Snus users switched to Camel Snus products. People who chose Camel Snus for treatment were significantly more likely to continue using it four weeks after treatment than those who chose Marlboro Snus (p=0.022) and Stonewall (p=0.042), but not compared to Ariva (p=0.088).
4. Discussion
This study demonstrates that smokers who are interested in trying oral tobacco products as a method for cigarette cessation differ in their preferences for oral tobacco products. What is most notable is that these smokers who have had high levels of nicotine exposure from smoking cigarettes do not necessarily select or endorse greater satisfaction for products with higher nicotine content. In fact, smokers did not like General Snus, the oral tobacco product that had the highest nicotine content. The dislike for this product may be due in part to the taste of the product, which was specifically developed for the tobacco users in Sweden.
Although no differences existed in preferences for U.S. oral products to be used during an abstinence phase, the product that produced the lowest number of cigarettes smoked during this period and the highest rate of point prevalence abstinence during follow-up was Camel Snus. Products such as Ariva and Marlboro Snus showed the lowest success for achieving abstinence, lowest rate of suppressing cigarette use or lowest rate of product use. These differences may be due to the levels of nicotine from the product. Per portion, Camel Snus has about 1.74 to 1.97 mg free nicotine, whereas products such as Stonewall have 0.28 to 0.57, Marlboro Snus has 0.14 to 0.38 and Ariva has 0.24 to 0.25 mg free nicotine (personal communication, Dr. Irina Stepanov). Biomarker levels tend to parallel the nicotine content. One study showed that Camel Snus leads to higher Cmax plasma nicotine levels (7.7 ng/ml) than Ariva (3.4 ng/ml) or Marlboro Snus (2.9 ng/ml; Cobb et al., 2010).
The abstinence findings are similar to a pilot study that compared medicinal oral nicotine replacement with Camel Snus and Taboka (which was an earlier version of Marlboro Snus). The results similarly showed that Camel Snus was associated with less cigarette smoking, greater product use and greater abstinence (although not statistically significant) than Taboka (a low nicotine product), but similar levels as 4 mg oral medicinal product (Kotlyar et al., 2010). When examining nicotine replacement therapies, studies have shown that 4 mg nicotine gum leads to a 2-fold higher rate of abstinence than 2 mg nicotine gum among more highly dependent smokers (Ebbert et al., 2004; Silagy et al., 2004). Together, these results demonstrate that lower nicotine oral tobacco products are not as effective in suppressing cigarette smoking and leading to abstinence than higher nicotine products and tend to be used less than higher nicotine products, perhaps because they are not sufficiently reinforcing (see Hatsukami et al., 2010 for study of cigarettes low in nicotine). The greater reinforcing effect of the higher dose product is also reflected by the greater number of subjects who continued to use Camel Snus products after the end of treatment. However, this continued use could also be a result of greater access to this product.
Subjective responses to the product also follow the dose and not formulation of the product during the treatment period. For example, the items’ satisfaction, withdrawal and craving relief, and enough nicotine were associated with higher scores among those who chose the higher nicotine containing products compared to the lower nicotine containing products. This observation was not as obvious during the sampling period, perhaps indicating a short exposure to a product using a cross-over design may not be sufficient for users to obtain adequate experience of the product. Nonetheless, in one laboratory study using a cross-over design, craving and Factor 1 of the Questionnaire of Smoking Urges (intention to smoke) measures were significantly decreased when Camel Snus was administered, but they did not decrease when Ariva or for the most part, when Marlboro Snus was administered (Cobb et al., 2010). In a laboratory pharmacokinetic study of different oral tobacco products, products with higher nicotine resulted in greater craving relief (Kotlyar et al., 2007). Still other studies have found no differences in response by dose. In a study that involved 5 days of product administration using a cross-over design (Blank and Eissenberg, 2010), it appears that no differences in responses were observed for craving, anticipation of withdrawal relief, or some withdrawal symptoms or sensory product characteristics between Ariva and Camel Snus. In a 4-week clinical trial in which smokers were randomized to Taboka, Camel Snus and medicinal nicotine products, no differences in craving and withdrawal were observed across the products (Kotlyar et al., 2010). Hence, the impact of dose on subjective responses to these products is still unresolved. In an effort to explore the reason for differences in results between our prior study (Kotlyar et al., 2010) and the present study, we re-analyzed the data from our present study examining the effects of products on craving when controlled for age, sex and cigarettes per day using our original mixed model and the same mixed model analysis employed in the prior study. Significant product effects were maintained. Comparisons in demographic and smoking history characteristics were also compared between these studies and no differences were observed. Dose-effects on subjective responses across oral tobacco products will require further investigation, including examining if factors such as motivation to quit, other subject characteristics, study design (e.g., length of product use, choice of product, instructions for use, multiple sites versus single site) play a role in outcome results (Hatsukami et al., 2009).
Another interesting finding was the differences in response to the products between Oregon and Minnesota. Minnesota subjects appeared to obtain greater satisfaction across products compared to Oregon subjects and were more concerned about developing dependence on Camel Snus. These findings suggest sample differences in response to these products, including the extent for achieving abstinence (e.g., lower socioeconomic status of the subjects in Oregon compared to Minnesota).
It should be noted that a limitation of these findings is that we reported unadjusted p values for multiple comparisons among four chosen products due to the fact that this was an exploratory investigation with a small sample size. The significant results should therefore be interpreted with caution.
In summary, this study shows that no oral tobacco product dominates in preference due to specific characteristics (tobacco lozenge or snus; high nicotine or low nicotine). However, smokers who chose the snus product with higher nicotine levels tended to report greater craving and withdrawal relief, more satisfaction, smoking less cigarettes and achieving greater short-term abstinence. Future studies may consider examining how these higher nicotine containing oral tobacco products compare to medicinal nicotine products as a complete substitution for smoking, the extent of toxicant exposure, and dependence on the product (which has been found to be high in Scandinavian smokers who have used snus to quit smoking, e.g., Lund et al., 2010).
Acknowledgments
Role of Funding Source: Study was solely supported by R01-CA135884. The funding source played no involvement in the study design, data collection and analysis and interpretation of the data or writing of the report. All tobacco products were purchased by researcher either in Oregon or Minnesota.
We thank Irina Stepnov for analyzing our product samples, Kathy Longley for help with the references and Dr. Robert Keenan for his edits.
Footnotes
Contributors: Drs. Dorothy Hatsukami and Severson were the primary investigators of this study; Joni Jensen was responsible for overseeing, coordinating and monitoring the study; Amanda Anderson and Berry Broadbent were the study coordinators and ran the study; Yan Zhang undertook the data analysis, Dr. Sharon Allen oversaw the medical status of the subjects. All read the draft of the manuscript which was written by Dr. Hatsukami and contributed to revisions. The manuscript has been approved by all authors.
Conflict of Interest: Dr. Dorothy Hatsukami has received funding from Nabi Biopharmaceuticals to test a nicotine immunotherapy in multi-site trials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accortt NA, Waterbor JW, Beall C, Howard G. Chronic disease mortality in a cohort of smokeless tobacco users. Am J Epidemiol. 2002;156:730–737. doi: 10.1093/aje/kwf106. [DOI] [PubMed] [Google Scholar]
- Bates C, Fagerstrom K, Jarvis MJ, Kunze M, McNeill A, Ramstrom L. European Union policy on smokeless tobacco: a statement in favour of evidence based regulation for public health. Tob Control. 2003;12:360–367. doi: 10.1136/tc.12.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Eissenberg T. Evaluating oral noncombustible potential-reduced exposure products for smokers. Nicotine Tob Res. 2010;12:336–343. doi: 10.1093/ntr/ntq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Weaver MF, Eissenberg T. Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tob Control. 2010;19:367–373. doi: 10.1136/tc.2008.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Rowland LC, Montori V, Vickers KS, Erwin PC, Dale LC, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database Syst Rev. 2004:1–22. doi: 10.1002/14651858.CD004306.pub2. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schildt EB. Should the European Union lift the ban on snus? Evidence from the Swedish experience. Addiction. 2003;98:1191–1195. doi: 10.1046/j.1360-0443.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- Gartner C, Hall W. Harm reduction policies for tobacco users. Int J Drug Policy. 2010;21:129–130. doi: 10.1016/j.drugpo.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Gartner CE, Hall WD, Vos T, Bertram MY, Wallace AL, Lim SS. Assessment of Swedish snus for tobacco harm reduction: an epidemiological modelling study. Lancet. 2007;369:2010–2014. doi: 10.1016/S0140-6736(07)60677-1. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hanson K, Briggs A, Parascandola M, Genkinger J, O’Connor R, Shields P. Clinical trials methods for evaluation of potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18:3143–3195. doi: 10.1158/1055-9965.EPI-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Lemmonds C, Tomar S. Smokeless tobacco use: harm reduction or induction approach? Prev Med. 2004;38:309–317. doi: 10.1016/j.ypmed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Koslowski LT, Frecker RC, Fagerström KO. The Fagerström Test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Hertsgaard LA, Lindgren BR, Jensen JA, Carmella SG, Stepanov I, Murphy SE, Hecht SS, Hatsukami DK. Effects of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20:91–100. doi: 10.1158/1055-9965.EPI-10-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, Smith EA, Hatsukami DK. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tob Control. 2007;16:138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund KE, McNeill A, Scheffels J. The use of snus for quitting smoking compared with medicinal products. Nicotine Tob Res. 2010;12:817–822. doi: 10.1093/ntr/ntq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstrom LM, Foulds J. Role of snus in initiation and cessation of tobacco smoking in Sweden. Tob Control. 2006;15:210–214. doi: 10.1136/tc.2005.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider NG, Olmstead RE, Nides M, Mody FV, Otte-Colquette P, Doan K, Patel S. Comparative testing of 5 nicotine systems: initial use and preferences. Am J Health Behav. 2004;28:72–86. doi: 10.5993/ajhb.28.1.8. [DOI] [PubMed] [Google Scholar]
- Severson HH, Forrester KK, Biglan A. Use of smokeless tobacco is a risk factor for cigarette smoking. Nicotine Tob Res. 2007;9:1331–1337. doi: 10.1080/14622200701705209. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;3:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Stegmayr B, Eliasson M, Rodu B. The decline of smoking in northern Sweden. Scand J Public Health. 2005;33:321–324. doi: 10.1080/14034940510032301. [DOI] [PubMed] [Google Scholar]
- Stenbeck M, Hagquist C, Rosen M. The association of snus and smoking behaviour: a cohort analysis of Swedish males in the 1990s. Addiction. 2009;104:1579–1585. doi: 10.1111/j.1360-0443.2009.02661.x. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob Res. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, Zhang X, Yusuf S. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647–658. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- Tomar SL. Snuff use and smoking in U.S. men: implications for harm reduction. Am J Prev Med. 2002;23:143–149. doi: 10.1016/s0749-3797(02)00491-9. [DOI] [PubMed] [Google Scholar]
- Tomar SL. Is use of smokeless tobacco a risk factor for cigarette smoking? The U.S experience. Nicotine Tob Res. 2003;5:561–569. doi: 10.1080/1462220031000118667. [DOI] [PubMed] [Google Scholar]
- Tomar SL. Epidemiologic perspectives on smokeless tobacco marketing and population harm. Am J Prev Med. 2007;33:S387–S397. doi: 10.1016/j.amepre.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Westman E, Levin E, Rose J. Smoking while wearing the nicotine patch: is smoking satisfying or harmful? Clin Res. 1992;40:871A. [Google Scholar]