Abstract
Purpose
The sigma-2 (σ2) receptor is a potential biomarker of proliferative status of solid tumors. Specific synthetic probes using N-substituted-9-azabicyclo[3.3.1]nonan-3α-yl carbamate analogs have been designed and implemented for experimental cancer diagnosis and therapy.
Procedures
We employed the fluorescently-labeled σ2 receptor probe, SW120, to evaluate σ2 receptor expression in human stem cells (SC), including: bone marrow stromal (BMSC), neural progenitor (NPC), amniotic fluid (AFSC), hematopoetic (HSC) and embryonic stem cells (ESC). We concurrently evaluated the intensity of SW120 and 5-ethynyl-2′-deoxyuridine (EdU) relative to passage number and multipotency.
Results
We substantiated significantly higher σ2 receptor density among proliferating SC relative to lineage-restricted cell types. Additionally, cellular internalization of the σ2 receptor in SC was consistent with receptor-mediated endocytosis and confocal microscopy indicated SW120 specific co-localization with a fluorescent marker of lysosomes in all SC imaged.
Conclusion
These results suggest that σ2 receptors may serve to monitor stem cell differentiation in future experimental studies.
Keywords: sigma 2 receptor, embryonic stem cell, amniotic fluid stem cell, bone marrow stromal cells, hematopoietic stem cell, differentiation, proliferation, EdU, Ki 67
Introduction
The inability to non-invasively image stem cell differentiation remains a challenge in monitoring the effects of cellular therapy. Advances in fluorescence and bioluminescence [1-5] or nuclear medicine techniques [5-9] that allows for serially tracking the biodistribution and viability of transplanted stem cells (SC) are inadequate for determining the differentiation state as compared to histological examination [10, 11]. Recent studies have demonstrated the capacity to genetically engineer lineage specific restrictions that are apparent with differentiation [12] as well as deriving real time pluripotency systems to monitor differentiation [13, 14]. However, these manipulations are technically challenging and elevate the risk of genetic abnormalities in SCs.
In pursuit of a non-invasive in vivo assessment of cellular transplantation that can serve as a reliable indication of underlying cellular differentiation, we have investigated the use of the σ2 receptor in determining the “stemness” of stem cells [15]. The σ2 receptor was initially reported as a subtype of the opiate receptor [16], and has subsequently been distinguished functionally and pharmacologically as a separate receptor system [17]. There are two functionally and pharmacologically distinct sigma binding sites, namely sigma-1 (σ1) and σ2 receptors [17]. The σ2 receptor is approximately 21.5 Kd and has not yet been cloned [18] whereas the σ1 receptor is about 25Kd and is found in biological systems including cell lines, primary cultures, and animals to alter and modulate voltage-regulated and ligand-gated ion channels, along with sodium potassium, N-methyl-D-aspartic acid and inositol trisphosphate receptors[19]. The σ2 receptor has been characterized through in vitro binding studies using radiotracers. It has been reported that there is a 10-fold higher expression in proliferating than in quiescent tumor cells [20] supporting the possible use of σ2 receptors as a potential receptor-based biomarker for the proliferative status of solid tumors [21]. Furthermore, natural ligands remain undetermined, however specific synthetic carbamate analogs have been designed to selectively bind to σ2 receptors. Moreover, the identification of radio-labeled and fluorescent σ2 receptor ligands that readily differentiate proliferating from quiescent tumor cells in experimental models holds the potential for future application in cancer diagnosis and therapy [22, 23].
Moreover, similar to cancer cells which are capable of sustaining tumor growth through infinite cell division [24, 25], SC are classically defined by the capacity to undergo many cell divisions while maintaining an undifferentiated state [26-28]. In this study, we sought to exploit this SC attribute in order to directly interrogate σ2 receptor expression in a variety of primary SC. All stem and corresponding differentiating/lineage-restricted cell types analyzed were of human origin exclusively. We have included embryonic (ESC), neural progenitor cells (NPC), amniotic fluid stem cells (AFSC), bone marrow stromal cells (BMSC) and hematopoetic stem cells (HSC) using a 7-nitrobenzo-2-oxa-1,3-diazole (NBD) labeled σ2 receptor specific ligand, SW120, with excitation and emission spectra similar to FITC (Fig 1)[17]. Studies employing confocal microscopy and flow cytometric analysis (FACS) were designed to identify the subcellular localization of SW120 in SC and lineage-restricted cell types. Additional correlation of SW120 positive populations with corresponding levels of EdU incorporation were made to serve as a direct index of cellular proliferation.
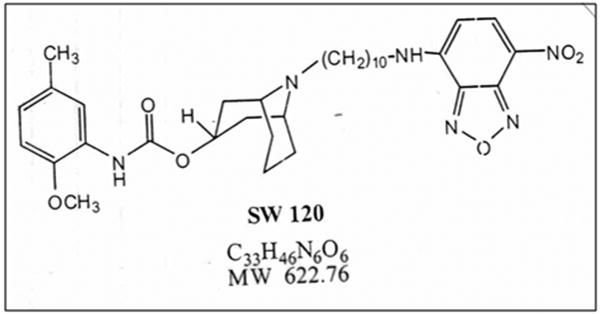
Figure 1. Chemical structure of SW120.
Exhibits an excitation wavelength of 465 nm and an emission wavelength of 520 nm. The Ki value for σ2 receptors is 11 nmol/L.
Materials and Methods
Cell Culture
C6 glioma cells were obtained from American Type Tissue Culture Collection (ATCC) and cultured in DMEM medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) under normoxic conditions (95% air, 5%CO2). All SC were cultured in a 37°C humidified hypoxic incubator (5% CO2, 1% O2) with the following media requirements: Amniotic Fluid Stem Cells (AFSC, Material Transfer Agreement A. Atala Wake Forest University) MEM alpha (Invitrogen Corp.,Carlsbad,CA); Chang B and Chang C sera 20% and 5% respectively (Irvine Scientific Inc. Santa Ana, CA) FBS 15%, L-glutamine 1%); Bone Marrow Stromal Cells (BMSC, Center for Bone Marrow Stromal Cell Transplantation, Clinical Center, NIH) (MEM alpha, FBS 20%); Neural Progenitor Cells (NPC) (Millipore Corporation, Billerica, MA), cultured only on mouse laminin (Millipore) and poly-ornithine (Sigma Aldrich, Saint Louis, MO) coated tissue culture plates and permanox chamber slides (Lab-Tek, Nagle Nunc International, Rochester, NY) Enstem Neuronal Expansion media (Millipore Corporation), 1% L-glutamine (Invitrogen), fibroblast growth factor (fgf) added fresh); and Hematopoietic stem cells (HSC, Cell Processing Section, Department of Transfusion Medicine, Clinical Center, NIH) Stem Line II stem cell basal media (Sigma-Aldrich, St. Louis, MO) containing 1× Glutamax and Stem Cell Factor at 40 ng/ml (Sigma-Aldrich), Flt3 ligand at 40 ng/ml (Sigma-Aldrich) and TPO at 10 ng/ml (Sigma-Aldrich). HSC were expanded for 5 days and maintained in T-25 flasks in suspension at ≤ 1 × 106cells/ ml with media addition every 2-3 days. ESCs (H9 cell line, WiCell, Madison, WI, passage 35 - 40) were maintained on a feeder layer of irradiated MEFs using medium consisting of 80% Knockout Dulbecco's modified Eagle's medium (KO-DMEM, Invitrogen, Carlsbad, CA) supplemented with 15% Fetal bovine serum (Invitrogen), 5% Knockout serum replacement (KSR, Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma, St.Louis, MO), 1% non-essential amino acids, 2 mM L-Alanyl-L-glutamine and 4 ng/ml basic fibroblast growth factor (bFGF, Invitrogen). Cell cultures were passaged using Collagenase IV (Invitrogen) every 6-7 days, only phenotypically uniform hESC colonies were collected. Subsequently, ESCs were transferred to feeder-independent culture conditions, using BD Matrigel hESC-qualified Matrix (BD Biosciences, San Jose, CA), and grown in mTeSR-1 (Stemcell Technologies, Vancouver, Canada) at 37°C and 5% CO2. Cell cultures were maintained and expanded following manufacturer's protocol. The medium was changed every day. For all confocal analyses, cells were grown on glass-bottom LabTek® two-well Chamber Slide (BD Biosciences) in the feeder-free conditions described above.
Lineage restricted cell types: Normal human astrocyte (Lonza, Walkerville, MD) cultures maintained with finite life span in vitro (for assay within 7-10 days) using Astrocyte Basal Medium and BulleKit (Lonza) containing proprietary concentrations: hEGF, insulin, ascorbic acid, GA-1000, L-glutamine and FBS.
Stem Cell Differentiation: NPC neuronal differentiation conditions required Enstem Neuronal Differentiation media (Millipore) changed every 3 days for 2 weeks with fresh addition of L-glutamine in coated 6 well plates. BMSC differentiation was accomplished using StemPro adipogenesis kit (Invitrogen) with media changes every 4 days for 10-14 days, followed by Oil Red O staining (Sigma Aldrich) briefly, cells were fixed with 4% PFA and stained with 0.3g oil Red O dissolved in 60 ml 2-propanol (diluted with 40 ml distilled water), filtered through Whatman #1 paper and stained for 1hr, rinsed with distilled water and viewed. For HSC following 5 day expansion, HSC colony-forming conditions (HSC-CFU) were implemented according to MethoCult (StemCell Technologies, Vancouver, BC, Canada) recommendations. Briefly, cells were added to Methocult GF H4534 Classic without EPO (StemCell Technologies) for support of granulocyte-macrophage (CFU-GM), macrophage (CFU-M) and granulocyte (CFU-G) and then plated at 5 × 103 cells/35 mm plate for 14 days at 37°C and 5% CO2, subsequently collected as directed for flow cytometry and proliferation assay.
Lastly, not all procedures could be carried out on ESC and HSC because of limited availability of the cell products. Supplemental Table 1 (Table S1) is a summary chart of the assays performed for the stem cells in this study. NPC differentiation was performed in chamber slides for the purpose of confocal microscopy SW120/Lysotracker assay only. Normal human astrocyte cultures were substituted for differentiating NPC to astrocytes and were obtained from Lonza (Walkerville, MD) for flow cytometry analysis. HSC differentiation was implemented for flow cytometry assay under semi-solid colony forming conditions not suitable for confocal imaging.
Ligand binding and cell labeling
SW120,7-nitrobenzo-2-oxa-1,3-diazole (NBD) labeled fluorescent probe, (MTA Mach, RH, Washington University School of Medicine, St. Louis, MO) was added at 10 nmol/L to cell culture media for 30 min incubation and 3 phosphate buffered saline pH 7.4 (PBS) washes for elimination of non-specific ligand binding. Figure 1 is the chemical structure of SW120. Following incubation with SW120 LysoTracker Red DND-99 (Invitrogen) lysosomal marker (150 nmol/L) was added for 30 min incubation in culture media under normal growth conditions. Cells were washed with PBS (3×), followed by fixation (4% paraformaldehyde) and mounting with cover-slips (Prolong Gold Antifade with DAPI, Invitrogen).
Cell Blocking
In vitro binding studies were conducted to determine the affinity of SW120 for σ2 and σ1 receptors. In order to observe whether SW120 binds to σ2 receptors or σ1 receptors in SC, a number of blocking experiments were performed in triplicate with σ2 receptor - (SW120) and σ1 receptor, (+) pentazocine (Fig S3b), specific ligands. BMSCs were pre-incubated with a range of concentrations of (+) pentazocine, (10, 100 μmol/L, 1, 10 nmol/L) in culture medium at 37°C for one hour followed by similar 30-minute exposure to 10 nmol/L SW120. Flow cytometry (AccuriC6, Ann Arbor, MI) was used to evaluate all resultant mean SW120 fluorescence intensity values of cells treated with either (+)-pentazocine or SV119 (Fig S3a). Flow cytometry (FACS) histograms were used in order to normalize to a percentage of the mean fluorescence intensity (MFI) of cells treated with SW120 alone.
The specificity of interaction of SW120 with the σ2 receptor was investigated through incubation of AFSCs with a range of concentrations (10, 100 nmol/L, 1, 10 μmol/L) of unconjugated σ2 ligand SV119 (MTA Mach, RH, Washington University School of Medicine, St. Louis, MO) [17] in culture media for 1hr at 37°C followed by a 30 minute exposure to SW120 10 nmol/L. To determine whether the cellular internalization of the σ2 receptor in stem cells is consistent with clathrin-mediated endocytosis, AFSC, BMSC NPC were pretreated in the presence of an inhibitor of endocytosis, phenylarsine oxide (PAO) (Sigma Aldrich) at 1,10, 50 and 100 μmol/L for 1 hour and then incubated in 10nm SW120 in the presence or absence of PAO for an additional 30 minutes. To assess σ2 receptor binding to SW120 in terminally differentiated cells, astrocyte, HSC-CFU and 14 days adipogenic differentiation of BMSC were incubated with SW120 at concentration 10 nmol/L for 30 minutes. All resultant SW120 MFI were obtained through FACs, as histograms were used in order to normalize to a percentage of the MFI of actively proliferating NPC or BMSC treated with SW120 alone.
Cellular Proliferation Assay
Cells were labeled using 5-ethynyl-2′-deoxyuridine (EdU) and the Click-iT Chemistry EdU Alexa Fluor 647 azide flow cytometry assay (Molecular Probes, Eugene, OR) per manufacturer's recommendation. Briefly, 12-18 hr incubation times in 5 μM EdU were derived through optimization of the assay per given cell type. Cells were then harvested, resuspended, fixed and permeabilized as directed. Following a 30 min incubation in fluor-azide 647 reaction cocktail, cells were thoroughly washed and evaluated by flow cytometry (Accuri C6, Ann Arbor, MI) using 640 nm excitation and red emission filter (FL4 photomultiplier tubes (PMTs) for detection and collection of fluorescence measurements).
Flow Cytometry
Cells (5 × 105) were plated in 6 well plates for overnight culture. Subsequent 30 min incubation with SW120 (10 nmol/L) followed (excitation: 465 nm, emission: 520 nm), cells were washed (3× PBS) to eliminate non-specific binding then immediately trypsinized and fixed (Cytofix, BD Biosciences,San Jose, CA). Flow cytometry assessment was performed with Accuri C6 (Ann Arbor, MI) implementing 488 nm laser for excitation of FITC with corresponding FL1 PMT for detection and collection of fluorescence measurements.
Additional staining was done for Cluster of Differentiation (CD) markers with unstained cells and cells stained with isotype controls for each antibody also included. The following conjugated antibodies and their corresponding isotype controls from BD Pharmingen, BD Biosciences,San Jose,CA were used per manufacturers' recommended dilutions for conjugated antibodies (generally 20 μl antibody per 100 μl stain buffer) : (anti–human CD14-IgG2a-PE, CD29-IgG2a-PE, CD34-IgG1κ-PE, CD44-IgG2b-PE, CD45-IgG2a-FITC, CD68-IgG2b κ-PE, CD73-IgG1 κ-PE, CD105-IgG1-APC, CD 117-PE anti- human monoclonal, CD133-IgG2b-APC, CD146-IgG1 κ-PE and also Oct 3/4-IgG2b-APC, MMP-1-IgG1-PE, We also used anti-human fibroblast antibody 5B5 IgG1-mouse monoclonal (1:100), (Abcam, Cambridge, MA) with Alexa Fluor 647 goat anti-mouse IgG secondary (1:5000) (Molecular Probes, Eugene, OR) and ki-67 (M-19) goat polyclonal IgG Alexa fluor 647(1:50), (Santa Cruz Biotechnology, Inc.). Briefly, we performed trypsinization, collection and centrifugation (3-5 min at 700 rpm) of cells with subsequent PBS rinse of cell pellet and 20 min room temperature (RT) fixation (Cytofix, BD Pharmingen, BD Biosciences, San Jose,CA), triton-x permeabilization was included for samples stained for Oct 3/4 and MMP-1 only (and was not indicated for either CD staining nor fibroblast surface antigen staining) an additional PBS rinse of fixed cells and resuspension at 1×106 per 100 ul stain buffer (BD Pharmingen, BD Biosciences,San Jose,CA) followed. Antibody staining was performed per manufacturer's recommendations at 20-30 min RT with subsequent PBS washes (3×) and resuspension in stain buffer for Flow cytometry assessment performed with Accuri C6 (Ann Arbor, MI). A total of 10,000 cells were analyzed at the FL1, FL2, FL3, FL4 detectors.
Apoptosis was also evaluated with Annexin V Alexa Fluor 647 (Invitrogen, Carlsbad, CA). Briefly, cells were incubated for 30 min or overnight with SW120 (10 nmol/L) and washed with 1× Annexin binding buffer (Invitrogen). Cells were resuspended at approximately 1 × 106 cells/ 100 ul binding buffer and 5 ul of Annexin V-Alexa-647 was added with gentle mixing and 15 min RT incubation. Next 400 ul 1× binding buffer was added to each sample on ice and FACS was done immediately. Propidium iodide (PI) (5 ug of 1 mg/ml stock) was added to samples immediately prior to flow analysis for accompanying cell viability information. A total of 10,000 events were analyzed, including unstained cells, cells stained with Annexin V-Alexa 647 alone and cells stained with PI alone (no Annexin V-Alexa 647) were included as controls. Analysis was performed with Accuri C6 using both 488 nm and 640 nm lasers for excitation of PE (propidium iodide) and APC (alexa fluor 647) respectively with corresponding FL1 and FL4 PMTs for detection and collection of fluorescence measurements. For all FACS the accompanying CFlow software by the manufacturer was used for data analysis.
Confocal Microscopy
SW120 was excited using the 488 nm line from an argon laser (Carl Zeiss LSM 710 confocal microscope, Jena, Germany) and emission collection was performed 494-552 nm band-pass filter. LysoTracker was excited using 561nm line of helium-neon laser and emission collection was performed using 560 nm long-pass filter. Multi-tracking minimized inter-channel cross-talk. Imaging parameters: no signal in the absence of compound, in presence of SW120 or LysoTracker only, signal in either the green or red channel only, respectively. Pixel dwell time: 1.58 μs, optical slice thickness 0.7 μm, image resolution 1024 × 1024. All co-localization analyses were performed using an Apochromat 63×/1.40 oil objective under identical master gain and pinhole settings in order to determine variation in fluorescence intensity across SC and upon differentiation. Resultant changes in MFI (green channel) between actively proliferating SC and differentiated cell conditions were interpreted using Zen 2008 software (Carl Zeiss).
Statistical Analysis
Assays were performed at least three times for each variable described. Data are reported as a mean ± SD. The statistically significant differences were derived through Student t test for unpaired data (2-tail) with significant p <0.05 (GraphPad Software, Inc., San Diego, CA).
Results
Identification of σ2 receptors in human stem cells
The presence of σ2 receptor activity in stem cells was identified through simple incubation of BMSC, AFSC, NPC, HSC and ESC in culture media containing SW120 (10 nmol/L) for a range of time points. Fluorescence intensity changes relative to control cells were observed following brief incubations of 1-5 min with SW120. The background fluorescence of an identical sample size per given cell type, that had not been incubated with SW120, was simultaneously recorded as the control reference in each case. Corresponding average increases in fluorescence intensity of approximately ≥10 fold were observed after longer exposure to SW120 with peak values obtained by 30 min (fig. 2a). Fluorescence intensity changes following incubation with SW120 for BMSC were highly influenced by passage number (Fig 2a BMSC*). We observed significant changes in the expression of BMSC surface markers CD44 and CD105 that in passage 2 cells were 99.6 and 87.3 percent expression and decreased to 21.87 ± 3.07 and 31.67 ± 1.92 at passage 25, respectively,(p values ≤ 0.001, (Table 2). Similarly CD29, CD73, and CD146 expression became undetectable in BMSC following 25 passages of cells in culture. We also observed that the specific cell donor source contributed considerable variability to the data (data not shown), perhaps indicative of underlying potential for multi-potency and onset of senescence per given culture. Due to the intersubject variability of BMSC (15), only a single donor per given cell type has been analyzed (tables 1,2,3).
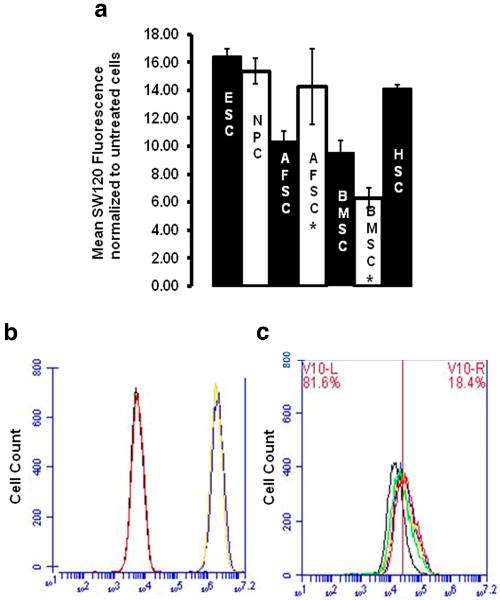
Figure 2. σ2 receptor specific binding of SW120 in SC.
a.) Evidence of σ2 receptor binding among a variety of SC. The change in mean fluorescence intensity (MFI) of SW120 compound upon binding to σ2 receptors in stem cells is depicted. MFI normalized by untreated control cells. (* Indicates late passage, AFSC passages: 35-45, BMSC: passages 20-25).
b) Specificity of SW120 interaction in SC with σ2 receptor as opposed to σ1 receptor, (SW120 concentration and incubation time,10 nmol/L,30 min throughout) with (+)pentazocine(10 μmol/L,60 min). Untreated AFSC (black), (+)pentazocine(red), SW120,(blue), (+)pentazocine/ SW120(yellow). c) Dose-specific inhibition following pretreatment SV119 (60 min), (vertical bar indicates MFI unstained cells, SW120 incubation time,concentration 10 nmol/L,30 min throughout) AFSC SW120 only (red), SW120/SV119(10 nmol/L,blue), SW120/SV119(100 nmol/L,yellow), SW120/SV119(1 μmol/L,green), SW120/SV119(10 μmol/L,black). Percentages represent maximum inhibition of SW120 MFI noted of 81.6% at SV119(10 μmol/L) in AFSC (comparable results in BMSC and NPC, data not shown). MFI derived using FACS histograms, background subtraction, normalization to percentage MFI SW120 only, 3 independent experiments, statistically significant differences noted (p < .001).
Table 2.
Comparative percentages of given phenotypic cell surface markers in BMSC: passage 2 (early) and passage 24 (late), Values displayed are significant (P value < 0.05) as a result of 3 independent assays.
| Marker | BMSC (early) | BMSC (late) |
|---|---|---|
| SW120 | 59.65 ± 2.44 | 17.13 ± 3.75 |
| EdU | 95.65 ± 2.58 | 46.45 +7.61 |
| CD29 | 87.3 ± 2.1 | negative |
| CD44 | 99.6 ± 0.6 | 21.87 ± 3.07 |
| CD73 | 98.5 ± 1.3 | negative |
| CD105 | 87.4 ± 5.9 | 31.67 ± 1.92 |
| CD146 | 81.3 ± 5.26 | negative |
Table 1.
Comparative percentages of given phenotypic cell surface markers in AFSC: passage 14 (early) and passage 40 (late), Values displayed are significant (P value < 0.05) as a result of 3 independent assays.
| Marker | AFSC (early) | AFSC (late) |
|---|---|---|
| SW120 | 56.02 ± 2.16 | 53.62 ± 2.86 |
| EdU | 62.47 ± 6.81 | 70.12 +4.36 |
| CD29 | 4.08 ± 0.90 | 3.53 ± 0.63 |
| CD44 | 23.63 ± 4.34 | 28.28 ± 5.77 |
| CD73 | negative | negative |
| CD105 | 44.37 ± 3.60 | 47.67 ± 6.24 |
| CD117 | 64.83 ± 3.47 | 56.87 ± 8.46 |
| Oct3/4 | 29.50 ± 4.56 | 30.67 ± 4.73 |
Table 3.
Comparative percentages of given phenotypic cell surface markers in HSC: HSC (expanded/actively proliferating) and HSC under colony forming conditions (HSC-CFU). Values displayed are significant (P value < 0.05) as a result of 3 independent assays.
| Marker | HSC | CFU |
|---|---|---|
| SW120 | 45.11 ± 5.23 | 1.75 ± 0.30 |
| EdU | 38.10 ± 8.16 | 22.76 ± 2.49 |
| CD34 | 87.3 ± 2.16 | 2.87 ± 0.21 |
| CD45 | 93.63 ± 4.42 | 7.2 ± 0.62 |
| CD68 | 2.07 ± 0.080 | 1.97 ± 0.40 |
| CD133 | 1.40 ± 0.098 | 3.13 ± 0.19 |
| CD14 | 0.6 ± 0.020 | 7.77 ± 0.45 |
Pharmacological characterization of SW120 fluorescent ligand in stem cells
The inhibition constant for binding of SW120 (excitation 465 nm, emission 520 nm) to σ2 receptors is 10 nmol/L [16]. The resultant fluorescence intensity of SC incubated with SW120 in the presence of σ1 receptor ligand, pentazocine (Fig S3b), did not alter SW120 affinity for the σ2 receptor (Fig 2b). Next, incubation of SC with SV119 (Fig S3a), the competitive inhibitor of SW120, at a wide range of concentrations prior to addition of SW120, prevented complete internalization of SW120 in a concentration dependent manner (Fig 2c). Inhibition of binding achieved by SV119 at the σ2 receptor was observed to be as follows: at 10 nmol/L 12.92 ± 3.37 % inhibition, likewise at 100 nmol/L 30.03 ± 5.28%, at 1μmol/L 42.0 ± 5.52%, at 10 μmol/L 81.6 ± 1.70% with a dose inhibition correlation of R2 = 0.94. Similar results were noted for BMSC and other SC analyzed (data not shown). A small percentage (≤ 20%) of incomplete blocking of σ2 receptor internalization was observed among SC despite comparable affinity of SW120 for the receptor (inhibition constant of inhibitor (Ki), 11 nmol/L) and that of the σ2 ligand SV119 (Ki 5.19 nmol/L). The incomplete blocking is most likely attributable to some degree of passive uptake of the ligand that has been reported in tumor cells [29]. The pharmacological data supports the observation that there is σ2 receptor selective binding of SW120 receptors in SC.
Identification of σ2 in human stem cells and lineage-restricted cells
The presence of σ2 receptors in stem cells (i.e., AFSC, HSC, BMSC, NPC and ESC) was evaluated by confocal microscopy following incubation of cells with SW120 and LysoTracker (Fig 3a). Confocal microscopy revealed the subcellular localization of SW120 within cells. Co-registration of SW120 with fluorescent lysosomal marker was observed in the SC (Fig 3a). The SW120 localization was not exclusive to endosomes since punctuate cytosol distribution of the σ2 receptor ligand was also observed similar to what was reported in proliferating cancer cells [29]. Evidence of drastic reduction in SW120 fluorescence under differentiation conditions was also obtained by examining at least 10 high power fields under confocal microscopy. A 75.6% reduction in signal intensity was observed in BMSC after 10 days under adipogenic conditions with the mean green channel fluorescence intensity of BMSC 4.87 ± 1.2 and following adipogenesis was 1.10 ± 0.60 (Fig 3a BMSC*). NPCs displayed nearly complete reduction of signal to background levels (mean green channel fluorescence intensity NPC was 7.15 ± 2.63 and following neurodifferentiation 0.13 ± 0.07, p = 0.002) when cells were cultured for 2 weeks in neurogenic conditions (Fig 3a NPC*). These results suggest highly significant changes (BMSC, 10 day adipogenesis p=0.001 and NPC, 2 week neurodifferentiation: p=0.002) in σ2 receptor expression among lineage restricted cells as compared to undifferentiated SC. AFSC incubated with an inhibitor of endocytosis, phenylarsine oxide PAO (10 μmol/L), prevented approximately 85% of SW120 internalization (p< 0.05), indicating that the majority of the σ2 receptors were internalized by endocytosis with the remainder potentially occurring through passive diffusion (Fig 3b), as previously suggested among tumor cells [23].
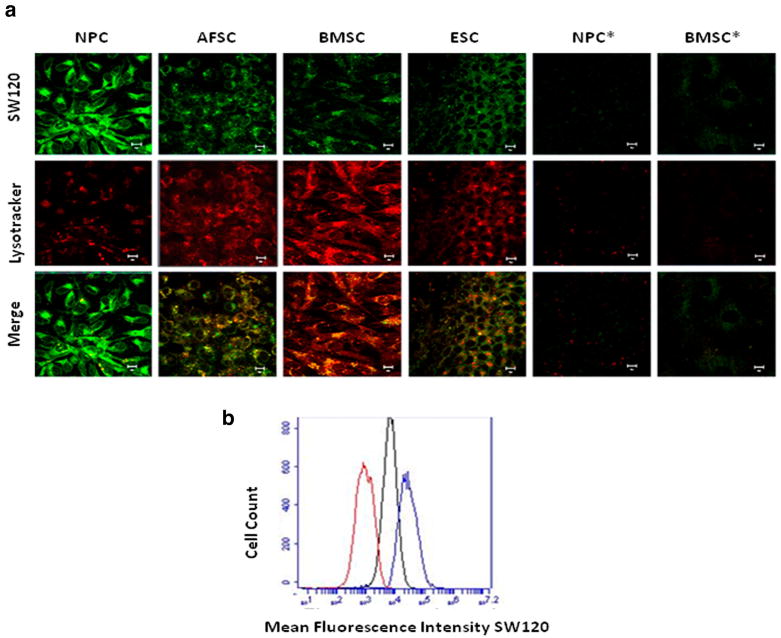
Figure 3. Subcellular localization σ2 receptors in SC versus lineage restricted cells types.
a) Colocalization with lysosomal marker (confocal microscopy(60× oil magnification/10 μm scale), SW120(10 nmol/L,30 min) and LysoTracker(150 nmol/L,60 min). Colocalization greater among SC relative to neuronal (NPC*) or adipogenic (BMSC*) differentiation conditions. b) Clathrin-mediated endocytosis, untreatedBMSC(red), SW120(10 nmol/L,30 min,blue), PAO(10μmol/L,60min/SW120(10 nmol/L,30 min,black) Experiments in triplicate, mean inhibition with PAO treatment 85.02 ± 2.4, p < 0.05.
FACS analysis was performed in order to assess the effect of differentiation on σ2 receptor expression. Astrocytes, BMSC under adipogenic differentiation culture conditions (at 14 days) and HSC-CFU following 14 days in culture were incubated with SW120. FACS revealed only 17.7%, 0.6% and 1.6% SW120 positive populations (p = 0.002 and p < 0.001) for astrocytes, adipocytes and HSC-CFU respectively (Fig 4d) as compared to 94.3%, 63.2% and 43.1% detected among NPCs, early passage BMSC and HSC under expansion conditions (Fig 4b). Corresponding assessment of morphological changes, lipid droplet accumulation and oil red positivity in BMSC under adipogenic culture conditions was used in confirmation of differentiation status (Fig S1). Similarly, HSC cultures under 14 day CFC revealed nearly a complete loss of expression of surface markers typically utilized for the enrichment of hematopoietic stem cell cultures. We specifically noted significant reduction in CD 34 and CD 45 expression levels by 97.7% and 95.3%, respectively, (p values ≤ 0.001, see Table 3). This perhaps is indicative of a significantly less homogenous HSC population. No significant differences were noted in the HSC surface expression under CFC for the lineage specific antigens, CD68, human monocyte/macrophage marker) [30], or CD133 [31]. However, there was a highly significant increase (i.e.,>10 fold, p value ≤0.001) in expression of lineage –specific, mature hematopoietic marker, CD14 (granulocyte colony-stimulating factor receptor), which may reflect involvement in GCSF-induced myeloid cell differentiation and myeloid cell type lineage restriction (Table 3). Furthermore, differentiation assays were not conducted with ESC, however, we anticipate that SW120 labeling and EdU incorporation in pluripotent ESC would be comparable to that noted for “broadly multipotent” AFSC[32].
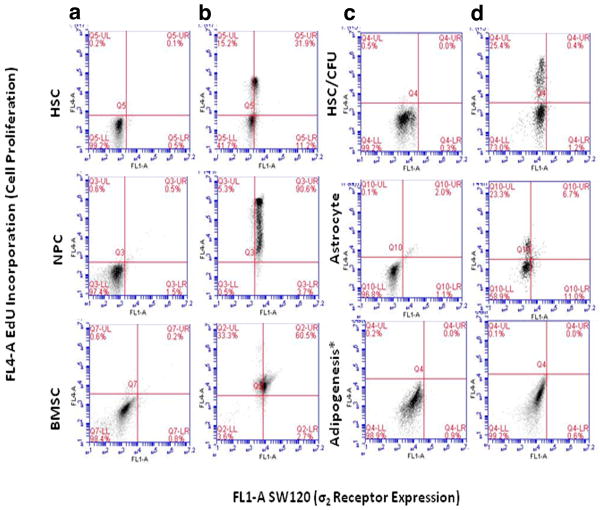
Figure 4. Differences in σ2 receptor expression and EdU incorporation in SC relative to corresponding lineage restricted cells types.
Following differentiation, highly significant changes noted among HSC-CFU, astrocytes and BMSC adipogenesis, p value<0.001, representative plots of SW120 (FL1-A) versus EdU incorporation (FL4-A) among SC and corresponding differentiated cell types. a) Stem cells only, b) SW120 (1hr)/EdU(12-18 hr), c) Cells only under corresponding differentiation conditions, D) Differentiation conditions with SW120 (1hr)/EdU (12-18 hr). Data reflect at least three independent assays, all results statistically significant, P≤0.05, Adipogenesis* data presented after 2 weeks differentiation, with greater variability noted at earlier time points.
σ2 Receptor as marker of Proliferative Status
EdU labeling was used to correlate the cell cycle with underlying σ2 receptor expression. Depending on SC type evaluated similar activity was noted for binding of SW120 and EdU incorporation (Fig. 5). We observed fluctuations in the proportion of SW120 positive (σ2 receptor positive) cells corresponding to changes in EdU incorporation (cellular proliferation). However, levels were not directly comparable in all cases and EdU positive fractions were generally higher than SW120 levels. These differences may be attributed to length of incubation time with maximal results attainable within 30-60 minutes for SW120 while variable proliferation rates existed among the SC and lineage restricted cell types. The greatest disparity was noted among BMSC which remained highly proliferative in culture even at late passages. For instance, the SW120 positive fraction of early passage BMSC was 59.65% whereas the level of EdU incorporation was 96.55%, as compared to later passages of BMSC in which SW120 uptake was 17.13% and the level of EdU incorporation was 46.45%. However, under such conditions considerable loss of stemness markers existed (tables 1 and 2). It is possible that SW120 expression levels more closely reflect this loss of stemness despite continued division in culture indicated by EdU incorporation. Perhaps, σ2 receptor activity may possibly serve as a more sensitive index of differentiation potential of the given cell population relative to that of proliferative marker EdU. Alternatively, additional staining performed for ki-67 antigen, a known marker of cellular proliferation [33, 34], more closely mimicked the trends noted for SW120 across the given cell types (Fig S4) and may serve to reinforce the current case for σ2 receptor as a biomarker of cellular proliferation. However, if SW120 fluorescence were to be used as a direct correlate of SC proliferation, the observed inconsistency may impact in vivo application and warrant additional consideration.
Figure 5. EdU labeling among SW120 positive cells.
Percentage SW120 positive cells in population (black), Percentage EdU incorporation same population (white), * denotes late passage cells, AFSC: passages 35-40, BMSC: passages 20-25. Per given stem cell type, similar trends noted as corresponding increases and decreases in proportion of SW120 positive (σ2 positive) cells reflect changes in EdU incorporation (cellular proliferation). Levels were not directly comparable in all cases and EdU values were generally higher than SW120 positive cellular fractions.
Phentotypic Changes with Prolonged Culture
Late passage BMSC were noted as having significantly decreased levels of a number of characteristic markers (table 1) compared to early passage cells. Unlike a number of other typical BMSC markers (i.e., CD90, CD44, CD73), CD 146 has been identified as unique to BMSC that is not expressed by fibroblasts [35]. Ninety percent of early passage (P2) BMSCs were CD146 positive, while CD146 expression fell below the level of detection in late passage MSC (P25). This decrease in SW120 despite elevated EdU incorporation might reflect the caveat of a lineage directed cell type continuing to proliferate. We also observed elevated levels of MMP-1 and fibroblast surface protein (greater than 80% and 20%, respectively among passage 25 BMSC) that were previously less than 25% and undetectable, respectively in passage 2 BMSC (data not shown). Of note, the discrepancy between extent of SW120 and EdU incorporation was not observed upon continual in vitro culture of AFSC. Early and late passages of AFSCs presented with relatively comparable amounts of SW120 labeling, EdU incorporation and corresponding cell surface markers (table 1). The early and late passage AFSCs, expressed considerably higher levels of CD105 (44.37±3.6, early and 47.67±6.24, late) and CD117 (64.83±3.47, early and 56.87±8.46, late) and far lower levels of CD29 (4.08±0.9, early and 3.53± 0.63, late), CD44 (23.63±4.34, early and 28.28±5.77, late) and CD73 (below detection limit for both passages) than originally reported by De Coppi et. al. suggesting underlying donor variability [32]. Although, we did not confirm differentiation potential of our AFSC at such late passages, the constant levels of detection for SW120 labeling and EdU observed are consistent with reports of broadly multipotent AFSC capable of 250 population doublings [32].
σ2 Receptor ligand-induced apoptosis in stem cells
There was no evidence of significant induction of apoptosis as a result of SW120 incubation with SC. We observed less than 10% annexin positivity when AFSC, BMSC and NPC were incubated repeatedly or for prolonged incubation with σ2 receptor ligand, SW120 (Fig S2b). FACS revealed on average a 30% increase in apoptotic cells following incubation of C6 glioma cells with SW120. These results indicate that in contrast to tumor cells where σ2 receptor selective ligands have been suggested to be associated with lysosomal leakage [36, 37] and ligand-induced cell death [22, 38], no similar indication is apparent with SC. Additionally, following 48-hour serum starvation, an average increase in apoptosis of 10-15% was noted in AFSC, BMSC and NPC (FigS2c) with 30% of C6 tumor cells becoming apoptotic (Fig S2c).
Discussion
The major finding of this study was that σ2 receptor expression was found significantly in various SC (e.g., ESC, BMSC, AFSC, NPC and HSC) as compared to lineage restricted cells by confocal microscopy and on flow cytometric analysis. These results suggest the use of σ2 receptors for ascertaining the proliferative status of multipotent stem cells. The most accurate method of measuring cellular proliferation is direct assessment of DNA synthesis. Thus, we used incorporation of EdU, a thymidine analog, into cellular DNA, subsequent “Click” reaction with a fluorescent azide and FACS analysis. This approach has been reported comparable to the “gold standard” BrdU as a fast, sensitive and reproducible means of evaluating cellular proliferation [39, 40]. We observed an average of 70% of σ2 receptor-positive SC were also identified as EdU positive. Lastly evidence of proliferation antigen Ki-67 labeling [33, 34], was also found almost exclusively among SW120 positive cell fractions in all SC types (Fig S4). Furthermore, the direct association of σ2 receptors with active cellular proliferation reinforces the current understanding of σ2 receptor as a biomarker of cellular proliferation in tumor cells.
In addition, we have demonstrated that σ2 receptor ligands are taken up by clathrin-medicated endocytosis and are found in lysosomes as well as in the cytoplasm in SC. Similar findings have been observed with proliferating tumor cells (e.g., MDA-MB 435) in which σ2 receptor fluorescently tagged ligands were observed in endosomes and in mitochondria as well as being associated with endoplasma reticulum [29]. The inability to more thoroughly prevent endocytosis of the σ2 receptor ligand by incubating SC with phenylarsine oxide may be attributed to the potential for passive diffusion of a small lipophilic molecule such as SW120 as well as inability to achieve higher concentrations or longer incubation times in the presence of PAO without provoking excessive cell death.
In the current study, we also demonstrated a significant difference in σ2 receptor expression in SC populations as compared to lineage restricted cells under differentiation conditions. BMSC grown in media to induce apidogenesis demonstrated a significant decrease in MFI following incubation with SW120. NPCs grown in media that is used to stimulate neuro-differentation resulted in an almost complete reduction in MFI. We also observed a marked decline in overlap with the lysosomal marker in differentiating cells compared to parent SC, supporting the likelihood that there is less specific σ2 receptor mediated cellular uptake of SW120.
Conclusions
These results suggest the possibility of using the SW120 ligand or a modified multimodal σ2 receptor imaging probe to monitor SC differentiation during the course of stem cell therapy. Clinical diagnosis of the success of stem cell therapy is currently limited to invasive biopsy, histological examination and perhaps indirect observation of presumed functional improvement in the patient. As such, development of non-invasive in vivo assessment methods is currently paramount if widespread implementation of cellular therapy is to ever be realized.
Supplementary Material
Supplemental Material
Figure S1. Phenotypic changes upon long term in vitro culture and differentiation of BMSC.
a) BMSC passage 2, b) BMSC passage 25, c) Oil red O positive BMSC following 14 day adipogenesis. Scale bars: 100 μm.
Table S1. Summary of the Assays performed for SC in this Study.
Not all procedures could be carried out on ESC and HSC because of limited availability of the cell products. *NPC differentiation was performed in chamber slides for the purpose of confocal microscopy SW120/Lysotracker assay only. Normal human astrocyte cultures were substituted for differentiating NPC to astrocytes and were obtained from Lonza (Walkerville, MD) for flow cytometry analysis. HSC differentiation was implemented for flow cytometry assay under semi-solid colony forming conditions not suitable for confocal imaging.
Figure S2. Apoptosis noted upon σ2 receptor binding in tumor cells not evident in BMSC or AFSC.
a BMSC, AFSC, NPC and comparative tumor cell line C6 glioma were incubated with annexinV-alexa-647 only 15min, b) BMSC, AFSC, NPC and C6 glioma incubated SW120 10 nmol/L,30 min, followed by annexinV-alexa-647,15 min, c) 48 hour serum starvation of BMSC, AFSC, NPC and C6 glioma, followed by annexinV-alexa-647,15 min and propidium iodide (10 μg/ml) staining. Bars indicate level fluorescence intensity of unstained cells. Fluorescence detection as follows: FL1-A: SW120, FL2-A: propidium iodide, FL4-A: annexin V-alexa-647. All samples evaluated with flow cytometry, experiment done in triplicate, values normalized to MFI unstained cells in annexin V binding buffer.
Figure S3. Molecular structures of additional Sigma Receptor-specific ligands
a) SV-119, unconjugated σ2 receptor ligand and b) (+) Pentazocine, σ1 receptor ligand.
Figure S4. Ki-67 biomarker of proliferative status demonstrated similar activity to SW120
Percentage of SW120+ cells in population (white), percentage SW120+/ki-67 double positive same population (grey). (*) denotes BMSC under 14 day adipogenic conditions and MDA-MB435 has been included as reference tumor cell line. Flow cytometric analysis identified the SW120 positive cellular fractions across the indicated SC types as almost exclusively ki-67 antigen positive. All samples evaluated in triplicate with values normalized to MFI of unstained cells in stain buffer.
Acknowledgments
This work was supported by the Intramural Research Program in the Clinical Center at the National Institutes of Health.
Abbreviations
- HSC
hematopoietic stem cell
- ESC
embryonic stem cell
- BMSC
bone marrow stromal cell
- NPC
neural progenitor cell
- AFSC
amniotic fluid stem cell
- CFU
colony forming unit
- CFC
colony forming conditions
- EdU
5-ethynyl-2′-deoxyuridine
- Ki-67
antigen Ki-67 cellular marker of proliferation
- PAO
phenylarsine oxide
- MFI
mean fluorescence intensity
- σ2 receptor
sigma-2 receptor
- NBD
7-nitrobenzo-2-oxa-1,3-diazole
- SW120
NBD labeled σ2 receptor specific ligand
- SV119
unconjugated σ2 receptor ligand
Footnotes
Authors contributions: J.H.: manuscript writing, designed all experiments, collection and assembly of data, data analysis and interpretation, I.P.: provision of study materials, edited manuscript, A.C.: collection and assembly of data, data analysis and interpretation C.Z.: provision of study materials, edited the manuscript, R.M.: conception and design, edited manuscript, provision of study materials, J.F.: conception and design, financial support, supervised project, final approval of manuscript
Conflict of Interest: The σ2 receptor ligands described in this paper have been licensed from Washington University (RH Mach, inventor) by Isotrace Technologies, Inc., St Charles, MO.
References
- 1.Cao F, et al. Noninvasive de novo imaging of human embryonic stem cell-derived teratoma formation. Cancer Res. 2009;69(7):2709–13. doi: 10.1158/0008-5472.CAN-08-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swijnenburg RJ, et al. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008;17(6):1023–9. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barberi T, et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13(5):642–8. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Chen X. Imaging mesenchymal stem cell migration and the implications for stem cell-based cancer therapies. Future Oncol. 2008;4(5):623–8. doi: 10.2217/14796694.4.5.623. [DOI] [PubMed] [Google Scholar]
- 5.Pomper MG, et al. Serial imaging of human embryonic stem-cell engraftment and teratoma formation in live mouse models. Cell Res. 2009;19(3):370–9. doi: 10.1038/cr.2008.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi D, et al. Hepatocyte-like cells from human mesenchymal stem cells engrafted in regenerating rat liver tracked with in vivo magnetic resonance imaging. Tissue Eng Part C Methods. 2008;14(1):15–23. doi: 10.1089/tec.2007.0329. [DOI] [PubMed] [Google Scholar]
- 7.Morgul MH, et al. Tracking of primary human hepatocytes with clinical MRI: initial results with Tat-peptide modified superparamagnetic iron oxide particles. Int J Artif Organs. 2008;31(3):252–7. doi: 10.1177/039139880803100309. [DOI] [PubMed] [Google Scholar]
- 8.Leiker M, et al. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant. 2008;17(8):911–22. doi: 10.3727/096368908786576444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee ES, et al. Microgel iron oxide nanoparticles for tracking human fetal mesenchymal stem cells through magnetic resonance imaging. Stem Cells. 2009;27(8):1921–31. doi: 10.1002/stem.112. [DOI] [PubMed] [Google Scholar]
- 10.Toma C, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 11.Jackson KA, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107(11):1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo P. A lentiviral vector with a short troponin-I promoter for tracking cardiomyocyte differentiation of human embryonic stem cells. Gene Ther. 2008;15(3):161–70. doi: 10.1038/sj.gt.3303017. [DOI] [PubMed] [Google Scholar]
- 13.Orban TI, et al. Applying a “double-feature” promoter to identify cardiomyocytes differentiated from human embryonic stem cells following transposon-based gene delivery. Stem Cells. 2009;27(5):1077–87. doi: 10.1002/stem.45. [DOI] [PubMed] [Google Scholar]
- 14.Zhong JF, et al. A real-time pluripotency reporter for human stem cells. Stem Cells Dev. 19(1):47–52. doi: 10.1089/scd.2008.0363. [DOI] [PubMed] [Google Scholar]
- 15.Balakumaran A, et al. Superparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their “stemness”. PLoS One. 5(7):e11462. doi: 10.1371/journal.pone.0011462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin WR, et al. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197(3):517–32. [PubMed] [Google Scholar]
- 17.Vangveravong S, et al. Synthesis of N-substituted 9-azabicyclo[3.3.1]nonan-3alpha-yl carbamate analogs as sigma2 receptor ligands. Bioorg Med Chem. 2006;14(20):6988–97. doi: 10.1016/j.bmc.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Mach RH, Wheeler KT. Development of molecular probes for imaging sigma-2 receptors in vitro and in vivo. Cent Nerv Syst Agents Med Chem. 2009;9(3):230–45. doi: 10.2174/1871524910909030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mach RH, et al. Sigma 2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997;57(1):156–61. [PubMed] [Google Scholar]
- 21.Wheeler KT, et al. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br J Cancer. 2000;82(6):1223–32. doi: 10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashiwagi H, et al. Sigma-2 receptor ligands potentiate conventional chemotherapies and improve survival in models of pancreatic adenocarcinoma. J Transl Med. 2009;7:24. doi: 10.1186/1479-5876-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mach RH, Dehdashti F, Wheeler KT. PET Radiotracers for Imaging the Proliferative Status of Solid Tumors. PET Clin. 2009;4(1):1–15. doi: 10.1016/j.cpet.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott RE, Wille JJ, Jr, Wier ML. Mechanisms for the initiation and promotion of carcinogenesis: a review and a new concept. Mayo Clin Proc. 1984;59(2):107–17. doi: 10.1016/s0025-6196(12)60244-4. [DOI] [PubMed] [Google Scholar]
- 25.Tzen CY, et al. Differentiation, cancer, and anticancer activity. Biochem Cell Biol. 1988;66(6):478–89. doi: 10.1139/o88-060. [DOI] [PubMed] [Google Scholar]
- 26.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–28. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 27.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 584(17):3826–30. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 28.Dwyer RM, Kerin MJ. Mesenchymal stem cells and cancer: tumor-specific delivery vehicles or therapeutic targets? Hum Gene Ther. 21(11):1506–12. doi: 10.1089/hum.2010.135. [DOI] [PubMed] [Google Scholar]
- 29.Zeng C, et al. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2007;67(14):6708–16. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]
- 30.Kim HW, et al. Human Macrophage ATP7A is Localized in the trans-Golgi Apparatus, Controls Intracellular Copper Levels, and Mediates Macrophage Responses to Dermal Wounds. Inflammation. doi: 10.1007/s10753-011-9302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehling UM, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–12. [PubMed] [Google Scholar]
- 32.De Coppi P, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 33.Gerdes J, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–5. [PubMed] [Google Scholar]
- 34.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Halfon S, et al. Markers Distinguishing Mesenchymal Stem Cells from Fibroblasts Are Downregulated with Passaging. Stem Cells Dev. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 36.Ostenfeld MS, et al. Anti-cancer agent siramesine is a lysosomotropic detergent that induces cytoprotective autophagosome accumulation. Autophagy. 2008;4(4):487–99. doi: 10.4161/auto.5774. [DOI] [PubMed] [Google Scholar]
- 37.Groth-Pedersen L, et al. Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine. Cancer Res. 2007;67(5):2217–25. doi: 10.1158/0008-5472.CAN-06-3520. [DOI] [PubMed] [Google Scholar]
- 38.Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002;62(1):313–22. [PubMed] [Google Scholar]
- 39.Zeng C, et al. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 1319:21–32. doi: 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin G, et al. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;11(7):864–73. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material
Figure S1. Phenotypic changes upon long term in vitro culture and differentiation of BMSC.
a) BMSC passage 2, b) BMSC passage 25, c) Oil red O positive BMSC following 14 day adipogenesis. Scale bars: 100 μm.
Table S1. Summary of the Assays performed for SC in this Study.
Not all procedures could be carried out on ESC and HSC because of limited availability of the cell products. *NPC differentiation was performed in chamber slides for the purpose of confocal microscopy SW120/Lysotracker assay only. Normal human astrocyte cultures were substituted for differentiating NPC to astrocytes and were obtained from Lonza (Walkerville, MD) for flow cytometry analysis. HSC differentiation was implemented for flow cytometry assay under semi-solid colony forming conditions not suitable for confocal imaging.
Figure S2. Apoptosis noted upon σ2 receptor binding in tumor cells not evident in BMSC or AFSC.
a BMSC, AFSC, NPC and comparative tumor cell line C6 glioma were incubated with annexinV-alexa-647 only 15min, b) BMSC, AFSC, NPC and C6 glioma incubated SW120 10 nmol/L,30 min, followed by annexinV-alexa-647,15 min, c) 48 hour serum starvation of BMSC, AFSC, NPC and C6 glioma, followed by annexinV-alexa-647,15 min and propidium iodide (10 μg/ml) staining. Bars indicate level fluorescence intensity of unstained cells. Fluorescence detection as follows: FL1-A: SW120, FL2-A: propidium iodide, FL4-A: annexin V-alexa-647. All samples evaluated with flow cytometry, experiment done in triplicate, values normalized to MFI unstained cells in annexin V binding buffer.
Figure S3. Molecular structures of additional Sigma Receptor-specific ligands
a) SV-119, unconjugated σ2 receptor ligand and b) (+) Pentazocine, σ1 receptor ligand.
Figure S4. Ki-67 biomarker of proliferative status demonstrated similar activity to SW120
Percentage of SW120+ cells in population (white), percentage SW120+/ki-67 double positive same population (grey). (*) denotes BMSC under 14 day adipogenic conditions and MDA-MB435 has been included as reference tumor cell line. Flow cytometric analysis identified the SW120 positive cellular fractions across the indicated SC types as almost exclusively ki-67 antigen positive. All samples evaluated in triplicate with values normalized to MFI of unstained cells in stain buffer.