Abstract
A thorough understanding of the lymphatic system and its interaction with cancer cells is crucial to our ability to fight cancer metastasis. Efforts to study the lymphatic system had previously been limited by the inability to visualize the lymphatic system in vivo in real time. Fluorescence imaging can address these limitations and allow for visualization of lymphatic delivery and trafficking of cancer cells and possibly therapeutic agents as well. Here, we review recent articles in which antibody-fluorophore conjugates are used to label the lymphatic network and fluorescent proteins to label cancer cells in the evaluation of lymphatic delivery and imaging.
2. INTRODUCTION
The lymphatic system plays a critical role in oncology and is often the first step in cancer metastasis. Lymphatic involvement is a key component of cancer staging and adversely affects overall prognosis. The dynamics by which tumor cells enter the lymphatic system, travel to distant sites, and eventually establish metastatic foci, remain poorly understood. However, approaches to this enigmatic topic have grown over the past few decades to address two main purposes: first, to image lymphatic drainage in order to identify lymph nodes that may be involved in the metastatic process and thus assist clinicians in the staging of cancer, and second, to potentially deliver therapeutics via the lymphatic system to prevent or curtail lymphatic spread of cancer cells. These objectives may be achieved partly by the utilization of specific antibodies to lymphatic components or to cancer cells conjugated to fluorophores that allow imaging of the lymphatic system in a novel and accurate way.
3. ANTIBODY-BASED LYMPHATIC IMAGING
Identification of the sentinel node has long been understood to be important in the staging of many cancers, including breast cancer and melanoma [1]. Agents used in this endeavor include blue dyes, such as isosulfan blue dye, and colloidal radiotracers, mainly Technetium-99 sulfur colloid [2, 3]. Both of these agents are locally injected intradermally, rely on the natural uptake of molecules in the interstitial spaces by lymphatic vessels, and subsequently travel to the first lymph node draining this particular area. However, since they lack specificity, they tend to further extend to other lymph nodes as well. A newer agent named Lymphoseek was developed specifically to map the sentinel node by lymphoscintigraphy [4]. It is a molecule consisting of a dextran backbone to which several units of DPTA and mannose are attached. The DPTA units allow binding of Technetium-99m to allow for its detection with the use of a gamma probe, and the mannose units bind to receptors found on the surface of macrophages that tend to accumulate in the sentinel lymph node. In that sense, Lymphoseek is a radiolabeled reporter that has the advantage of being specific for the sentinel node. However, like all currently available tracers, by itself, it offers no information as to the presence of cancer cells in these sentinel nodes.
The use of specific antibodies for detection of lymphatic metastasis was first studied by Weinstein et al. [5, 6]. At the time, the authors established that monoclonal antibodies (Mab) injected intradermally entered the lymphatics, traveled to the regional lymph nodes, and accumulated in the metastatic lymph nodes. Their method of detection of the antibodies relied on radio-labeling. This immunolymphoscintigraphy model became a key element in several subsequent studies looking to further elucidate the process of lymphatic delivery of substances and tumor cell trafficking. Today, efforts to more clearly define this process are enhanced by the development of fluorescence imaging. For instance, Sharma and colleagues were able to quantitate lymphatic flow with the use of a near-infrared (NIR) fluorophore injected intradermally [7]. Fluorescence imaging has advantages over previous tracers such as blue dyes and colloid radiotracers in that it allows live, real-time imaging of the lymphatic system, and permits evaluation of lymphatic function as well. A combination of specific antibodies and fluorescence imaging should provide a deeper insight into the crucial interaction between the lymphatic system and cancer cells.
Although other agents are used to perform lymphatic mapping, they are non-specific and require local injection at the tumor site. Licha et al. sought to evaluate whether systemic, intravenous injection of a fluorophore-conjugated antibody directed against glycoproteins found on the luminal side of specialized high endothelial venules (HEV) in lymph nodes, such as MECA-79 antibody, would allow recirculation of this antibody to the peripheral lymph nodes [8]. After verifying its specificity via microautoradiography and organ distribution analysis, the authors conjugated MECA-79 antibody with a near-IR cyanine fluorescent dye with absorption and fluorescence peaks at 750 nm and 771 nm, respectively. A second conjugate with near identical properties was created that contained a control antibody instead. Both conjugates were injected intravenously via the tail vein at equal doses in nude mice. Fluorescence images were then obtained of both groups of mice at multiple time points.
The authors found that the MECA-79 antibody-fluorophore conjugate bound specifically to lymph nodes as early as 10 minutes after injection, with maximal contrast being achieved at 24 hours post-injection. By comparison, the control antibody-fluorophore conjugate failed to concentrate in lymph nodes on imaging. Lymph nodes were also collected from animals sacrificed 24 minutes after injection of the MECA-79 antibody cyanine dye conjugate. The tissues were semiquantitatively analyzed for fluorescence signal intensity, and as expected, displayed higher fluorescence intensities than nodes collected from mice injected with the control antibody dye conjugate. This study identifies MECA-79 antibody as an appropriate imaging probe for fluorescence imaging of lymph nodes.
While MECA-79 antibody allows homing of the contrast agents into lymph nodes, it is not directed against cancer cells, and is thus non-specific. To address this issue, Sampath and colleagues recently developed dual-labeled trastuzumab, a monoclonal antibody specific for the human epidermal growth factor receptor HER2 present on the surface of up to 30% of breast cancer [9]. HER2 is associated with a poor prognosis, and trastuzumab has been shown to have therapeutic value in the treatment of HER2-positive breast cancer [10]. In this study, the authors conjugated the antibody to both a nuclear radiotracer and a near-IR fluorophore, IRDye 800 [9]. The specificity of this compound was tested in vivo by both nuclear and fluorescence imaging of nude mice bearing HER2-overexpressing human breast cancer xenografts. The conjugate was injected intravenously, and under both imaging modalities, aggregated most strongly to the tumor region, as demonstrated by the strongest signals. When scintigraphy and fluorescence images were compared, the signal-to-noise ratio was found to be significantly greater in fluorescence imaging. This segment of the study demonstrated that cancer-specific antibodies can target cancer cells when delivered intravenously.
In the second part of the study, the authors sought to evaluate the potential of using antibody-based optical imaging for lymph node imaging. The compound was injected intradermally, and exhibited a similar pattern of lymphatic uptake and drainage as with fluorophore alone, albeit at different accumulation and clearance rates. However, the mice in which this was performed did not bear tumors, so that the use of this anti-HER2 antibody in this particular situation was non-specific. A similar experiment in a HER2-overexpressing-tumor-bearing mouse would be interesting in identifying tumor cell-bearing lymph nodes only.
4. ANTIBODY INHIBITION OF LYMPHATIC PROLIFERATION IN CANCER
Vascular endothelial growth factor (VEGF)-C expression has previously been shown to be associated with lymphangiogenesis and lymphatic metastasis via its interaction with both VEGF receptors 2 and 3 (VEGFR-2 and VEGFR-3) [11, 12]. Hoshida et al. carried out a set of elegant experiments designed to study the effects of VEGF-C on lymphatic development, morphology, and function, combining fluorescence lymphangiography and VEGFR-2 and VEGFR-3 specific antibodies [13]. In this study, fibrosarcoma and melanoma cells were stably engineered to over-express VEGF-C and transduced to express green fluorescent protein (GFP). The lymphatic vessels in the ear of nude mice were delineated and studied with both blue dye and fluorescence lymphangiography. Next, VEGF-C overexpressing fibrosarcoma and melanoma cells were implanted at the tip of the ear, and a number of parameters were measured. First, the authors demonstrated that these cells were associated with an increased incidence of lymphatic metastasis when compared with mock-transduced control cancer cells. This is likely partly due to VEGF-C-induced enlargement of peritumor lymphatic vessels, which were measured with epifluorescence and multiphoton laser scanning intravital microscopy. This finding was confirmed on histochemical staining with LYVE-1 and podoplanin antibodies (both are specific markers of lymphatic endothelial cells), and is responsible for an overall increased lymph flow rate. Furthermore, VEGF-C appears to create abnormal lymph flow patterns.
Using the same fluorescence imaging modality, the authors were able to acquire static images of GFP-expressing cells shed from the tumors into the peritumor lymphatics and eventually into the draining lymph node. Their finding revealed a 200-fold increase in tumor cell accumulation in the lymph node in tumors overexpressing VEGF-C compared to control tumors 10 days after tumor implantation, with no difference in migratory, proliferative, or survival properties among the cancer cells themselves.
The roles of VEGFR-2 and VEGFR-3 were further investigated in this study with the use of monoclonal antibodies. Interestingly, the authors identified a preventive role for VEGFR-3 blockade, while VEGFR-2 blockade may have a therapeutic role. Blockage of VEGFR-3 reduced lymphatic hyperplasia and tumor cell delivery to the lymphatic system, but failed to inhibit growth of cancer cells seeded in the lymph node. Blockade of VEGFR-2, on the other hand, successfully inhibited growth of both primary tumors and lymph node metastases.
5. IMAGING OF LYMPHATIC CANCER CELL TRAFFICKING IN VIVO
The dynamic processes by which cancer cells invade the lymphatic system and travel within the lymphatic vessels to the draining lymph node and between nodes remain poorly understood. We have developed real-time imaging of cancer cell trafficking in lymphatic vessels. Cancer cells dually-labeled with GFP in the nucleus and RFP in the cytoplasm or singly-labeled with GFP or RFP only were injected into the inguinal lymph node of nude mice. The labeled cancer cells trafficked through lymphatic vessels where they were imaged via a skin flap in real time at the cellular level until they entered the axillary lymph node. The bright fluorescence of the cancer cells and the real-time microscopic imaging capability of the Olympus OV100 small-animal imaging system enabled imaging of the trafficking cancer cells in the lymphatics. Using this imaging strategy, two different cancer cell lines, one expressing GFP and the other expressing RFP, were simultaneously injected in the inguinal lymph node. Fluorescence imaging readily distinguished the two color-coded cell lines and their different abilities to survive in the lymphatic system. Using this imaging technology, we also investigated the role of pressure on tumor-cell shedding into lymphatic vessels. Pressure was generated by placing 25- and 250-g weights for 10 s on the bottom surface of a tumor-bearing footpad [14]. Tumor cell fragments, single cells, and emboli shed from the footpad tumor were easily distinguished with the labeled cells and OV100 imaging system. Increasing pressure on the tumor increased the numbers of shed cells, fragments, and emboli. Pressure also deformed the shed emboli, increasing their maximum major axis. Imaging lymphatic trafficking of cancer cells can reveal critical steps of lymph node metastasis.
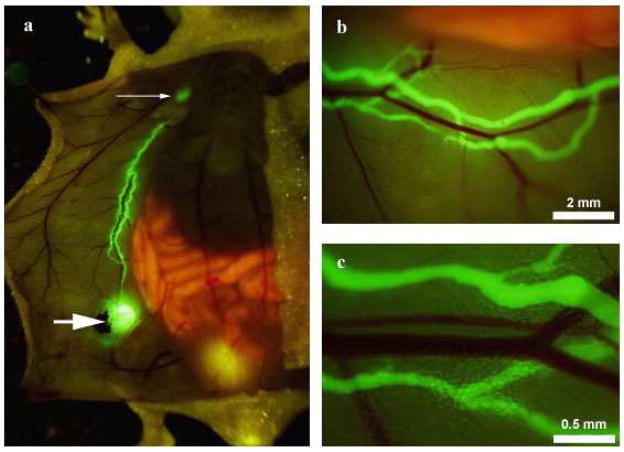
Recently, our laboratory published a study that combines fluorophore-conjugated lymphatic specific antibody LYVE-1 with fluorescent-protein-labeled cancer cells to achieve in vivo color-coded imaging of tumor cell trafficking in lymphatic vessels and lymph nodes [15]. LYVE-1 is a hyaluronic acid receptor that is specific for lymphatic endothelial cells, and anti-LYVE-1 antibodies had previously been used for immunohistochemical staining. In our study, anti-LYVE-1 antibodies were conjugated with AlexaFluor 488 fluorescent to image lymphatic vessels. A skin flap model in a nude mouse was performed to expose the inguinal and axillary lymph nodes and connecting lymphatic vessels. The conjugate was then directly injected into the inguinal lymph node, allowing strong, distinct, and long-lasting visualization of the draining inguinal lymphatics as well as the afferent and axillary lymphatics (Fig. 1). The signal persisted up to 48 hours and was strongest at 4 hours. In comparison, control conjugated IgG or FITC-dextran administration to the inguinal lymph node had much less durable signals, with no signal visible at 12 hours post-injection. Specificity of anti-LYVE-1-antibody was verified ex vivo by fluorescence microscopy.
Fig. 1.
In vivo fluorescence imaging of the ventral skin lymphatic system in a skin flap mouse model. AlexaFluor-conjugated anti-LYVE-1 antibody was injected into the inguinal lymph node (A, large arrow); under fluorescence imaging, the inguinal lymph node, afferent lymphatics, and axillary lymph node (A, small arrow) display a strong, durable fluorescent signal that did not stain adjacent blood vessels (B,C).
From ref. 15.
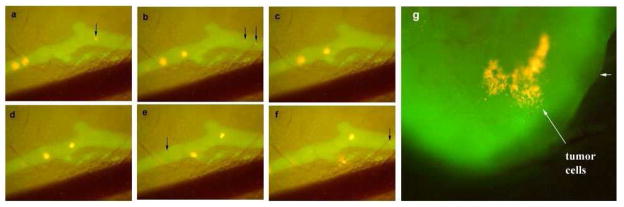
We next injected RFP-expressing XPA-1 pancreatic cancer cells into the inguinal lymph node and surrounding tissues. Fluorescence video imaging allowed real-time visualization of tumor cell trafficking through the lymphatic system. The contrast of RFP-expressing cancer cells on the bright green background of the lymphatic system afforded by AlexaFluor 488 made it easy to clearly observe the cancer cells in real time as they traveled from the inguinal node to the axillary node (Fig. 2). Our study was the first to demonstrate in vivo cancer cell trafficking in fluorescence lymphangiography [15]. This powerful tool may offer significant advantages in future studies of the interaction between the lymphatic system and cancer cells.
Fig. 2.
Time-sequential images of the same area of abdominal wall lymphatics (A-F) clearly show RFP-expressing XPA-1 cancer cells travel through the fluorescently labeled lymphatic vessel. These cells could be seen accumulating in the axillary lymph node (G). Again, the lymphatic system was highlighted by administration of AlexaFluor-conjugated anti-LYVE-1 antibody.
From ref. 15.
6. CONCLUSIONS
The unique interaction of cancer cells with the lymphatic network is a complicated process that includes the involvement of lymphatic drainage and metastasis. Our understanding of this process is enhanced with the use of fluorescence imaging. This review makes it clear that antibody-fluorophore conjugates and fluorescent-protein-labeled cancer cells can play an important role in understanding the interaction of cancer cells and the lymphatic system. MECA-79-antibody-fluorophore conjugates can identify lymph nodes, trastuzumab-fluorophore conjugates can localize specifically to HER2-expressing cancer cells, VEGFR-2 and VEGFR-3 antibodies can inhibit lymphatic hyperplasia and cancer cell seeding into the lymphatic system, and anti-LYVE-1-fluorophore conjugates can clearly delineate the lymphatic system and allow in vivo visualization of RFP-expressing cancer cells trafficking. The use of dual-photon imaging and red-shifted fluorophores can increase the depth capabilities of fluorescence imaging allowing more non-invasive imaging of the interaction of cancer cells and the lymphatic system. The combination of these technologies with target-specific antibodies should provide us with an ever-growing understanding of the critical interplay between cancer cells and the lymphatic system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. J Nucl Med. 2004;45:1509–1518. [PubMed] [Google Scholar]
- 2.Cochran AJ, Essner R, Rose DM, Glass EC. Principles of sentinel lymph node identification: background and clinical implications. Langenbecks Arch Surg. 2000;385:252–260. doi: 10.1007/s004230000143. [DOI] [PubMed] [Google Scholar]
- 3.Mariani G, Moresco L, Viale G, Villa G, Bagnasco M, Canavese G, Buscombe J, Strauss HW, Paganelli G. Radioguided sentinel lymph node biopsy in breast cancer surgery. J Nucl Med. 2001;42:1198–1215. [PubMed] [Google Scholar]
- 4.Wallace AM, Hoh CK, Vera DR, Darrah DD, Schulteis G. Lymphoseek: a molecular radiopharmaceutical for sentinel node detection. Ann Surg Onc. 2003;10:531–538. doi: 10.1245/aso.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein JN, Parker RJ, Keenan AM, Dower SK, Morse HC, III, Sieber SM. Monoclonal antibodies in the lymphatics: toward the diagnosis and therapy of tumor metastases. Science. 1982;218:1334–1337. doi: 10.1126/science.7146917. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JN, Stellar MA, Keenan AM, Covell DG, Key ME, Sieber SM, Oldham RK, Hwang KM, Parker RJ. Monoclonal antibodies in the lymphatics: selective delivery to lymph node metastases of a solid tumor. Science. 1983;222:423–426. doi: 10.1126/science.6623082. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Wang W, Rasmussen JC, Joshi A, Houston JP, Adams KE, Cameron A, Ke S, Kwon S, Mawad ME, Sevick-Muraca EM. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol. 2007;292:H3109–H3118. doi: 10.1152/ajpheart.01223.2006. [DOI] [PubMed] [Google Scholar]
- 8.Licha K, Debus N, Emig-Vollmer S, Hofmann B, Hasbach M, Stibenz D, Sydow S, Schirner M. Optical molecular imaging of lymph nodes using a targeted vascular contrast agent. J Biomed Opt. 2005;10:41205. doi: 10.1117/1.2007967. [DOI] [PubMed] [Google Scholar]
- 9.Sampath L, Wang W, Sevick-Muraca EM. Near infrared fluorescent optical imaging for nodal staging. J Biomed Opt. 2008;13:41312. doi: 10.1117/1.2953498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeon CH, Pegram MD. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Invest New Drugs. 2005;23:391–409. doi: 10.1007/s10637-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 12.Kadambi A, Mouta Carreira C, Yun CO, Padera TP, Dolmans DE, Carmeliet P, Fukurama D, Jain RK. Vascular endothelial growth factor (VEGF)-C differentially affects tumor vascular function and leukocyte recruitment: role of VEGF-receptor 2 and host VEGF-A. [PubMed] [Google Scholar]
- 13.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Jiang P, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007;67:8223–8228. doi: 10.1158/0008-5472.CAN-07-1237. [DOI] [PubMed] [Google Scholar]
- 15.McElroy M, Hayashi K, Garmy-Susini B, Kaushal S, Varner JA, Moossa AR, Hoffman RM, Bouvet M. Fluorescent LYVE-1 antibody to image dynamically lymphatic trafficking of cancer cells in vivo. J Surg Res. 2009;151:68–73. doi: 10.1016/j.jss.2007.12.769. [DOI] [PMC free article] [PubMed] [Google Scholar]