Abstract
The objective of this work was to delineate the effect of hydrophilic and hydrophobic polymeric additives on sol–gel transition and release profile of timolol maleate (TM) from poly (ethylene glycol)–poly (ε-caprolactone)– poly (ethylene glycol) (PEG–PCL–PEG)-based thermosensitive hydrogel. Polycaprolactone (hydrophobic additive) and polyvinyl alcohol (PVA) (hydrophilic additive) reduced critical gel concentration of PEG–PCL–PEG triblock polymer. The effect of PCL on sol–gel transition was more pronounced than PVA. However, with PCL no statistically significant difference in release profile was observed. The effect of PVA on release profile was more pronounced, which reduced the cumulative percentage release of TM from 86.4±0.8% to 73.7±1.8% over 316 h. Moreover, cytotoxicity of the hydrogel was also investigated utilizing rabbit primary corneal epithelial culture cells. No significant cytotoxicity of hydrogel alone or in presence of additives was observed. So, polymeric additive strategy serves as a valuable tool for optimizing TM release kinetics from PEG–PCL–PEG hydrogel matrix.
Keywords: Hydrogel, Sol–gel transition, Drug release, Polymeric additive
Introductions
Hydrogel composed of biodegradable polymers such as polylactide (PLA), poly (dl-lactide-co-glycolide) (PLGA), or polycaprolactone (PCL) has wide application in the field of drug delivery due to excellent biocompatibility of these polymers with living tissues [1–3]. These systems provide controlled drug release via diffusion as well as degradation mechanisms [4–6]. Several articles have reported the development of thermoreversible hydrogels composed of poly (ethylene glycol) (PEG) as a hydrophilic block and PLA, PLGA, or PCL as a hydrophobic block [7–14].
PCL is a biodegradable and biocompatible polymer approved by FDA for application in drug delivery systems and medical devices [15, 16]. Recent studies have explored the applications of PCL-based hydrogels in sustained release formulations [3, 17–20]. PCL-based triblock polymer, i.e., poly (ethylene glycol)–poly (ε-caprolactone)–poly (ethylene glycol) (PEG–PCL–PEG) remains in solution state at room temperature (25 °C) and forms a transparent gel at body temperature (37 °C). This change in physical state is rapid and reversible, which makes thermosensitive hydrogel an attractive means for drug delivery [21]. This unique thermoreversible property could be utilized for sustained delivery of timolol maleate (TM) in the treatment of ocular hypertension or glaucoma.
Currently, the only non-invasive mode of treating glaucoma requires once or twice daily administration. PCL-based hydrogel formulation of TM could eliminate daily dosing, thereby improving therapy and patient compliance. Topical beta-blockers, particularly TM is one of the major drug for treating glaucoma and ocular hypertension because of their excellent intra-ocular-pressure-lowering efficacy. TM is a selective β-adrenergic blocker and is widely indicated in the treatment of glaucoma [22–24]. Typically, drug release from a hydrogel depends initially on diffusion. In later time points it depends upon a combination of diffusion and degradation mechanisms [4]. Drug release from PEG–PCL–PEG hydrogel depends primarily on the diffusion mechanism due to extremely slow degradation rate of PCL. It also has the limitation of burst release due to a porous matrix. Such initial burst release can provide high drug levels causing systemic and local toxicities [25, 26]. In attempts to achieve sustain release and lower burst release of TM from the hydrogel, we have utilized a novel polymeric additive strategy.
Earlier studies focused on synthesis and release of hydrophilic and hydrophobic model drugs from PEG–PCL–PEG thermosensitive hydrogel [27]. However, it has been observed that hydrogel applications in therapeutic delivery are limited by burst release due to porous structure and frontal diffusion processes. So in this study we evaluated effects of hydrophilic and hydrophobic polymeric additives on sol–gel transition and drug release from hydrogel. Hydrogel matrix is porous in nature and TM molecules can diffuse through the pores of polymeric chains. Polymeric additives can easily fit into the spaces of a hydrogel matrix and modify drug release rate.
PCL was selected as a hydrophobic and polyvinyl alcohol (PVA) as a hydrophilic additive. These additives are already approved by FDA for application in human [15]. The rationale behind selecting PCL and PVA as additives depends on their molecular property to provide better packing of the hydrogel matrix through intra- and intermolecular interactions between the triblock polymeric chains.
Hence, the objective of this study was to delineate the effects of polymeric additives with hydrophilic and hydrophobic properties on the gelling behavior and release profiles of TM from PCL-based triblock polymer. In addition, the effects of parameters such as drug loading and molecular weight of polymer on the release profile of TM were also investigated.
Material and methods
Materials
Timolol maleate, monomethoxy poly (ethylene glycol) (Mw=550 and 750), ε-caprolactone, stannous octoate, polyvinyl alcohol, and polycaprolactone diol (PCL) were obtained from Sigma chemical company (St. Louis, MO). Hexamethylene diisocyanate (HMDI) was procured from Acros organics (Morris Plains, NJ). All chemicals were used without any further purification.
Methods
Synthesis of triblock polymer PEG–PCL–PEG
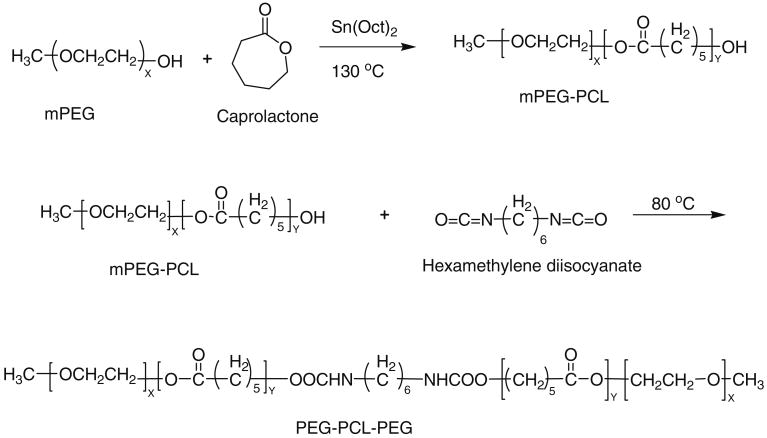
PEG–PCL diblock polymer was synthesized by ring opening polymerization of ε-caprolactone utilizing either monomethoxy poly (ethylene glycol) (mPEG) 750 or 550 as an initiator and stannous octoate as the catalyst. The diblock polymer was further coupled using HMDI as a coupling agent to synthesize the triblock polymer PEG–PCL–PEG. The resulting polymer was purified by dissolving in methylene chloride followed by precipitation from diethyl ether.
Briefly, the synthesis of PEG–PCL–PEG (Fig. 1) was carried out as follows: the mPEG was dried under vacuum for 3 h before copolymerization. Then calculated amount of mPEG (0.01 mol), ε-caprolactone (0.1 mol), and stannous octoate (0.5 wt.%) were added to the reaction mixture. The reaction was performed for 24 h at 130 °C. Then coupling of diblock polymer was allowed to proceed by adding HMDI (0.01 mol) and the reaction was continued for 6 h at 60 °C. The final product was dissolved in 20 ml of methylene chloride and purified by fractional precipitation with petroleum ether. The purified polymer product was vacuum dried for 48 h to remove residual solvent.
Fig. 1. Synthetic scheme of PEG–PCL–PEG.
Characterization of polymeric material
-
NMR
1H NMR spectroscopy was performed to characterize the composition of polymer. Spectra were recorded by dissolving polymeric material in CDCl3 and then analyzing the proton NMR spectra recorded using Varian-400 NMR instrument.
-
Gel permeation chromatography analysis
Gel permeation chromatography (GPC) analysis was performed with the refractive index detector (Waters 410) to determine molecular weight and its distribution. Polymeric material (2 mg/ml) was dissolved in Tetrahydrofuran (THF). Analysis was performed with THF as eluting solvent at a flow rate of 1 ml/min utilizing Styragel HR-3 column maintained at 35 °C. Polystyrenes with narrow molecular weight distribution were used as standards for GPC analysis.
-
Fourier transform infrared spectroscopy
The Fourier transform infrared spectroscopy (FTIR) spectra were recorded with a Nicolet-100 infrared spectrophotometer at a resolution of 4 s−1. Polymer was dissolved in methylene chloride and casted on KBr plates.
Characterization of thermosensitive gel
-
Sol–gel transition
Sol–gel transition studies were performed by test tube inversion method. First, the polymer was solubilized in PBS by storing overnight at 4 °C. Then 0.5 ml of polymer solution in 4 ml tube was placed in water bath and temperature was increased from 20 to 60 °C with 1 °C increment at each step. The gel formation was characterized visually by inverting the tube. A physical state of flow was characterized as sol phase, whereas a state of no flow was characterized as gel phase. Similar studies were performed by incorporating PCL or PVA (5 wt.%) as an additive to the hydrogel matrix. Viscosities of hydrogel alone and in the presence of additives were determined by cone and plate viscometer at 750 rpm and 37 °C.
-
Scanning electron microscopy
Aqueous polymeric solutions of PCEC II alone and with additives were allowed to gel at 37 °C and then lyophilized for 48 h. The lyophilized hydrogels were sputtered with gold/palladium at 0.6 kV. The samples were examined by FEG ESEM XL 30 electron microscope.
In vitro release
TM dissolved in PEG–PCL–PEG triblock polymeric aqueous solution (0.4 ml) was injected into 10 ml vial with 12-mm internal diameter. Vials were kept in shaker bath at 37 °C with a stroke rate of 30 rpm. The polymeric solution was allowed to gel for 2 min. Then 5 ml of the release medium containing phosphate buffer (pH 7.4) and sodium azide (0.02 wt.%) were added in order to prevent microbial contamination during a release study. The entire release medium was replaced with fresh buffer at predetermined intervals to mimic the sink condition. Samples were stored at −20 °C until further analysis. The release samples were analyzed by high-performance liquid chromatography with a phenomex C18 column at a flow rate of 1 ml/min. The mobile phase was composed of 20 mM phosphate buffer with pH adjusted to 2.5. The analyte was measured at 294 nm. All experiments were conducted in triplicate.
Effect of molecular weight
mPEG of two different molecular weights, i.e., mPEG 550 and 750 were utilized to delineate the effect of molecular weight on release kinetics of TM from the gel matrix. Release study was performed with 25 wt.% of hydrogel with 1.0 wt.% drug loading.
Effect of drug loading
The effect of drug loading on the release behavior was evaluated with two different concentrations of TM (0.5 and 1 wt.%) in the 25 wt.% hydrogel. A method as described previously was followed for release study.
Effect of PVA as additive
PVA (Mw=30,000–70,000) was used as a hydrophilic additive to modulate TM release rate. It was incorporated along with drug and polymer during hydrogel preparation. Incorporation of PVA up to 5 wt.% concentration in hydrogel matrix retained reversible sol–gel transition. Therefore, optimized concentration (5 wt.%) of additive was incorporated in 20 wt.% hydrogel with 1% drug loading.
Effect of PCL as additive
PCL of low molecular weight (Mw=550) was utilized as a hydrophobic additive. PCL was selected to explore the role of hydrophobic interactions. In order to compare the effect of hydrophobic and hydrophilic additives on sol–gel transition and release profile of TM, similar methods followed for PVAwere utilized for the hydrogel preparation and release study.
Drug release mechanisms
Drug release parameters were computed by three different methods utilizing First order, Higuchi, and Korsmeyer equations. Release data were fitted into the model equations in order to determine release mechanism.
First order equation:
Mt represents the cumulative amount of drug released at time t, k is the release rate constant obtained by plotting LnMt against time.
Higuchi equation:
KH denotes the Higuchi release rate constant obtained by plotting cumulative percent drug released against the square root of time.
Korsmeyer–Peppas equation:
Mt and M∞ represent the cumulative amount of drug released at time t and at the equilibrium, respectively. The constant k is the kinetic constant and n is the release exponent that indicates the drug release mechanism. Values of n<0.5 indicates Fickian (ideal) diffusion mechanism and values of 0.5<n<1.0 suggest non-Fickian diffusion. When value of n is greater than 1.0, it represents case II transport or zero-order release kinetics. Drug release data is employed to obtain the release exponent for Mt/M∞≤0.6.
Cytotoxicity studies
Cell viability studies were performed utilizing MTS assays in which novel tetrazolium compound, 3-(4, 5-dimethylthiazole-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was reduced into a colored formazan product by live cells. Rabbit primary corneal epithelial culture cells (rPCEC) were grown in the culture media comprising MEM, 10% FBS, HEPES, sodium bicarbonate, penicillin, and streptomycin sulfate. Cells were maintained under a humidified atmosphere of 5% CO2 at 37 °C and then subcultured and seeded in 96-well culture plates at a density of 10,000 cells/well. After 24 h incubation, fresh media containing hydrogels of different concentrations ranging from 0.5 to 20 mg/ml were added alone and with 5 wt.% (PVA or PCL) polymeric additives. Triton X-100 (0.1%) was used as positive control while the blank (without treatment) was considered as negative control. Following 24 h incubation, 100 μL serum-free medium containing 20 μL of MTS solution was added to the 96 well plates and were incubated at 37 °C and 5% CO2 for 4 h. After incubation, the absorbance of each well was measured at 450 nm by an ELISA plate reader. Absorbance was directly proportional to viability of cells which was calculated by following equation.
Statistical analysis
All release experiments were conducted in triplicates and the results were reported as mean ± standard deviation. Statistical analysis of the effect of additives, molecular weight, and drug loading on release rates were compared by one-way ANOVA. Statistical package for social science version 11 was used to compare mean of each group. A level of p<0.05 was considered statistically significant in all cases.
Result and discussion
Synthesis and characterization of PEG–PCL–PEG
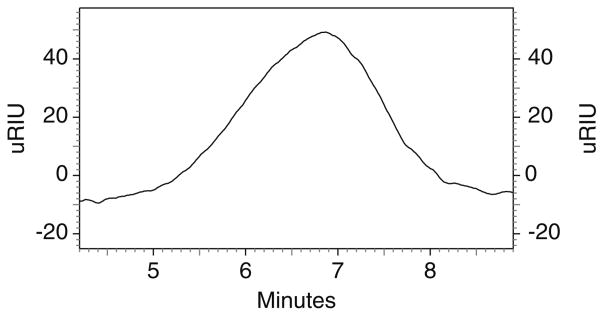
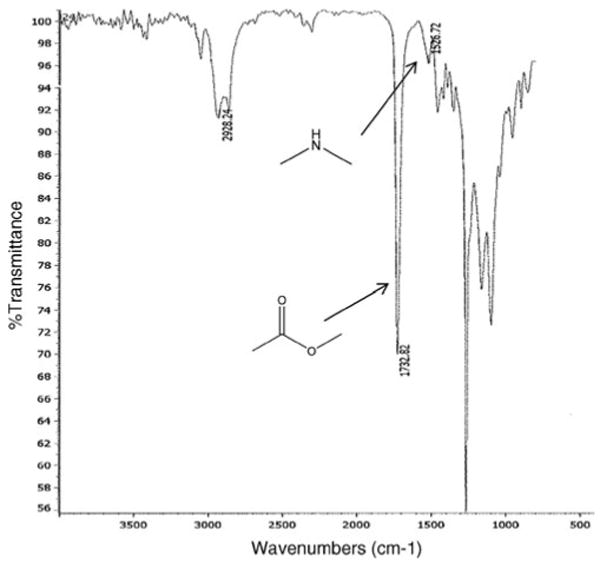
PEG–PCL–PEG was synthesized by ring opening polymerization reaction utilizing stannous octoate as the catalyst. 1H NMR spectra (Fig. 2) shows two main peaks at 3.60 (– OCH2CH2–) and 4.06 (–OCH2CH2CH2CH2CH2CH2CO–) that corresponds to mPEG and PCL, respectively, confirmed the formation of triblock polymer. Gel permeation chromatography analysis (Fig. 3) also points to narrow polydispersity between 1.41 and 1.47 (Table 1) and molecular weight between 5,000 and 7,000. FTIR spectrum (Fig. 4) indicated appearance of absorption band at 1,526 cm−1 characteristic of −NH group and at 1,732 cm−1 characteristic of C = O group, confirms the formation of triblock polymer. Absence of absorption band at 2,270–2,285 cm−1 due to −NCO stretching confirms complete conjugation of HMDI.
Fig. 2. 1H-NMR spectra of PECE copolymer in CDCl3.
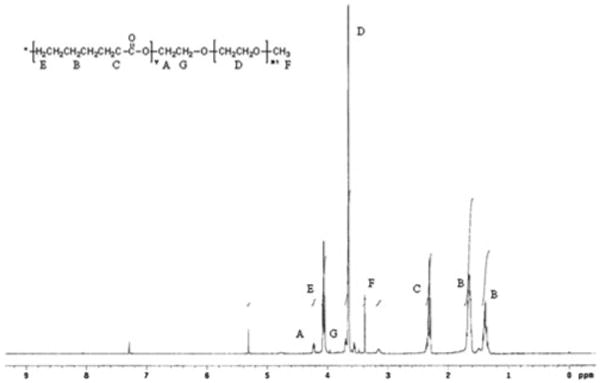
Fig. 3. Gel permeation chromatogram of PCEC II.
Table 1. Molecular weight distribution.
| Polymer | Total Mn (theoretical) | Mn | Mw | Polydispersity |
|---|---|---|---|---|
| PEG750–PCL3750–PEG750 | 5,250 | 4,568 | 6,754 | 1.47 |
| PEG550–PCL2420–PEG550 | 3,500 | 3,995 | 5,640 | 1.41 |
Fig. 4. FTIR spectrum of PEG–PCL–PEG.
Sol–gel transition studies
Sol–gel transition studies revealed that PEG550– PCL2420–PEG550 (PCEC I) has 20 wt.% critical gelling concentration (CGC) whereas PEG750–PCL3750–PEG750 (PCEC II) has CGC of 25 wt.%. Such difference in CGC can be attributed to a difference in the hydrophilicity–hydrophobicity ratio between the two polymers. This observation is in agreement with findings of other investigators [21]. In this study, we evaluated the role of both hydrophilic and hydrophobic polymeric additives on sol–gel transition studies. Addition of PVA and PCL increased the viscosity of hydrogel and improved the gelling efficiency possibly by providing better packing of the gel matrix. We observed that viscosity of 25 wt.% PCEC II hydrogel alone was 0.51±0.03 poise whereas incorporation of 5 wt.% PCL and PVA in 20 wt.% PCEC II has increased the viscosity of hydrogels to 0.83±0.02 and 2.59±0.04 poise, respectively. As shown in Fig. 5a and b, both hydrophilic and hydrophobic additives have lowered the CGC of PCEC II. Addition of PVA (Fig. 5a) in PCEC II decreased CGC from 25 to 22 wt.%. Previous studies reported that incorporation of PEG in the PCL-based hydrogel lowers the CGC [27]. However, addition of PCL (Fig. 5b) decreased CGC to 18 wt.%. We observed more pronounced effect of PCL on sol–gel transition in comparison to PVA on PCEC II because PCL is a hydrophobic polymer and promotes better micellar aggregation for the formation of gel. However, addition of PVA or PCL in PCEC I did not produce any significant change in the sol–gel transition phenomenon. PCEC I was relatively more hydrophobic gel due to lower molecular weight of PEG block in comparison to PCEC II and have higher micellar aggregation tendency. Therefore, incorporation of additives did not further alter hydrophobic aggregation, which is the primary mechanism responsible for sol–gel transition.
Fig. 5.
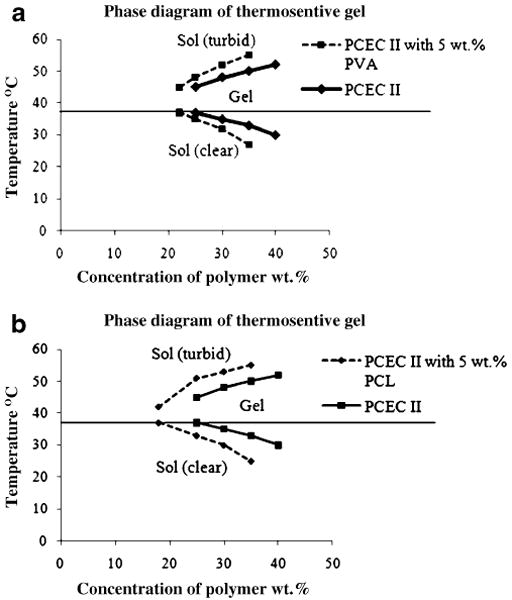
Sol–gel transition phase diagram. a PCEC II triblock copolymer aqueous solutions alone and with 5 wt.% PVA; b PCEC II triblock copolymer aqueous solutions alone and with 5 wt.% PCL
Images in Fig. 6 illustrate that additives also reduced the transparency of the gel, which confirmed the formation of a denser gel matrix. Figure 7 shows the scanning electron microscopy (SEM) of PCEC II alone and with PCL or PVA as additives. Figure 7a represents the porous matrix of hydrogel alone at 37 °C. Addition of PVA provided less porous surface morphology (Fig. 7c) than PCEC II alone. We speculate that high-molecular-weight PVA uniformly covers the porous matrix of hydrogel and thereby may reduce the diffusion of drug molecules from the hydrogel matrix. However, addition of PCL promoted the aggregation of polymeric chains at 37 °C but did not alter the porosity of the hydrogel matrix (Fig. 7b) due to its low molecular weight.
Fig. 6.
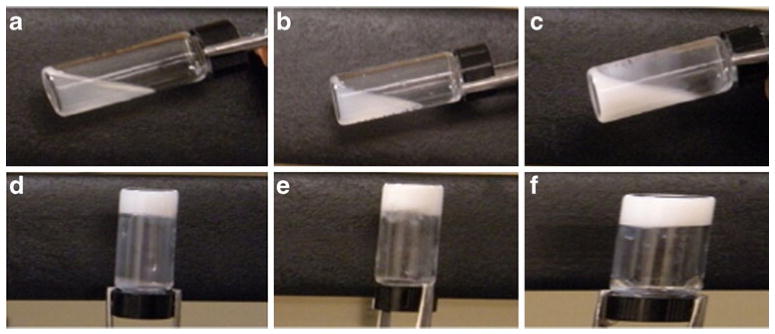
Photograph of PCEC II at 25 °C a alone, b with 5 wt.% PVA, and c with 5 wt.% PCL and at 37 °C d alone, e with 5 wt.% PVA, and f with 5 wt.% PCL
Fig. 7.
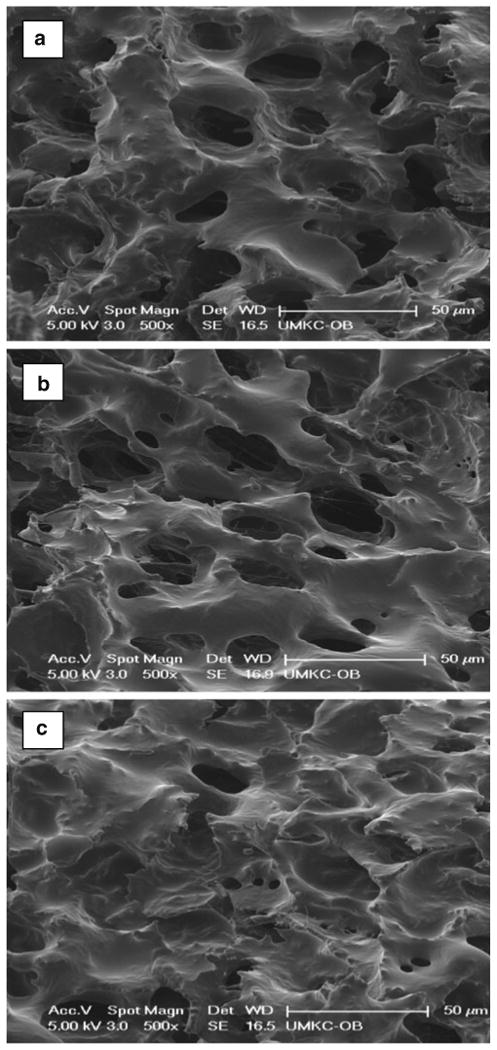
SEM of PCEC II a alone, b with 5 wt.% PCL, c with 5 wt.% PVA
In vitro release studies
We investigated the effect of different parameters such as molecular weight, drug loading, hydrogel concentration, and polymeric additives on the release profile of TM form the hydrogel matrix.
Effect of molecular weight
Release of TM from the hydrogel matrix depended on the molecular weight of polymer (p<0.05) as shown in Fig. 8a. Release rate of TM was slower with PCEC II (Mw=5,250) than PCEC I (Mw=3,500). For instance, in the first 48 h cumulative percentage drug release from PCEC I was 78.0± 2.8% whereas in PCEC II it was 41.3±2.3%. The reason for this behavior can be attributed to higher molecular weight of PCEC II with relatively longer PCL block length that may have restricted the permeation of water molecules across the polymeric matrix. Hence, slower hydration of gel resulted in decreased diffusion coefficient of the drug across the hydrogel matrix. These results suggest that the release rate of TM may have been affected by two major factors, i.e., hydrophilic/hydrophobic balance and total molecular weight of the polymer. TM (pKa 9.2) exists predominantly in ionized from at pH 7.4. We have observed that addition of PVA or PCL did not change the pH (7.4) of aqueous polymeric solution and pH of release medium was constant due to extremely slow degradation of PCL.
Fig. 8.
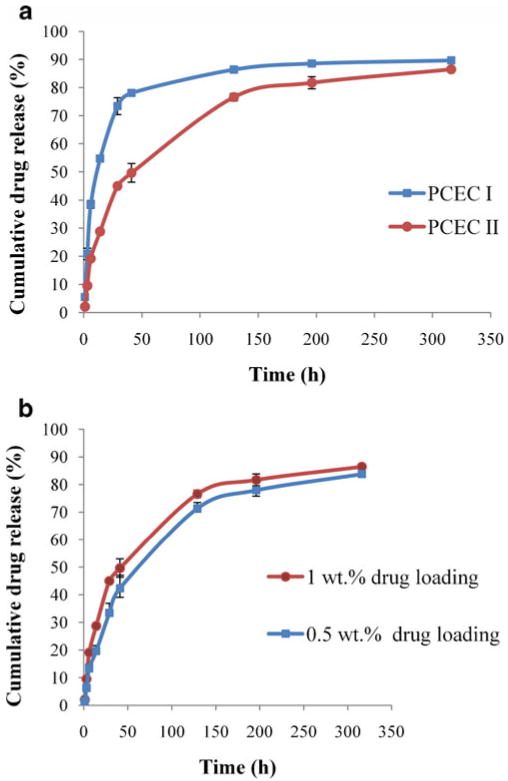
Effect of molecular weight and drug loading. a release of TM from PCEC I
 and PCEC II
and PCEC II
 triblock copolymer (25 wt. %) hydrogel; b release of TM
triblock copolymer (25 wt. %) hydrogel; b release of TM
 0.5 and
0.5 and
 1.0 wt.% from PCEC II triblock copolymer (25 wt.%) hydrogel in PBS buffer (pH 7.4) at 37 °C. The values are represented as mean ± standard deviation of n=3
1.0 wt.% from PCEC II triblock copolymer (25 wt.%) hydrogel in PBS buffer (pH 7.4) at 37 °C. The values are represented as mean ± standard deviation of n=3
Effect of drug loading and hydrogel concentration
Figure 8b suggested that no statistically significant difference in release rate of TM was observed with two different concentrations, i.e., 0.5 to 1.0 wt.% in 25 wt.% PCEC II hydrogel, due to higher aqueous solubility of TM. Other studies also reported minor change in release rates of hydrophilic molecules with higher drug content in the hydrogel [5, 28]. It was reported that physicochemical property of the active drug such as charge, hydrophilicity, and molecular size also affects the release rate [29–31]. Hydrophilic drug molecules tend to partition in the PEG domain of the hydrogel whereas hydrophobic drug molecules partition into PCL core. Therefore, TM disperses in PEG domain and easily diffused out in the release medium irrespective of drug loading.
Hydrogel concentration played a significant role in TM release. Release of TM from 25 wt.% polymer is slower than 20 wt.% due to volume effect of gel (data not shown). Since 25 wt.% hydrogel forms a dense matrix, 20 wt.% was selected for the incorporation of different polymeric additives to observe the effect of polymeric additives on the diffusion process.
Effect of polyvinyl alcohol as additive
PVA is a long-chain synthetic polymer incorporated in ophthalmic formulations as additive. It acts as a wetting agent and reduces surface and interfacial tension [32]. Moreover, addition of PVA is advantageous due to its biocompatibility with ocular tissues. Addition of PVA may reduce the burst release by promoting hydrophobic aggregation of the polymer chains in the gel matrix. Therefore, we have selected PVA as an additive. Figure 9a illustrates that release of TM was altered by PVA (p<0.05). Incorporation of PVA in the hydrogel matrix lowered TM release from 86.4±0.8% to 73.7±1.8% in 316 h. Moreover, it was observed that burst release in initial 24 h significantly minimized to 22.3±1.9% from 44.9±0.4% in presence of PVA. The effect of different percentage of PVA on release profile of TM was also evaluated. In case of 1 wt.% PVA any significant difference was not noticed in the release profile (data not shown). However, as shown in Fig. 9a, 5 wt.% PVA caused drug release rate from hydrogel to be significantly reduced in the initial phase. Slower drug release from 5 wt.% PVA added hydrogel may be accounted by restricted diffusion of TM across less porous hydrogel matrix. We hypothesize that PVA being a hydrophilic polymer tends to localize with hydrophilic block of the hydrogel where majority of TM molecules are dispersed. Higher percentage of PVA favors micellar aggregation due to presence of polar hydroxyl groups. This mechanism of aggregation was also evaluated previously. A study reported that PVA as additive promoted the gelling of methylcellulose based hydrogel. This study suggested the role of PVA as a cosolute, which was dominant over pseudo-surfactancy effect of other additives [33]. Therefore, in this study, long-chain PVA molecules had successfully modulated the drug release profile.
Fig. 9.
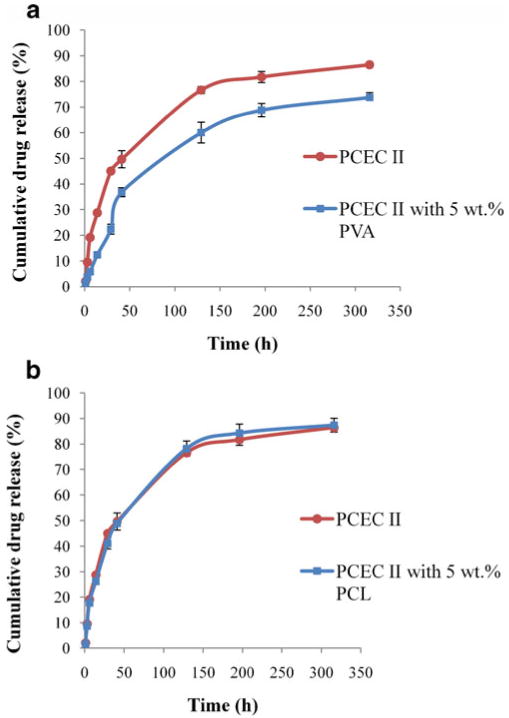
Effect of polymeric additives. a release of TM from PCEC II (25 wt.%)
 and PCEC II (20 wt.%) with 5 wt.% PVA
and PCEC II (20 wt.%) with 5 wt.% PVA
 ; b release of TM from PCEC II (25 wt.%)
; b release of TM from PCEC II (25 wt.%)
 and PCEC II (20 wt.%) with 5 wt.% PCL
and PCEC II (20 wt.%) with 5 wt.% PCL
 hydrogel in PBS buffer (pH 7.4) at 37 °C. The values are represented as mean ± standard deviation of n=3
hydrogel in PBS buffer (pH 7.4) at 37 °C. The values are represented as mean ± standard deviation of n=3
Effect of polycaprolactone as additive
Release of TM from hydrogel depends on diffusion- and degradation-mediated processes. However, degradation of PCL-based hydrogel is extremely slow [34]. In order to modulate diffusion-mediated release by hydrophobic additive, low-molecular-weight PCL (Mw=550), was incorporated in the hydrogel matrix. In this study, first we tried to incorporate the high-molecular-weight PCL but due to more hydrophobicity it was not soluble in the hydrogel polymeric matrix. Therefore, we utilized low-molecular-weight PCL, which can be easily solubilized in the hydrogel solution. To elucidate the role of PCL and compare the TM release profile with PVA added hydrogel, 5 wt.% PCL was selected as additive for the release study. As shown in Fig. 9b, release rate was not affected by the addition of PCL and no statistically significant difference was observed with 5 wt.% PCL. We presume that PCL being a hydrophobic polymer tends to partition with the hydrophobic block of hydrogel. However, as we discussed earlier majority of TM disperses in the hydrophilic block, addition of PCL did not significantly modulate the drug release profile. Other attempts of dissolving higher percentage of PCL remain unsuccessful due to excessive hydrophobic interactions.
Drug release kinetics
Drug release from hydrogel generally follows diffusion/degradation or a combination [4]. Analysis of drug release kinetics, as shown in Table 2, correlated well with Higuchi and Korsmeyer models. This observation suggests that drug release depended primarily on diffusion from the hydrogel matrix rather than on bulk erosion process of the polymer (first order). In addition, analysis of first 60% release data, according to Korsmeyer model suggested that addition of polymeric additives (PVA or PCL) can significantly lower the release rate constant. However, the effect of PVA on release kinetics was more pronounced than PCL. In hydrogel alone, KKP value was 3.03 whereas with PVA, KKP was found to be 1.368 and for PCL it was 2.455. A diffusion exponent 0.685<n<0.869 suggests anomalous diffusion mechanism from all the matrices.
Table 2. Kinetic parameters.
| Polymer | First order | Higuchi | Korsmeyer–Peppas | ||||
|---|---|---|---|---|---|---|---|
| r2 | ko (h−1) | r2 | kH (h−1/2) | r2 | KKP (h−n) | n | |
| PCCE-I | 0.298 | 0.0046±0.0002 | 0.702 | 4.433±0.107 | 0.923 | 7.889±0.578 | 0.685 |
| PCEC II | 0.425 | 0.0071±0.0001 | 0.923 | 5.154±0.067 | 0.941 | 3.033±0.108 | 0.819 |
| PCEC II + PCL | 0.428 | 0.0078±0.0001 | 0.933 | 5.382±0.185 | 0.933 | 2.455±0.165 | 0.869 |
| PCEC II + PVA | 0.560 | 0.0092±0.0002 | 0.961 | 4.811 ±0.195 | 0.995 | 1.368±0.184 | 0.852 |
Cytotoxicity studies
Cell viability response showed that PCEC II alone and in presence of 5% additives is not toxic to rPCEC cells. Figure 10 suggested that cytotoxicity of hydrogel was concentration dependent. However, at low concentrations no statistically significant difference from control was observed. Cell viability studies suggest that polycaprolactone-based hydrogels alone and with polymeric additives are biocompatible with the corneal epithelial cells. Similar results were reported with human lens epithelial cells [35].
Fig. 10.
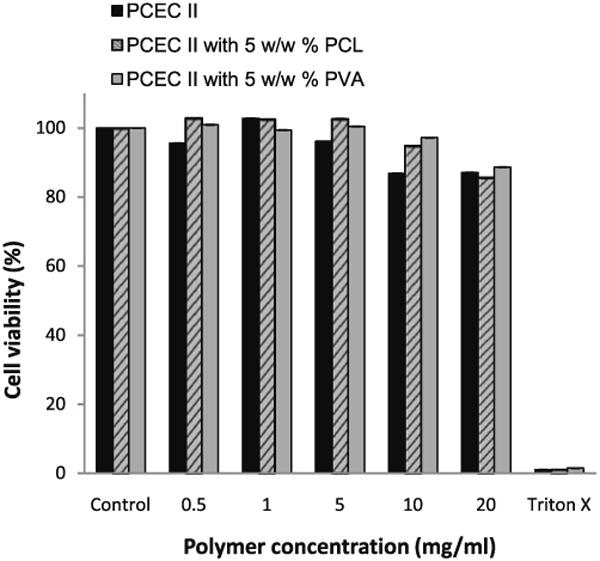
Rabbit primary corneal epithelial culture cell viability study. Cell survival decreased with increase of concentration of PECE hydrogel. The values are represented as mean ± standard deviation of n=6
Conclusions
PEG–PCL–PEG of different compositions were synthesized. The additives strategy was applicable to modulate the sol–gel transition and TM release kinetics from hydrogel. Effect of PCL on the CGC was more pronounced than PVA. Release of TM was sustained longer in case of high-molecular-weight PCEC II. PVA as additive played a dominant role in regulating drug release kinetics whereas no significant difference in drug release was observed with PCL. Cell viability studies confirmed that hydrogel alone or in presence of polymeric additives was biocompatible to corneal epithelial cells. Therefore, additive-based drug delivery may be a promising strategy for optimizing sustained drug delivery systems in the treatment of ocular hypertension and glaucoma.
Acknowledgments
This study was supported by NIH R01EY09171-14 and a grant from Saint Luke's Hospital Foundation, Kansas City
References
- 1.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garripelli VK, Kim JK, Namgung R, Kim WJ, Repka MA, Jo S. A novel thermosensitive polymer with pH-dependent degradation for drug delivery. Acta Biomater. 6(2):477–485. doi: 10.1016/j.actbio.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh DP, Nguyen MK, Pi BS, Kim MS, Chae SY, Lee KC, Kim BS, Kim SW, Lee DS. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials. 2008;29(16):2527–2534. doi: 10.1016/j.biomaterials.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Wu DQ, Chu CC. Biodegradable hydrophobic–hydrophilic hybrid hydrogels: swelling behavior and controlled drug release. J Biomater Sci Polym Ed. 2008;19(4):411–429. doi: 10.1163/156856208783719536. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wu X, Han Y, Mo F, Duan Y, Li S. Novel thymopentin release systems prepared from bioresorbable PLA-PEG-PLA hydrogels. Int J Pharm. 386(1–2):15–22. doi: 10.1016/j.ijpharm.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 6.Soares JS, Zunino P. A mixture model for water uptake, degradation, erosion and drug release from polydisperse polymeric networks. Biomaterials. 31(11):3032–3042. doi: 10.1016/j.biomaterials.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Li S, Ghzaoui AE, Nouailhas H, Zhuo R. Synthesis and gelation properties of PEG–PLA–PEG triblock copolymers obtained by coupling monohydroxylated PEG–PLA with adipoyl chloride. Langmuir. 2007;23(5):2778–2783. doi: 10.1021/la0629025. [DOI] [PubMed] [Google Scholar]
- 8.Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG–PLGA–PEG triblock copolymers. J Control Release. 2000;63(1–2):155–163. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Seo KS, Khang G, Cho SH, Lee HB. Preparation of poly(ethylene glycol)-block-poly(caprolactone) copolymers and their applications as thermo-sensitive materials. J Biomed Mater Res A. 2004;70(1):154–158. doi: 10.1002/jbm.a.30049. [DOI] [PubMed] [Google Scholar]
- 10.Kan P, Lin XZ, Hsieh MF, Chang KY. Thermogelling emulsions for vascular embolization and sustained release of drugs. J Biomed Mater Res B Appl Biomater. 2005;75(1):185–192. doi: 10.1002/jbm.b.30286. [DOI] [PubMed] [Google Scholar]
- 11.Kang YM, Lee SH, Lee JY, Son JS, Kim BS, Lee B, Chun HJ, Min BH, Kim JH, Kim MS. A biodegradable, injectable, gel system based on MPEG-b-(PCL-ran-PLLA) diblock copolymers with an adjustable therapeutic window. Biomaterials. 31(9):2453–2460. doi: 10.1016/j.biomaterials.2009.11.115. [DOI] [PubMed] [Google Scholar]
- 12.Wei X, Gong C, Gou M, Fu S, Guo Q, Shi S, Luo F, Guo G, Qiu L, Qian Z. Biodegradable poly(epsilon-caprolactone)-poly (ethylene glycol) copolymers as drug delivery system. Int J Pharm. 2009;381(1):1–18. doi: 10.1016/j.ijpharm.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Qiao M, Chen D, Ma X, Liu Y. Injectable biodegradable temperature-responsive PLGA–PEG–PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1–2):103–112. doi: 10.1016/j.ijpharm.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Pieper R, Webster DC, Singh J. Triblock copolymers: synthesis, characterization, and delivery of a model protein. Int J Pharm. 2005;288(2):207–218. doi: 10.1016/j.ijpharm.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Mohan N, Nair PD. Polyvinyl alcohol-poly(caprolactone) semi IPN scaffold with implication for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;84(2):584–594. doi: 10.1002/jbm.b.30906. [DOI] [PubMed] [Google Scholar]
- 16.Rohner D, Hutmacher DW, Cheng TK, Oberholzer M, Hammer B. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J Biomed Mater Res B Appl Biomater. 2003;66(2):574–580. doi: 10.1002/jbm.b.10037. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z, Hao J, You Y, Liu Y, Wang Z, Deng X. Biodegradable and thermoreversible hydrogels of poly(ethylene glycol)-poly(epsilon-caprolactone-co-glycolide)-poly(ethylene glycol) aqueous solutions. J Biomed Mater Res A. 2008;87(1):45–51. doi: 10.1002/jbm.a.31699. [DOI] [PubMed] [Google Scholar]
- 18.Jones DS, McLaughlin DW, McCoy CP, Gorman SP. Physicochemical characterisation and biological evaluation of hydrogel-poly(epsilon-caprolactone) interpenetrating polymer networks as novel urinary biomaterials. Biomaterials. 2005;26(14):1761–1770. doi: 10.1016/j.biomaterials.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Hua F, Lee DS. Thermoreversible gelation of biodegradable poly(epsilon-caprolactone) and poly(ethylene glycol) multiblock copolymers in aqueous solutions. J Control Release. 2001;73(2–3):315–327. doi: 10.1016/s0168-3659(01)00297-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Cao M, Li H, Li L, Xu W. Synthesis and characterization of thermo-sensitive semi-IPN hydrogels based on poly(ethylene glycol)-co-poly(epsilon-caprolactone) macromer, N-isopropylacrylamide, and sodium alginate. Carbohydr Res. 345(3):425–431. doi: 10.1016/j.carres.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Hwang MJ, Suh JM, Bae YH, Kim SW, Jeong B. Caprolactonic poloxamer analog: PEG–PCL–PEG. Biomacromolecules. 2005;6(2):885–890. doi: 10.1021/bm049347a. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman TJ, Kaufman HE. Timolol, dose response and duration of action. Arch Ophthalmol. 1977;95(4):605–607. doi: 10.1001/archopht.1977.04450040071009. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman TJ, Kaufman HE. Timolol. A beta-adrenergic blocking agent for the treatment of glaucoma. Arch Ophthalmol. 1977;95(4):601–604. doi: 10.1001/archopht.1977.04450040067008. [DOI] [PubMed] [Google Scholar]
- 24.Peeters A, Schouten JS, Severens JL, Hendrikse F, Prins MH, Webers CA. Latanoprost versus timolol as first choice therapy in patients with ocular hypertensionA cost-effectiveness analysis. Acta Ophthalmol. doi: 10.1111/j.1755-3768.2009.01857.x. [DOI] [PubMed] [Google Scholar]
- 25.Munroe WP, Rindone JP, Kershner RM. Systemic side effects associated with the ophthalmic administration of timolol. Drug Intell Clin Pharm. 1985;19(2):85–89. doi: 10.1177/106002808501900201. [DOI] [PubMed] [Google Scholar]
- 26.Cheong HI, Johnson J, Cormier M, Hosseini K. In vitro cytotoxicity of eight beta-blockers in human corneal epithelial and retinal pigment epithelial cell lines: comparison with epidermal keratinocytes and dermal fibroblasts. Toxicol In Vitro. 2008;22(4):1070–1076. doi: 10.1016/j.tiv.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Gong CY, Dong PW, Shi S, Fu SZ, Yang JL, Guo G, Zhao X, Wei YQ, Qian ZY. Thermosensitive PEG–PCL–PEG hydrogel controlled drug delivery system: sol–gel–sol transition and in vitro drug release study. J Pharm Sci. 2009;98(10):3707–3717. doi: 10.1002/jps.21694. [DOI] [PubMed] [Google Scholar]
- 28.Huynh DP, Im GJ, Chae SY, Lee KC, Lee DS. Controlled release of insulin from pH/temperature-sensitive injectable pentablock copolymer hydrogel. J Control Release. 2009;137(1):20–24. doi: 10.1016/j.jconrel.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Kim SW, Bae YH, Okano T. Hydrogels: swelling, drug loading, and release. Pharm Res. 1992;9(3):283–290. doi: 10.1023/a:1015887213431. [DOI] [PubMed] [Google Scholar]
- 30.Henke M, Brandl F, Goepferich AM, Tessmar JK. Size-dependent release of fluorescent macromolecules and nanoparticles from radically cross-linked hydrogels. Eur J Pharm Biopharm. 74:184–192. doi: 10.1016/j.ejpb.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Makino K, Hiyoshi J, Ohshima H. Effects of thermosensitivity of poly (N-isopropylacrylamide) hydrogel upon the duration of a lag phase at the beginning of drug release from the hydrogel. Colloids Surf B Biointerfaces. 2001;20(4):341–346. doi: 10.1016/s0927-7765(00)00201-0. [DOI] [PubMed] [Google Scholar]
- 32.Reddy IK, Aziz W, Sause RB. Artificial tear formulations, irrigating solutions and contact lens products. In: Reddy IK, editor. Ocular therapeutics and drug delivery: a multi-disciplinary approach. Technomic; Lancaster: 1996. pp. 171–212. [Google Scholar]
- 33.Kundu PKM, Sinha M, Choe S, Chattopadhayay D. Effect of alcoholic, glycolic, and polyester resin additives on the gelation of dilute solution (1%) of methylcellulose. Carbohydr Polym. 2003;51:57–61. [Google Scholar]
- 34.Jiang Z, Hao J, You Y, Gu Q, Cao W, Deng X. Biodegradable thermogelling hydrogel of P(CL-GL)–PEG–P (CL-GL) triblock copolymer: degradation and drug release behavior. J Pharm Sci. 2009;98(8):2603–2610. doi: 10.1002/jps.21613. [DOI] [PubMed] [Google Scholar]
- 35.Yin H, Gong C, Shi S, Liu X, Wei Y, Qian Z. Toxicity evaluation of biodegradable and thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J Biomed Mater Res B Appl Biomater. 92(1):129–137. doi: 10.1002/jbm.b.31498. [DOI] [PubMed] [Google Scholar]